Magnetic Resonance Imaging of Multiple Cerebral and Spinal Cavernous Malformations of a Patient with Dementia and Tetraparesis
Abstract
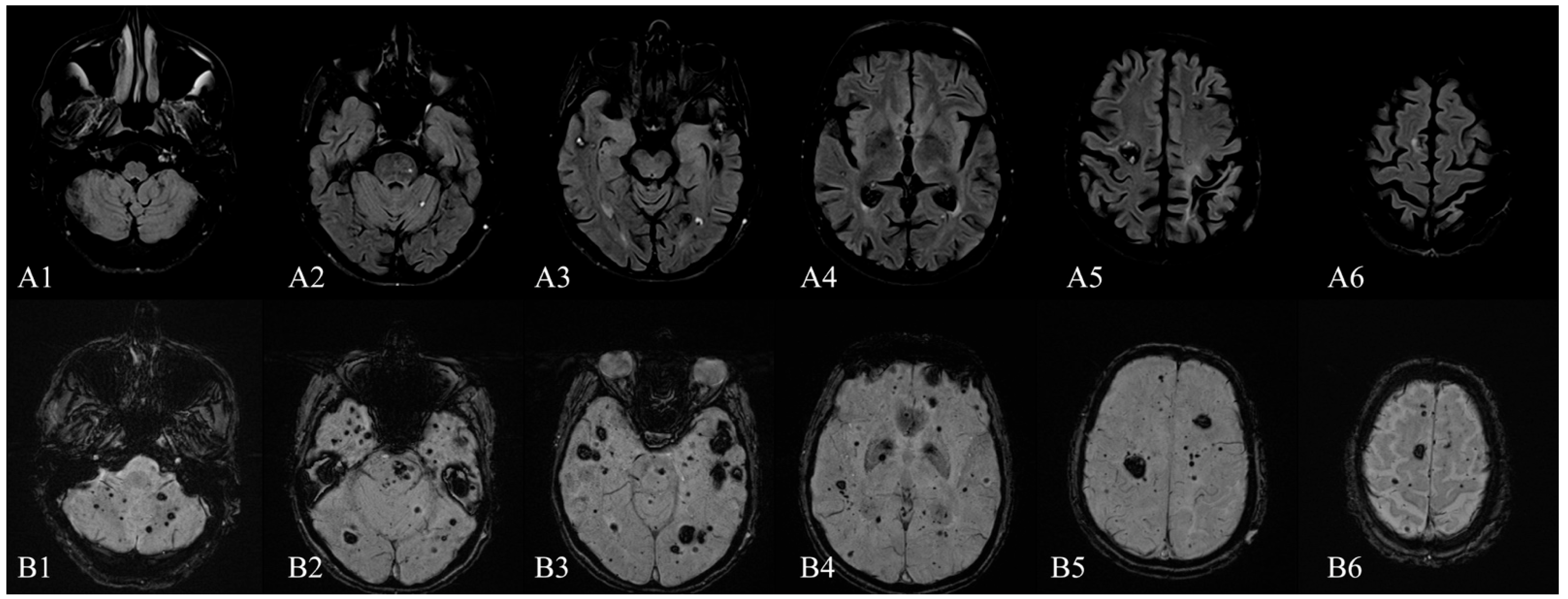
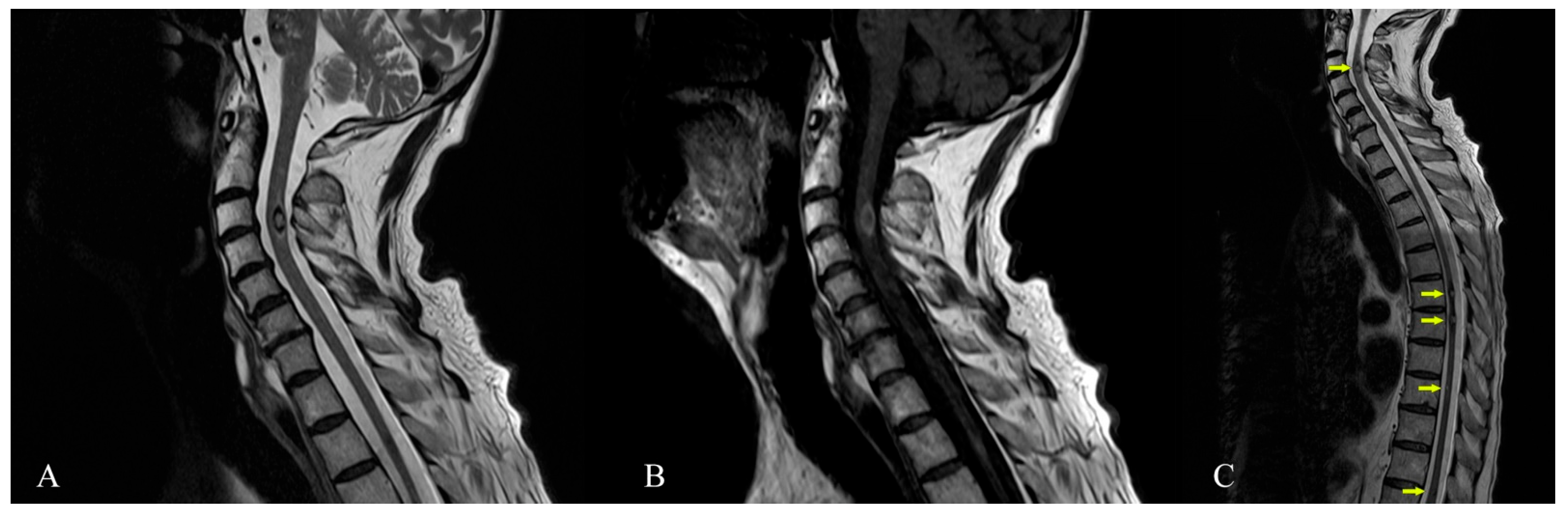
:


Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Campbell, P.G.; Jabbour, P.; Yadla, S.; Awad, I.A. Emerging clinical imaging techniques for cerebral cavernous malformations: A systematic review. Neurosurg. Focus 2010, 29, E6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Coban, A.; Gurses, C.; Bilgic, B.; Sencer, S.; Karasu, A.; Bebek, N.; Baykan, B.; Hepgul, K.T.; Gokyigit, A. Sporadic multiple cerebral cavernomatosis: Report of a case and review of literature. Neurologist 2008, 14, 46–49. [Google Scholar] [CrossRef] [PubMed]
- Trentadue, M.; Mucelli, R.P.; Piovan, E.; Pizzini, F.B. Incidental optochiasmatic cavernoma: Case report of an unusual finding on 3 Tesla MRI. Neuroradiol. J. 2016, 29, 289–294. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gastelum, E.; Sear, K.; Hills, N.; Roddu, E.; Dominica, R.; Chettout, N.; Hess, C.; Cotter, J.; Daphne, H.-K.; Fullerton, H.; et al. Rates and characteristics of radiographically detected intracerebral cavernous malformations after cranial radiation therapy in pediatric cancer patients. J. Child Neurol. 2015, 30, 842–849. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zabramski, J.M.; Henn, J.S.; Coons, S. Pathology of cerebral vascular malformations. Neurosurg. Clin. N. Am. 1999, 10, 395–410. [Google Scholar] [CrossRef]
- Mespreuve, M.; Vanhoenacker, F.; Lemmerling, M. Familial multiple cavernous malformation syndrome: MR features in this uncommon but silent threat. J. Belgian Soc. Radiol. 2016, 100, 51. [Google Scholar]
- Cisneros, O.; Rehmani, R.; Garcia de de Jesus, K. Cerebellar cavernous malformation (Cavernoma): A case report. Cureus 2019, 11, e4371. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Toll, A.; Parera, E.; Gimenez-Arnau, A.M.; Pou, A.; Lloreta, J.; Limaye, N.; Vikkula, M.; Pujol, R.M. Cutaneous venous malformations in familial cerebral cavernomatosis caused by KRIT1 gene mutations. Dermatology 2009, 218, 307–313. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kivelev, J.; Laakso, A.; Niemela, M.; Hernesniemi, J. A proposed grading system of brain and spinal cavernomas. Neurosurgery 2011, 69, 807–814. [Google Scholar] [CrossRef] [PubMed]
- Parsch, D.; Freund, M.; Gerner, H. Complete paraplegia due to multiple intracerebral and spinal cavernomas. Spinal Cord 1999, 37, 62–64. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cohen-Gadol, A.A.; Jacob, J.T.; Edwards, D.A.; Krauss, W.E. Coexistence of intracranial and spinal cavernous malformations: A study of prevalence and natural history. J. Neurosurg. 2006, 104, 376–381. [Google Scholar] [CrossRef] [PubMed]
- Batra, S.; Lin, D.; Recinos, P.F.; Zhang, J.; Rigamonti, D. Cavernous malformations: Natural history, diagnosis and treatment. Nat. Rev. Neurol. 2009, 5, 659–670. [Google Scholar] [CrossRef] [PubMed]
- Mouchtouris, N.; Chalouhi, N.; Chitale, A.; Starke, R.M.; Tjoumakaris, S.I.; Rosenwasser, R.H.; Jabbour, P.M. Management of cerebral cavernous malformations: From diagnosis to treatment. Sci. World J. 2015, 2015, 808314. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zabramski, J.M.; Wascher, T.M.; Spetzler, R.F.; Johnson, B.; Golfinos, J.; Drayer, B.P.; Brown, B.; Rigamonti, D.; Brown, G. The natural history of familial cavernous malformations: Results of an ongoing study. J. Neurosurg. 1994, 80, 422–432. [Google Scholar] [CrossRef] [PubMed]
- Zafar, A.; Quadri, S.A.; Farooqui, M.; Ikram, A.; Robinson, M.; Hart, B.L.; Mabray, M.C.; Vigil, C.; Tang, A.T.; Kahn, M.L.; et al. Familial cerebral cavernous malformations. Stroke 2019, 50, 1294–1301. [Google Scholar] [CrossRef] [PubMed]
- Schneble, H.-M.; Soumare, A.; Hervé, D.; Bresson, D.; Guichard, J.-P.; Riant, F.; Tournier-Lasserve, E.; Tzourio, C.; Chabriat, H.; Stapf, C. Antithrombotic therapy and bleeding risk in a prospective cohort study of patients with cerebral cavernous malformations. Stroke 2012, 43, 3196–3199. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schwammenthal, Y.; Kurnik, D. Letter by Kurnik et al regarding Article, “Antithrombotic therapy and bleeding risk in a prospective cohort study of patients with cerebral cavernous malformations”. Stroke 2013, 44, e52. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nikoubashman, O.; Rocco, F.D.; Davagnanam, I.; Mankad, K.; Zerah, M.; Wiesmann, M. Prospective hemorrhage rates of cerebral cavernous malformations in children and adolescents based on MRI appearance. Am. J. Neuroradiol. 2015, 36, 2177–2179. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Akers, A.; Salman, R.A.-S.; Awad, I.A.; Dahlem, K.; Flemming, K.; Hart, B.; Kim, H.; Jusue-Torres, I.; Kondziolka, D.; Lee, C.; et al. Synopsis of guidelines for the clinical management of cerebral cavernous malformations: Consensus recommendations based on systematic literature review by the Angioma Alliance Scientific Advisory Board Clinical Experts Panel. Neurosurgery 2017, 80, 665–680. [Google Scholar] [CrossRef] [Green Version]
- Sun, I.; Pamir, M.N. Spinal cavernomas: Outcome of surgically treated 10 patients. Front. Neurol. 2017, 8, 672. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ohnishi, Y.; Nakajima, N.; Takenaka, T.; Fujiwara, S.; Miura, S.; Terada, E.; Yamada, S.; Kishima, H. Conservative and surgical management of spinal cord cavernous malformations. World Neurosurg. X 2020, 5, 100066. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.S.; Kim, K.H.; Lee, M.H.; Lee, J.-I. Stereotactic radiosurgery for brainstem cavernous malformations: An updated systematic review and meta-analysis. World Neurosurg. 2019, 130, e648–e659. [Google Scholar] [CrossRef] [PubMed]
- Amin-Hanjani, S.; Ogilvy, C.S.; Candia, G.J.; Lyons, S.; Chapman, P.H. Stereotactic radiosurgery for cavernous malformations: Kjellberg’s experience with proton beam therapy in 98 cases at the Harvard Cyclotron. Neurosurgery 1998, 42, 1229–1236. [Google Scholar]
- Ghosh, R.; Dubey, S.; Roy, D.; Lahiri, D.; Ray, B.K.; Finsterer, J. Superficial siderosis due to multiple cavernomas: An uncommon cause of early-onset dementia. Psychogeriatrics 2021, 21, 434–437. [Google Scholar] [CrossRef]
- Ohara, K.; Shinjo, T.; Nishii, R.; Takeda, K.; Kokai, M.; Morita, Y. A case of intra-axial multiple cavernous angiomas, presented with dementia and cerebellar signs. Seishin Shinkeigaku Zasshi 2002, 104, 585–594. [Google Scholar] [PubMed]
- Kageyama, Y.; Kodama, Y.; Yamamoto, S.; Tadano, M.; Ichikawa, K. A case of multiple intracranial cavernous angiomas presented with dementia and parkinsonism—clinical and MRI study for 10 years. Rinsho Shinkeigaku 2000, 40, 1105–1109. [Google Scholar] [PubMed]
- Blanco-Rojas, L.; Arboix, A.; Canovas, D.; Grau-Olivares, M.; Carles Oliva Morera, J.; Parra, O. Cognitive profile in patients with a first-ever lacunar infarct with and without silent lacunes: A comparative study. BMC Neurol. 2013, 13, 203. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Holmes, G.L. Cognitive impairment in epilepsy: The role of network abnormalities. Epileptic Disord. 2015, 17, 101–116. [Google Scholar] [CrossRef]
- Lawrence, A.J.; Zeestraten, E.A.; Benjamin, P.; Lambert, C.P.; Morris, R.G.; Barrick, T.R.; Markus, H.S. Longitudinal decline in structural networks predicts dementia in cerebral small vessel disease. Neurology 2018, 90, 1898–1910. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, L.; Biesbroek, J.M.; Shi, L.; Liu, W.; Kuijf, H.J.; Chu, W.W.; Abrigo, J.M.; Lee, R.K.; Leung, T.W.; Lau, A.Y.; et al. Strategic infarct location for post-stroke cognitive impairment: A multivariate lesion-symptom mapping study. J. Cereb. Blood Flow Metab. 2018, 38, 1299–1311. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.H.; Park, J.S.; Ahn, H.-J.; Seo, S.W.; Lee, J.-M.; Kim, S.T.; Han, S.-H.; Na, D.L. Voxel-based analysis of diffusion tensor imaging in patients with subcortical vascular cognitive impairment: Correlates with cognitive and motor deficits. J. Neuroimaging 2011, 21, 317–324. [Google Scholar] [CrossRef] [PubMed]
- Duering, M.; Gesierich, B.; Seiler, S.; Pirpamer, L.; Gonik, M.; Hofer, E.; Jouvent, E.; Duchesnay, E.; Chabriat, H.; Ropele, S.; et al. Strategic white matter tracts for processing speed deficits in age-related small vessel disease. Neurology 2014, 82, 1946–1950. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Selden, N.R.; Gitelman, D.R.; Salamon-Murayama, N.; Parrish, T.B.; Mesulam, M.M. Trajectories of cholinergic pathways within the cerebral hemispheres of the human brain. Brain 1998, 121, 2249–2257. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, G.H.; Lee, J.H.; Seo, S.W.; Ye, B.S.; Cho, H.; Kim, H.J.; Noh, Y.; Yoon, C.W.; Chin, J.H.; Oh, S.J.; et al. Seoul criteria for PiB(−) subcortical vascular dementia based on clinical and MRI variables. Neurology 2014, 82, 1529–1536. [Google Scholar] [CrossRef] [PubMed]
- Cohn-Hokke, P.E.; Holstege, H.; Weiss, M.M.; van der Flier, W.M.; Barkhof, F.; Sistermans, E.A.; Pijnenburg, Y.A.L.; van Swieten, J.C.; Meijers-Heijboer, H.; Scheltens, P. A novel CCM2 variant in a family with non-progressive cognitive complaints and cerebral microbleeds. Am. J. Med. Genet. Part B Neuropsychiatr. Genet. Publ. 2016, 174, 220–226. [Google Scholar] [CrossRef] [PubMed] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Antonescu, F.; Butnariu, I.; Cojocaru, F.M.; Anghel, D.N.; Antonescu-Ghelmez, D.; Tuță, S. Magnetic Resonance Imaging of Multiple Cerebral and Spinal Cavernous Malformations of a Patient with Dementia and Tetraparesis. Diagnostics 2022, 12, 677. https://doi.org/10.3390/diagnostics12030677
Antonescu F, Butnariu I, Cojocaru FM, Anghel DN, Antonescu-Ghelmez D, Tuță S. Magnetic Resonance Imaging of Multiple Cerebral and Spinal Cavernous Malformations of a Patient with Dementia and Tetraparesis. Diagnostics. 2022; 12(3):677. https://doi.org/10.3390/diagnostics12030677
Chicago/Turabian StyleAntonescu, Florian, Ioana Butnariu, Florentina Melania Cojocaru, Daniela Nicoleta Anghel, Dana Antonescu-Ghelmez, and Sorin Tuță. 2022. "Magnetic Resonance Imaging of Multiple Cerebral and Spinal Cavernous Malformations of a Patient with Dementia and Tetraparesis" Diagnostics 12, no. 3: 677. https://doi.org/10.3390/diagnostics12030677
APA StyleAntonescu, F., Butnariu, I., Cojocaru, F. M., Anghel, D. N., Antonescu-Ghelmez, D., & Tuță, S. (2022). Magnetic Resonance Imaging of Multiple Cerebral and Spinal Cavernous Malformations of a Patient with Dementia and Tetraparesis. Diagnostics, 12(3), 677. https://doi.org/10.3390/diagnostics12030677





