In Elderly Anemic Patients without Endoscopic Signs of Bleeding Are Duodenal Biopsies Always Necessary to Rule Out Celiac Disease?
Abstract
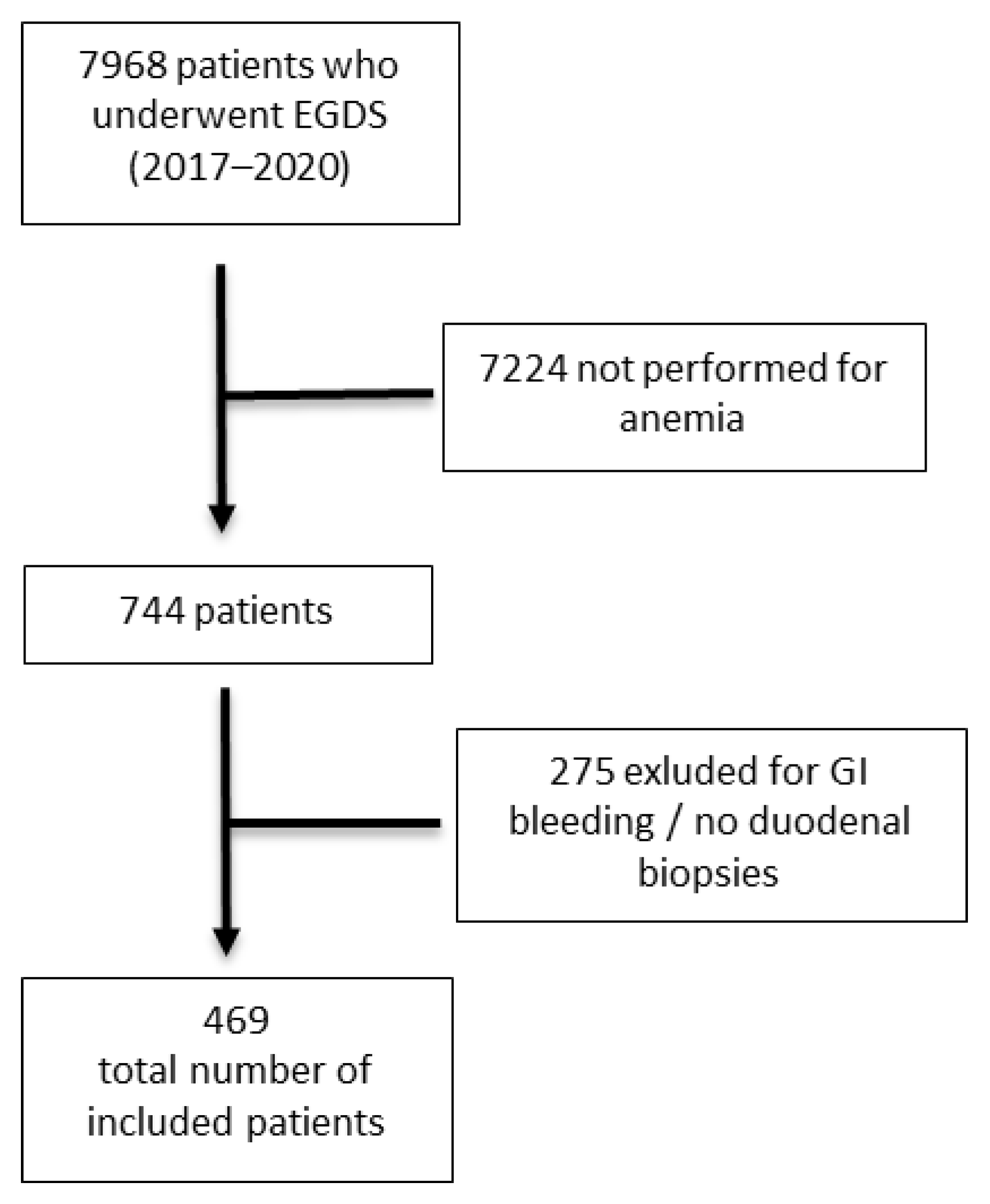
:1. Introduction
2. Materials and Methods
2.1. Study Population and Design of the Study
2.2. Endoscopy and Histopathology
2.3. Statistic Analysis
3. Results
3.1. Histopathological Findings and Endoscopic Features for Age
3.2. Frequency of Histopathological Duodenal Alterations for Age Groups
3.3. Strength of Association between Endoscopically and Histopathologically Normal Mucosa and Increasing Age
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- McLean, E.; Cogswell, M.; Egli, I.; Wojdyla, D.; de Benoist, B. Worldwide prevalence of anaemia, WHO Vitamin and Mineral Nutrition Information System, 1993–2005. Public Health Nutr. 2009, 12, 444–454. [Google Scholar] [CrossRef] [Green Version]
- Guralnik, J.M.; Eisenstaedt, R.S.; Ferrucci, L.; Klein, H.G.; Woodman, R.C. Prevalence of anemia in persons 65 years and older in the United States: Evidence for a high rate of unexplained anemia. Blood 2004, 104, 2263–2268. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ko, C.W.; Siddique, S.M.; Patel, A.; Harris, A.; Sultan, S.; Altayar, O.; Falck-Ytter, Y. AGA Clinical Practice Guidelines on the Gastrointestinal Evaluation of Iron Deficiency Anemia. Gastroenterology 2020, 159, 1085–1094, Comment on Gastroenterology 2021, 160, 2616. [Google Scholar] [CrossRef] [PubMed]
- Broide, E.; Matalon, S.; Kriger-Sharabi, O.; Richter, V.; Shirin, H.; Leshno, M. Cost effectiveness of routine duodenal biopsies in iron deficiency anemia. World J. Gastroenterol. 2016, 22, 7813–7823. [Google Scholar] [CrossRef] [PubMed]
- Ludvigsson, J.F.; Bai, J.C.; Biagi, F.; Card, T.R.; Ciacci, C.; Ciclitira, P.J.; Green, P.H.; Hadjivassiliou, M.; Holdoway, A.; van Heel, D.A.; et al. Diagnosis and management of adult coeliac disease: Guidelines from the British Society of Gastroenterology. Gut 2014, 63, 1210–1228. [Google Scholar] [CrossRef] [PubMed]
- Rashtak, S.; Murray, J.A. Celiac disease in the elderly. Gastroenterol. Clin. N. Am. 2009, 38, 433–446. [Google Scholar] [CrossRef] [PubMed]
- Cappello, M.; Morreale, G.C.; Licata, A. Elderly Onset Celiac Disease: A Narrative Review. Clin. Med. Insights Gastroenterol. 2016, 9, 41–49. [Google Scholar] [CrossRef] [Green Version]
- Husnoo, N.; Ahmed, W.; Shiwani, M.H. Duodenal biopsies for the diagnosis of coeliac disease: Are we adhering to current guidance? BMJ Open Gastroenterol. 2017, 4, e000140. [Google Scholar] [CrossRef] [PubMed]
- Baccini, F.; Spiriti, M.A.; Vannella, L.; Monarca, B.; Delle Fave, G.; Annibale, B. Unawareness of gastrointestinal symptomatology in adult coeliac patients with unexplained iron-deficiency anaemia presentation. Aliment. Pharmacol. Ther. 2006, 23, 915–921. [Google Scholar] [CrossRef] [PubMed]
- Sonnenberg, A.; Turner, K.O.; Genta, R.M. Seasonal Variation of Duodenal Intraepithelial Lymphocytosis. Clin. Gastroenterol. Hepatol. 2020, 18, 2136–2138.e1. [Google Scholar] [CrossRef] [PubMed]
- Borchard, F. Chemisch-reaktive Gastritis [Chemical-reactive gastritis]. Pathologe 2001, 22, 44–55. [Google Scholar] [CrossRef] [PubMed]
- Goddard, A.F.; McIntyre, A.S.; Scott, B.B.; British Society of Gastroenterology. Guidelines for the management of iron deficiency anaemia. Gut 2000, 46 (Suppl. 3–4), IV1–IV5, Correction in Gut 2000, 47, 872. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goddard, A.F.; James, M.W.; McIntyre, A.S.; Scott, B.B.; British Society of Gastroenterology. Guidelines for the management of iron deficiency anaemia. Gut 2011, 60, 1309–1316. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rubio-Tapia, A.; Hill, I.D.; Kelly, C.P.; Calderwood, A.H.; Murray, J.A.; American College of Gastroenterology. ACG clinical guidelines: Diagnosis and management of celiac disease. Am. J. Gastroenterol. 2013, 108, 656–677. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Beg, S.; Ragunath, K.; Wyman, A.; Banks, M.; Trudgill, N.; Pritchard, M.D.; Riley, S.; Anderson, J.; Griffiths, H.; Bhandari, P.; et al. Quality standards in upper gastrointestinal endoscopy: A position statement of the British Society of Gastroenterology (BSG) and Association of Upper Gastrointestinal Surgeons of Great Britain and Ireland (AUGIS). Gut 2017, 66, 1886–1899, Correction in Gut 2017, 66, 2188. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Murray, J.A.; Van Dyke, C.; Plevak, M.F.; Dierkhising, R.A.; Zinsmeister, A.R.; Melton, L.J., 3rd. Trends in the identification and clinical features of celiac disease in a North American community, 1950–2001. Clin. Gastroenterol. Hepatol. 2003, 1, 19–27. [Google Scholar] [CrossRef] [PubMed]
- Hankey, G.L.; Holmes, G.K. Coeliac disease in the elderly. Gut 1994, 35, 65–67. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Freeman, H.J. Adult celiac disease in the elderly. World J. Gastroenterol. 2008, 14, 6911–6914. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Licata, A.; Cappello, M.; Arini, A.; Florena, A.M.; Randazzo, C.; Butera, G.; Almasio, P.L.; Craxì, A. Serology in adults with celiac disease: Limited accuracy in patients with mild histological lesions. Intern. Emerg. Med. 2012, 7, 337–342. [Google Scholar] [CrossRef] [PubMed]


| N (469) | % | |
|---|---|---|
| Females | 315 | 67% |
| Median age, years | 54.7 (18–93) | |
| Provenience: | ||
| -Outpatients | 268 | 57.1% |
| -Emergency room | 22 | 4.5% |
| -Inpatients | 119 | 25.4% |
| Gastrointestinal symptoms | 55 | 11.7% |
| Odds Ratio | 95% CI | |
|---|---|---|
| Age under 50 years | 1 | |
| Age over 50 years | 20,565 | 10,547 to 40,101 |
| Presence of endoscopic findings in duodenum | 1 | |
| Absence of endoscopic findings in duodenum | 35,426 | 15,132 to 82,938 |
| Age under 60 years | 1 | |
| Age over 60 years | 30,187 | 14,278 to 63,825 |
| Presence of endoscopic findings in duodenum | 1 | |
| Absence of endoscopic findings in duodenum | 35,007 | 14,929 to 82,090 |
| Age under 70 years | 1 | |
| Age over 70 years | 30,184 | 11,485 to 79,328 |
| Presence of endoscopic findings in duodenum | 1 | |
| Absence of endoscopic findings in duodenum | 36,409 | 15,643 to 84,741 |
| Age under 80 years | 1 | |
| Age over 80 years | 42,501 | 0.5620 to 321,424 |
| Presence of endoscopic findings in duodenum | 1 | |
| Absence of endoscopic findings in duodenum | 40,749 | 17,674 to 93,948 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pivetta, G.; Coluccio, C.; Dilaghi, E.; Lahner, E.; Pilozzi, E.; Carabotti, M.; Corleto, V.D. In Elderly Anemic Patients without Endoscopic Signs of Bleeding Are Duodenal Biopsies Always Necessary to Rule Out Celiac Disease? Diagnostics 2022, 12, 678. https://doi.org/10.3390/diagnostics12030678
Pivetta G, Coluccio C, Dilaghi E, Lahner E, Pilozzi E, Carabotti M, Corleto VD. In Elderly Anemic Patients without Endoscopic Signs of Bleeding Are Duodenal Biopsies Always Necessary to Rule Out Celiac Disease? Diagnostics. 2022; 12(3):678. https://doi.org/10.3390/diagnostics12030678
Chicago/Turabian StylePivetta, Giulia, Chiara Coluccio, Emanuele Dilaghi, Edith Lahner, Emanuela Pilozzi, Marilia Carabotti, and Vito Domenico Corleto. 2022. "In Elderly Anemic Patients without Endoscopic Signs of Bleeding Are Duodenal Biopsies Always Necessary to Rule Out Celiac Disease?" Diagnostics 12, no. 3: 678. https://doi.org/10.3390/diagnostics12030678
APA StylePivetta, G., Coluccio, C., Dilaghi, E., Lahner, E., Pilozzi, E., Carabotti, M., & Corleto, V. D. (2022). In Elderly Anemic Patients without Endoscopic Signs of Bleeding Are Duodenal Biopsies Always Necessary to Rule Out Celiac Disease? Diagnostics, 12(3), 678. https://doi.org/10.3390/diagnostics12030678






