Optic Nerve Sheath Diameter Ultrasound: A Non-Invasive Approach to Evaluate Increased Intracranial Pressure in Critically Ill Pediatric Patients
Abstract
:1. Introduction
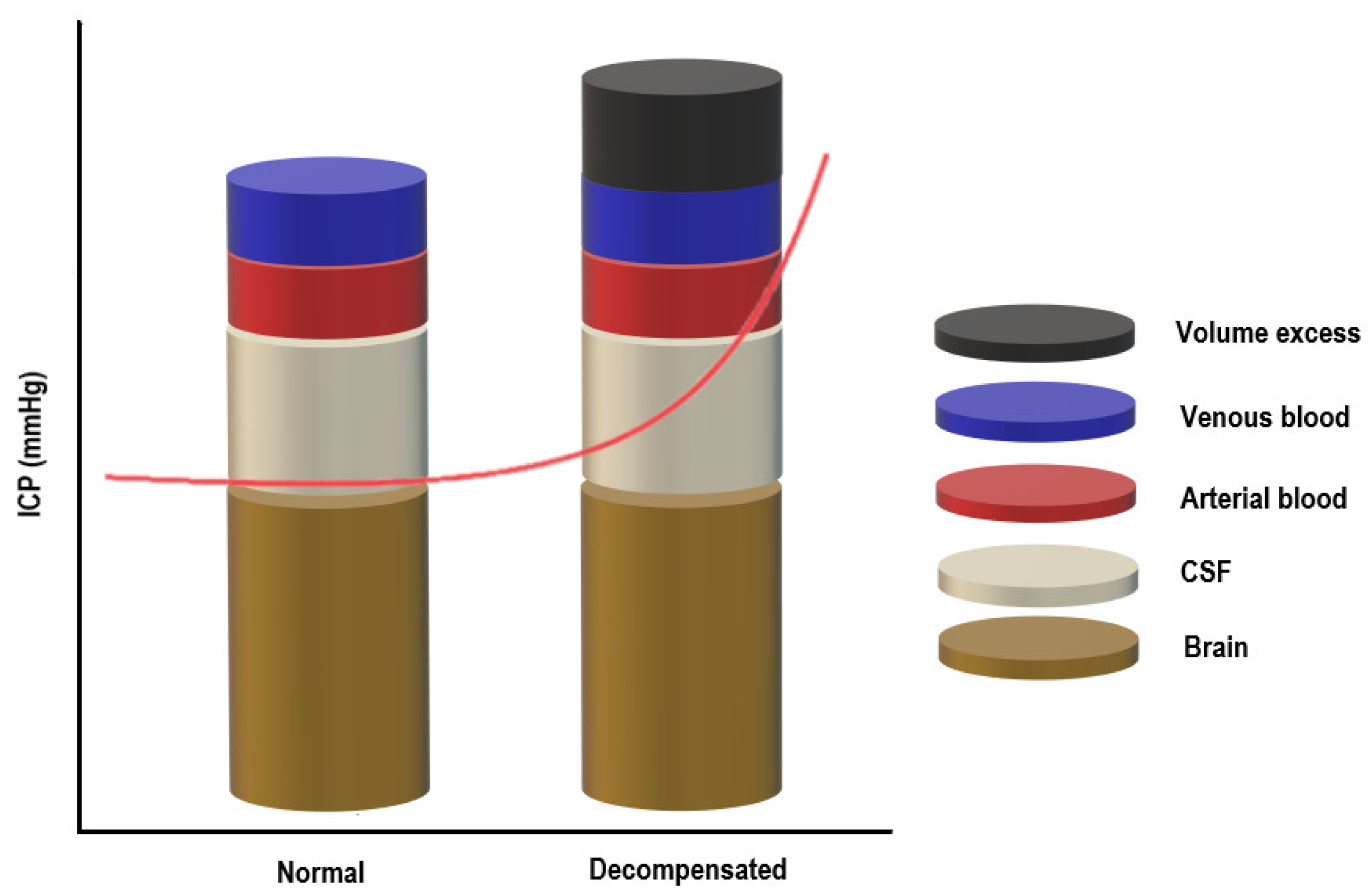
2. Pathophysiology of Raised Intracranial Pressure (ICP)
3. Optic Nerve and Its Measurement
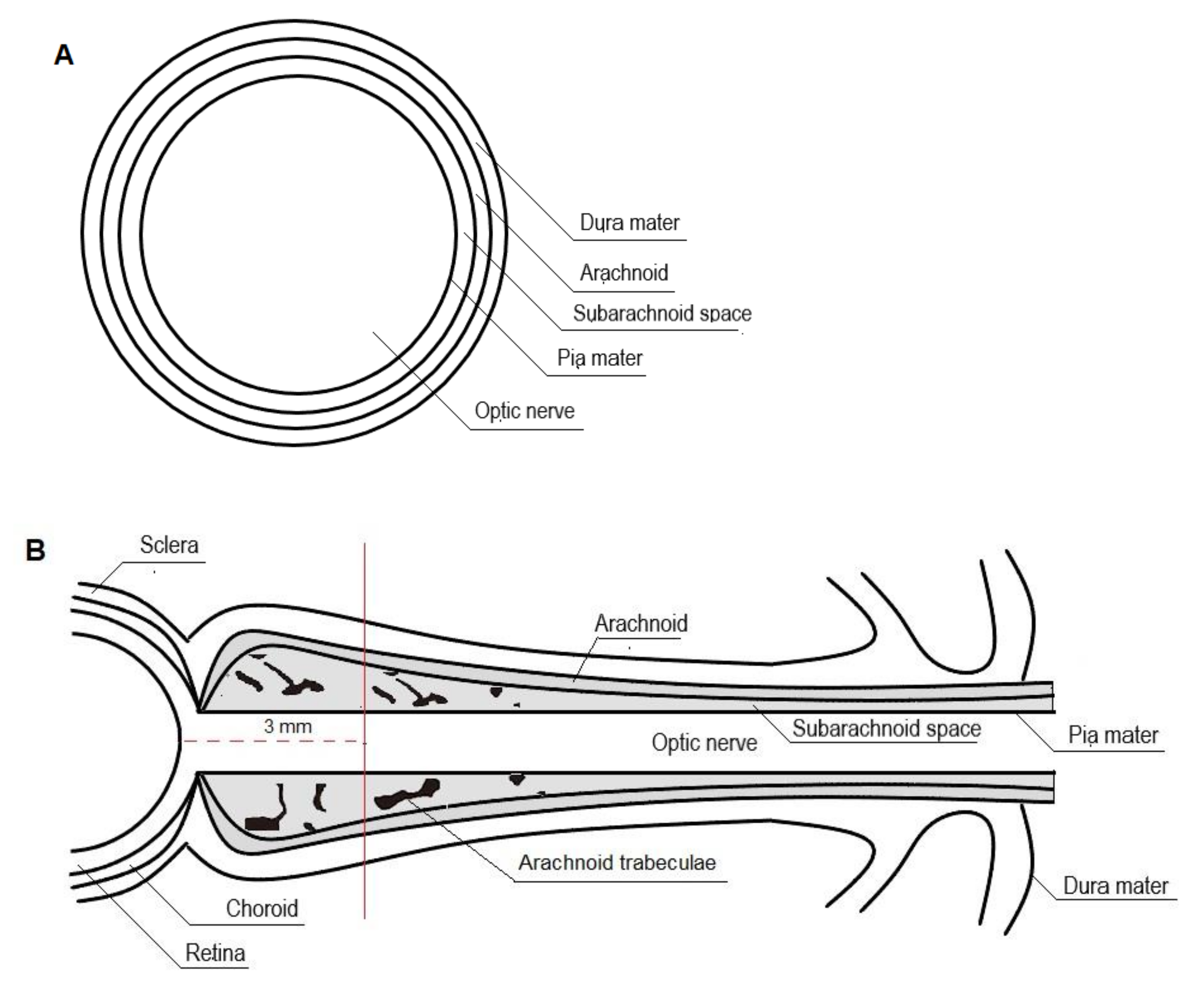
3.1. Anatomy and Physiology of Optic Nerve
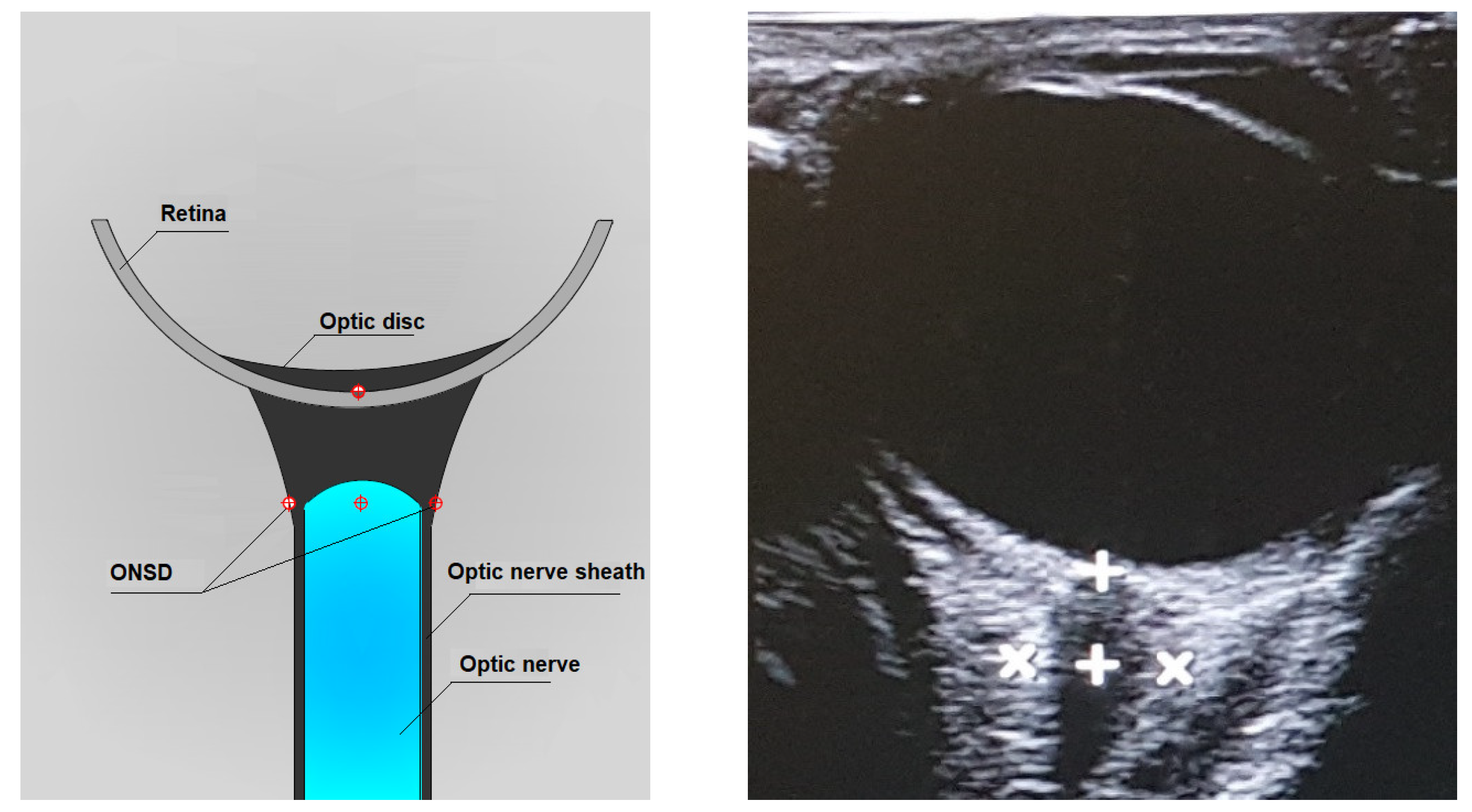
3.2. Ultrasonographic Technique for Optic Nerve Sheath Diameter (ONSD) Measurement
4. Optic Nerve Sheath Diameter (ONSD) Measurements in Children
5. ONSD Measurements in Neonates
6. Discussion and Future Directions
7. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Marmarou, A.; Anderson, R.L.; Ward, J.D.; Choi, S.C.; Young, H.F.; Eisenberg, H.M.; Foulkes, M.A.; Marshall, L.F.; Jane, J.A. Impact of ICP instability and hypotension on outcome in patients with severe head trauma. J. Neurosurg. 1991, 75, S59–S66. [Google Scholar] [CrossRef]
- Chesnut, R.M.; Marshall, L.F.; Klauber, M.R.; Blunt, B.A.; Baldwin, N.; Eisenberg, H.M.; Jane, J.A.; Marmarou, A.; Foulkes, M.A. The role of secondary brain injury in determining outcome from severe head injury. J. Trauma 1993, 34, 216–222. [Google Scholar] [CrossRef] [PubMed]
- Juul, N.; Morris, G.F.; Marshall, S.B.; Marshall, L.F. Intracranial hypertension and cerebral perfusion pressure: Influence on neurological deterioration and outcome in severe head injury. J. Neurosurg. 2000, 92, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Balestreri, M.; Czosnyka, M.; Hutchinson, P.; Steiner, L.A.; Hiler, M.; Smielewski, P.; Pickard, J.D. Impact of Intracranial Pressure and Cerebral Perfusion Pressure on Severe Disability and Mortality After Head Injury. Neurocritical Care 2006, 4, 008–013. [Google Scholar] [CrossRef]
- Myburgh, J.A.; Cooper, D.J.; Finfer, S.R.; Venkatesh, B.; Jones, D.; Higgins, A.; Bishop, N.; Higlett, T. Epidemiology and 12-Month Outcomes from Traumatic Brain Injury in Australia and New Zealand. J. Trauma Acute Care Surg. 2008, 64, 854–862. [Google Scholar] [CrossRef]
- Kochanek, P.M.; Tasker, R.C.; Carney, N.; Totten, A.M.; Adelson, P.D.; Selden, N.R.; Davis-O’Reilly, C.; Hart, E.L.; Bell, M.J.; Bratton, S.L.; et al. Guidelines for the Management of Pediatric Severe Traumatic Brain Injury, Third Edition: Update of the Brain Trauma Foundation Guidelines, Executive Summary. Neurosurgery 2019, 84, 1169–1178. [Google Scholar] [CrossRef] [Green Version]
- Langlois, J.A.; Rutland-Brown, W.; Wald, M.M. The Epidemiology and Impact of Traumatic Brain Injury: A brief overview. J. Head Trauma Rehabil. 2006, 21, 375–378. [Google Scholar] [CrossRef] [Green Version]
- Bruce, D.A.; Alavi, A.; Bilaniuk, L.; Dolinskas, C.; Obrist, W.; Uzzell, B. Diffuse cerebral swelling following head injuries in children: The syndrome of “malignant brain edema”. J. Neurosurg. 1981, 54, 170–178. [Google Scholar] [CrossRef]
- Muizelaar, J.P.; Marmarou, A.; DeSalles, A.A.F.; Ward, J.D.; Zimmerman, R.S.; Li, Z.; Choi, S.C.; Young, H.F. Cerebral blood flow and metabolism in severely head-injured children. J. Neurosurg. 1989, 71, 63–71. [Google Scholar] [CrossRef]
- Hakim, S.; Venegas, J.G.; Burton, J.D. The physics of the cranial cavity, hydrocephalus and normal pressure hydrocephalus: Me-chanical interpretation and mathematical model. Surg. Neurol. 1976, 5, 187–210. [Google Scholar]
- Shapiro, H.M. Intracranial hypertension: Therapeutic and anesthetic considerations. J. Am. Soc. Anesthesiol. 1975, 43, 445–471. [Google Scholar] [CrossRef]
- Lidofsky, S.D.; Bass, N.M.; Prager, M.C.; Washington, D.E.; Read, A.E.; Wright, T.L.; Ascher, N.L.; Roberts, J.P.; Scharschmidt, B.F.; Lake, J. Intracranial pressure monitoring and liver transplantation for fulminant hepatic failure. Hepatology 1992, 16, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Bingaman, W.E.; Frank, J.I. Malignant Cerebral Edema and Intracranial Hypertension. Neurol. Clin. 1995, 13, 479–509. [Google Scholar] [CrossRef]
- Harary, M.; Dolmans, R.G.F.; Gormley, W.B. Intracranial Pressure Monitoring—Review and Avenues for Development. Sensors 2018, 18, 465. [Google Scholar] [CrossRef] [Green Version]
- Czosnyka, M. Monitoring and interpretation of intracranial pressure. J. Neurol. Neurosurg. Psychiatry 2004, 75, 813–821. [Google Scholar] [CrossRef]
- Padayachy, L.C.; Figaji, A.A.; Bullock, M.R. Intracranial pressure monitoring for traumatic brain injury in the modern era. Child’s Nerv. Syst. 2010, 26, 441–452. [Google Scholar] [CrossRef]
- Talving, P.; Karamanos, E.; Teixeira, P.G.; Skiada, D.; Lam, L.; Belzberg, H.; Inaba, K.; Demetriades, D. Intracranial pressure monitoring in severe head injury: Compliance with Brain Trauma Foundation guidelines and effect on outcomes: A prospective study. J. Neurosurg. 2013, 119, 1248–1254. [Google Scholar] [CrossRef] [Green Version]
- Bauer, D.F.; Razdan, S.N.; Bartolucci, A.A.; Markert, J.M. Meta-Analysis of Hemorrhagic Complications From Ventriculostomy Placement by Neurosurgeons. Neurosurgery 2011, 69, 255–260. [Google Scholar] [CrossRef] [Green Version]
- Binz, D.D.; Toussaint, L.G.; Friedman, J.A. Hemorrhagic Complications of Ventriculostomy Placement: A Meta-Analysis. Neurocritical Care 2009, 10, 253–256. [Google Scholar] [CrossRef]
- Bekar, A.; Doğan, Ş.; Abaş, F.; Caner, B.; Korfalı, G.; Kocaeli, H.; Yılmazlar, S. Risk factors and complications of intracranial pressure monitoring with a fiberoptic device. J. Clin. Neurosci. 2009, 16, 236–240. [Google Scholar] [CrossRef]
- Holloway, K.; Barnes, T.; Choi, S.; Bullock, R.; Marshall, L.F.; Eisenberg, H.M.; Jane, J.A.; Ward, J.D.; Young, H.F.; Marmarou, A. Ventriculostomy infections: The effect of monitoring duration and catheter exchange in 584 patients. J. Neurosurg. 1996, 85, 419–424. [Google Scholar] [CrossRef] [Green Version]
- Robba, C.; Bacigaluppi, S.; Cardim, D.; Donnelly, J.; Bertuccio, A.; Czosnyka, M. Non-invasive assessment of intracranial pressure. Acta Neurol. Scand. 2015, 134, 4–21. [Google Scholar] [CrossRef]
- Padayachy, L.C. Non-invasive intracranial pressure assessment. Child’s Nerv. Syst. 2016, 32, 1587–1597. [Google Scholar] [CrossRef]
- Kayhanian, S.; Young, A.M.H.; Piper, R.; Donnelly, J.; Scoffings, D.; Garnett, M.R.; Fernandes, H.M.; Smielewski, P.; Czosnyka, M.; Hutchinson, P.; et al. Radiological Correlates of Raised Intracranial Pressure in Children: A Review. Front. Pediatr. 2018, 6, 32. [Google Scholar] [CrossRef] [Green Version]
- Price, D.A.; Grzybowski, A.; Eikenberry, J.; Januleviciene, I.; Vercellin, A.C.V.; Mathew, S.; Siesky, B.; Harris, A. Review of non-invasive intracranial pressure measurement techniques for ophthalmology applications. Br. J. Ophthalmol. 2019, 104, 887–892. [Google Scholar] [CrossRef]
- Singh, Y.; Tissot, C.; Fraga, M.V.; Yousef, N.; Cortes, R.G.; Lopez, J.; Sanchez-De-Toledo, J.; Brierley, J.; Mayordomo-Colunga, J.; Raffaj, D.; et al. International evidence-based guidelines on Point of Care Ultrasound (POCUS) for critically ill neonates and children issued by the POCUS Working Group of the European Society of Paediatric and Neonatal Intensive Care (ESPNIC). Crit. Care 2020, 24, 65. [Google Scholar] [CrossRef] [Green Version]
- Bortcosh, W.; Shaahinfar, A.; Sojar, S.; Klig, J.E. New directions in point-of-care ultrasound at the crossroads of paediatric emergency and critical care. Curr. Opin. Pediatr. 2018, 30, 350–358. [Google Scholar] [CrossRef]
- O’Brien, A.J.; Brady, R.M. Point-of-care ultrasound in paediatric emergency medicine. J. Paediatr. Child Health 2016, 52, 174–180. [Google Scholar] [CrossRef]
- Moore, C.L.; Copel, J.A. Point-of-Care Ultrasonography. N. Engl. J. Med. 2011, 364, 749–757. [Google Scholar] [CrossRef] [Green Version]
- Liu, D.; Kahn, M. Measurement and Relationship of Subarachnoid Pressure of the Optic Nerve to Intracranial Pressures in Fresh Cadavers. Am. J. Ophthalmol. 1993, 116, 548–556. [Google Scholar] [CrossRef]
- Helmke, K.; Hansen, H.C. Fundamentals of transorbital sonographic evaluation of optic nerve sheath expansion under intracranial hypertension. Pediatr. Radiol. 1996, 26, 701–705. [Google Scholar] [CrossRef]
- Maude, R.R.; Hossain, M.A.; Hassan, M.U.; Osbourne, S.; Langan Abu Sayeed, K.; Rezaul Karim, M.; Samad, R.; Borooah, S.; Dhillon, B.; Day, N.P.J.; et al. Transorbital sonographic evaluation of normal optic nerve sheath diameter in healthy volunteers in Bangladesh. PLoS ONE 2013, 8, e81013. [Google Scholar] [CrossRef]
- Hansen, H.-C.; Helmke, K. Validation of the optic nerve sheath response to changing cerebrospinal fluid pressure: Ultrasound findings during intrathecal infusion tests. J. Neurosurg. 1997, 87, 34–40. [Google Scholar] [CrossRef]
- Moretti, R.; Pizzi, B. Ultrasonography of the optic nerve in neurocritically ill patients. Acta Anaesthesiol. Scand. 2011, 55, 644–652. [Google Scholar] [CrossRef]
- Launey, Y.; Nesseler, N.; Le Maguet, P.; Malledant, Y.; Seguin, P. Effect of Osmotherapy on Optic Nerve Sheath Diameter in Patients with Increased Intracranial Pressure. J. Neurotrauma 2014, 31, 984–988. [Google Scholar] [CrossRef]
- Toscano, M.; Spadetta, G.; Pulitano, P.; Rocco, M.; Di Piero, V.; Mecarelli, O.; Vicenzini, E. Optic Nerve Sheath Diameter Ultrasound Evaluation in Intensive Care Unit: Possible Role and Clinical Aspects in Neurological Critical Patients’ Daily Monitoring. BioMed Res. Int. 2017, 2017, 1621428. [Google Scholar] [CrossRef] [Green Version]
- Dubourg, J.; Javouhey, E.; Geeraerts, T.; Messerer, M.; Kassai, B. Ultrasonography of optic nerve sheath diameter for detection of raised intracranial pressure: A systematic review and meta-analysis. Intensiv. Care Med. 2011, 37, 1059–1068. [Google Scholar] [CrossRef]
- Rajajee, V.; Vanaman, M.; Fletcher, J.J.; Jacobs, T.L. Optic Nerve Ultrasound for the Detection of Raised Intracranial Pressure. Neurocritical Care 2011, 15, 506–515. [Google Scholar] [CrossRef]
- Geeraerts, T.; Merceron, S.; Benhamou, D.; Vigué, B.; Duranteau, J. Non-invasive assessment of intracranial pressure using ocular sonography in neurocritical care patients. Intensiv. Care Med. 2008, 34, 2062–2067. [Google Scholar] [CrossRef]
- Geeraerts, T.; Launey, Y.; Martin, L.; Pottecher, J.; Vigué, B.; Duranteau, J.; Benhamou, D. Ultrasonography of the optic nerve sheath may be useful for detecting raised intracranial pressure after severe brain injury. Intensiv. Care Med. 2007, 33, 1704–1711. [Google Scholar] [CrossRef]
- Tayal, V.S.; Neulander, M.; Norton, H.J.; Foster, T.; Saunders, T.; Blaivas, M. Emergency Department Sonographic Measurement of Optic Nerve Sheath Diameter to Detect Findings of Increased Intracranial Pressure in Adult Head Injury Patients. Ann. Emerg. Med. 2007, 49, 508–514. [Google Scholar] [CrossRef]
- Malayeri, A.A.; Bavarian, S.; Mehdizadeh, M. Sonographic Evaluation of Optic Nerve Diameter in Children with Raised Intracranial Pressure. J. Ultrasound Med. 2005, 24, 143–147. [Google Scholar] [CrossRef] [Green Version]
- Hansen, G.; Sellers, E.A.; Beer, D.L.; Vallance, J.K.; Clark, I. Optic Nerve Sheath Diameter Ultrasonography in Pediatric Patients with Diabetic Ketoacidosis. Can. J. Diabetes 2016, 40, 126–130. [Google Scholar] [CrossRef]
- Helmke, K.; Burdelski, M.; Hansen, H.-C. Detection and monitoring of intracranial pressure dysregulation in liver failure by ultrasound. Transplantation 2000, 70, 392–395. [Google Scholar] [CrossRef]
- Hall, M.K.; Spiro, D.M.; Sabbaj, A.; Moore, C.L.; Hopkins, K.L.; Meckler, G. Bedside optic nerve sheath diameter ultrasound for the evaluation of suspected pediatric ventriculoperitoneal shunt failure in the emergency department. Child’s Nerv. Syst. 2013, 29, 2275–2280. [Google Scholar] [CrossRef]
- McAuley, D.; Paterson, A.; Sweeney, L. Optic nerve sheath ultrasound in the assessment of paediatric hydrocephalus. Child’s Nerv. Syst. 2009, 25, 87–90. [Google Scholar] [CrossRef]
- Newman, W.D.; Hollman, A.S.; Dutton, G.N.; Carachi, R. Measurement of optic nerve sheath diameter by ultrasound: A means of detecting acute raised intracranial pressure in hydrocephalus. Br. J. Ophthalmol. 2002, 86, 1109–1113. [Google Scholar] [CrossRef] [Green Version]
- Driessen, C.; Van Veelen, M.-L.C.; Lequin, M.; Joosten, K.F.M.; Mathijssen, I.M.J. Nocturnal Ultrasound Measurements of Optic Nerve Sheath Diameter Correlate with Intracranial Pressure in Children with Craniosynostosis. Plast. Reconstr. Surg. 2012, 130, 448e–451e. [Google Scholar] [CrossRef]
- Murphy, S.; Cserti-Gazdewich, C.; Dhabangi, A.; Musoke, C.; Nabukeera-Barungi, N.; Price, D.; King, M.E.; Romero, J.; Noviski, N.; Dzik, W. Ultrasound findings in Plasmodium falciparum malaria: A pilot study. Pediatr. Crit. Care Med. 2011, 12, e58–e63. [Google Scholar] [CrossRef]
- Monro, A. Observations on the Structure and Functions of the Nervous System; Creech and Johnson: Edinbourgh, UK, 1783. [Google Scholar]
- Kellie, G. Appearances observed in the dissection of two individuals: Death from cold and congestion of the brain. Trans. Med. Chir. Soc. Edinbrugh 1824, 1, 84. [Google Scholar]
- Budday, S.; Ovaert, T.C.; Holzapfel, G.A.; Steinmann, P.; Kuhl, E. Fifty Shades of Brain: A Review on the Mechanical Testing and Modeling of Brain Tissue. Arch. Comput. Methods Eng. 2019, 27, 1187–1230. [Google Scholar] [CrossRef] [Green Version]
- Greenberg, M. (Ed.) Neuromonitoring. In Handbook of Neurosurgery, 8th ed.; Thieme: New York, NY, USA, 2016; pp. 856–881. [Google Scholar]
- Morton, R.; Ellenbogen, R. Intracranial hypertension. In Principles of Neurological Surgery, 3rd ed.; Saunders/Elsevier: Philadelphia, PA, USA, 2012; pp. 311–323. [Google Scholar]
- Lassen, N.A. Cerebral Blood Flow and Oxygen Consumption in Man. Physiol. Rev. 1959, 39, 183–238. [Google Scholar] [CrossRef] [Green Version]
- Lassen, N.A. Control of Cerebral Circulation in Health and Disease. Circ. Res. 1974, 34, 749–760. [Google Scholar] [CrossRef] [Green Version]
- Drummond, J.C. The Lower Limit of Autoregulation. Anesthesiologists 1997, 86, 1431–1433. [Google Scholar] [CrossRef]
- Latorre, J.G.S.; Greer, D.M. Management of Acute Intracranial Hypertension. Neurologist 2009, 15, 193–207. [Google Scholar] [CrossRef]
- Armstead, W.M. Cerebral Blood Flow Autoregulation and Dysautoregulation. Anesthesiol. Clin. 2016, 34, 465–477. [Google Scholar] [CrossRef] [Green Version]
- Meng, L.; Gelb, A.W. Regulation of Cerebral Autoregulation by Carbon Dioxide. Anesthesiology 2015, 122, 196–205. [Google Scholar] [CrossRef] [Green Version]
- Kinoshita, K. Traumatic brain injury: Pathophysiology for neurocritical care. J. Intensiv. Care 2016, 4, 29. [Google Scholar] [CrossRef] [Green Version]
- Youmans, J.R. (Ed.) Neurological Surgery, 4th ed.; WB Saunders: Philadelphia, PA, USA, 1996; Volume 3. [Google Scholar]
- Sanz-García, A.; Pérez-Romero, M.; Pastor, J.; Sola, R.G.; Vega-Zelaya, L.; Monasterio, F.; Torrecilla, C.; Vega, G.; Pulido, P.; Ortega, G.J. Identifying causal relationships between EEG activity and intracranial pressure changes in neurocritical care patients. J. Neural Eng. 2018, 15, 066029. [Google Scholar] [CrossRef]
- Donnelly, J.; Budohoski, K.P.; Smielewski, P.; Czosnyka, M. Regulation of the cerebral circulation: Bedside assessment and clinical implications. Crit. Care 2016, 20, 129. [Google Scholar] [CrossRef] [Green Version]
- Cavus, E.; Bein, B.; Dörges, V.; Stadlbauer, K.-H.; Wenzel, V.; Steinfath, M.; Hanss, R.; Scholz, J. Brain tissue oxygen pressure and cerebral metabolism in an animal model of cardiac arrest and cardiopulmonary resuscitation. Resuscitation 2006, 71, 97–106. [Google Scholar] [CrossRef]
- Bowton, D.L.; Bertels, N.H.; Prough, D.S.; Stump, D.A. Cerebral blood flow is reduced in patients with sepsis syndrome. Crit. Care Med. 1989, 17, 399–403. [Google Scholar] [CrossRef]
- Lundberg, N. Continuous recording and control of ventricular fluid pressure in neurosurgical practice. Acta Psychiatr. Scand. Suppl. 1960, 36, 13764297. [Google Scholar] [CrossRef] [Green Version]
- Hawryluk, G.W.J.; Nielson, J.L.; Huie, J.R.; Zimmermann, L.; Saigal, R.; Ding, Q.; Hirschi, R.; Zeiler, F.A.; Ferguson, A.R.; Manley, G.T. Analysis of Normal High-Frequency Intracranial Pressure Values and Treatment Threshold in Neurocritical Care Patients. JAMA Neurol. 2020, 77, 1150–1158. [Google Scholar] [CrossRef]
- Albeck, M.J.; Børgesen, S.E.; Gjerris, F.; Schmidt, J.F.; Sorensen, P.S. Intracranial pressure and cerebrospinal fluid outflow conductance in healthy subjects. J. Neurosurg. 1991, 74, 597–600. [Google Scholar] [CrossRef] [Green Version]
- Chapman, P.H.; Cosman, E.R.; Arnold, M.A. The relationship between ventricular fluid pressure and body position in normal subjects and subjects with shunts. Neurosurgery 1990, 26, 181–189. [Google Scholar] [CrossRef]
- Carney, N.; Totten, A.M.; O’Reilly, C.; Ullman, J.S.; Hawryluk, G.W.; Bell, M.J.; Bratton, S.L.; Chesnut, R.; Harris, O.A.; Kissoon, N.; et al. Guidelines for the Management of Severe Traumatic Brain Injury, Fourth Edition. Neurosurgery 2016, 80, 6–15. [Google Scholar] [CrossRef]
- Welch, K. The intracranial pressure in infants. J. Neurosurg. 1980, 52, 693–699. [Google Scholar] [CrossRef]
- Mohseni-Bod, H.; Drake, J.; Kukreti, V. Management of raised intracranial pressure in children with traumatic brain injury. J. Pediatr. Neurosci. 2014, 9, 207–215. [Google Scholar] [CrossRef]
- Cruz, J.; Nakayama, P.; Imamura, J.H.; Rosenfeld, K.G.; de Souza, H.S.; Giorgetti, G.V.F. Cerebral Extraction of Oxygen and Intracranial Hypertension in Severe, Acute, Pediatric Brain Trauma: Preliminary Novel Management Strategies. Neurosurgery 2002, 50, 774–780. [Google Scholar] [CrossRef]
- Adelson, P.D.; Ragheb, J.; Muizelaar, J.P.; Kanev, P.; Brockmeyer, D.; Beers, S.R.; Brown, S.D.; Cassidy, L.D.; Chang, Y.; Levin, H. Phase II Clinical Trial of Moderate Hypothermia after Severe Traumatic Brain Injury in Children. Neurosurgery 2005, 56, 740–754. [Google Scholar] [CrossRef]
- Chambers, I.R.; Treadwell, L.; Mendelow, A.D. Determination of threshold levels of cerebral perfusion pressure and intracranial pressure in severe head injury by using receiver operating—Characteristic curves: An observational study in 291 patients. J. Neurosurg. 2001, 94, 412–416. [Google Scholar] [CrossRef]
- Jagannathan, J.; Okonkwo, D.O.; Yeoh, H.K.; Dumont, A.S.; Saulle, D.; Haizlip, J.; Barth, J.T.; Jane, J.A. Long-term outcomes and prognostic factors in pediatric patients with severe traumatic brain injury and elevated intracranial pressure. J. Neurosurg. Pediatr. 2008, 2, 240–249. [Google Scholar] [CrossRef] [Green Version]
- Michaud, L.J.; Rivara, F.P.; Grady, M.S.; Reay, D.T. Predictors of Survival and Severity of Disability after Severe Brain Injury in Children. Neurosurgery 1992, 31, 254–264. [Google Scholar] [CrossRef]
- Esparza, J.; M-Portillo, J.; Sarabia, M.; Roger, R.; Lamas, E. Outcome in children with severe head injuries. Child’s Nerv. Syst. 1985, 1, 109–114. [Google Scholar] [CrossRef]
- Spencer, W.H. Ophthalmic Pathology: An Atlas and Textbook, 3rd ed.; WB Saunders: Philadelphia, PA, USA, 1986; pp. 2337–2458. [Google Scholar]
- Barr, R.; Gean, A. Craniofacial trauma. In Fundamentals of Diagnostic Radiology, 2nd ed.; Brant, W., Helms, C., Eds.; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 1999; pp. 49–61. [Google Scholar]
- O’Rahilly, R. The early development of the eye in staged embryos. Contrib. Embryol. 1966, 38, 1–42. [Google Scholar]
- Rothman, M.I.; Zoarski, G.H. The orbit. In Textbook of Radiology and Imaging, 7th ed.; Sutton, D., Ed.; Churchill Living-Stone: London, UK, 2003; pp. 1573–1595. [Google Scholar]
- Killer, H.E.; Laeng, H.R.; Flammer, J.; Groscurth, P. Architecture of arachnoid trabeculae, pillars, and septa in the subarachnoid space of the human optic nerve: Anatomy and clinical considerations. Br. J. Ophthalmol. 2003, 87, 777–781. [Google Scholar] [CrossRef] [Green Version]
- Geeraerts, T.; Duranteau, J.; Benhamou, D. Ocular sonography in patients with raised intracranial pressure: The papilloedema revisited. Crit. Care 2008, 12, 150. [Google Scholar] [CrossRef]
- Wood, J.H. Physiology, pharmacology, and dynamics ofcerebrospinal fluid. In Neurobiology Ofcerebrospinal Fluid; Wood, J.H., Ed.; Plenum Press: New York, NJ, USA, 1989; pp. 1–16. [Google Scholar]
- Gausas, R.E.; Gonnering, R.; Lemke, B.N.; Dortzbach, R.K.; Sherman, D.D. Identification of Human Orbital Lymphatics. Ophthalmic Plast. Reconstr. Surg. 1999, 15, 252–259. [Google Scholar] [CrossRef]
- Hayreh, S.S. Pathogenesis of oedema of the optic disc (papilloedema): A preliminary report. Br. J. Ophthalmol. 1964, 48, 522–543. [Google Scholar] [CrossRef] [Green Version]
- Gangemi, M.; Cennamo, G.; Maiuri, F.; D’Andrea, F. Echographic measurement of the optic nerve in patients with intracranial hypertension. Min -Minim. Invasive Neurosurg. 1987, 30, 53–55. [Google Scholar] [CrossRef]
- Hansen, H.C.; Helmke, K. The subarachnoid space surrounding the optic nerves. An ultrasound study of the optic nerve sheath. Surg. Radiol. Anat. 1996, 18, 323–328. [Google Scholar] [CrossRef]
- Soldatos, T.; Karakitsos, D.; Chatzimichail, K.; Papathanasiou, M.; Gouliamos, A.; Karabinis, A. Optic nerve sonography in the diagnostic evaluation of adult brain injury. Crit. Care 2008, 12, R67. [Google Scholar] [CrossRef] [Green Version]
- Moretti, R.; Pizzi, B.; Cassini, F.; Vivaldi, N. Reliability of Optic Nerve Ultrasound for the Evaluation of Patients with Spontaneous Intracranial Hemorrhage. Neurocritical Care 2009, 11, 406–410. [Google Scholar] [CrossRef]
- Ossoinig, K.C. Standardized echography: Basic principles, clinical applications, and results. Int. Ophthalmol. Clin. 1979, 19, 127–210. [Google Scholar] [CrossRef]
- DiBernardo, C.W.; Greenberg, E. Ophthalmic Ultrasound: A Diagnostic Atlas; ThiemeMedical Publishers: New York, NY, USA, 2007. [Google Scholar]
- Romagnuolo, L.; Tayal, V.; Tomaszewski, C.; Saunders, T.; Norton, H.J. Optic nerve sheath diameter does not change with patient position. Am. J. Emerg. Med. 2005, 23, 686–688. [Google Scholar] [CrossRef]
- Soldatos, T.; Chatzimichail, K.; Papathanasiou, M.; Gouliamos, A. Optic nerve sonography: A new window for the non-invasive evaluation of intracranial pressure in brain injury. Emerg. Med. J. 2009, 26, 630–634. [Google Scholar] [CrossRef]
- Bäuerle, J.; Lochner, P.; Kaps, M.; Nedelmann, M. Intra- and Interobsever Reliability of Sonographic Assessment of the Optic Nerve Sheath Diameter in Healthy Adults. J. Neuroimaging 2012, 22, 42–45. [Google Scholar] [CrossRef]
- Ballantyne, S.; O’Neill, G.; Hamilton, R.; Hollman, A. Observer variation in the sonographic measurement of optic nerve sheath diameter in normal adults. Eur. J. Ultrasound 2002, 15, 145–149. [Google Scholar] [CrossRef]
- Steinborn, M.; Fiegler, J.; Ruedisser, K.; Hapfelmeier, A.; Denne, C.; Macdonald, E.; Hahn, H. Measurement of the Optic Nerve Sheath Diameter in Children: Comparison Between Transbulbar Sonography and Magnetic Resonance Imaging. Ultraschall Med. Eur. J. Ultrasound 2011, 33, 569–573. [Google Scholar] [CrossRef]
- Steinborn, M.; Fiegler, J.; Kraus, V.; Denne, C.; Hapfelmeier, A.; Wurzinger, L.; Hahn, H. High Resolution Ultrasound and Magnetic Resonance Imaging of the Optic Nerve and the Optic Nerve Sheath: Anatomic Correlation and Clinical Importance. Ultraschall Med. Eur. J. Ultrasound 2010, 32, 608–613. [Google Scholar] [CrossRef] [PubMed]
- Fielding, J. Ocular ultrasound. Clin. Radiol. 1996, 51, 533–544. [Google Scholar] [CrossRef]
- Ballantyne, J.; Hollman, A.; Hamilton, R.; Bradnam, M.; Carachi, R.; Young, D.; Dutton, G. Transorbital optic nerve sheath ultrasonography in normal children. Clin. Radiol. 1999, 54, 740–742. [Google Scholar] [CrossRef]
- Rehman Siddiqui, N.U.; Haque, A.; Abbas, Q.; Jurair, H.; Salam, B.; Sayani, R. Ultrasonographic optic nerve sheath diameter Meas-urement for raised intracranial pressure in a Tertiary care centre of a developing country. J. Ayub. Med. Coll Abbottabad. 2018, 30, 495–500. [Google Scholar]
- Irazuzta, J.E.; Brown, M.E.; Akhtar, J. Bedside Optic Nerve Sheath Diameter Assessment in the Identification of Increased Intracranial Pressure in Suspected Idiopathic Intracranial Hypertension. Pediatr. Neurol. 2016, 54, 35–38. [Google Scholar] [CrossRef]
- Aslan, N.; Yildizdas, D.; Ozcan, N.; Horoz, O.O.; Mert, G.G.; Sertdemir, Y.; Altunbasak, S. Optic Nerve Sheath Diameter and Retinal Artery Resistive Index Measurements with Bedside Ophthalmic Ultrasound in Pediatric Patients with Pseudotumor Cerebri Syndrome. J. Pediatr. Intensiv. Care 2020, 9, 181–187. [Google Scholar] [CrossRef]
- Padayachy, L.C.; Padayachy, V.; Galal, U.; Pollock, T.; Fieggen, A.G. The relationship between transorbital ultrasound measurement of the optic nerve sheath diameter (ONSD) and invasively measured ICP in children. Child’s Nerv. Syst. 2016, 32, 1779–1785. [Google Scholar] [CrossRef]
- Kerscher, S.R.; Schöni, D.; Hurth, H.; Neunhoeffer, F.; Haas-Lude, K.; Wolff, M.; Schuhmann, M.U. The relation of optic nerve sheath diameter (ONSD) and intracranial pressure (ICP) in pediatric neurosurgery practice—Part I: Correlations, age-dependency and cut-off values. Child’s Nerv. Syst. 2020, 36, 99–106. [Google Scholar] [CrossRef]
- Robba, C.; Cardim, D.; Czosnyka, M.; Abecasis, F.; Pezzato, S.; Buratti, S.; Moscatelli, A.; Sortica, C.; Racca, F.; Pelosi, P.; et al. Ultrasound non-invasive intracranial pressure assessment in paediatric neurocritical care: A pilot study. Child’s Nerv. Syst. 2019, 36, 117–124. [Google Scholar] [CrossRef]
- Fontanel, L.; Pensiero, S.; Ronfani, L.; Rosolen, V.; Barbi, E. Optic Nerve Sheath Diameter Ultrasound: Optic Nerve Growth Curve and Its Application to Detect Intracranial Hypertension in Children. Am. J. Ophthalmol. 2019, 208, 421–428. [Google Scholar] [CrossRef]
- Steinborn, M.; Friedmann, M.; Makowski, C.; Hahn, H.; Hapfelmeier, A.; Juenger, H. High resolution transbulbar sonography in children with suspicion of increased intracranial pressure. Child’s Nerv. Syst. 2016, 32, 655–660. [Google Scholar] [CrossRef] [PubMed]
- Aslan, N.; Yıldızdaş, D.; Horoz, Ö.Ö.; Özsoy, M.; Yöntem, A.; Çetinalp, E.; Mert, G.G. Evaluation of ultrasonographic optic nerve sheath diameter and central retinal artery Doppler indices by point-of-care ultrasound in pediatric patients with increased intracranial pressure. Turk. J. Pediatr. 2021, 63, 300–306. [Google Scholar] [CrossRef] [PubMed]
- Le, A.; Hoehn, M.E.; Smith, M.E.; Spentzas, T.; Schlappy, D.; Pershad, J. Bedside Sonographic Measurement of Optic Nerve Sheath Diameter as a Predictor of Increased Intracranial Pressure in Children. Ann. Emerg. Med. 2009, 53, 785–791. [Google Scholar] [CrossRef]
- Gravendeel, J.; Rosendahl, K. Cerebral biometry at birth and at 4 and 8 months of age. A prospective study using US. Pediatr. Radiol. 2010, 40, 1651–1656. [Google Scholar] [CrossRef] [PubMed]
- Ardell, S.; Daspal, S.; Holt, T.; Hansen, G. Optic Nerve Sheath Diameter for Preterm Infants: A Pilot Study. Neonatology 2019, 116, 1–5. [Google Scholar] [CrossRef]
- Yapicioglu, H.; Aslan, N.; Sertdemir, Y.; Yildizdas, D.; Gulasi, S.; Mert, K. Determination of normal values of optic nerve sheath diameter in newborns with bedside ultrasonography. Early Hum. Dev. 2020, 145, 104986. [Google Scholar] [CrossRef]
- Lochner, P.; Leone, M.A.; Coppo, L.; Nardone, R.; Zedde, M.L.; Cantello, R.; Brigo, F. B-mode transorbital ultrasononography for the diagnosis of acute optic neuritis. A systematic review. Clin. Neurophysiol. 2015, 127, 803–809. [Google Scholar] [CrossRef]
- Sargsyan, A.E.; Blaivas, M.; Geeraerts, T.; Karakitsos, D. Ocular ultrasound in the intensivecare unit. In Critical Care Ultrasound, 1st ed.; Lumb, P., Karakitsos, D., Eds.; Elsevier Saunders: Philadelphia, PA, USA, 2014; ISBN 978-1-4557-5357-4. [Google Scholar]
- Bloria, S.D.; Bloria, P.; Luthra, A. Is it the time to standardize the procedure of ultrasound guided optic nerve sheath diameter measurement? Saudi J. Anaesth. 2019, 13, 255–256. [Google Scholar] [CrossRef]
- Lochner, P.; Czosnyka, M.; Naldi, A.; Lyros, E.; Pelosi, P.; Mathur, S.; Fassbender, K.; Robba, C. Optic nerve sheath diameter: Present and future perspectives for neurologists and critical care physicians. Neurol. Sci. 2019, 40, 2447–2457. [Google Scholar] [CrossRef]
- Pansell, J.; Bell, M.; Rudberg, P.; Friman, O.; Cooray, C. Optic nerve sheath diameter measurement by ultrasound: Evaluation of a standardized protocol. J. Neuroimaging 2021, 32, 104–110. [Google Scholar] [CrossRef]
- Zeiler, F.A.; Ziesmann, M.T.; Goeres, P.; Unger, B.; Park, J.; Karakitsos, D.; Blaivas, M.; Vergis, A.; Gillman, L.M. A unique method for estimating the reliability learning curve of optic nerve sheath diameter ultrasound measurement. Crit. Ultrasound J. 2016, 8, 9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| Population | ICP Reference Values |
|---|---|
| Adults | <10–15 mm Hg |
| Children | 3–7 mm Hg |
| Term infants | 1.5–6 mm Hg |
| Author | Included Children (n) | Normal Mean ONSD Value (mm) | Cut-Off Value for ONSD (mm) | ||
|---|---|---|---|---|---|
| Ballantyne et al. [102] | 5 (0–2 months) | 2.57 (SD 0.30) | >4 (<1 year) | ||
| 9 (2–3 months) | 2.95 (SD 0.35) | >4.5 (1–15 years) | |||
| 5 (3–12 months) | 3.21 (SD 0.22) | ||||
| 9 (1–2 years) | 2.99 (SD 0.23) | ||||
| 17 (2–3 years) | 3.03 (SD 0.20) | ||||
| 18 (3–4 years) | 3.15 (SD 0.28) | ||||
| 16 (4–5 years) | 3.23 (SD 0.38) | ||||
| 10 (5–10 years) | 2.98 (SD 0.16) | ||||
| 13 (10–15 years) | 3.26 (SD 0.35) | ||||
| Rehman Siddiqui et al. [103] | 48 | Patients with signs of raised ICP | Patients with no signs of raised ICP | ||
| 8 (1 month–1 year) | 4.64 (SD 0.48) n = 3 | 4.32 (SD 0.71) n = 5 | >4 (SE 100% SP 60%) | ||
| 21 (1–10 years) | 6.44 (SD 0.65) n = 10 | 5.03 (SD 0.82) n = 11 | >4.71 (SE 100% SP 63.6%) | ||
| 19 (10–16 years) | 6.28 (SD 0.62) n = 13 | 5.46 (SD 0.91) n = 6 | >5.43 (SE 100% SP 66.7%) | ||
| Irazuzta et al. [104] | 13 (12–18 years) | Patients with CSF OP greater than 20 cm 2O | Patients with CSF OP less than 20 cm H2O | >4.5 mm (SE 100%) | |
| n = 3 | n = 10 | ||||
| 5.5 ± 1.2 (right eye) | 3.9 ± 0.1 mm (right eye) | ||||
| 5.4 ± 1 (left eye) | 3.7 ± 0.2 mm (left eye) | ||||
| Aslan et al. [105] | 22 (7–17 years) | PTCS group | Control group | Not reported in the article | |
| (84–204 months) | |||||
| PTCS group | n = 7 | n = 15 | |||
| (30–204 months) | 6.7 (SD 0.5) (right eye) | 5.3 (SD 0.2) (right eye) | |||
| Control group | 6.7 (SD 0.6) (left eye) | 5.2 (SD 0.3) (left eye) | |||
| Padayachy et al. [106] | 174 | Not reported in the article | ICP threshold of 20 mm Hg | ||
| Overall | 5.5 | ||||
| 56 (≤1 year) | 5.16 (SE 80%, SP 76.1%) | ||||
| 118 (>1 year) | 5.75 (SE 85.9%, SP 70.4%) | ||||
| 62 (open AF) | 5.16 (SE 85.7%, SP 75%) | ||||
| 112 (closed AF) | 5.80 (SE 85%, SP 73.1%) | ||||
| Kerscher et al. [107] | 72 | Not reported in the article | ICP threshold of 20 mm Hg | ||
| Overall | 5.57 (SE 81.3%, SP 62.5) | ||||
| 21 (≤1 year) | 4.99 (SE 50%, SP 58.8%) | ||||
| 51 (>1 year) | 5.75 (SE 91.7, SP 66.7) | ||||
| ICP threshold of 15 mm Hg | |||||
| Overall | 5.28 (SE 90.9%, SP 69.2%) | ||||
| 21 (≤1 year) | 4.99 (SE 71.4%, SP 71.4%) | ||||
| 51 (>1 year) | 5.57 (SE 80%, SP 69.2%) | ||||
| Robba et al. [108] | 10 (4–14 years) | 3.70 (4.50–3.40) Median (IQR) | ICP threshold of 20 mm Hg | ||
| 4.75 (SE 0.956, SP 0.938) | |||||
| ICP threshold of 15 mm Hg | |||||
| 3.85 (SE 0.811, SP 0.939) | |||||
| Fontanel et al. [109] | 215 (0–18 years) | ||||
| ≥4.1 (SE 100%, SP 83.9%) | |||||
| 29 IHT | |||||
| 0 (<1 year) | |||||
| 0 (1–4 years) | |||||
| 29 (>4 years) | IHT | Healthy | ODD | ||
| n = 29 | n = 165 | n = 21 | (>4 y) | ||
| 165 healthy | |||||
| 21 (<1 year) | 4.9 (4.5–5.1) (>4 y) | 4.0 (3.8–4.1) (>4 y) | 4.0 (3.8–4.0) (>4 y) | ≥4.1 (SE 100%, SP 89.3%) | |
| 29 (1–4 years) | |||||
| 115 (>4 years) | |||||
| Median (IQR) | Median (IQR) | Median (IQR) | ≥4.4 (SE 100%, SP 98.8%) (11–18 y) | ||
| 21 optic disc drusen (ODD) | |||||
| 1 (<1 year) | |||||
| 1 (1–4 years) | |||||
| 19 (>4 years) | |||||
| Steinborn et al. [110] | 81 (3–17.8 years) | Increased ICP | Normal ICP | >6 (SE 82%, SP 74%) | |
| n = 25 | n = 56 | ||||
| 6.85 (SD 0.81) | 5.77 (SD 0.48) | ||||
| Malayeri et al. [42] | 156 | Case group | Control group | Not reported in the article | |
| n = 78 | n = 78 | ||||
| 34 (<4 years) | 5.55 (SD 0.68) (<4 y) | 3.00 (SD 0.67) (<4 y) | |||
| 44 (>4 years) | 5.68 (SD 0.71) (>4 y) | 3.60 (SD 0.42) (>4 y) | |||
| Case group increased ICP | |||||
| 32 (<4 years) | |||||
| 46 (>4 years) | |||||
| Control group normal ICP | |||||
| Aslan et al. [111] | 57 (3–204 months) | Increased ICP | Normal ICP | 5.8 (SE 66%, SP 100%) | |
| (suspected clinically or radiologically) | |||||
| n = 38 | n = 19 | ||||
| 5.9 (SD 0.8) | 5.2 (SD 0.3) | ||||
| Le et al. [112] | 64 (0–18 years) | Increased ICP | Suspected ICP | >4 (<1 year) | |
| Suspected or confirmed increased ICP | (cranial imaging or direct measurement) | >4.5 (>1 year) | |||
| n = 24 | n = 40 | (SE 83%, SP 38%) | |||
| Not reported in the article | Not reported in the article | ||||
| Author | Included Neonates (n) | Normal Mean ONSD Value (mm) | Cut-Off Value for ONSD (mm) | |
|---|---|---|---|---|
| Ballantyne et al. [102] | 5 (0–2 months) | 2.57 (SD 0.30) | >4 (<1 year) | |
| 9 (2–3 months) | 2.95 (SD 0.35) | >4.5 (1–15 years) | ||
| 5 (3–12 months) | 3.21 (SD 0.22) | |||
| 9 (1–2 years) | 2.99 (SD 0.23) | |||
| 17 (2–3 years) | 3.03 (SD 0.20) | |||
| 18 (3–4 years) | 3.15 (SD 0.28) | |||
| 16 (4–5 years) | 3.23 (SD 0.38) | |||
| 10 (5–10 years) | 2.98 (SD 0.16) | |||
| 13 (10–15 years) | 3.26 (SD 0.35) | |||
| Gravendeel J et al. [113] | 120 (37–42 weeks of gestation) | Not reported in the article | ||
| 0–4 days | 3.9 (3.1–4.7) | |||
| 4 months | 5.5 (4.5–6.5) | |||
| 8 months | 5.8 (5.0–6.6) | |||
| 95% reference intervals Males | ||||
| 0–4 days | 3.7 (2.7–4.7) | |||
| 4 months | 5.3 (4.3–6.3) | |||
| 8 months | 5.6 (4.6–6.6) | |||
| 95% reference intervals Females | ||||
| Ardell S et al. [114] | 12 preterm infants (29–36 weeks postconceptional age) | Right eye | Left eye | Not reported in the article |
| 29 weeks | 2.1 (SD 0.1) | 2.1 (SD 0.2) | ||
| 30 weeks | 2.2 (SD 0.1) | 2.3 (SD 0.2) | ||
| 31 weeks | 2.4 (SD 0.1) | 2.4 (SD 0.1) | ||
| 32 weeks | 2.7 (SD 0.3) | 2.7 (SD 0.4) | ||
| 33 weeks | 2.7 (SD 0.2) | 2.7 (SD 0.3) | ||
| 34 weeks | 2.9 (SD 0.2) | 3.0 (SD 0.3) | ||
| 35 weeks | 3.2 (SD 0.4) | 3.2 (SD 0.3) | ||
| 36 weeks | 3.1 (SD 0.3) | 3.2 (SD 0.2) | ||
| Yapicioglu et al. [115] | 554 | Not reported in the article | ||
| 22 (23 weeks 0 day–28 weeks 6 days) | 2.6 (SD 0.3)—Distance 2 mm | |||
| 2.7 (SD 0.3)—Distance 2.5 mm | ||||
| 64 (29 weeks 0 day–32 weeks 6 days) | 3.0 (SD 0.2)—Distance 2 mm | |||
| 3.1 (SD 0.2)—Distance 2.5 mm | ||||
| 167 (33 weeks 0 day–36 weeks 6 days) | 3.3 (SD 0.2)—Distance 2 mm | |||
| 3.5 (SD 0.2)—Distance 2.5 mm | ||||
| 3.6 (SD 0.2)—Distance 3 mm | ||||
| 301 (37 weeks 0 day–41 weeks 6 days) | 4.0 (SD 0.2)—Distance 3 mm | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cannata, G.; Pezzato, S.; Esposito, S.; Moscatelli, A. Optic Nerve Sheath Diameter Ultrasound: A Non-Invasive Approach to Evaluate Increased Intracranial Pressure in Critically Ill Pediatric Patients. Diagnostics 2022, 12, 767. https://doi.org/10.3390/diagnostics12030767
Cannata G, Pezzato S, Esposito S, Moscatelli A. Optic Nerve Sheath Diameter Ultrasound: A Non-Invasive Approach to Evaluate Increased Intracranial Pressure in Critically Ill Pediatric Patients. Diagnostics. 2022; 12(3):767. https://doi.org/10.3390/diagnostics12030767
Chicago/Turabian StyleCannata, Giulia, Stefano Pezzato, Susanna Esposito, and Andrea Moscatelli. 2022. "Optic Nerve Sheath Diameter Ultrasound: A Non-Invasive Approach to Evaluate Increased Intracranial Pressure in Critically Ill Pediatric Patients" Diagnostics 12, no. 3: 767. https://doi.org/10.3390/diagnostics12030767
APA StyleCannata, G., Pezzato, S., Esposito, S., & Moscatelli, A. (2022). Optic Nerve Sheath Diameter Ultrasound: A Non-Invasive Approach to Evaluate Increased Intracranial Pressure in Critically Ill Pediatric Patients. Diagnostics, 12(3), 767. https://doi.org/10.3390/diagnostics12030767





