Early Markers in Resistant Schizophrenia: Effect of the First Antipsychotic Drug
Abstract
:1. Background
2. Material and Methods
- Prospective monitoring for a period of at least 12 weeks.
- Administration of at least two antipsychotic medication trials at a dose corresponding to or greater than 600 mg chlorpromazine equivalents.
- Reduction of symptoms when assessed with the PANSS and BPRS by less than 20% for the observed period of time.
- The assessment of social dysfunction using the SOFAS (Social and Occupational Functioning Assessment Scale) is below 60 [46].
- Mental retardation.
- Presence of organic brain damage.
- Concomitant progressive neurological or severe somatic diseases.
- Expressed personality change.
- Score of MMSI below 25 points.
3. Results
- -
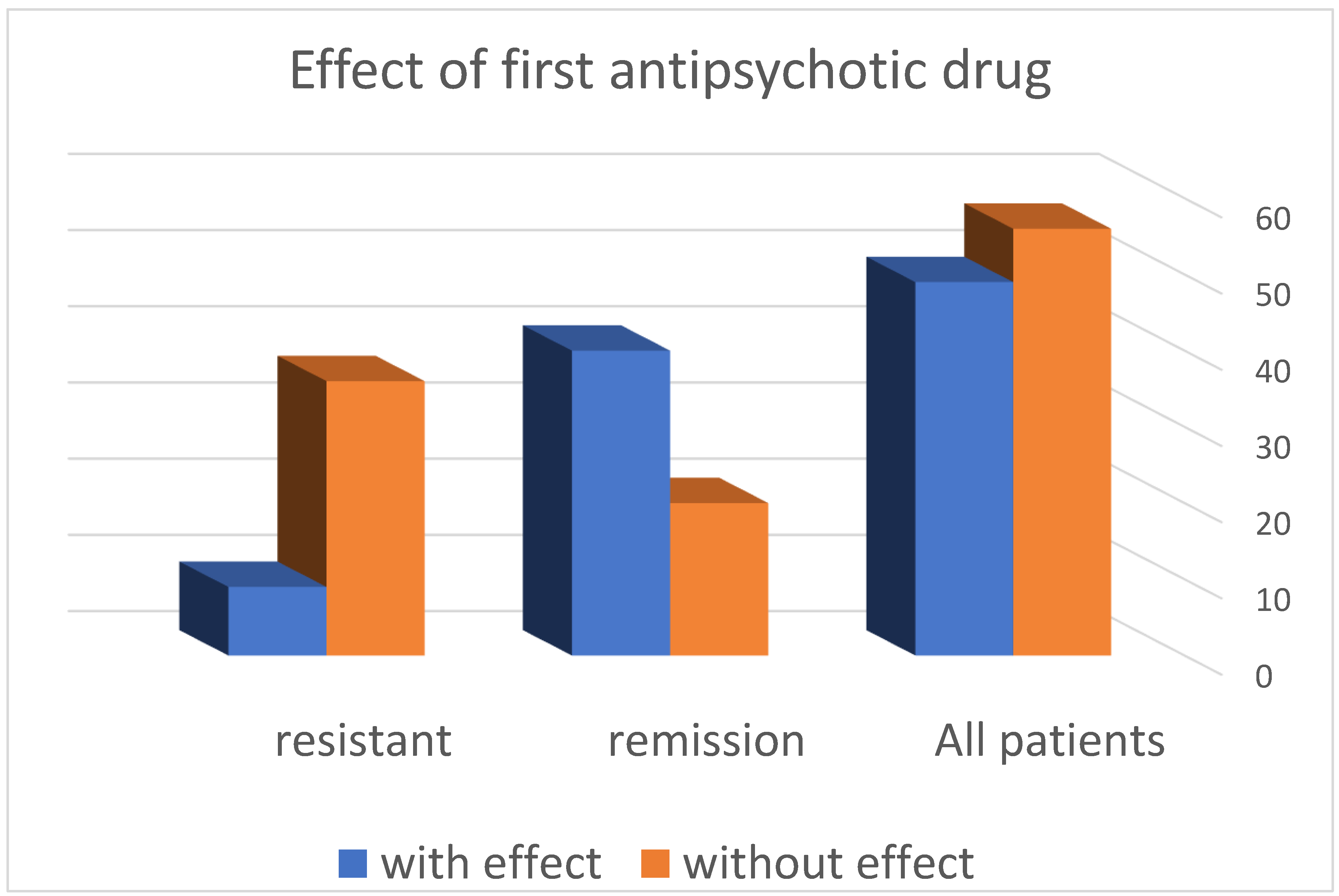
- The distribution of patients according to the response to the first antipsychotic drug in the resistance group shows that in 36 (80%) patients, no effect was observed, and in the remaining 9 (20%), such an effect was registered.
- -
- In the group of patients in remission, it was found that in 20 (33.33%) patients, no effect was observed when using the first neuroleptic, and in the remaining 40 (66.57%), an effect was found.
- -
4. Discussion
5. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Nicola, V.; Stoyanov, D. Psychiatry in Crisis: At the Crossroads of Social Sciences, the Humanities, and Neuroscience; Springer Nature: Berlin, Germany, 2019; ISBN 13 978-3030551391. [Google Scholar]
- Tanaka, M.; Vécsei, L. Editorial of Special Issue “Crosstalk between Depression, Anxiety, and Dementia: Comorbidity in Behavioral Neurology and Neuropsychiatry. Biomedicines 2021, 9, 517. [Google Scholar] [CrossRef] [PubMed]
- Howes, O.; Kapur, S. The Dopamine Hypothesis of Schizophrenia: Version III—The Final Common Pathway. Schizophr. Bull. 2009, 35, 549–562. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moustafa, S.R.; Al-Rawi, K.F.; Stoyanov, D.; Al-Dujaili, A.H.; Supasitthumrong, T.; Al-Hakeim, H.K.; Maes, M. The Endogenous Opioid System in Schizophrenia and Treatment Resistant Schizophrenia: Increased Plasma Endomorphin 2, and κ and μ Opioid Receptors Are Associated with Interleukin-6. Diagnostics 2020, 10, 633. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, M.; Tóth, F.; Polyák, H.; Szabó, Ágnes; Mándi, Y.; Vécsei, L. Immune Influencers in Action: Metabolites and Enzymes of the Tryptophan-Kynurenine Metabolic Pathway. Biomedicines 2021, 9, 734. [Google Scholar] [CrossRef]
- Correia, B.; Nani, J.; Ricardo, R.W.; Stanisic, D.; Costa, T.; Hayashi, M.; Tasic, L. Effects of Psychostimulants and Antipsychotics on Serum Lipids in an Animal Model for Schizophrenia. Biomedicines 2021, 9, 235. [Google Scholar] [CrossRef]
- Nyatega, C.O.; Qiang, L.; Adamu, M.J.; Younis, A.; Kawuwa, H.B. Altered Dynamic Functional Connectivity of Cuneus in Schizophrenia Patients: A Resting-State fMRI Study. Appl. Sci. 2021, 11, 11392. [Google Scholar] [CrossRef]
- Candini, M.; Battaglia, S.; Benassi, M.; di Pellegrino, G.; Frassinetti, F. The physiological correlates of interpersonal space. Sci. Rep. 2021, 11, 2611. [Google Scholar] [CrossRef]
- Battaglia, S.; Harrison, B.J.; Fullana, M.A. Does the human ventromedial prefrontal cortex support fear learning, fear extinction or both? A commentary on subregional contributions. Mol. Psychiatry 2021. [Google Scholar] [CrossRef]
- Stoyanov, D.; Kandilarova, S.; Borgwardt, S.; Stieglitz, R.-D.; Hugdahl, K.; Kostianev, S. Psychopathology Assessment Methods Revisited: On Translational Cross-Validation of Clinical Self-Evaluation Scale and fMRI. Front. Psychiatry 2018, 9, 21. [Google Scholar] [CrossRef] [Green Version]
- Stoyanov, D.; Aryutova, K.; Kandilarova, S.; Paunova, R.; Arabadzhiev, Z.; Todeva-Radneva, A.; Kostianev, S.; Borgwardt, S. Diagnostic Task Specific Activations in Functional MRI and Aberrant Connectivity of Insula with Middle Frontal Gyrus Can Inform the Differential Diagnosis of Psychosis. Diagnostics 2021, 11, 95. [Google Scholar] [CrossRef]
- Koch, M.; Schmiedt-Fehr, C.; Mathes, B. Neuropharmacology of altered brain oscillations in schizophrenia. Int. J. Psychophysiol. 2016, 103, 62–68. [Google Scholar] [CrossRef] [PubMed]
- Veerman, S.R.T.; Schulte, P.F.J.; De Haan, L. The glutamate hypothesis: A pathogenic pathway from which pharmacological interventions have emerged. Pharmacopsychiatry 2014, 47, 121–130. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Angelopoulos, E. Brain functional connectivity and the pathophysiology of schizophrenia. Psychiatriki 2014, 25, 91–94. [Google Scholar] [PubMed]
- De Pablo, G.S.; Woods, S.W.; Drymonitou, G.; de Diego, H.; Fusar-Poli, P. Prevalence of Individuals at Clinical High-Risk of Psychosis in the General Population and Clinical Samples: Systematic Review and Meta-Analysis. Brain Sci. 2021, 11, 1544. [Google Scholar] [CrossRef]
- Weber, S.; Johnsen, E.; Kroken, R.A.; Løberg, E.-M.; Kandilarova, S.; Stoyanov, D.; Kompus, K.; Hugdahl, K. Dynamic Functional Connectivity Patterns in Schizophrenia and the Relationship With Hallucinations. Front. Psychiatry 2020, 11, 227. [Google Scholar] [CrossRef]
- Dzyubenko, E.; Juckel, G.; Faissner, A. The antipsychotic drugs olanzapine and haloperidol modify network connectivity and spontaneous activity of neural networks in vitro. Sci. Rep. 2017, 7, 11609. [Google Scholar] [CrossRef] [Green Version]
- Towlson, E.K.; Vértes, P.E.; Müller-Sedgwick, U.; Ahnert, S.E. Brain Networks Reveal the Effects of Antipsychotic Drugs on Schizophrenia Patients and Controls. Front. Psychiatry 2019, 10, 611. [Google Scholar] [CrossRef]
- Miller, A.L.; Chiles, J.A.; Chiles, J.K.; Crismon, M.L.; Rush, A.J.; Shon, S.P. The Texas Medication Algorithm Project (TMAP) schizophrenia algorithms. J. Clin. Psychiatry 1999, 60, 649–657. [Google Scholar] [CrossRef]
- May, P.R.; Van Putten, T.; Yale, C. Predicting outcome of antipsychotic drug treatment from early response. Am. J. Psychiatry 1980, 137, 1088–1089. [Google Scholar] [CrossRef]
- Correll, C.U.; Malhotra, A.K.; Kaushik, S.; McMeniman, M.; Kane, J.M. Early Prediction of Antipsychotic Response in Schizophrenia. Am. J. Psychiatry 2003, 160, 2063–2065. [Google Scholar] [CrossRef]
- Agid, O.; Kapur, S.; Arenovich, T.; Zipursky, R.B. Delayed-onset hypothesis of antipsychotic action: A hypothesis tested and rejected. Arch. Gen. Psychiatry 2003, 60, 1228–1235. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leucht, S.; Busch, R.; Hamann, J.; Kissling, W.; Kane, J.M. Early-onset hypothesis of antipsychotic drug action: A hypothesis tested, confirmed and extended. Biol. Psychiatry 2005, 57, 1543–1549. [Google Scholar] [CrossRef] [PubMed]
- Overall, J.E.; Gorham, D.R. The Brief Psychiatric Rating Scale. Psychol. Rep. 1962, 10, 799–812. [Google Scholar] [CrossRef]
- Ascher-Svanum, H.; Nyhuis, A.W.; Faries, D.E.; Kinon, B.J.; Baker, R.W.; Shekhar, A. Clinical, functional, and economic ramifications of early nonresponse to antipsychotics in the naturalistic treatment of schizophrenia. Schizophr. Bull. 2008, 34, 1163–1171. [Google Scholar] [CrossRef] [Green Version]
- Kinon, B.J.; Chen, L.; Ascher-Svanum, H.; Stauffer, V.L.; Kollack-Walker, S.; Sniadecki, J.L.; Kane, J.M. Predicting response to atypical antipsychotics based on early response in the treatment of schizophrenia. Schizophr. Res. 2008, 102, 230–240. [Google Scholar] [CrossRef]
- Leucht, S.; Davis, J.M.; Engel, R.; Kane, J.M.; Wagenpfeil, S. Defining ‘Response’ in Antipsychotic Drug Trials: Recommendations for the Use of Scale-Derived Cutoffs. Neuropsychopharmacology 2007, 32, 1903–1910. [Google Scholar] [CrossRef]
- Jones, P.B.; Barnes, T.R.E.; Davies, L.; Dunn, G.; Lloyd, H.; Hayhurst, K.P.; Murray, R.; Markwick, A.; Lewis, S. Randomized controlled trial of the effect on Quality of Life of second- vs first-generation antipsychotic drugs in schizophrenia: Cost Utility of the Latest Antipsychotic Drugs in Schizophrenia Study (CUTLASS 1). Arch. Gen. Psychiatry 2006, 63, 1079–1087. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Canive, J.M.; A Miller, G.; Irwin, J.G.; Moses, S.N.; Thoma, R.J.; Edgar, J.C.; Sherwood, A.; Torres, F.; LaNoue, M.; Lewis, S.; et al. Efficacy of olanzapine and risperidone in schizophrenia: A randomized double-blind crossover design. Psychopharmacol. Bull. 2006, 39, 105–116. [Google Scholar]
- Simpson, G.M.; O’Gorman, C.J.; Loebel, A.; Yang, R. Long-term improvement in efficacy and safety after switching to ziprasidone in stable outpatients with schizophrenia. CNS Spectr. 2008, 13, 898–905. [Google Scholar] [CrossRef]
- Wang, X.; Savage, R.; Borisov, A.; Rosenberg, J.; Woolwine, B.; Tucker, M.; May, R.; Feldman, J.; Nemeroff, C.B.; Miller, A.H. Efficacy of risperidone versus olanzapine in patients with schizophrenia previously on chronic conventional antipsychotic therapy: A switch study. J. Psychiatr. Res. 2006, 40, 669–676. [Google Scholar] [CrossRef]
- Essock, S.M.; Covell, N.H.; Davis, S.M.; Stroup, T.S.; Rosenheck, R.A.; Lieberman, J.A. Effectiveness of switching antipsychotic medications. Am. J. Psychiatry 2006, 163, 2090–2095. [Google Scholar] [CrossRef] [PubMed]
- Rosenheck, R.A.; Davis, S.; Covell, N.; Essock, S.; Swartz, M.; Stroup, S.; McEvoy, J.; Lieberman, J. Does switching to a new antipsychotic improve outcomes? Data from the CATIE Trial. Schizophr. Res. 2009, 107, 22–29. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, H.; Kamata, M.; Yoshida, K.; Ishigooka, J.; Higuchi, H. Switching to olanzapine after unsuccessful treatment with risperidone during the first episode of schizophrenia: An open-label trial. J. Clin. Psychiatry 2006, 67, 1577–1582. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, H.; Yoshida, K.; Ishigooka, J.; Higuchi, H. Switching to risperidone after unsuccessful treatment of olanzapine in the first-episode schizophrenia: An open trial. Prog. Neuropsychopharmacol. Biol. Psychiatry 2006, 30, 1067–1072. [Google Scholar] [CrossRef] [PubMed]
- Kay, S.R.; Fiszbein, A.; Opler, L.A. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr. Bull. 1987, 13, 261–276. [Google Scholar] [CrossRef]
- Kinon, B.J.; Chen, L.; Ascher-Svanum, H.; Stauffer, V.L.; Kollack-Walker, S.; Zhou, W.; Kapur, S.; Kane, J.M. Early response to antipsychotic drug therapy as a clinical marker of subsequent response in the treatment of schizophrenia. Neuropsychopharmacology 2010, 35, 581–590. [Google Scholar] [CrossRef]
- Lally, J.; MacCabe, J.H. Antipsychotic medication in schizophrenia: A review. Br. Med. Bull. 2015, 114, 169–179. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Volavka, J.; Citrome, L. Oral antipsychotics for the treatment of schizophrenia: Heterogeneity in efficacy and tolerability should drive decision-making. Expert Opin. Pharmacother. 2009, 10, 1917–1928. [Google Scholar] [CrossRef] [PubMed]
- Lieberman, J.A.; McEvoy, J.P.; Swartz, M.S.; Rosenheck, R.A.; Perkins, D.O.; Keefe, R.S.; Davis, S.M.; Davis, C.E.; Lebowitz, B.D.; Severe, J.; et al. Effectiveness of antipsychotic drugs in patients with chronic schizophrenia. N. Engl. J. Med. 2005, 353, 1209–1223. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kayo, M.; Tassell, I.; Hiroce, V.; Menezes, A.; Elkis, H. Does lack of improvement in the first two weeks predict treatment resistance in recent-onset psychosis? Clinics 2012, 67, 1479–1482. [Google Scholar] [CrossRef]
- Lieberman, J.; Jody, D.; Geisler, S.; Alvir, J.; Loebel, A.; Szymanski, S.; Woerner, M.; Borenstein, M. Time course and biologic correlates of treatment response in first-episode schizophrenia. Arch. Gen. Psychiatry 1993, 50, 369–376. [Google Scholar] [CrossRef] [PubMed]
- Kwan, P.; Brodie, M.J. Early identification of refractory epilepsy. N. Engl. J. Med. 2000, 342, 314–319. [Google Scholar] [CrossRef] [PubMed]
- American Psychiatric Association (APA). Diagnostic and Statistical Manual of Mental Disorders; American Psychiatric Association: Washington, DC, USA, 2013; ISBN 978-0-89042-554-1. [Google Scholar]
- Howes, O.D.; McCutcheon, R.; Agid, O.; De Bartolomeis, A.; Van Beveren, N.J.; Birnbaum, M.L.; Bloomfield, M.; Bressan, R.A.; Buchanan, R.W.; Carpenter, W.T.; et al. Consensus Guidelines on Diagnosis and Terminology. Am. J. Psychiatry 2017, 174, 216–229. [Google Scholar] [CrossRef] [PubMed]
- Morosini, P.L.; Magliano, L.; Brambilla, L.; Ugolini, S.; Pioli, R. Development, reliability and acceptability of a new version of the DSM-IV Social and Occupational Functioning Assessment Scale (SOFAS) to assess routine social functioning. Acta Psychiatr. Scand. 2000, 101, 323–329. [Google Scholar] [CrossRef] [PubMed]
- Kinon, B.J.; Kane, J.M.; Chakos, M.; Munne, R. Possible predictors of neuroleptic-resistant schizophrenic relapse: Influence of negative symptoms and acute extrapyramidal side effects. Psychopharmacol. Bull. 1993, 29, 365–369. [Google Scholar] [PubMed]
- Wolf, P.; Trimble, M.R. Biological antagonism and epileptic psychosis. Br. J. Psychiatry 1985, 146, 272–276. [Google Scholar] [CrossRef] [PubMed]
- Emsley, R.; Rabinowitz, J.; Medori, R. Time course for antipsychotic treatment response in first-episode schizophrenia. Am. J. Psychiatry 2006, 163, 743–745. [Google Scholar] [CrossRef] [PubMed]
- Agid, O.; Arenovich, T.; Sajeev, G.; Zipursky, R.B.; Kapur, S.; Foussias, G.; Remington, G. An algorithm-based approach to first-episode schizophrenia: Response rates over 3 prospective antipsychotic trials with a retrospective data analysis. J. Clin. Psychiatry 2011, 72, 1439–1444. [Google Scholar] [CrossRef] [PubMed]
- Mousavi, S.G.; Rostami, H.; Sharbafchi, M.R.; Boroujeni, A.S.; Mahaki, B. Onset of action of atypical and typical antipsychotics in the treatment of acute psychosis. J. Res. Pharm. Pract. 2013, 2, 138–144. [Google Scholar] [CrossRef] [Green Version]
- Uçok, A.; Gaebel, W. Side effects of atypical antipsychotics: A brief overview. World Psychiatry 2008, 7, 58–62. [Google Scholar] [CrossRef]
- Stroup, T.S.; Gray, N. Management of common adverse effects of antipsychotic medications. World Psychiatry 2018, 17, 341–356. [Google Scholar] [CrossRef] [PubMed]
- Dibben, C.R.; Khandaker, G.M.; Underwood, B.R.; O’Loughlin, C.; Keep, C.; Mann, L.; Jones, P.B. First-generation antipsychotics: Not gone but forgotten. BJPsych Bull. 2016, 40, 93–96. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leucht, S.; Tardy, M.; Komossa, K.; Heres, S.; Kissling, W.; Salanti, G.; Davis, J.M. Antipsychotic drugs versus placebo for relapse prevention in schizophrenia: A systematic review and meta-analysis. Lancet 2012, 379, 2063–2071. [Google Scholar] [CrossRef]
- Palotai, M.; Telegdy, G.; Tanaka, M.; Zsolt Bagosi, Z.; Miklós Jászberényi, M. Neuropeptide AF induces anxiety-like and antidepressant-like behavior in mice. Behav. Brain Res. 2014, 274, 264–269. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Borgomaneri, S.; Battaglia, S.; Avenanti, A.; Pellegrino, G.D. Don’t Hurt Me No More: State-dependent Transcranial Magnetic Stimulation for the treatment of specific phobia. J. Affect. Disord. 2021, 286, 78–79. [Google Scholar] [CrossRef] [PubMed]
- Chervyakov, A.V.; Chernyavsky, A.Y.; Sinitsyn, D.O.; Piradov, M. Possible Mechanisms Underlying the Therapeutic Effects of Transcranial Magnetic Stimulation. Front. Hum. Neurosci. 2015, 9, 303. [Google Scholar] [CrossRef]
| First Antipsychotic Medication | |||
|---|---|---|---|
| Count | With Effect | Without Effect | |
| All patients | 105 | 49 (46.7%) | 56 (53.3%) |
| Resistant Sch | 45 | 9 (20%) | 36 (80%) |
| Remission Sch | 60 | 40 (66.57%) | 20 (33.33%) |
| Value | Asymptotic Standard Error | Approximate T | Approximate Significance | ||
|---|---|---|---|---|---|
| Interval-by-Interval | Pearson’s R | 0.463 | 0.084 | 5.300 | 0.000 |
| Ordinal-by-Ordinal | Spearman Correlation | 0.463 | 0.084 | 5.300 | 0.000 |
| No. of Valid Cases | 105 | ||||
| Chi-Square Tests | |||||
|---|---|---|---|---|---|
| Value | df | Asymptotic Significance (2-Sided) | Exact Sig. (2-Sided) | Exact Sig. (1-Sided) | |
| Pearson’s Chi-Square | 22.500 | 1 | 0.000 | ||
| Continuity Correction | 20.664 | 1 | 0.000 | ||
| Likelihood Ratio | 23.676 | 1 | 0.000 | ||
| Fisher’s Exact Test | 0.000 | 0.000 | |||
| Linear-by-Linear | 22.286 | 1 | 0.000 | ||
| No. of Valid Cases | 105 | ||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Panov, G.P. Early Markers in Resistant Schizophrenia: Effect of the First Antipsychotic Drug. Diagnostics 2022, 12, 803. https://doi.org/10.3390/diagnostics12040803
Panov GP. Early Markers in Resistant Schizophrenia: Effect of the First Antipsychotic Drug. Diagnostics. 2022; 12(4):803. https://doi.org/10.3390/diagnostics12040803
Chicago/Turabian StylePanov, Georgi Panov. 2022. "Early Markers in Resistant Schizophrenia: Effect of the First Antipsychotic Drug" Diagnostics 12, no. 4: 803. https://doi.org/10.3390/diagnostics12040803
APA StylePanov, G. P. (2022). Early Markers in Resistant Schizophrenia: Effect of the First Antipsychotic Drug. Diagnostics, 12(4), 803. https://doi.org/10.3390/diagnostics12040803





