P-Wave Beat-to-Beat Analysis to Predict Atrial Fibrillation Recurrence after Catheter Ablation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Measurements
2.1.1. Standard P-Wave Indices
2.1.2. P-Wave Beat-to-Beat Analysis
2.1.3. Echocardiographic Study
2.1.4. Clinical Scores
2.2. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
| Variable | Female (n = 34) | Male (n = 104) | p Value |
|---|---|---|---|
| Age | 61.3 ± 9.2 | 57.9 ± 8.9 | 0.057 |
| AF duration (months) | 76.8 ± 77.1 | 72 ± 68.7 | 0.976 |
| Heart failure | 1 (2.9%) | 3 (2.9%) | 0.510 |
| Stroke/TIA | 2 (5.9%) | 6 (5.8%) | 0.980 |
| CAD | 1 (2.9%) | 9 (8.7%) | 0.265 |
| Hypertension | 15 (44.1%) | 49 (47.1%) | 0.761 |
| Diabetes mellitus | 6 (17.6%) | 6 (5.8%) | 0.033 |
| Dyslipidemia | 10 (29.4%) | 32 (30.8%) | 0.881 |
| COPD | 1 (2.9%) | 4 (3.8%) | 0.806 |
| Ablation type (RF) | 16 (47.1%) | 64 (61.5%) | 0.138 |
| AF type (paroxysmal) | 30 (88.2%) | 91 (87.5%) | 0.910 |
| Redo | 3 (8.8%) | 6 (5.8%) | 0.531 |
| Buddle brunch block | 2 (5.9%) | 10 (9.6%) | 0.502 |
| AADs | 26 (76.5%) | 78 (75%) | 0.863 |
| Smoking (current) | 4 (11.8%) | 27 (26%) | 0.085 |
| BMI > 30 | 17 (50%) | 36 (34.6%) | 0.109 |
| Metabolic s. | 6 (17.6%) | 13 (12.5%) | 0.450 |
| EF | 57.8 ± 5.1 | 59.6 ± 4.3 | 0.098 |
| LA diameter | 38.8 ± 5.9 | 41.9 ± 4.7 | 0.010 |
| LA area | 20.9 ± 4.2 | 22.9 ± 3.5 | 0.005 |
| LA volume | 65 ± 18 | 72.6 ± 15.9 | 0.029 |
| BSA | 1.8 ± 0.2 | 2.1 ± 0.2 | <0.001 |
| BMI | 29.8 ± 6.7 | 28.7 ± 3.4 | 0.334 |
| LAVI | 34.7 ± 11.3 | 34.3 ± 7.5 | 0.832 |
References
- Virani, S.S.; Alonso, A.; Aparicio, H.J.; Benjamin, E.J.; Bittencourt, M.S.; Callaway, C.W.; Carson, A.P.; Chamberlain, A.M.; Cheng, S.; Delling, F.N.; et al. Heart Disease and Stroke Statistics-2021 Update A Report from the American Heart Association. Circulation 2021, 143, E254–E743. [Google Scholar] [CrossRef]
- Hindricks, G.; Potpara, T.; Dagres, N.; Bax, J.J.; Boriani, G.; Dan, G.A.; Fauchier, L.; Kalman, J.M.; Lane, D.A.; Lettino, M.; et al. 2020 ESC Guidelines for the Diagnosis and Management of Atrial Fibrillation Developed in Collaboration with the European Association for Cardio-Thoracic Surgery (EACTS): The Task Force for the Diagnosis and Management of Atrial Fibrillation of the European Society of Cardiology (ESC) Developed with the Special Contribution of the European Heart Rhythm Association (EHRA) of the ESC. Eur. Heart J. 2021, 42, 373–498. [Google Scholar] [CrossRef]
- Nielsen, J.C.; Johannessen, A.; Raatikainen, P.; Hindricks, G.; Walfridsson, H.; Pehrson, S.M.; Englund, A.; Hartikainen, J.; Mortensen, L.S.; Hansen, P.S. Long-Term Efficacy of Catheter Ablation as First-Line Therapy for Paroxysmal Atrial Fibrillation: 5-Year Outcome in a Randomised Clinical Trial. Heart 2017, 103, 368–376. [Google Scholar] [CrossRef] [PubMed]
- Blomström-Lundqvist, C.; Gizurarson, S.; Schwieler, J.; Jensen, S.M.; Bergfeldt, L.; Kennebäck, G.; Rubulis, A.; Malmborg, H.; Raatikainen, P.; Lönnerholm, S.; et al. Effect of Catheter Ablation vs Antiarrhythmic Medication on Quality of Life in Patients with Atrial Fibrillation: The CAPTAF Randomized Clinical Trial. JAMA-J. Am. Med. Assoc. 2019, 321, 1059–1068. [Google Scholar] [CrossRef] [PubMed]
- Ganesan, A.N.; Shipp, N.J.; Brooks, A.G.; Kuklik, P.; Lau, D.H.; Lim, H.S.; Sullivan, T.; Roberts-Thomson, K.C.; Sanders, P. Long-Term Outcomes of Catheter Ablation of Atrial Fibrillation: A Systematic Review and Meta-Analysis. J. Am. Heart Assoc. 2013, 2, e004549. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Canpolat, U.; Aytemir, K.; Yorgun, H.; Şahiner, L.; Kaya, E.B.; Oto, A. A Proposal for a New Scoring System in the Prediction of Catheter Ablation Outcomes: Promising Results from the Turkish Cryoablation Registry. Int. J. Cardiol. 2013, 169, 201–206. [Google Scholar] [CrossRef] [PubMed]
- Wójcik, M.; Berkowitsch, A.; Greiss, H.; Zaltsberg, S.; Pajitnev, D.; Deubner, N.; Hamm, C.W.; Pitschner, H.F.; Kuniss, M.; Neumann, T. Repeated Catheter Ablation of Atrial Fibrillation: How to Predict Outcome? Circ. J. Off. J. Jpn. Circ. Soc. 2013, 77, 2271–2279. [Google Scholar] [CrossRef] [Green Version]
- Kornej, J.; Hindricks, G.; Kosiuk, J.; Arya, A.; Sommer, P.; Husser, D.; Rolf, S.; Richter, S.; Huo, Y.; Piorkowski, C.; et al. Comparison of CHADS2, R2CHADS2, and CHA 2DS2-VASc Scores for the Prediction of Rhythm Outcomes after Catheter Ablation of Atrial Fibrillation the Leipzig Heart Center AF Ablation Registry. Circ. Arrhythmia Electrophysiol. 2014, 7, 281–287. [Google Scholar] [CrossRef] [Green Version]
- Kornej, J.; Hindricks, G.; Shoemaker, M.B.; Husser, D.; Arya, A.; Sommer, P.; Rolf, S.; Saavedra, P.; Kanagasundram, A.; Patrick Whalen, S.; et al. The APPLE Score: A Novel and Simple Score for the Prediction of Rhythm Outcomes after Catheter Ablation of Atrial Fibrillation. Clin. Res. Cardiol. Off. J. Ger. Card. Soc. 2015, 104, 871–876. [Google Scholar] [CrossRef] [Green Version]
- Kosiuk, J.; Dinov, B.; Kornej, J.; Acou, W.-J.; Schönbauer, R.; Fiedler, L.; Buchta, P.; Myrda, K.; Gąsior, M.; Poloński, L.; et al. Prospective, Multicenter Validation of a Clinical Risk Score for Left Atrial Arrhythmogenic Substrate Based on Voltage Analysis: DR-FLASH Score. Heart Rhythm 2015, 12, 2207–2212. [Google Scholar] [CrossRef]
- Winkle, R.A.; Jarman, J.W.E.; Mead, R.H.; Engel, G.; Kong, M.H.; Fleming, W.; Patrawala, R.A. Predicting Atrial Fibrillation Ablation Outcome: The CAAP-AF Score. Heart Rhythm 2016, 13, 2119–2125. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mujović, N.; Marinković, M.; Marković, N.; Shantsila, A.; Lip, G.Y.H.; Potpara, T.S. Prediction of Very Late Arrhythmia Recurrence after Radiofrequency Catheter Ablation of Atrial Fibrillation: The MB-LATER Clinical Score. Sci. Rep. 2017, 7, 40828. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mesquita, J.; Ferreira, A.M.; Cavaco, D.; Moscoso Costa, F.; Carmo, P.; Marques, H.; Morgado, F.; Mendes, M.; Adragão, P. Development and Validation of a Risk Score for Predicting Atrial Fibrillation Recurrence after a First Catheter Ablation Procedure–ATLAS Score. EP Eur. 2018, 20, f428–f435. [Google Scholar] [CrossRef] [PubMed]
- Letsas, K.P.; Efremidis, M.; Giannopoulos, G.; Deftereos, S.; Lioni, L.; Korantzopoulos, P.; Vlachos, K.; Xydonas, S.; Kossyvakis, C.; Sideris, A. CHADS2 and CHA2DS2-VASc Scores as Predictors of Left Atrial Ablation Outcomes for Paroxysmal Atrial Fibrillation. Europace 2014, 16, 202–207. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jud, F.N.; Obeid, S.; Duru, F.; Haegeli, L.M. A Novel Score in the Prediction of Rhythm Outcome after Ablation of Atrial Fibrillation: The SUCCESS Score. Anatol. J. Cardiol. 2019, 21, 142–149. [Google Scholar] [CrossRef]
- Jastrzębski, M.; Kiełbasa, G.; Fijorek, K.; Bednarski, A.; Kusiak, A.; Sondej, T.; Bednarek, A.; Wojciechowska, W.; Rajzer, M. Comparison of Six Risk Scores for the Prediction of Atrial Fibrillation Recurrence after Cryoballoon-Based Ablation and Development of a Simplified Method, the 0-1-2 PL Score. J. Arrhythmia 2021, 37, 956–964. [Google Scholar] [CrossRef]
- Choi, J.H.; Kwon, H.J.; Kim, H.R.; Park, S.J.; Kim, J.S.; On, Y.K.; Park, K.M. Electrocardiographic Predictors of Early Recurrence of Atrial Fibrillation. Ann. Noninvasive Electrocardiol. 2021, 26, e12884. [Google Scholar] [CrossRef]
- Tachmatzidis, D.; Filos, D.; Chouvarda, I.; Tsarouchas, A.; Mouselimis, D.; Bakogiannis, C.; Lazaridis, C.; Triantafyllou, K.; Antoniadis, A.P.; Fragakis, N.; et al. Beat-to-Beat P-Wave Analysis Outperforms Conventional P-Wave Indices in Identifying Patients with a History of Paroxysmal Atrial Fibrillation during Sinus Rhythm. Diagnostics 2021, 11, 1694. [Google Scholar] [CrossRef]
- Wiesel, J.; Fitzig, L.; Herschman, Y.; Messineo, F.C. Detection of Atrial Fibrillation Using a Modified Microlife Blood Pressure Monitor. Am. J. Hypertens. 2009, 22, 848–852. [Google Scholar] [CrossRef] [Green Version]
- Lewis, M.; Parker, D.; Weston, C.; Bowes, M. Screening for Atrial Fibrillation: Sensitivity and Specificity of a New Methodology. Br. J. Gen. Pract. J. R. Coll. Gen. Pract. 2011, 61, 38–39. [Google Scholar] [CrossRef] [Green Version]
- von Elm, E.; Altman, D.G.; Egger, M.; Pocock, S.J.; Gøtzsche, P.C.; Vandenbroucke, J.P. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) Statement: Guidelines for Reporting Observational Studies. J. Clin. Epidemiol. 2008, 61, 344–349. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Salah, A.; Zhou, S.; Liu, Q.; Yan, H. P Wave Indices to Predict Atrial Fibrillation Recurrences Post Pulmonary Vein Isolation. Arq. Bras. Cardiol. 2013, 101, 519–527. [Google Scholar] [CrossRef] [PubMed]
- Yıldırım, E.; Günay, N.; Bayam, E.; Keskin, M.; Ozturkeri, B.; Selcuk, M. Relationship between Paroxysmal Atrial Fibrillation and a Novel Electrocardiographic Parameter P Wave Peak Time. J. Electrocardiol. 2019, 57, 81–86. [Google Scholar] [CrossRef]
- German, D.M.; Kabir, M.M.; Dewland, T.A.; Henrikson, C.A.; Tereshchenko, L.G. Atrial Fibrillation Predictors: Importance of the Electrocardiogram. Ann. Noninvasive Electrocardiol. 2016, 21, 20–29. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- van Beeumen, K.; Houben, R.; Tavernier, R.; Ketels, S.; Duytschaever, M. Changes in P-Wave Area and P-Wave Duration after Circumferential Pulmonary Vein Isolation. EP Eur. 2010, 12, 798–804. [Google Scholar] [CrossRef] [PubMed]
- Park, J.K.; Park, J.; Uhm, J.S.; Joung, B.; Lee, M.H.; Pak, H.N. Low P-Wave Amplitude (<0.1 MV) in Lead I Is Associated with Displaced Inter-Atrial Conduction and Clinical Recurrence of Paroxysmal Atrial Fibrillation after Radiofrequency Catheter Ablation. Europace 2016, 18, 384–391. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.; Zheng, Z.; Wu, B.; Tang, L.; Xie, X.; Dong, R.; Luo, Y.; Li, S.; Zhu, J.; Liu, J. Predictive Value of P Wave Terminal Force in Lead V1 for Atrial Fibrillation: A Meta-analysis. Ann. Noninvasive Electrocardiol. 2020, 25, e12739. [Google Scholar] [CrossRef]
- Bayés de Luna, A.; Platonov, P.; Cosio, F.G.; Cygankiewicz, I.; Pastore, C.; Baranowski, R.; Bayés-Genis, A.; Guindo, J.; Viñolas, X.; Garcia-Niebla, J.; et al. Interatrial Blocks. A Separate Entity from Left Atrial Enlargement: A Consensus Report. J. Electrocardiol. 2012, 45, 445–451. [Google Scholar] [CrossRef]
- Alexander, B.; Milden, J.; Hazim, B.; Haseeb, S.; Bayes-Genis, A.; Elosua, R.; Martínez-Sellés, M.; Yeung, C.; Hopman, W.; Bayes de Luna, A.; et al. New Electrocardiographic Score for the Prediction of Atrial Fibrillation: The MVP ECG Risk Score (Morphology-Voltage-P-Wave Duration). Ann. Noninvasive Electrocardiol. 2019, 24, e12669. [Google Scholar] [CrossRef] [Green Version]
- Eranti, A.; Carlson, J.; Kenttä, T.; Holmqvist, F.; Holkeri, A.; Haukilahti, M.A.; Kerola, T.; Aro, A.L.; Rissanen, H.; Noponen, K.; et al. Orthogonal P-Wave Morphology, Conventional P-Wave Indices, and the Risk of Atrial Fibrillation in the General Population Using Data from the Finnish Hospital Discharge Register. EP Eur. 2020, 22, 1173–1181. [Google Scholar] [CrossRef]
- Filos, D.; Chouvarda, I.; Tachmatzidis, D.; Vassilikos, V.; Maglaveras, N. Beat-to-Beat P-Wave Morphology as a Predictor of Paroxysmal Atrial Fibrillation. Comput. Methods Programs Biomed. 2017, 151, 111–121. [Google Scholar] [CrossRef] [PubMed]
- Lang, R.M.; Badano, L.P.; Victor, M.A.; Afilalo, J.; Armstrong, A.; Ernande, L.; Flachskampf, F.A.; Foster, E.; Goldstein, S.A.; Kuznetsova, T.; et al. Recommendations for Cardiac Chamber Quantification by Echocardiography in Adults: An Update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J. Am. Soc. Echocardiogr. Off. Publ. Am. Soc. Echocardiogr. 2015, 28, 1–39. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Creed, J.; Gerke, T.; Berglund, A. MatSurv: Survival Analysis and Visualization in MATLAB. J. Open Source Softw. 2020, 5, 1830. [Google Scholar] [CrossRef]
- Vittinghoff, E.; McCulloch, C.E. Relaxing the Rule of Ten Events per Variable in Logistic and Cox Regression. Am. J. Epidemiol. 2007, 165, 710–718. [Google Scholar] [CrossRef] [Green Version]
- Conte, G.; Luca, A.; Yazdani, S.; Caputo, M.L.; Regoli, F.; Moccetti, T.; Kappenberger, L.; Vesin, J.-M.M.; Auricchio, A. Usefulness of P-Wave Duration and Morphologic Variability to Identify Patients Prone to Paroxysmal Atrial Fibrillation. Am. J. Cardiol. 2017, 119, 275–279. [Google Scholar] [CrossRef]
- Censi, F.; Corazza, I.; Reggiani, E.; Calcagnini, G.; Mattei, E.; Triventi, M.; Boriani, G. P-Wave Variability and Atrial Fibrillation. Sci. Rep. 2016, 6, 26799. [Google Scholar] [CrossRef] [PubMed]
- Nakatani, Y.; Sakamoto, T.; Yamaguchi, Y.; Tsujino, Y.; Kataoka, N.; Kinugawa, K. Coefficient of Variation of P-Wave Duration Measured Using an Automated Measurement System Predicts Recurrence of Atrial Fibrillation. J. Electrocardiol. 2019, 53, 79–84. [Google Scholar] [CrossRef] [PubMed]
- García Iglesias, D.; Roqueñi Gutiérrez, N.; de Cos, F.J.; Calvo, D. Analysis of the High-Frequency Content in Human QRS Complexes by the Continuous Wavelet Transform: An Automatized Analysis for the Prediction of Sudden Cardiac Death. Sensors 2018, 18, 560. [Google Scholar] [CrossRef] [Green Version]
- Morlet, D.; Peyrin, F.; Desseigne, P.; Touboul, P.; Rubel, P. Wavelet Analysis of High-Resolution Signal-Averaged ECGs in Postinfarction Patients. J. Electrocardiol. 1993, 26, 311–320. [Google Scholar] [CrossRef]
- Vassilikos, V.P.; Dakos, G.; Chouvarda, I.; Karagounis, L.; Karvounis, H.; Maglaveras, N.; Mochlas, S.; Spanos, P.; Louridas, G.; Karvounis, C.; et al. Can P Wave Wavelet Analysis Predict Atrial Fibrillation after Coronary Artery Bypass Grafting? Pacing Clin. Electrophysiol. 2003, 26, 305–309. [Google Scholar] [CrossRef]
- Xia, X.; Couderc, J.-P.; Mcnitt, S.; Zareba, W. Predicting Effectiveness of Cardiac Resynchronization Therapy Based on QRS Decomposition Using the Meyer Orthogonal Wavelet Transformation. Comput. Cardiol. 2010, 37, 983–986. [Google Scholar]
- Vassilikos, V.P.; Mantziari, L.; Dakos, G.; Kamperidis, V.; Chouvarda, I.; Chatzizisis, Y.S.; Kalpidis, P.; Theofilogiannakos, E.; Paraskevaidis, S.; Karvounis, H.; et al. QRS Analysis Using Wavelet Transformation for the Prediction of Response to Cardiac Resynchronization Therapy: A Prospective Pilot Study. J. Electrocardiol. 2014, 47, 59–65. [Google Scholar] [CrossRef] [PubMed]
- Batchvarov, V.N.; Bortolan, G.; Christov, I.I.; Bastiaenen, R.; Raju, H.; Naseef, A.; Behr, E.R. ECG Wavelet Analysis for the Detection of Gene Mutations in Patients with Brugada Syndrome. In Proceedings of the Computing in Cardiology, Hangzhou, China, 18–21 September 2011; Volume 38, pp. 785–788. [Google Scholar]
- Takayama, H.; Yodogawa, K.; Katoh, T.; Takano, T. Evaluation of Arrhythmogenic Substrate in Patients With Hypertrophic Cardiomyopathy Using Wavelet Transform Analysis. Circ. J. 2006, 70, 69–74. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yi, G.; Hnatkova, K.; Mahon, N.G.; Keeling, P.J.; Reardon, M.; Camm, A.J.; Malik, M. Predictive Value of Wavelet Decomposition of the Signal-Averaged Electrocardiogram in Idiopathic Dilated Cardiomyopathy. Eur. Heart J. 2000, 21, 1015–1022. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Girasis, C.; Vassilikos, V.; Efthimiadis, G.K.; Papadopoulou, S.L.; Dakos, G.; Dalamaga, E.G.; Chouvarda, I.; Giannakoulas, G.; Kamperidis, V.; Paraskevaidis, S.; et al. Patients with Hypertrophic Cardiomyopathy at Risk for Paroxysmal Atrial Fibrillation: Advanced Echocardiographic Evaluation of the Left Atrium Combined with Non-Invasive P-Wave Analysis. Eur. Heart J. Cardiovasc. Imaging 2013, 14, 425–434. [Google Scholar] [CrossRef] [Green Version]
- Dakos, G.; Konstantinou, D.; Chatzizisis, Y.S.; Chouvarda, I.; Filos, D.; Paraskevaidis, S.; Mantziari, L.; Maglaveras, N.; Karvounis, H.; Vassilikos, V. P Wave Analysis with Wavelets Identifies Hypertensive Patients at Risk of Recurrence of Atrial Fibrillation: A Case-Control Study and 1 Year Follow-Up. J. Electrocardiol. 2015, 48, 845–852. [Google Scholar] [CrossRef]
- Dakos, G.; Chatzizisis, Y.S.; Konstantinou, D.; Chouvarda, I.; Filos, D.; Paraskevaidis, S.; Mantziari, L.; Maglaveras, N.; Karvounis, H.; Styliadis, I.; et al. Wavelet-Based Analysis of P Waves Identifies Patients with Lone Atrial Fibrillation: A Cross-Sectional Pilot Study. Int. J. Cardiol. 2014, 174, 389–392. [Google Scholar] [CrossRef]
- Vassilikos, V.; Dakos, G.; Chatzizisis, Y.S.; Chouvarda, I.; Karvounis, C.; Maynard, C.; Maglaveras, N.; Paraskevaidis, S.; Stavropoulos, G.; Styliadis, C.I.; et al. Novel Non-Invasive P Wave Analysis for the Prediction of Paroxysmal Atrial Fibrillation Recurrences in Patients without Structural Heart Disease: A Prospective Pilot Study. Int. J. Cardiol. 2011, 153, 165–172. [Google Scholar] [CrossRef]
- Pezzuto, S.; Gharaviri, A.; Schotten, U.; Potse, M.; Conte, G.; Caputo, M.L.; Regoli, F.; Krause, R.; Auricchio, A. Beat-to-Beat P-Wave Morphological Variability in Patients with Paroxysmal Atrial Fibrillation: An in Silico Study. Europace 2018, 20, III26–III35. [Google Scholar] [CrossRef]
- Filos, D.; Korosoglou, P.; Tachmatzidis, D.; Maglaveras, N.; Vassilikos, V.; Chouvarda, I. Multiple P-Wave Morphologies in Paroxysmal Atrial Fibrillation Patients During Sinus Rhythm: A Simulation Study. In Proceedings of the Computing in Cardiology Conference (Cinc), Maastricht, The Netherlands, 9 September 2018; pp. 1–4. [Google Scholar]
- Pranata, R.; Yonas, E.; Vania, R. Prolonged P-Wave Duration in Sinus Rhythm Pre-Ablation Is Associated with Atrial Fibrillation Recurrence after Pulmonary Vein Isolation—A Systematic Review and Meta-Analysis. Ann. Noninvasive Electrocardiol. 2019, 24, e12653. [Google Scholar] [CrossRef] [Green Version]
- Chen, Q.; Mohanty, S.; Trivedi, C.; Gianni, C.; della Rocca, D.G.; Canpolat, U.; Burkhardt, J.D.; Sanchez, J.E.; Hranitzky, P.; Gallinghouse, G.J.; et al. Association between Prolonged P Wave Duration and Left Atrial Scarring in Patients with Paroxysmal Atrial Fibrillation. J. Cardiovasc. Electrophysiol. 2019, 30, 1811–1818. [Google Scholar] [CrossRef] [PubMed]
- O’Neal, W.T.; Zhang, Z.M.; Loehr, L.R.; Chen, L.Y.; Alonso, A.; Soliman, E.Z. Electrocardiographic Advanced Interatrial Block and Atrial Fibrillation Risk in the General Population. Am. J. Cardiol. 2016, 117, 1755–1759. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martínez-Sellés, M.; Elosua, R.; Ibarrola, M.; de Andrés, M.; Díez-Villanueva, P.; Bayés-Genis, A.; Baranchuk, A.; Bayés-De-Luna, A. Advanced Interatrial Block and P-Wave Duration Are Associated with Atrial Fibrillation and Stroke in Older Adults with Heart Disease: The BAYES Registry. Europace 2020, 22, 1001–1008. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.T.; Zhao, D.Q.; Li, F.F.; Wu, R.; Fan, X.W.; Hu, G.L.; Bai, M.F.; Yang, H.T.; Yan, L.J.; Liu, J.J.; et al. Advanced Interatrial Block Predicts Recurrence of Atrial Fibrillation after Accessory Pathway Ablation in Patients with Wolff-Parkinson-White Syndrome. Clin. Cardiol. 2019, 42, 806–811. [Google Scholar] [CrossRef] [Green Version]
- Holmqvist, F.; Olesen, M.S.; Tveit, A.; Enger, S.; Tapanainen, J.; Jurkko, R.; Havmöller, R.; Haunsø, S.; Carlson, J.; Svendsen, J.H.; et al. Abnormal Atrial Activation in Young Patients with Lone Atrial Fibrillation. Europace 2011, 13, 188–192. [Google Scholar] [CrossRef]
- Efremidis, M.; Letsas, K.P.; Georgopoulos, S.; Karamichalakis, N.; Vlachos, K.; Lioni, L.; Bazoukis, G.; Saplaouras, A.; Sakellaropoulou, A.; Kolokathis, A.M.; et al. Safety, Long-Term Outcomes and Predictors of Recurrence Following a Single Catheter Ablation Procedure for Atrial Fibrillation. Acta Cardiol. 2018, 74, 319–324. [Google Scholar] [CrossRef]
- Kosich, F.; Schumacher, K.; Potpara, T.; Lip, G.Y.; Hindricks, G.; Kornej, J. Clinical Scores Used for the Prediction of Negative Events in Patients Undergoing Catheter Ablation for Atrial Fibrillation. Clin. Cardiol. 2019, 42, 320–329. [Google Scholar] [CrossRef]
- Maheshwari, A.; Norby, F.L.; Roetker, N.S.; Soliman, E.Z.; Koene, R.J.; Rooney, M.R.; O’Neal, W.T.; Shah, A.M.; Claggett, B.L.; Solomon, S.D.; et al. Refining Prediction of Atrial Fibrillation-Related Stroke Using the P2-CHA2DS2-VASc Score: ARIC and MESA. Circulation 2019, 139, 180–191. [Google Scholar] [CrossRef]
- Marrouche, N.F.; Brachmann, J.; Andresen, D.; Siebels, J.; Boersma, L.; Jordaens, L.; Merkely, B.; Pokushalov, E.; Sanders, P.; Proff, J.; et al. Catheter Ablation for Atrial Fibrillation with Heart Failure. N. Engl. J. Med. 2018, 378, 417–427. [Google Scholar] [CrossRef]
- Liang, J.J.; Callans, D.J. Ablation for Atrial Fibrillation in Heart Failure with Reduced Ejection Fraction. Card. Fail. Rev. 2018, 4, 33–37. [Google Scholar] [CrossRef] [Green Version]
- Mogensen, U.M.; Jhund, P.S.; Abraham, W.T.; Desai, A.S.; Dickstein, K.; Packer, M.; Rouleau, J.L.; Solomon, S.D.; Swedberg, K.; Zile, M.R.; et al. Type of Atrial Fibrillation and Outcomes in Patients With Heart Failure and Reduced Ejection Fraction. J. Am. Coll. Cardiol. 2017, 70, 2490–2500. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, R.K.; Williams, S.E.; Niederer, S.A.; O’Neill, M.D. Atrial Fibrillation Ablation in Patients with Heart Failure: One Size Does Not Fit All. Arrhythmia Electrophysiol. Rev. 2018, 7, 84–90. [Google Scholar] [CrossRef] [PubMed]
- Zink, M.D.; Chua, W.; Zeemering, S.; di Biase, L.; de Luna Antoni, B.; David, C.; Hindricks, G.; Haeusler, K.G.; Al-Khalidi, H.R.; Piccini, J.P.; et al. Predictors of Recurrence of Atrial Fibrillation within the First 3 Months after Ablation. EP Eur. 2020, 22, 1337–1344. [Google Scholar] [CrossRef]
- Botto, G.L.; Tortora, G.; Casale, M.C.; Canevese, F.L.; Maria Brasca, F.A. Impact of the Pattern of Atrial Fibrillation on Stroke Risk and Mortality. Arrhythmia Electrophysiol. Rev. 2021, 10, 68–76. [Google Scholar] [CrossRef] [PubMed]
- Zylla, M.M.; Brachmann, J.; Lewalter, T.; Hoffmann, E.; Kuck, K.H.; Andresen, D.; Willems, S.; Eckardt, L.; Tebbenjohanns, J.; Spitzer, S.G.; et al. Sex-Related Outcome of Atrial Fibrillation Ablation: Insights from the German Ablation Registry. Heart Rhythm 2016, 13, 1837–1844. [Google Scholar] [CrossRef] [PubMed]
- Donnellan, E.; Aagaard, P.; Kanj, M.; Jaber, W.; Elshazly, M.; Hoosien, M.; Baranowski, B.; Hussein, A.; Saliba, W.; Wazni, O. Association Between Pre-Ablation Glycemic Control and Outcomes among Patients with Diabetes Undergoing Atrial Fibrillation Ablation. JACC Clin. Electrophysiol. 2019, 5, 897–903. [Google Scholar] [CrossRef] [PubMed]
- Chao, T.F.; Ambrose, K.; Tsao, H.M.; Lin, Y.J.; Chang, S.L.; Lo, L.W.; Hu, Y.F.; Tuan, T.C.; Suenari, K.; Li, C.H.; et al. Relationship between the CHADS(2) Score and Risk of Very Late Recurrences after Catheter Ablation of Paroxysmal Atrial Fibrillation. Heart Rhythm 2012, 9, 1185–1191. [Google Scholar] [CrossRef]
- Bhargava, M.; di Biase, L.; Mohanty, P.; Prasad, S.; Martin, D.O.; Williams-Andrews, M.; Wazni, O.M.; Burkhardt, J.D.; Cummings, J.E.; Khaykin, Y.; et al. Impact of Type of Atrial Fibrillation and Repeat Catheter Ablation on Long-Term Freedom from Atrial Fibrillation: Results from a Multicenter Study. Heart Rhythm 2009, 6, 1403–1412. [Google Scholar] [CrossRef]
- Jiang, H.; Wang, W.; Wang, C.; Xie, X.; Hou, Y. Association of Pre-Ablation Level of Potential Blood Markers with Atrial Fibrillation Recurrence after Catheter Ablation: A Meta-Analysis. EP Europace 2017, 19, 392–400. [Google Scholar] [CrossRef]
- Perez, M.V.; Mahaffey, K.W.; Hedlin, H.; Rumsfeld, J.S.; Garcia, A.; Ferris, T.; Balasubramanian, V.; Russo, A.M.; Rajmane, A.; Cheung, L.; et al. Large-Scale Assessment of a Smartwatch to Identify Atrial Fibrillation. N. Engl. J. Med. 2019, 381, 1909. [Google Scholar] [CrossRef]
- Shen, M.J.; Arora, R.; Jalife, J. Atrial Myopathy. JACC Basic Transl. Sci. 2019, 4, 640. [Google Scholar] [CrossRef] [PubMed]
| Score | Study | Year | Parameters | Range |
|---|---|---|---|---|
| BASE-AF2 | Canpolat et al. | 2013 | AF duration, AF type, BMI, ERAF, LA diameter, current smoking | 0–6 |
| ALARMEc | Wójcik et al. | 2013 | AF type, eGFR, LA area, metabolic s, hypertrophic/dilated cardiomyopathy | 0–5 |
| CHA2DS2-VASc | Letsas et al. | 2014 | CHF, HTN, Age, DM, stroke/TIA/thromboembolism, vascular disease, gender | 0–9 |
| APPLE | Kornej et al. | 2015 | age, AF type, eGFR, LA diameter, LVEF | 0–5 |
| DR-FLASH | Kosiuk et al. | 2015 | age, AF type, eGFR, LA diam, gender, HTN, DM | 0–7 |
| CAAP-AF | Winkle et al. | 2016 | age, AF type, LA diameter, gender, CAD, number of antiarrhythmics failed | 0–13 |
| MB-LATER | Mujovic et al. | 2017 | AF type, LA diameter, gender, BBB, ERAF | 0–6 |
| ATLAS | Mesquita et al. | 2017 | age, AF type, LAVI, gender, current smoking | low risk < 6, high risk > 10 |
| SUCCESS | Jud et al. | 2019 | age, AF type, eGFR, LA diameter, LVEF, previous ablations | APPLE score plus 1 point for each previous ablation |
| 0-1-2 PL | Jastrzębski et al. | 2021 | AF type, LA diameter | 0–2 |
| Variable | |
|---|---|
| Age | 58.7 ± 9.1 |
| Male sex (%) | 104 (75.4) |
| Hypertension (%) | 64 (46.4) |
| Diabetes (%) | 12 (8.7) |
| Dyslipidemia (%) | 42 (30.4) |
| Stroke/TIA | 8 (5.8) |
| Coronary Artery Disease | 10 (7.2) |
| Heart Failure | 4 (2.9) |
| Chronic obstructive pulmonary disease | 5 (3.6) |
| Paroxysmal AF | 121 (87.7) |
| Persistent AF | 13 (9.4) |
| Long-standing persistent AF | 4 (2.9) |
| Body mass index (kg/m2) | 29.0 ± 4.4 |
| Parameter | Free from AF Recurrence (n = 100) | AF Recurrence (n = 38) | Univariate Analysis HR (95% CI) | p Value |
|---|---|---|---|---|
| Age (years) | 58.0 ± 9.5 | 60.6 ± 7.5 | 1.22 (0.64–2.31) | 0.539 |
| Female sex | 20 (20.0%) | 14 (36.8%) | 2.26 (1.05–4.89) | 0.038 |
| Heart failure | 1 (1.0%) | 3 (7.9%) | 3.41 (1.05–11.1) | 0.028 |
| CAD | 7 (7.0%) | 3 (7.9%) | 1.00 (0.31–3.25) | 0.999 |
| HTN | 45 (45.0%) | 19 (50.0%) | 1.34 (0.71–2.55) | 0.355 |
| Stroke/TIA | 3 (3.0%) | 5 (13.2%) | 3.34 (1.30–8.62) | 0.007 |
| Diabetes mellitus | 8 (8.0%) | 4 (10.5%) | 1.17 (0.39–3.53) | 0.736 |
| Dyslipidemia | 30 (30.0%) | 12 (31.6%) | 1.18 (0.58–2.4) | 0.626 |
| Metabolic s. | 13 (13.0%) | 6 (15.8%) | 1.24 (0.48–3.18) | 0.620 |
| BMI | 28.7 ± 4.1 | 29.5 ± 5.1 | 0.85 (0.45–1.63) | 0.623 |
| BMI > 30 kg/m2 | 36 (36.0%) | 17 (44.7%) | 1.31 (0.68–2.53) | 0.395 |
| COPD | 4 (4.0%) | 1 (2.6%) | 0.68 (0.13–3.59) | 0.700 |
| Smoking (current) | 22 (22.0%) | 9 (23.7%) | 1.03 (0.49–2.19) | 0.932 |
| AF duration (months) | 71.1 ± 71.0 | 78.7 ± 70.3 | 1.8 (0.95–3.41) | 0.066 |
| AF type (paroxysmal) | 89 (89.0%) | 32 (84.2%) | 0.68 (0.25–1.86) | 0.375 |
| Ablation type (RF) | 60 (60.0%) | 20 (52.6%) | 0.64 (0.33–1.24) | 0.155 |
| ERAF | 8 (8.0%) | 19 (50%) | 7.41 (3.88–14.09) | <0.001 |
| History of previous ablation | 8 (8.0%) | 1 (2.6%) | 0.33 (0.10–1.09) | 0.240 |
| Bundle branch block | 8 (8.0%) | 4 (10.5%) | 1.14 (0.38–3.39) | 0.801 |
| Antiarrhythmic drugs failure | 79 (79.0%) | 25 (65.8%) | 0.61 (0.29–1.29) | 0.140 |
| LV Ejection fraction (%) | 59.5 ± 4.3 | 58.2 ± 5.1 | 0.54 (0.28–1.04) | 0.063 |
| LA diameter (mm) | 41.2 ± 5.6 | 41.0 ± 4.1 | 0.94 (0.49–1.79) | 0.845 |
| LA area (cm2) | 22.7± 3.7 | 21.8 ± 3.7 | 0.79 (0.41–1.5) | 0.456 |
| LA volume (ml) | 72.1 ± 17.5 | 67.0 ± 13.5 | 0.69 (0.36–1.33) | 0.257 |
| LA Volume Index (ml/m2) | 35.0 ± 8.3 | 32.8 ± 8.9 | 1.09 (0.57–2.08) | 0.786 |
| Parameter | Free from AF Recurrence (n = 100) | AF Recurrence (n = 38) | Univariate Analysis HR (95% CI) | p Value |
|---|---|---|---|---|
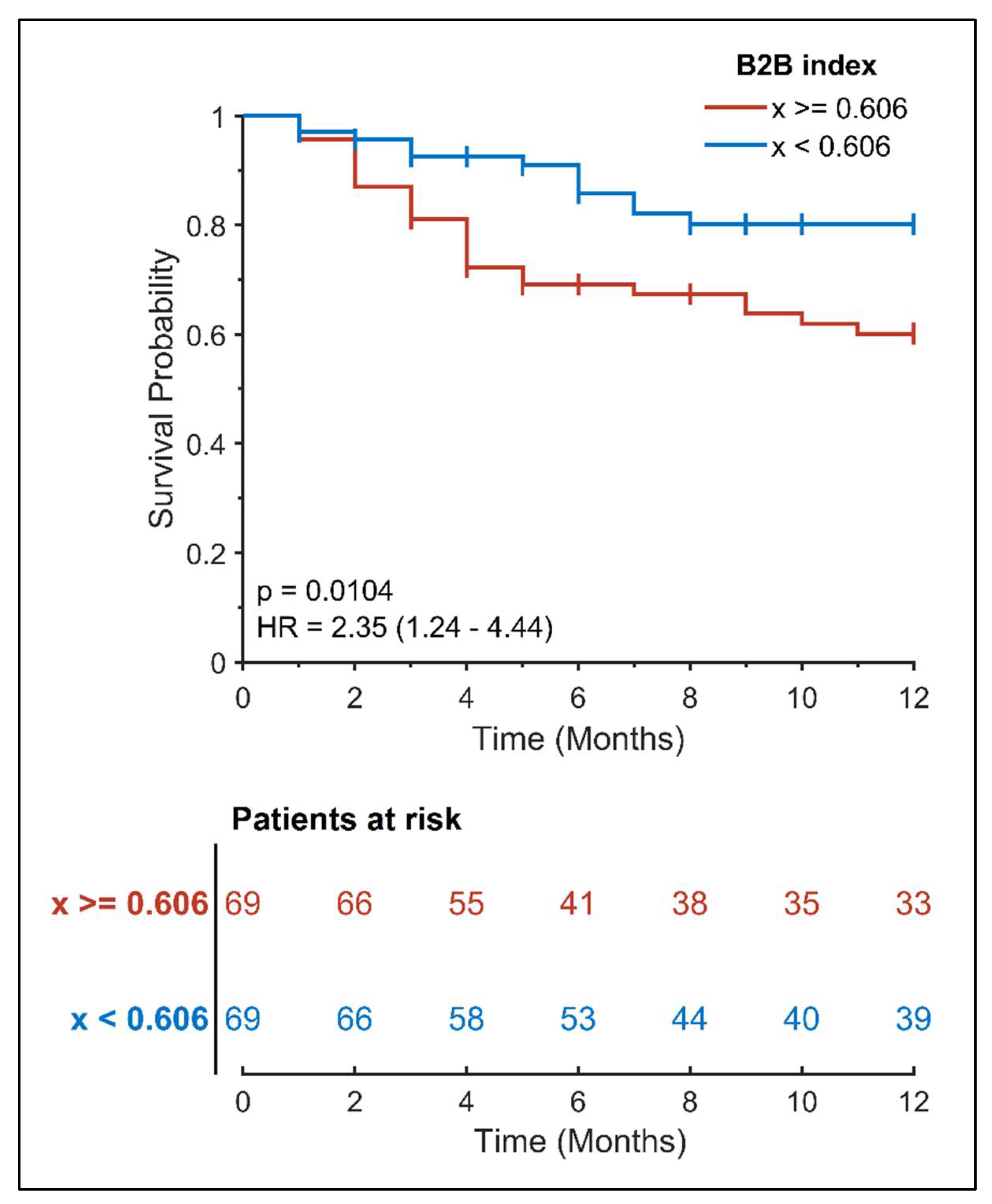
| B2B index | 0.59 ± 0.11 | 0.65 ± 0.13 | 2.35 (1.24–4.44) | 0.010 |
| P-wave duration, X-axis | 133.6 ± 17.5 | 134.4 ± 25.6 | 0.97 (0.51–1.84) | 0.925 |
| P-wave duration, Y-axis | 146.4 ± 18.4 | 146.8 ± 19.5 | 1.19 (0.63–2.25) | 0.588 |
| P-wave duration, Z-axis | 138.4 ± 20.2 | 140.4 ± 17.2 | 1.6 (0.85–3.02) | 0.147 |
| P-wave duration, lead II | 122.3 ± 12.2 | 124.1 ± 10.4 | 1.93 (1.04–3.59) | 0.040 |
| PR duration, lead II | 196.3 ± 30.8 | 196.4 ± 24.8 | 1.12 (0.59–2.12) | 0.721 |
| P-wave peak time, lead II | 67.6 ± 13.2 | 65.2 ±17.0 | 0.78 (0.42–1.48) | 0.449 |
| P-wave dispersion | 24.0 ± 13.4 | 28.4 ± 14.2 | 1.31 (0.69–2.48) | 0.400 |
| P-wave area, lead I | 5.0 ± 2.2 | 4.1 ± 2.4 | 0.56 (0.3–1.06) | 0.075 |
| P-wave area, lead II | 7.4 ± 3.3 | 7.1 ± 2.9 | 0.76 (0.4–1.43) | 0.383 |
| P-wave voltage lead I | 83.0 ± 37.2 | 65.4 ± 38.0 | 0.69 (0.36–1.3) | 0.247 |
| P-wave axis | 51.0 ± 14.3 | 57.6 ± 17.1 | 1.67 (0.89–3.16) | 0.116 |
| PTFV1 | 2.5 ± 2.3 | 2.0 ± 1.7 | 0.82 (0.44–1.56) | 0.541 |
| Orthogonal Type | 0.156 | |||
| Type 1 | 10 (10.0%) | 3 (7.9%) | 0.74 (0.26–2.08) | 0.604 |
| Type 2 | 81 (81.0%) | 27 (71.1%) | 0.71 (0.33–1.52) | 0.324 |
| Type 3 | 2 (2.0%) | 3 (7.9%) | 3.17 (0.44–23.10) | 0.039 |
| Interatrial Block | 0.097 | |||
| No IAB | 49 (49.0%) | 13 (34.2%) | 0.59 (0.30–1.16) | 0.128 |
| Partial IAB | 41 (41.0%) | 17 (44.7%) | 1.07 (0.56–2.02) | 0.841 |
| Advanced IAB | 10 (10.0%) | 8 (21.1) | 2.38 (1.08–5.24) | 0.031 |
| MVP score | 3.3 ± 1.0 | 3.5 ± 1.0 | 1.54 (0.81–2.94) | 0.203 |
| Score | Free from AF Recurrence (n = 100) | AF Recurrence (n = 38) | Univariate Analysis HR (95% CI) | p Value |
|---|---|---|---|---|
| CHA2DS2-VASc ≥ 2 | 35 (35%) | 22 (57.9%) | 2.24 (1.16–4.32) | 0.010 |
| ALARMEc ≥ 1 | 52 (52%) | 19 (50%) | 1.01 (0.53–1.93) | 0.971 |
| APPLE ≥ 1 | 58 (58%) | 23 (60.5%) | 1.15 (0.60–2.21) | 0.674 |
| DR-FLASH ≥ 2 | 44 (44%) | 19 (50%) | 1.38 (0.721–2.65) | 0.314 |
| CAAP-AF ≥ 4 | 43 (43%) | 18 (47.4%) | 1.25 (0.66–2.41) | 0.481 |
| ATLAS ≥ 5 | 49 (49%) | 25 (65.8%) | 1.92 (1.01–3.66) | 0.054 |
| SUCCESS ≥ 1 | 63 (63%) | 23 (60.5%) | 0.97 (0.50–1.89) | 0.924 |
| 0-1-2 PL ≥ 1 | 33 (33%) | 9 (23.7%) | 0.76 (0.37–1.53) | 0.453 |
| Variable | Hazard Ratio | Hazard Ratio 95% Boundary | p-Value |
|---|---|---|---|
| B2B index | 2.13 | 1.06–4.28 | 0.033 |
| Heart failure | 3.58 | 1.08–11.86 | 0.037 |
| Stroke/TIA | 3.37 | 1.30–8.71 | 0.012 |
| Advanced IAB | 2.22 | 0.98–5.01 | 0.056 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tachmatzidis, D.; Tsarouchas, A.; Mouselimis, D.; Filos, D.; Antoniadis, A.P.; Lysitsas, D.N.; Mezilis, N.; Sakellaropoulou, A.; Giannopoulos, G.; Bakogiannis, C.; et al. P-Wave Beat-to-Beat Analysis to Predict Atrial Fibrillation Recurrence after Catheter Ablation. Diagnostics 2022, 12, 830. https://doi.org/10.3390/diagnostics12040830
Tachmatzidis D, Tsarouchas A, Mouselimis D, Filos D, Antoniadis AP, Lysitsas DN, Mezilis N, Sakellaropoulou A, Giannopoulos G, Bakogiannis C, et al. P-Wave Beat-to-Beat Analysis to Predict Atrial Fibrillation Recurrence after Catheter Ablation. Diagnostics. 2022; 12(4):830. https://doi.org/10.3390/diagnostics12040830
Chicago/Turabian StyleTachmatzidis, Dimitrios, Anastasios Tsarouchas, Dimitrios Mouselimis, Dimitrios Filos, Antonios P. Antoniadis, Dimitrios N. Lysitsas, Nikolaos Mezilis, Antigoni Sakellaropoulou, Georgios Giannopoulos, Constantinos Bakogiannis, and et al. 2022. "P-Wave Beat-to-Beat Analysis to Predict Atrial Fibrillation Recurrence after Catheter Ablation" Diagnostics 12, no. 4: 830. https://doi.org/10.3390/diagnostics12040830







