A Comprehensive Review of Methods and Equipment for Aiding Automatic Glaucoma Tracking
Abstract
:1. Introduction
- Step 1: the collection of the patient’s history and an ophthalmological examination, refractometry to verify the degree of axis, verification of eye movements, biomicroscopy for visualization from the anterior part of the eye, direct ophthalmoscopy for visualization of the fundus of the eye or fundus biomicroscopy, tonometry (measurement of eye pressure) and a presumptive diagnosis of pathologies that must include glaucoma screening;
- Step 2: the negative cases will have semiannual or annual returns, and the positive cases are forwarded for subsidiary examinations and treated. Patients undergoing treatment are monitored and may require treatment adjustments according to their evolution.
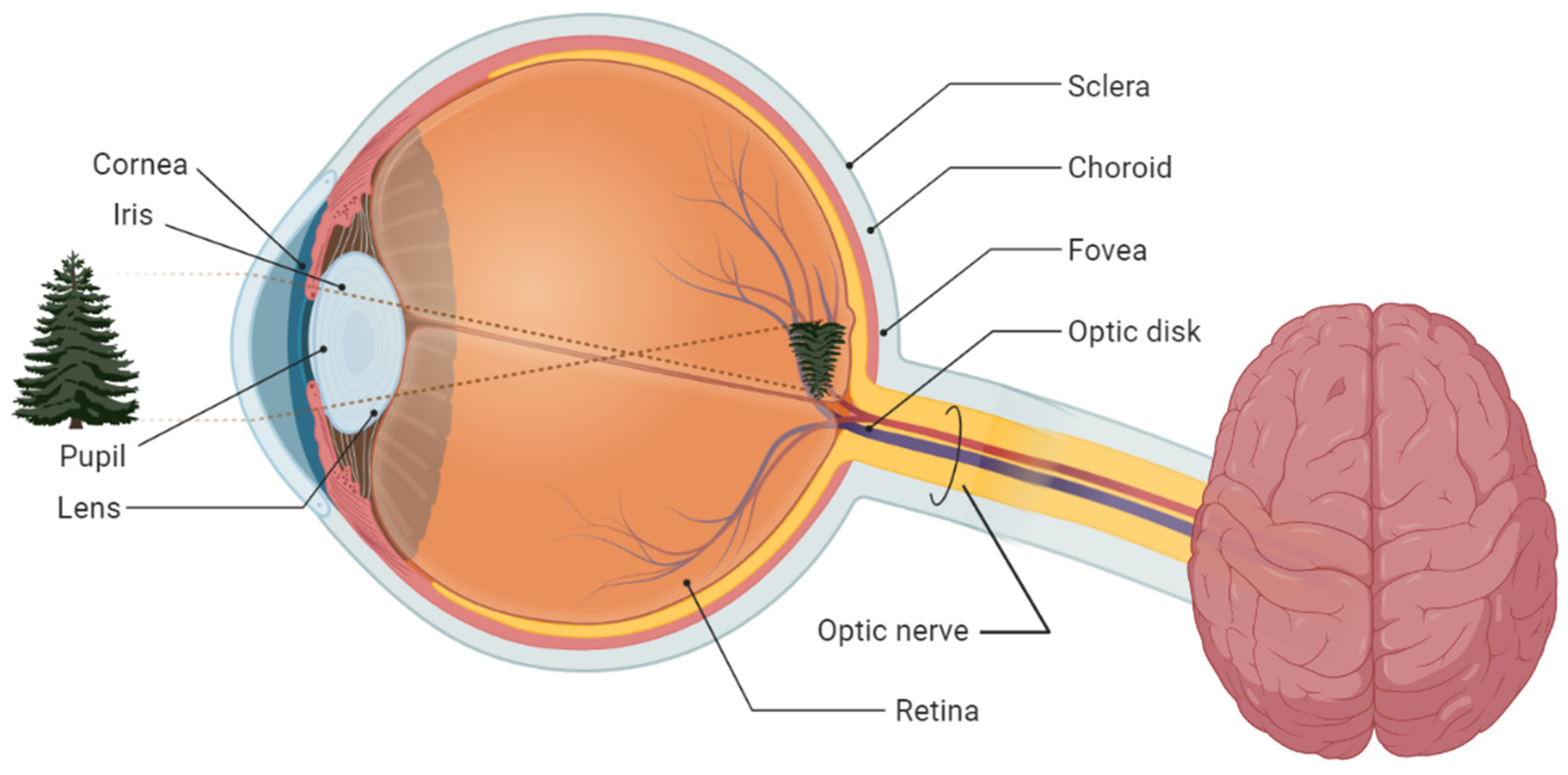
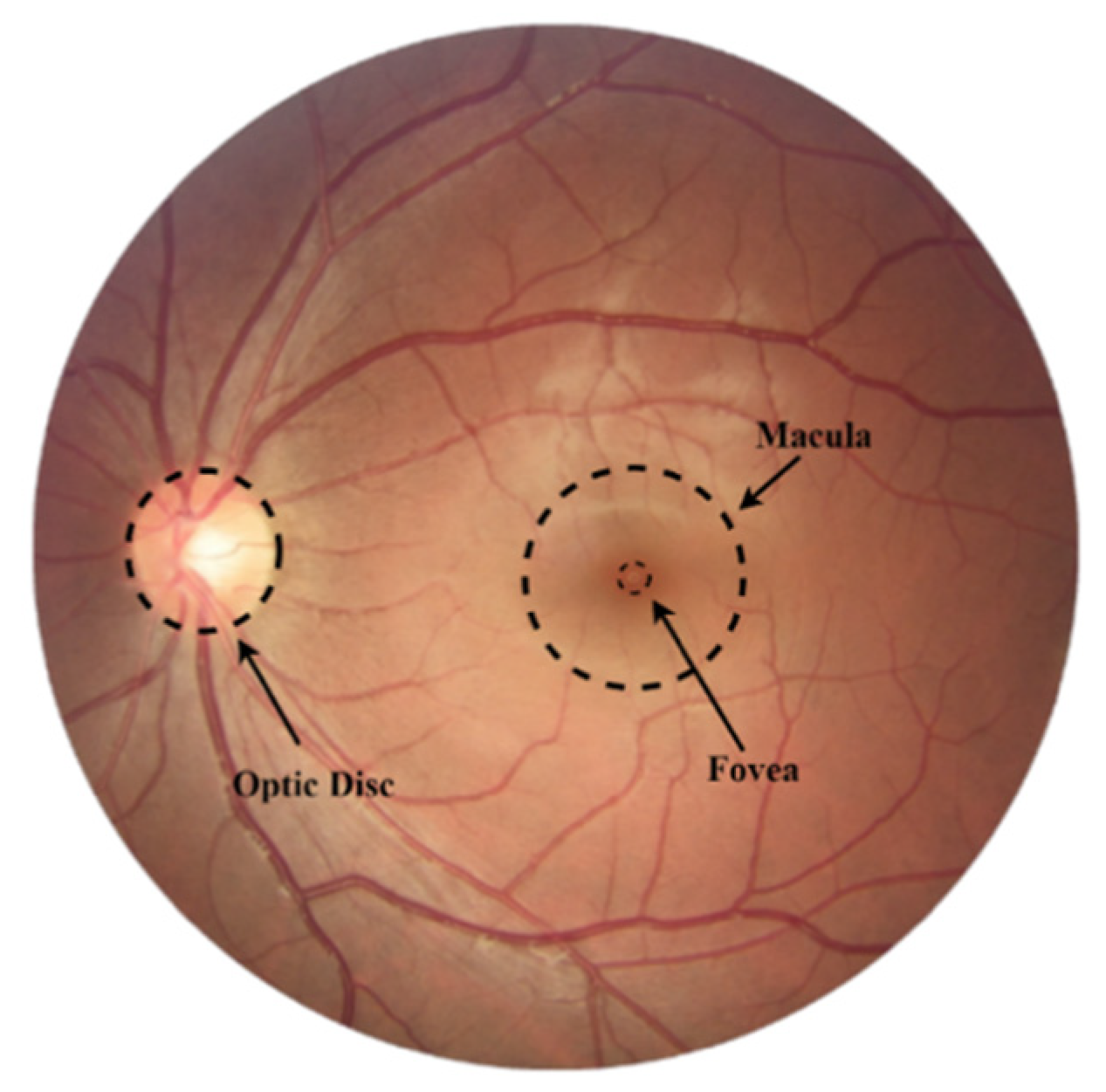
2. Fundamentals of Glaucoma Screening
2.1. Glaucoma Tracking
- ▪
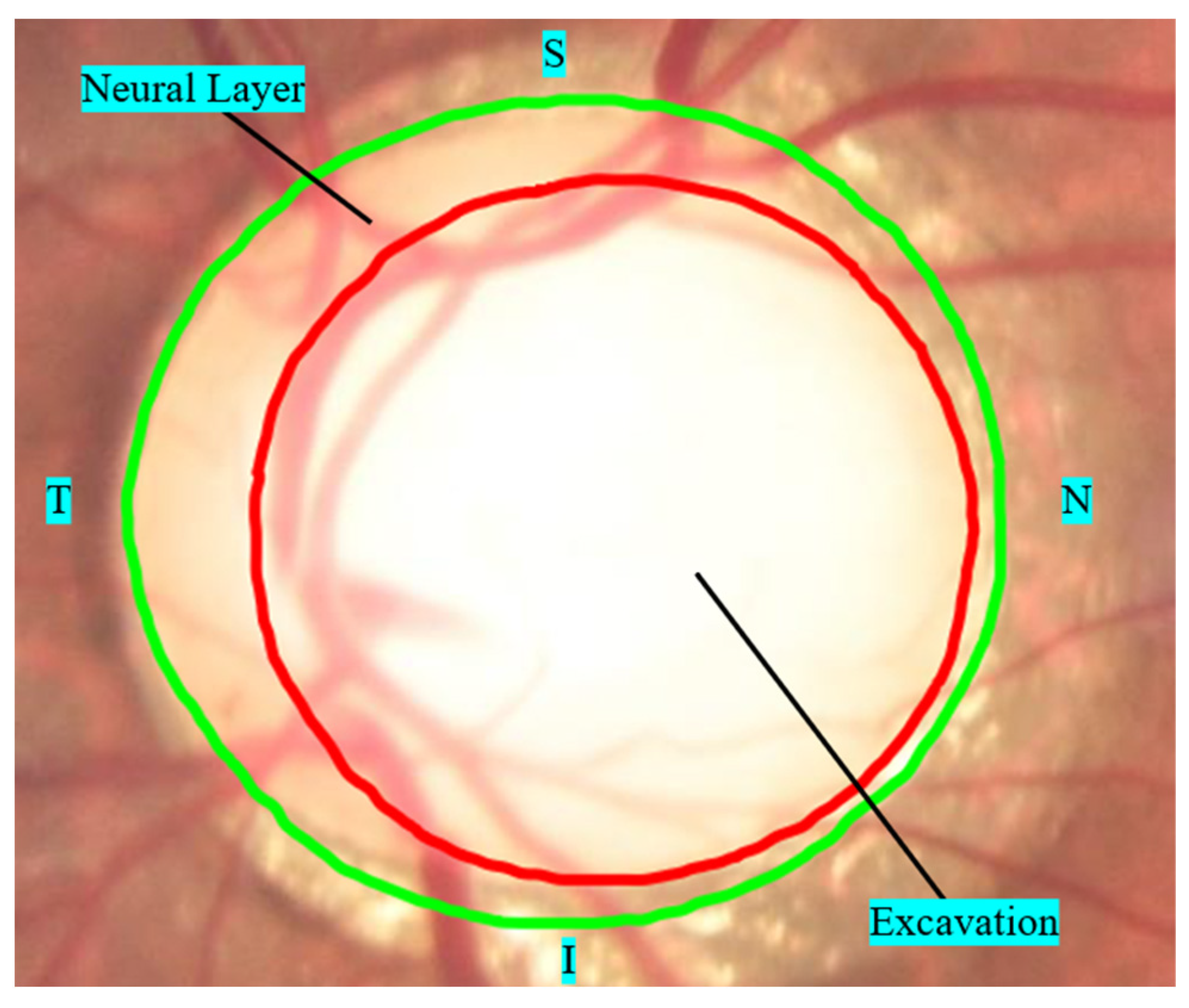
- The vertical axis of the cup is greater than 65% of the vertical axis of the outer edge of the papilla in large papillae (>1.5 mm) and 50% of the vertical axis in small papillae (<1.5 mm).
- ▪
- The limit of papilla cupping is less than 20% of the outer boundary of the papilla, including the localized area of neural layer atrophy (notch).
- ▪
- Asymmetry of the internal excavation is greater than 20% between the optic papillae of the right and left eyes.
- ▪
- Defects in the nerve fiber layer in the peripapillary region, associated or not with a suspicious thinning of the nerve fiber layer, as exemplified in Figure 5c, where an area of atrophy is visible in the path of the fibers from 7:00 and 8:00.
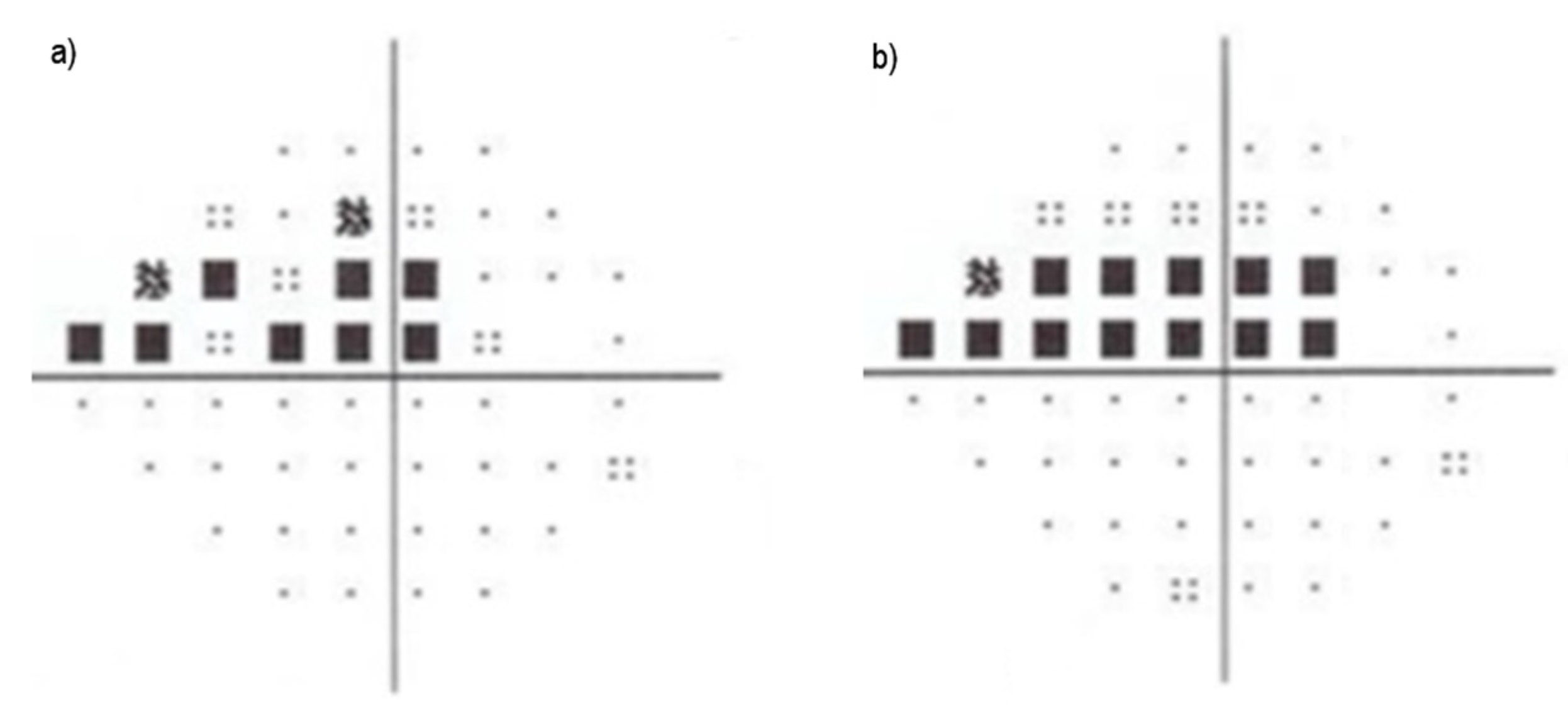
2.2. Metrics Used for Glaucoma Assessment
3. Retinal Visualization Equipment
3.1. Retinographer
3.2. Optical Coherence Tomography (OCT)
3.3. Heidelberg Retinal Tomography (HRT)
3.4. Lenses
3.5. Slit Lamp
3.6. Direct Ophthalmoscope
3.7. Binocular Indirect Ophthalmoscope
3.8. Mobile Devices
3.9. Other Equipment
- Advantages: with low cost and easy handling, they can be incorporated into smartphone cameras, with low price, good quality, and the capability of transmission to other specialists, yet without diagnostic disadvantages regarding stereoscopic photographs.
- Disadvantages: However, taking photographs using portable cameras requires some extra technical training from a professional for the correct alignment of ocular structures.
4. Current Studies for Automatic Tracking of Glaucoma
- Inferior, Superior, Nasal, and Temporal rule (ISNT)—characterizes the healthiness of the optic disc based on the thickness in certain regions (inferior, superior, nasal, and temporal poles). It can be an early symptom of disease if this pattern is disrupted (in this order), either by a change in diameter or area [42,56,101].
5. Challenges for Glaucoma Screening
5.1. Lacking Data, Need to Standardize
5.2. Integration of Historical and Anamnesis Data
5.3. Development of Methods “That Learn Continuously”
5.4. Development of “Explainable AI” Methods
5.5. Development of Methods to Detect Multiple Diseases
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Christopher, M.; Belghith, A.; Bowd, C.; Proudfoot, J.A.; Goldbaum, M.H.; Weinreb, R.N.; Girkin, C.A.; Liebmann, J.M.; Zangwill, L.M. Performance of Deep Learning Architectures and Transfer Learning for Detecting Glaucomatous Optic Neuropathy in Fundus Photographs. Sci. Rep. 2018, 8, 16685. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stein, J.D.; Kim, D.S.; Niziol, L.M.; Talwar, N.; Nan, B.; Musch, D.C.; Richards, J.E. Differences in Rates of Glaucoma among Asian Americans and Other Racial Groups, and among Various Asian Ethnic Groups. Ophthalmology 2011, 118, 1031–1037. [Google Scholar] [CrossRef] [Green Version]
- Weinreb, R.N.; Leung, C.K.S.; Crowston, J.G.; Medeiros, F.A.; Friedman, D.S.; Wiggs, J.L.; Martin, K.R. Primary Open-Angle Glaucoma. Nat. Rev. Dis. Primers 2016, 2, 16067. [Google Scholar] [CrossRef]
- Yamamoto, S.; Sawaguchi, S.; Iwase, A.; Yamamoto, T.; Abe, H.; Tomita, G.; Tomidokoro, A.; Araie, M. Primary Open-Angle Glaucoma in a Population Associated with High Prevalence of Primary Angle-Closure Glaucoma. Ophthalmology 2014, 121, 1558–1565. [Google Scholar] [CrossRef] [PubMed]
- Gupta, D. Glaucoma Diagnosis and Management; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2005; ISBN 0-7817-5403-8. [Google Scholar]
- Pan, Y.; Varma, R. Natural History of Glaucoma. Indian J. Ophthalmol. 2011, 59, S19. [Google Scholar]
- Duncan, R.O.; Sample, P.A.; Weinreb, R.N.; Bowd, C.; Zangwill, L.M. Retinotopic Organization of Primary Visual Cortex in Glaucoma: Comparing FMRI Measurements of Cortical Function with Visual Field Loss. Prog. Retin. Eye Res. 2007, 26, 38–56. [Google Scholar] [CrossRef] [Green Version]
- Tham, Y.-C.; Li, X.; Wong, T.Y.; Quigley, H.A.; Aung, T.; Cheng, C.-Y. Global Prevalence of Glaucoma and Projections of Glaucoma Burden through 2040. Ophthalmology 2014, 121, 2081–2090. [Google Scholar] [CrossRef]
- Galloway, N.R.; Amoaku, W.M.; Galloway, P.H.; Browning, A.C. Common Eye Diseases and Their Management; Springer: Berlin/Heidelberg, Germany, 2016; ISBN 3-319-32869-7. [Google Scholar]
- Fatima Bokhari, S.T.; Sharif, M.; Yasmin, M.; Fernandes, S.L. Fundus Image Segmentation and Feature Extraction for the Detection of Glaucoma: A New Approach. CMIR 2017, 14, 77–87. [Google Scholar] [CrossRef]
- Tan, N.Y.Q.; Friedman, D.S.; Stalmans, I.; Ahmed, I.I.K.; Sng, C.C.A. Glaucoma Screening: Where Are We and Where Do We Need to Go? Curr. Opin. Ophthalmol. 2020, 31, 91–100. [Google Scholar] [CrossRef]
- Danesh-Meyer, H.V.; Yoon, J.J.; Lawlor, M.; Savino, P.J. Visual Loss and Recovery in Chiasmal Compression. Prog. Retin. Eye Res. 2019, 73, 100765. [Google Scholar] [CrossRef]
- Kestenbaum, A. Clinical Methods of Neuro-Ophthalmologic Examination; Elsevier: Amsterdam, The Netherlands, 2013; ISBN 1-4832-2549-6. [Google Scholar]
- Salam, A.A.; Khalil, T.; Akram, M.U.; Jameel, A.; Basit, I. Automated Detection of Glaucoma Using Structural and Non Structural Features. SpringerPlus 2016, 5, 1519. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mehta, P.; Petersen, C.A.; Wen, J.C.; Banitt, M.R.; Chen, P.P.; Bojikian, K.D.; Egan, C.; Lee, S.-I.; Balazinska, M.; Lee, A.Y.; et al. Automated Detection of Glaucoma with Interpretable Machine Learning Using Clinical Data and Multimodal Retinal Images. Am. J. Ophthalmol. 2021, 231, 154–169. [Google Scholar] [CrossRef] [PubMed]
- Gedde, S.J.; Lind, J.T.; Wright, M.M.; Chen, P.P.; Muir, K.W.; Vinod, K.; Li, T.; Mansberger, S.L. Primary Open-Angle Glaucoma Suspect Preferred Practice Pattern®. Ophthalmology 2021, 128, P151–P192. [Google Scholar] [CrossRef] [PubMed]
- Gordon, M.O.; Kass, M.A. What We Have Learned From the Ocular Hypertension Treatment Study. Am. J. Ophthalmol. 2018, 189, xxiv–xxvii. [Google Scholar] [CrossRef]
- Al-Aswad, L.A.; Elgin, C.Y.; Patel, V.; Popplewell, D.; Gopal, K.; Gong, D.; Thomas, Z.; Joiner, D.; Chu, C.-K.; Walters, S.; et al. Real-Time Mobile Teleophthalmology for the Detection of Eye Disease in Minorities and Low Socioeconomics At-Risk Populations. Asia-Pac. J. Ophthalmol. 2021, 10, 461–472. [Google Scholar] [CrossRef]
- Yum, H.R.; Park, S.H.; Shin, S.Y. Change of Ocular Parameters in Children with Large Cup-to-Disc Ratio and Interocular Cup-to-Disc Ratio Asymmetry. Graefe’s Arch. Clin. Exp. Ophthalmol. 2021, 259, 3453–3459. [Google Scholar] [CrossRef]
- Mahabadi, N.; Foris, L.A.; Tripathy, K. Open Angle Glaucoma. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2021. [Google Scholar]
- Christopher, M.; Bowd, C.; Belghith, A.; Goldbaum, M.H.; Weinreb, R.N.; Fazio, M.A.; Girkin, C.A.; Liebmann, J.M.; Zangwill, L.M. Deep Learning Approaches Predict Glaucomatous Visual Field Damage from OCT Optic Nerve Head En Face Images and Retinal Nerve Fiber Layer Thickness Maps. Ophthalmology 2020, 127, 346–356. [Google Scholar] [CrossRef]
- Xie, Q.; Faust, K.; Van Ommeren, R.; Sheikh, A.; Djuric, U.; Diamandis, P. Deep Learning for Image Analysis: Personalizing Medicine Closer to the Point of Care. Crit. Rev. Clin. Lab. Sci. 2019, 56, 61–73. [Google Scholar] [CrossRef]
- Schmidt-Erfurth, U.; Sadeghipour, A.; Gerendas, B.S.; Waldstein, S.M.; Bogunović, H. Artificial Intelligence in Retina. Prog. Retin. Eye Res. 2018, 67, 1–29. [Google Scholar] [CrossRef]
- Gómez-Valverde, J.J.; Antón, A.; Fatti, G.; Liefers, B.; Herranz, A.; Santos, A.; Sánchez, C.I.; Ledesma-Carbayo, M.J. Automatic Glaucoma Classification Using Color Fundus Images Based on Convolutional Neural Networks and Transfer Learning. Biomed. Opt. Express 2019, 10, 892. [Google Scholar] [CrossRef] [Green Version]
- Rank, E.A.; Nevlacsil, S.; Muellner, P.; Hainberger, R.; Salas, M.; Gloor, S.; Duelk, M.; Sagmeister, M.; Kraft, J.; Leitgeb, R.A.; et al. In Vivo Human Retinal Swept Source Optical Coherence Tomography and Angiography at 830 Nm with a CMOS Compatible Photonic Integrated Circuit. Sci. Rep. 2021, 11, 21052. [Google Scholar] [CrossRef] [PubMed]
- Maheshwari, S.; Kanhangad, V.; Pachori, R.B.; Bhandary, S.V.; Acharya, U.R. Automated Glaucoma Diagnosis Using Bit-Plane Slicing and Local Binary Pattern Techniques. Comput. Biol. Med. 2019, 105, 72–80. [Google Scholar] [CrossRef] [PubMed]
- Stella Mary, M.C.V.; Rajsingh, E.B.; Naik, G.R. Retinal Fundus Image Analysis for Diagnosis of Glaucoma: A Comprehensive Survey. IEEE Access 2016, 4, 4327–4354. [Google Scholar] [CrossRef]
- Werginz, P.; Wang, B.-Y.; Chen, Z.C.; Palanker, D. On Optimal Coupling of the ‘Electronic Photoreceptors’ into the Degenerate Retina. J. Neural Eng. 2020, 17, 045008. [Google Scholar] [CrossRef]
- Claro, M.L.; Veras, R.D.M.S.; Santana, A.M.; Vogado, L.H.S.; Sousa, L.P. Diagnóstico de Glaucoma Utilizando Atributos de Textura e CNN’s Pré-Treinadas. RITA 2018, 25, 82. [Google Scholar] [CrossRef]
- Hagiwara, Y.; Koh, J.E.W.; Tan, J.H.; Bhandary, S.V.; Laude, A.; Ciaccio, E.J.; Tong, L.; Acharya, U.R. Computer-Aided Diagnosis of Glaucoma Using Fundus Images: A Review. Comput. Methods Programs Biomed. 2018, 165, 1–12. [Google Scholar] [CrossRef]
- Neto, A.; Camara, J.; Cunha, A. Evaluations of Deep Learning Approaches for Glaucoma Screening Using Retinal Images from Mobile Device. Sensors 2022, 22, 1449. [Google Scholar] [CrossRef]
- Malik, S.; Kanwal, N.; Asghar, M.N.; Sadiq, M.A.A.; Karamat, I.; Fleury, M. Data Driven Approach for Eye Disease Classification with Machine Learning. Appl. Sci. 2019, 9, 2789. [Google Scholar] [CrossRef] [Green Version]
- Leung, E.W.; Medeiros, F.A.; Weinreb, R.N. Prevalence of Ocular Surface Disease in Glaucoma Patients. J. Glaucoma 2008, 17, 350–355. [Google Scholar] [CrossRef] [Green Version]
- Bock, R.; Meier, J.; Nyúl, L.G.; Hornegger, J.; Michelson, G. Glaucoma Risk Index:Automated Glaucoma Detection from Color Fundus Images. Med. Image Anal. 2010, 14, 471–481. [Google Scholar] [CrossRef] [Green Version]
- Takwoingi, Y.; Botello, A.P.; Burr, J.M.; Azuara-Blanco, A.; Garway-Heath, D.F.; Lemij, H.G.; Sanders, R.; King, A.J.; Deeks, J.J. For the Surveillance for Ocular Hypertension Study Group External Validation of the OHTS-EGPS Model for Predicting the 5-Year Risk of Open-Angle Glaucoma in Ocular Hypertensives. Br. J. Ophthalmol. 2014, 98, 309–314. [Google Scholar] [CrossRef] [PubMed]
- Bajwa, M.N.; Malik, M.I.; Siddiqui, S.A.; Dengel, A.; Shafait, F.; Neumeier, W.; Ahmed, S. Correction to: Two-Stage Framework for Optic Disc Localization and Glaucoma Classification in Retinal Fundus Images Using Deep Learning. BMC Med. Inform. Decis. Mak. 2019, 19, 139. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thakur, N.; Juneja, M. Survey on Segmentation and Classification Approaches of Optic Cup and Optic Disc for Diagnosis of Glaucoma. Biomed. Signal Processing Control. 2018, 42, 162–189. [Google Scholar] [CrossRef]
- Karvonen, E.; Stoor, K.; Luodonpää, M.; Hägg, P.; Lintonen, T.; Liinamaa, J.; Tuulonen, A.; Saarela, V. Diagnostic Performance of Modern Imaging Instruments in Glaucoma Screening. Br. J. Ophthalmol. 2020, 104, 1399. [Google Scholar] [CrossRef] [PubMed]
- Jung, Y.; Park, H.-Y.L.; Park, Y.R.; Park, C.K. Usefulness of 10-2 Matrix Frequency Doubling Technology Perimetry for Detecting Central Visual Field Defects in Preperimetric Glaucoma Patients. Sci. Rep. 2017, 7, 14622. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mastropasqua, R.; D’Aloisio, R.; Agnifili, L.; Zuppardi, E.; Di Marzio, G.; Di Nicola, M.; Porreca, A.; Guarini, D.; Totta, M.; Brescia, L. Functional and Structural Reliability of Optic Nerve Head Measurements in Healthy Eyes by Means of Optical Coherence Tomography Angiography. Medicina 2020, 56, 44. [Google Scholar] [CrossRef] [Green Version]
- Fung, T.H.; Amoaku, W.M. Patient Management in Clinical Practice. In Viva and OSCE Exams in Ophthalmology; Springer: Berlin/Heidelberg, Germany, 2020; pp. 63–165. [Google Scholar]
- Jonas, J.B.; Panda-Jonas, S. The Optic Nerve Head in High Myopia/Abnormalities of the Intrapapillary and Parapapillary Region. In Pathologic Myopia; Springer: Berlin/Heidelberg, Germany, 2021; pp. 167–176. [Google Scholar]
- Cheng, K.K.; Tatham, A.J. Spotlight on the Disc-Damage Likelihood Scale (DDLS). Clin. Ophthalmol. 2021, 15, 4059. [Google Scholar] [CrossRef]
- Akil, H.; Chopra, V.; Al-Sheikh, M.; Falavarjani, K.G.; Huang, A.S.; Sadda, S.R.; Francis, B.A. Swept-Source OCT Angiography Imaging of the Macular Capillary Network in Glaucoma. Br. J. Ophthalmol. 2018, 102, 515–519. [Google Scholar] [CrossRef]
- Gupta, N.; Aung, T.; Congdon, N.; Dada, T.; Lerner, F.; Olawoye, S. ICO Guidelines for Glaucoma Eye Care. Int. Counc. Ophthalmol. 2016. Available online: http://www.glaucomaclinic.com/wp-content/uploads/2016/12/ICOGlaucomaGuidelines.pdf (accessed on 20 March 2022).
- Sampaolesi, R.; Sampaolesi, J.R.; Zárate, J. Concept of Glaucoma Pathogenesis. In The Glaucomas; Springer: Berlin/Heidelberg, Germany, 2014; pp. 13–27. [Google Scholar]
- Khunger, M.; Choudhury, T.; Satapathy, S.C.; Ting, K.-C. Automated Detection of Glaucoma Using Image Processing Techniques. In Emerging Technologies in Data Mining and Information Security; Abraham, A., Dutta, P., Mandal, J.K., Bhattacharya, A., Dutta, S., Eds.; Advances in Intelligent Systems and Computing; Springer: Singapore, 2019; Volume 814, pp. 323–335. ISBN 9789811315008. [Google Scholar]
- Yin, F.; Liu, J.; Wong, D.W.K.; Tan, N.M.; Cheung, C.; Baskaran, M.; Aung, T.; Wong, T.Y. Automated Segmentation of Optic Disc and Optic Cup in Fundus Images for Glaucoma Diagnosis. In Proceedings of the 2012 25th IEEE International Symposium on Computer-Based Medical Systems (CBMS), Rome, Italy, 20–22 June 2012; pp. 1–6. [Google Scholar]
- Almazroa, A.; Burman, R.; Raahemifar, K.; Lakshminarayanan, V. Optic Disc and Optic Cup Segmentation Methodologies for Glaucoma Image Detection: A Survey. J. Ophthalmol. 2015, 2015, 180972. [Google Scholar] [CrossRef] [Green Version]
- Orlando, J.I.; Fu, H.; Barbosa Breda, J.; van Keer, K.; Bathula, D.R.; Diaz-Pinto, A.; Fang, R.; Heng, P.-A.; Kim, J.; Lee, J.; et al. REFUGE Challenge: A Unified Framework for Evaluating Automated Methods for Glaucoma Assessment from Fundus Photographs. Med. Image Anal. 2020, 59, 101570. [Google Scholar] [CrossRef]
- Haleem, M.S.; Han, L.; van Hemert, J.; Li, B. Automatic Extraction of Retinal Features from Colour Retinal Images for Glaucoma Diagnosis: A Review. Comput. Med. Imaging Graph. 2013, 37, 581–596. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Joshi, G.D.; Sivaswamy, J.; Krishnadas, S.R. Optic Disk and Cup Segmentation From Monocular Color Retinal Images for Glaucoma Assessment. IEEE Trans. Med. Imaging 2011, 30, 1192–1205. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Jia, X.; Hu, M.; Zhao, L. Computer-aided diagnosis of glaucoma using fundus images. In Proceedings of the 2014 International Conference on Mechatronics, Electronic, Industrial and Control Engineering (MEIC-14), Shenyang, China, 15–17 November 2014; Atlantis Press: Paris, France, 2014; pp. 915–920. [Google Scholar]
- Noronha, K.P.; Acharya, U.R.; Nayak, K.P.; Martis, R.J.; Bhandary, S.V. Automated Classification of Glaucoma Stages Using Higher Order Cumulant Features. Biomed. Signal Processing Control. 2014, 10, 174–183. [Google Scholar] [CrossRef]
- de Carvalho Junior, A.S.V.; Carvalho, E.D.; de Carvalho Filho, A.O.; de Sousa, A.D.; Corrêa Silva, A.; Gattass, M. Automatic Methods for Diagnosis of Glaucoma Using Texture Descriptors Based on Phylogenetic Diversity. Comput. Electr. Eng. 2018, 71, 102–114. [Google Scholar] [CrossRef]
- Thakkar, K.; Chauhan, K.; Sudhalkar, A.; Gulati, R.; Ophthalmologist, M.S. Detection of Glaucoma from Retinal Fundus Images by Analysing ISNT Measurement and Features of Optic Cup and Blood Vessels. Int. J. Eng. Technol. Sci. Res. IJETSR 2017, 4, 2394–3386. [Google Scholar]
- Ajeesha, A.A.; Arun Kumar, M.N. Efficient Optic Disc Segmentation and Peripappilary Atropy Detection in Digital Fundus Images. Int. J. Innov. Res. Sci. Technol. 2016, 3, 213–222. [Google Scholar]
- Miller, S.E.; Thapa, S.; Robin, A.L.; Niziol, L.M.; Ramulu, P.Y.; Woodward, M.A.; Paudyal, I.; Pitha, I.; Kim, T.N.; Newman-Casey, P.A. Glaucoma Screening in Nepal: Cup-to-Disc Estimate With Standard Mydriatic Fundus Camera Compared to Portable Nonmydriatic Camera. Am. J. Ophthalmol. 2017, 182, 99–106. [Google Scholar] [CrossRef]
- Viquez, K.D.C.; Arandjelović, O.; Blaikie, A.; Hwang, I.A. Synthesising Wider Field Images from Narrow-Field Retinal Video Acquired Using a Low-Cost Direct Ophthalmoscope (Arclight) Attached to a Smartphone. In Proceedings of the 2017 IEEE International Conference on Computer Vision Workshops, ICCVW 2017, Venice, Italy, 22–29 October 2017; pp. 90–98. [Google Scholar] [CrossRef] [Green Version]
- Wintergerst, M.W.M.; Brinkmann, C.K.; Holz, F.G.; Finger, R.P. Undilated versus Dilated Monoscopic Smartphone-Based Fundus Photography for Optic Nerve Head Evaluation. Sci. Rep. 2018, 8, 2–8. [Google Scholar] [CrossRef] [Green Version]
- MacCormick, I.J.C.; Williams, B.M.; Zheng, Y.; Li, K.; Al-Bander, B.; Czanner, S.; Cheeseman, R.; Willoughby, C.E.; Brown, E.N.; Spaeth, G.L.; et al. Correction: Accurate, Fast, Data Efficient and Interpretable Glaucoma Diagnosis with Automated Spatial Analysis of the Whole Cup to Disc Profile. PLoS ONE 2019, 14, e0209409. [Google Scholar] [CrossRef] [Green Version]
- Claro, M.; Santos, L.; Silva, W.; Araújo, F.; Santana, A.D.A. Automatic Detection of Glaucoma Using Disc Optic Segmentation and Feature Extraction. In Proceedings of the 2015 41st Latin American Computing Conference, CLEI 2015, Arequipa, Peru, 19–23 October 2015. [Google Scholar] [CrossRef]
- Santos, L.; Araújo, F.; Claro, M.; Silva, W.; Silva, R.; Drumond, P. Implementação e Comparação de Um Método de Detecção e Segmentação Automática Do Disco Óptico Em Diferentes Bases de Imagens Da Retina. In Proceedings of the XII Simpósio Brasileiro de Automação Inteligente (SBAI) 2015, Sao Paulo, Brazil, 25–28 October 2015; pp. 1–6. [Google Scholar]
- Odstrcilik, J.; Kolar, R.; Budai, A.; Hornegger, J.; Jan, J.; Gazarek, J.; Kubena, T.; Cernosek, P.; Svoboda, O.; Angelopoulou, E. Retinal Vessel Segmentation by Improved Matched Filtering: Evaluation on a New High-resolution Fundus Image Database. IET Image Processing 2013, 7, 373–383. [Google Scholar] [CrossRef]
- Khan, S.M.; Liu, X.; Nath, S.; Korot, E.; Faes, L.; Wagner, S.K.; Keane, P.A.; Sebire, N.J.; Burton, M.J.; Denniston, A.K. A Global Review of Publicly Available Datasets for Ophthalmological Imaging: Barriers to Access, Usability, and Generalisability. Lancet Digit. Health 2021, 3, e51–e66. [Google Scholar] [CrossRef]
- Randive, S.N.; Senapati, R.K.; Rahulkar, A.D. A Review on Computer-Aided Recent Developments for Automatic Detection of Diabetic Retinopathy. J. Med. Eng. Technol. 2019, 43, 87–99. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Fong, S.; Wang, H.; Hu, Q.; Lin, C.; Huang, S.; Shi, J.; Lan, K.; Tang, R.; Wu, Y.; et al. Artificial Intelligence in Ophthalmopathy and Ultra-Wide Field Image: A Survey. Expert Syst. Appl. 2021, 182, 115068. [Google Scholar] [CrossRef]
- Melo, D.; Soares, F.; Felgueiras, S.; Gonçalves, J.; Vieira, P. A New Compact Optical System Proposal and Image Quality Comparison Against Other Affordable Non-Mydriatic Fundus Cameras. In Biomedical Engineering Systems and Technologies; Cliquet, A., Wiebe, S., Anderson, P., Saggio, G., Zwiggelaar, R., Gamboa, H., Fred, A., Bermúdez, I., Badia, S., Eds.; Communications in Computer and Information Science; Springer International Publishing: Cham, Switzerland, 2019; Volume 1024, pp. 26–48. ISBN 978-3-030-29195-2. [Google Scholar]
- Rauscher, F.G.; Azmanis, P.; Körber, N.; Koch, C.; Hübel, J.; Vetterlein, W.; Werner, B.; Thielebein, J.; Dawczynski, J.; Wiedemann, P. Optical Coherence Tomography as a Diagnostic Tool for Retinal Pathologies in Avian Ophthalmology. Investig. Ophthalmol. Vis. Sci. 2013, 54, 8259–8269. [Google Scholar] [CrossRef] [PubMed]
- Fujihara, F.M.F.; de Arruda Mello, P.A.; Lindenmeyer, R.L.; Pakter, H.M.; Lavinsky, J.; Benfica, C.Z.; Castoldi, N.; Picetti, E.; Lavinsky, D.; Finkelsztejn, A. Individual Macular Layer Evaluation with Spectral Domain Optical Coherence Tomography in Normal and Glaucomatous Eyes. Clin. Ophthalmol. 2020, 14, 1591. [Google Scholar] [CrossRef] [PubMed]
- Tong, L.; Chan, Y.-H.; Gazzard, G.; Loon, S.-C.; Fong, A.; Selvaraj, P.; Healey, P.R.; Tan, D.; Wong, T.Y.; Saw, S.M. Heidelberg Retinal Tomography of Optic Disc and Nerve Fiber Layer in Singapore Children: Variations with Disc Tilt and Refractive Error. Investig. Ophthalmol. Vis. Sci. 2007, 48, 4939. [Google Scholar] [CrossRef]
- Kim, K.; Jang, K.-W.; Ryu, J.-K.; Jeong, K.-H. Biologically Inspired Ultrathin Arrayed Camera for High-Contrast and High-Resolution Imaging. Light Sci. Appl. 2020, 9, 28. [Google Scholar] [CrossRef] [Green Version]
- Armstrong, G.W.; Kalra, G.; De Arrigunaga, S.; Friedman, D.S.; Lorch, A.C. Anterior Segment Imaging Devices in Ophthalmic Telemedicine. Semin. Ophthalmol. 2021, 36, 149–156. [Google Scholar] [CrossRef]
- Lee, S.Y. Assessment of Blue Light Exposure in the Occupational Visual Field. Ph.D. Thesis, University of Adelaide, Adelaide, Australia, 2020. [Google Scholar]
- Douglass, A.; Cham, K.M.; Jaworski, A.; Maillet, A.; Cochrane, A.; Armitage, J.A. The Equivalency of the Binocular Indirect Ophthalmoscope Simulator to Peer Practice: A Pilot Study. Optom. Vis. Sci. 2021, 98, 1239–1247. [Google Scholar] [CrossRef]
- Liu, Z.; Ao, M.; Wang, W.; Hou, Z. Effectiveness of EYESI Binocular Indirect Ophthalmoscope Simulation Training System as an Assessment and Training Platform for Ophthalmic Residents. Ophthalmol. China 2020, 29, 230. [Google Scholar] [CrossRef]
- Feitosa-Santana, C.; Fernandes Costa, M.; Ballalai Ferraz, H.; Andrade, L.A.F.; Moura, A.L.; Amaro, E.; Hamer, R.D.; Fix Ventura, D. Visual Losses in Early-Onset and Late-Onset Parkinson’s Disease. J. Opt. Soc. Am. A 2020, 37, A285–A293. [Google Scholar] [CrossRef] [PubMed]
- Ichhpujani, P.; Thakur, S. Smartphones and Telemedicine in Ophthalmology. In Smart Resources in Ophthalmology; Springer: Berlin/Heidelberg, Germany, 2018; pp. 247–255. [Google Scholar]
- Nesemann, J.; Seider, M.I.; Maamar, R.N.; Ayele, B.; Varnado, N.E.; Cotter, S.Y.; Margolis, T.; Fletcher, D.A.; Emerson, P.M.; Keenan, J. Validation of Modified Smartphone Camera against Single Lens Reflex (SLR) Camera and in-Person Grading of Trachoma. Investig. Ophthalmol. Vis. Sci. 2020, 61, 3997. [Google Scholar]
- Bright, T.; Kuper, H.; Macleod, D.; Musendo, D.; Irunga, P.; Yip, J.L. Population Need for Primary Eye Care in Rwanda: A National Survey. PLoS ONE 2018, 13, e0193817. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dao, D.K.T.; Shah, N.; Tamhankar, M.; Tapino, P.; Pan, W.; Ying, G.-S.; Willett, K.; VanderBeek, B.L.; Brucker, A.J. Smartphone Ophthalmoscopy (D-Eye System) for Detection of Optic Nerve Pathology and Cup-to-Disc Ratio in an Outpatient Clinical Setting. Investig. Ophthalmol. Vis. Sci. 2017, 58, 4836. [Google Scholar]
- Hunt, B.; Ruiz, A.J.; Pogue, B.W. Smartphone-Based Imaging Systems for Medical Applications: A Critical Review. J. Biomed. Opt. 2021, 26, 040902. [Google Scholar] [CrossRef]
- Lee, D.-G.; Jang, Y.; Seo, Y.-S. Intelligent Image Synthesis for Accurate Retinal Diagnosis. Electronics 2020, 9, 767. [Google Scholar] [CrossRef]
- PHELCOM Technologies. Available online: https://phelcom.com/ (accessed on 20 November 2021).
- VisoScope 20D. Available online: https://odocseyecare.shop/products/visoscope-2-0 (accessed on 20 November 2021).
- Pictor PlusTM Fundus Camera. Available online: https://www.volk.com/products/pictor-plus-fundus-camera (accessed on 20 November 2021).
- INview® for IPod Touch Including IPod Touch. Available online: https://www.volk.com/products/inview-for-ipod-touch-including-ipod-touch (accessed on 20 November 2021).
- IEXAMINER. Available online: https://www.welchallyn.com/en/microsites/iexaminer.html#form (accessed on 20 November 2021).
- Digital Retinal Camera|The Direct Ophthalmoscope for Your IPhone|Portable Digital Retinal Camera|D-EYE. Available online: https://www.ophthalmologyweb.com/5740-Digital-Retinal-Camera/9505029-D-EYE-Smartphone-Portable-Retinal-Camera/ (accessed on 20 November 2021).
- Pujari, A.; Saluja, G.; Agarwal, D.; Selvan, H.; Sharma, N. Clinically Useful Smartphone Ophthalmic Imaging Techniques. Graefe’s Arch. Clin. Exp. Ophthalmol. 2021, 259, 279–287. [Google Scholar] [CrossRef]
- Cicinelli, M.V.; Cavalleri, M.; Brambati, M.; Lattanzio, R.; Bandello, F. New Imaging Systems in Diabetic Retinopathy. Acta Diabetol. 2019, 56, 981–994. [Google Scholar] [CrossRef]
- Aumann, S.; Donner, S.; Fischer, J.; Müller, F. Optical Coherence Tomography (OCT): Principle and Technical Realization. In High Resolution Imaging in Microscopy and Ophthalmology; Springer: Cham, Switzerland, 2019; pp. 59–85. [Google Scholar] [CrossRef] [Green Version]
- Park, J.-H.; Yoo, C.; Kim, Y.Y. Localized Retinal Nerve Fiber Layer Defect Location Among Red-Free Fundus Photographs, En Face Structural Images, and Cirrus HD-OCT Maps. J. Glaucoma 2019, 28, 1054–1060. [Google Scholar] [CrossRef]
- Rogers, T.W.; Jaccard, N.; Carbonaro, F.; Lemij, H.G.; Vermeer, K.A.; Reus, N.J.; Trikha, S. Evaluation of an AI System for the Automated Detection of Glaucoma from Stereoscopic Optic Disc Photographs: The European Optic Disc Assessment Study. Eye 2019, 33, 1791–1797. [Google Scholar] [CrossRef] [Green Version]
- Wynne, N.; Carroll, J.; Duncan, J.L. Promises and Pitfalls of Evaluating Photoreceptor-Based Retinal Disease with Adaptive Optics Scanning Light Ophthalmoscopy (AOSLO). Prog. Retin. Eye Res. 2021, 83, 100920. [Google Scholar] [CrossRef] [PubMed]
- Hill, D. Retinal Characteristics of Myopic Eyes in a Semi-Rural UK Population; Aston University: Birmingham, UK, 2019. [Google Scholar]
- Vilela, M.A.; Valença, F.M.; Barreto, P.K.; Amaral, C.E.; Pellanda, L.C. Agreement between Retinal Images Obtained via Smartphones and Images Obtained with Retinal Cameras or Fundoscopic Exams–Systematic Review and Meta-Analysis. Clin. Ophthalmol. 2018, 12, 2581. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Myers, J.S.; Fudemberg, S.J.; Lee, D. Evolution of Optic Nerve Photography for Glaucoma Screening: A Review. Clin. Exp. Ophthalmol. 2018, 46, 169–176. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tatham, A.J.; Weinreb, R.N.; Zangwill, L.M.; Liebmann, J.M.; Girkin, C.A.; Medeiros, F.A. The Relationship between Cup-to-Disc Ratio and Estimated Number of Retinal Ganglion Cells. Investig. Ophthalmol. Vis. Sci. 2013, 54, 3205–3214. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fernandez-Granero, M.A.; Sarmiento, A.; Sanchez-Morillo, D.; Jiménez, S.; Alemany, P.; Fondón, I. Automatic CDR Estimation for Early Glaucoma Diagnosis. J. Healthc. Eng. 2017, 2017, 5953621. [Google Scholar] [CrossRef] [Green Version]
- Chan, E.W.; Liao, J.; Foo, R.C.M.; Loon, S.C.; Aung, T.; Wong, T.Y.; Cheng, C.-Y. Diagnostic Performance of the ISNT Rule for Glaucoma Based on the Heidelberg Retinal Tomograph. Trans. Vis. Sci. Tech. 2013, 2, 2. [Google Scholar] [CrossRef] [Green Version]
- Kara-José, A.C.; Melo, L.A.S.; Esporcatte, B.L.B.; Endo, A.T.N.H.; Leite, M.T.; Tavares, I.M. The Disc Damage Likelihood Scale: Diagnostic Accuracy and Correlations with Cup-to-Disc Ratio, Structural Tests and Standard Automated Perimetry. PLoS ONE 2017, 12, e0181428. [Google Scholar] [CrossRef]
- Bock, R.; Meier, J.; Michelson, G.; Nyúl, L.G.; Hornegger, J. Classifying Glaucoma with Image-Based Features from Fundus Photographs. In Joint Pattern Recognition Symposium; Springer: Berlin/Heidelberg, Germany, 2007; pp. 355–364. [Google Scholar]
- Claro, D.L.; Melo, R.D.; Veras, S. Glaucoma Diagnosis Using Texture Attributes and Pre-Trained CNN’s. Rev. Inf. Te Orica Apl.-RITA-ISSN 2018, 2175, 2745. [Google Scholar]
- Litjens, G.; Kooi, T.; Bejnordi, B.E.; Setio, A.A.A.; Ciompi, F.; Ghafoorian, M.; van der Laak, J.A.W.M.; van Ginneken, B.; Sánchez, C.I. A Survey on Deep Learning in Medical Image Analysis. Med. Image Anal. 2017, 42, 60–88. [Google Scholar] [CrossRef] [Green Version]
- Zhou, T.; Ruan, S.; Canu, S. A Review: Deep Learning for Medical Image Segmentation Using Multi-Modality Fusion. Array 2019, 3–4, 100004. [Google Scholar] [CrossRef]
- Yanase, J.; Triantaphyllou, E. A Systematic Survey of Computer-Aided Diagnosis in Medicine: Past and Present Developments. Expert Syst. Appl. 2019, 138, 112821. [Google Scholar] [CrossRef]
- Ahn, J.M.; Kim, S.; Ahn, K.-S.; Cho, S.-H.; Lee, K.B.; Kim, U.S. A Deep Learning Model for the Detection of Both Advanced and Early Glaucoma Using Fundus Photography. PLoS ONE 2018, 13, e0207982. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Panda, R.; Puhan, N.B.; Rao, A.; Padhy, D.; Panda, G. Recurrent Neural Network Based Retinal Nerve Fiber Layer Defect Detection in Early Glaucoma. In Proceedings of the 2017 IEEE 14th International Symposium on Biomedical Imaging (ISBI 2017), Melbourne, Australia, 18–21 April 2017; pp. 692–695. [Google Scholar]

| Type | Collected Data |
|---|---|
| External eye inspection | eye movements, opacities, and eye volume change |
| Eye complaints | visual blurring, presence of colored halos, vision loss |
| Personal background | existence of chronic diseases (e.g., diabetes, hypertension, neurological and rheumatologic conditions) and use of medications (e.g., steroids, which increase the incidence of glaucoma) |
| Family background | incidence of glaucoma in first-degree relatives |
| Eye exam | eyeglasses, funduscopy with direct visualization of the optical papilla; biomicroscopy performed with the aid of a slit lamp that cuts the light at different angles and allows the verification of ocular structures |
| Equipment | Advantages | Disadvantages | Costs | Portability |
|---|---|---|---|---|
| Retinographer | Single image capture Excellent contrast and detail | Pupillary dilation may be needed Expensive Image quality more susceptible to media opacities, motion artefact, and image processing Cannot quantify membrane thickness and presence of edema | USD $50–USD $100K | No |
| OCT | Monitors evolution of macular thickness Improved resolution More images taken Eye tracking feature Portable | Expensive Limited penetration power Transverse resolution must to be similar to axial resolution | USD $10K–USD $50K | Yes |
| HRT | 3D construction of optic nerve head Automatically tracks progression of the disease using contour lines sketched during previous patient examinations Real-time quality control during image acquisition Sophisticated analysis software for glaucoma detection and progression Large race-specific normative database that has been shown to improve diagnostics | Measurements rely on a reference plane based on the placement of a user-defined contour line Stereometric measurements can be influenced by moderate changes in IOP | >USD $5K | Yes |
| Lenses | Cheap Helps to increase angles of visualization | Requires other equipment for attachment | USD $3K–USD $5K | Yes |
| Slit Lamp | Easy examination of the eye structures in detail resolution improved in higher models by maximizing the quality of the lenses | Discomfort in some photophobic patients | >USD $5K | Yes |
| Direct Ophthalmoscope | High magnification Portable Check anterior and fundus Cheap | Pupillary dilation may be required Cannot use with ocular opacities Not quick | USD $0.2K–USD $0.3K | Yes |
| Binocular Indirect Ophthalmoscope | Greater area of the fundus Easier to use Ideal for defining the extent and height of retina High-quality stereoscopic image Portable Either magnification or field of view can be prioritised by varying the choice of the condensing lens | Not good for defining the relative depth of the lesion vertically and horizontally inverted image Complicates the recording of fundus abnormalities Level of magnification is relatively low High level of retinal illuminance can be uncomfortable for the patient | USD $1K–USD $3K | Yes |
| Equipment | Costs (k$) | Mydriatic | Field Angle | Lens (Diopter) | Resolution | Smartphone | CAD Software |
|---|---|---|---|---|---|---|---|
| Phelcom Eyer [84] | 4 | No | 45° | N/D | 12 MP | Samsung Galaxy S9 | Integration with EyerCloud system |
| visoScope [85] | 0.25/0.5 | N/D | 50° | 20 | N/D | iPhone | N/D |
| Volk Pictor Plus [86] | 6.8 | No | 40° | N/D | 2560 × 1920 pixels | No | N/D |
| Volk iNview [87] | 1 | N/D | 50° | N/D | 8 MP | iPhone 6 and 6S | N/D |
| Welch Allyn iExaminer [88] | N/D | No | 25° | N/D | 8 MP | iPhone 6 and 6S | N/D |
| D-EYE™ [89] | 4 | N/D | miosis 6° mydriasis 20° | N/D | 8 MP | iPhone | N/D |
| Indicator | References | Nr of Papers | Explanation |
|---|---|---|---|
| CDR | [19,31,58,81,99,100] | 6 | Ratio between the vertical or horizontal diameter of cupping and the papilla. |
| CDAR | [19,31,58,81,99,100] | 6 | Ratio between the area of cupping and the papilla. |
| ISNT | [42,56,102] | 3 | Characterization of the healthiness of the optic disc based on the thickness in certain regions (inferior, superior, nasal, and temporal poles). |
| DDLS | [43,102] | 2 | Comparison of the diameter of the neural rim and the optic disc and the shortest distance between the optical disc contour and the excavation. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Camara, J.; Neto, A.; Pires, I.M.; Villasana, M.V.; Zdravevski, E.; Cunha, A. A Comprehensive Review of Methods and Equipment for Aiding Automatic Glaucoma Tracking. Diagnostics 2022, 12, 935. https://doi.org/10.3390/diagnostics12040935
Camara J, Neto A, Pires IM, Villasana MV, Zdravevski E, Cunha A. A Comprehensive Review of Methods and Equipment for Aiding Automatic Glaucoma Tracking. Diagnostics. 2022; 12(4):935. https://doi.org/10.3390/diagnostics12040935
Chicago/Turabian StyleCamara, José, Alexandre Neto, Ivan Miguel Pires, María Vanessa Villasana, Eftim Zdravevski, and António Cunha. 2022. "A Comprehensive Review of Methods and Equipment for Aiding Automatic Glaucoma Tracking" Diagnostics 12, no. 4: 935. https://doi.org/10.3390/diagnostics12040935
APA StyleCamara, J., Neto, A., Pires, I. M., Villasana, M. V., Zdravevski, E., & Cunha, A. (2022). A Comprehensive Review of Methods and Equipment for Aiding Automatic Glaucoma Tracking. Diagnostics, 12(4), 935. https://doi.org/10.3390/diagnostics12040935









