Abstract
Seroprevalence studies are essential to get an accurate estimate of the actual SARS-CoV-2 diffusion within populations. We report on the findings of the first serosurvey conducted in Tunis prior to the implementation of mass vaccination and analyzed factors associated with seropositivity. A household cross sectional survey was conducted (March–April 2021) in Tunis, spanning the end of the second wave and the beginning of the third wave of COVID-19. SARS-CoV-2 specific immunoglobulin G (IgG) antibodies to the spike (S-RBD) or the nucleocapsid (N) proteins were detected by in-house ELISA tests. The survey included 1676 individuals from 431 households. The mean age and sex ratio were 43.3 ± 20.9 years and 0.6, respectively. The weighted seroprevalence of anti-N and/or anti-S-RBD IgG antibodies was equal to 38.0% (34.6–41.5). In multivariate analysis, age under 10, no tobacco use, previous diagnosis of COVID-19, a history of COVID-19 related symptoms and contact with a COVID-19 case within the household, were independently associated with higher SARS-CoV-2 seroprevalence. More than one third of people living in Tunis obtained antibodies to SARS-CoV-2. Further studies are needed to monitor changes in these figures as Tunisian population is confronted to the subsequent epidemic waves and to guide the vaccine strategy.
1. Introduction
The severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) was first detected in Wuhan city, China in December 2019 [1]. Since then, the virus has rapidly spread throughout the world causing 126 million cases of infection and nearly 2.8 million deaths by the end of March 2021 [2]. By mid-January 2022, the World Health Organization registered nearly 319 million cases worldwide with more than 5 million deaths [3].
In Tunisia, the first case of coronavirus disease-19 (COVID-19) was identified in 2 March 2020. The spread of infection in the country was slowed down by the strict measures imposed by the national authorities after this introduction, such as a nationwide lockdown and borders closures. As a consequence, the total number of cases and deaths registered between March and June 2020 was very modest (29 cases with no deaths and 1087 cases with 49 deaths until 17 March 2020 and 7 June 2020 respectively) [4,5]. However, after border reopening, the number of COVID-19 cases and deaths dramatically increased, shaping two COVID-19 waves: the first in August–December 2020 and the second in January–March 2021 [6]. Until 28 March 2021, 251,169 cumulative cases and 8760 COVID-19 related deaths were reported in Tunisia [7].
The monitoring of SARS-CoV-2 infection is mainly based on laboratory confirmed symptomatic cases and contacts. Hence, the true number of infected people is certainly much higher than the official reported figures mainly due to the often-asymptomatic forms of the disease. Also, some symptomatic people avoid getting tested for COVID-19 and seeking medical care for various reasons such as fear of stigma, logistical barriers or belief that COVID-19 does not exist [8,9]. A meta-analysis conducted by Chen et al. [10] at the global level included 404 serological studies published between December 2019 and December 2020 and carried out among either healthcare workers, close contacts or general population. Most of the included surveys used convenience sampling and chemiluminescence immunoassays for laboratory analysis. Based on the results of 82 seroprevalence studies of higher quality conducted among general population without known exposure to confirmed or suspected COVID-19 individuals, the estimated seroprevalence was equal to 8%. Besides, the estimated serology detected infections to confirmed cases ratio was equal to 11.1, stressing the very large burden of unreported SARS-CoV-2 infection.
Population-based seroprevalence surveys were recommended by the World Health Organization to determine as accurately as possible, the extent of the COVID-19 infection in the population [11]. Such data will provide valuable information to health authorities to tailoring prevention strategies, including vaccination.
As the extent of the SARS-CoV-2 dissemination in Tunisian communities was not sufficiently documented, we assessed the seroprevalence of SARS-CoV-2 infection among the general population in the governorate of Tunis after the second epidemic wave (from 21 March to 10 April 2021), just before the start of the vaccination campaign, and analyzed factors associated with seropositivity.
2. Materials and Methods
2.1. Study Design and Population
A cross-sectional household survey was conducted between 21 March and 10 April 2021 in anticipation of the start of mass vaccination among the general population. This period coincided with the end of the second wave and the beginning of the third epidemic wave of COVID-19 in Tunisia and was dominated by the circulation of the alpha variant [6]. The study took place in the city of Tunis in the two urban areas of El Omrane (41,781 inhabitants) and La Goulette (57,660 inhabitants) that were characterized by contrasted incidence of COVID-19 (COVID-19 incidence in El Omrane was equal to 1213 per 100,000, population which corresponds to a low to intermediate incidence and in La Goulette to 2289 per 100,000 population which corresponds to a high incidence). The two areas have similar socioeconomic characteristics, including mainly moderate-income communities.
Tunisia is located in Northern Africa at the southern shore of the Mediterranean Sea. The country population is 11.747 million according to the estimates of the National Institute of Statistics for 2020 [12] with almost 10% located in Tunis, the capital city (1,074,126 inhabitants).
The study included all persons who were permanent residents in the selected houses and who gave their consent to participate to the study. We did not include households that were unreachable after three visits of investigators. Households in which at least one member refused to participate to the survey or refused blood sampling were excluded. Nevertheless, for children younger than five years, blood test refusal was not considered as a reason for household exclusion.
2.2. Sampling Procedure
Households were included based on a two-stage cluster sampling. First, each study area was stratified by communities (El Omrane comprises 6 communities: France ville, Bir Atigue, Cité des oliviers, Ras Tabia, Jbel Lahmer and Oued el Sebai, and La Goulette comprises 5 communities: La Goulette, La Goulette casino, Khaireddine, Cité Essalama and Taieb El Mhiri). Within each community, a variable number of clusters of about five households proportional to its population size, were randomly selected. In each cluster, households were chosen using systematic sampling of every fifth household after a random starting point and a random direction. Then all individuals in the selected household were enrolled after being properly informed.
2.3. Sample Size
Assuming a design effect of 2, a prevalence equal to 50%, a precision of 0.05, a population size of 40,000, and a 95% confidence interval, the calculated sample size was equal to 760 individuals in each area, which corresponds to about 190 households with an average household size equal to 4.
2.4. Data Collection
In each study area, four teams of two trained investigators (one for questionnaire administration and the other for blood sampling) performed the data collection.
The face-to-face standardized questionnaire (Appendix A) included questions related to sociodemographic characteristics, lifestyle habits and medical history, compliance with barrier measures, risk factors for exposure to the SARS-CoV-2, history of COVID-19 infection and COVID-19 related symptoms.
Blood samples of about one to two milliliters were taken on a serum tube from each person. The collected sera were used for serological analysis using two in-house semi-quantitative SARS-CoV-2 ELISA tests developed and validated at Institut Pasteur in Tunis. The two tests detect immunoglobulin G (IgG) antibodies to the receptor-binding domain of the spike protein (S-RBD) or the nucleocapsid (N) proteins of the SARS-CoV-2 respectively. N and S-RBD recombinant proteins were produced in E. coli BL21 (DE3) and the eukaryotic expression system Sf9 respectively. ELISA assays were subsequently optimized and validated using 108 sera from RT PCR confirmed COVID-19 patients and 72 prepandemic sera from the Tunisian population collected between 2013 and 2018. Individual data are expressed as a ratio of the Optical density (OD) of the test sample to that of a reference sample selected because it gives an OD just at the level of the cut-off value of the test. This allows better comparability of the results generated by different assays by different sera.
Using a receiver operating curve, developed assays displayed have very high performances (AUC: 0.966 and 0.98, respectively, p < 0.0001). This resulted in a specificity of 93% and a sensitivity of 95% for the anti-S-RBD test and a specificity of 93% and a sensitivity of 94% for the anti-N test [13].
2.5. Data Analyses
Qualitative variables were summarized in terms of frequencies and percentages and quantitative ones in terms of means and standard deviation.
In order to facilitate data interpretation, we dichotomized the responses to the four-point Likert type scale questions related to compliance with preventive measures (namely, social distancing, hand hygiene and wear of facial mask). Hence, the responses «Always» and «Often» were grouped together on the one side, and «Never» and «Occasionally» on the other side.
Contact with a COVID-19 case within the household was defined as a contact with at least one member of the household who was previously diagnosed with COVID-19 or who was tested seropositive to the SARS-CoV-2 in the present study. COVID-19 related symptoms included respiratory symptoms, fever, chills, digestive symptoms, headache, conjunctivitis, weakness, myalgia, arthralgia, anosmia, agueusia, sore throat and loss of consciousness.
The overall seroprevalence was calculated on the basis of the detection of IgG antibodies to either the S-RBD or the N proteins of the SARS-CoV-2. This crude prevalence was afterwards weighted, using the post stratification weight method, for age and sex of the population in “El Omrane” and “La Goulette” using data published in the 2014 Tunisia population and housing census [14]. The prevalence of IgG antibodies against S-RBD and N proteins were also adjusted for ELISA test performance using the following formula [15]:
The Chi-square test for bivariate analysis and multivariable logistic regression for multivariate analysis were used to identify factors associated with SARS-CoV-2 anti-S-RBD IgG antibodies and/or anti-N IgG antibodies seropositivity prevalence.
A p-value ≤ 0.05 indicates statistical significance for all analyses.
3. Results
During this survey, 290 households were visited by investigators in each study area. In “La Goulette”, 60 were not reached at their homes after three consecutive visits of investigators and 40 were excluded given that at least one household member (aged older than 5 years) refused to participate in the survey. In “El Omrane”, 30 households were visited by investigators, each three times, with no response, and 19 had at least one member who refused to participate in the study.
Overall, 1676 individuals from 431 households (190 in “La Goulette” and 241 in “EL Omrane”) were included in the survey. Most were female (62.5%). The mean age of participants was equal to 43.3 ± 20.9 years ranging from 1 to 100 years. Nearly a quarter (25.3%) were aged 60 years and above and 22.4% were smokers.
More than half of surveyed individuals (53.9%) were either employees or students; 58.6% did not have any underlying medical conditions and majority of them lived in an independent house (90.9%) (Table 1). The mean number of persons per room was equal to 1.4 ± 0.7.

Table 1.
Sociodemographic characteristics and lifestyle habits of the study population, Tunis, Tunisia.
Nearly two thirds of participants (68.4%) reported that they frequently comply with preventive measures and around the third (37.2%) declared private cars as the mean of transport they usually use. More details are presented in Appendix B.
Among all included individuals, 10.1% (95% confidence interval (CI): 8.8–11.6) reported they had already been tested for SARS-CoV-2, of whom, 42.9% (35.7–50.5) tested positive. Hence, only 4.4% (3.5–5.4) of all participants knew they had been already infected by the pandemic virus.
The weighted and test-performance adjusted prevalence of IgG antibodies against the N or the S-RBD proteins were equal to 26.6% (22.9–30.8) and 25.1% (22.2–28.4) respectively. The overall weighted seroprevalence (i.e., reactivity with S-RBD and/or N) was equal to 38.0% (34.6–41.5). At the level of each study area, it was equal to 41.9% (38.0–45.9) in “El Omrane” and to 34.0% (28.5–39.9) in “La Goulette” (More details are presented in Table 2). Applying these percentages to the total population of each study area, we found that the estimated number of infected individuals in “El Omrane” and “La Goulette” were by March 2021, 34.5 and 14.8 times higher than the reported cumulative COVID-19 cases in each area respectively.

Table 2.
Prevalence of Immunoglobulin G antibodies in the governorate of Tunis Tunisia.
Among all seropositive participants, more than three quarter (79.2% (75.1–82.8)) were asymptomatic.
In univariate analysis, seropositivity prevalence varied significantly according to age groups (p < 10−3). Indeed, participants younger than 10 years had the highest seroprevalence of SARS-CoV-2 infection: 51.1% (35.2–66.8) still, none of them were previously diagnosed as a COVID-19 case and only 7.1% developed COVID-19 related symptoms. A higher seroprevalence at 47.9% (41.2–54.6) was also characteristic of the group of adolescents 10–20 years old.
Seropositivity was higher among participants who were in contact (i) with a COVID-19 case within the same household (OR = 2.3 (1.8–2.9)), (ii) with those who had reported any symptom compatible with a COVID-19 infection (OR = 2.0 (1.5–2.6)) and with those who were previously tested positive for COVID-19 (OR = 4.3 (2.4–7.9)). In addition, current tobacco smokers had lower SARS-CoV-2 seroprevalence than non-smokers (OR = 0.5 (0.4–0.6)) (Table 3).

Table 3.
Seroprevalence to SARS-CoV-2 according to study variables Tunis, Tunisia.
In multivariate analysis, young age, previous diagnosis of COVID-19 infection, COVID-19 related symptoms, no tobacco use and contact with a COVID-19 case within the household were independently associated with SARS-CoV-2 seropositivity (Table 4).

Table 4.
Predictors of seropositivity among Tunisian participants as a result of multiple logistic regression analysis.
4. Discussion
In the present study, we conducted a cross-sectional survey to assess the seroprevalence of SARS-CoV-2 in people living in two urban areas of Tunis: El Omrane and La Goulette. The two study areas were selected because they expressed contrasted incidence (intermediate to low versus high) of COVID-19, based on the cumulative incidence since the pandemic’s onset. The weighted prevalence of seropositivity in the study population, defined by the detection of IgG antibodies against the N and/or the S-RBD proteins, was equal to 38.0% (34.6–41.5). In multivariate analysis, we found that younger age, smoking status, previous confirmed COVID-19 infection, history of COVID-19 related symptoms, and contact with a COVID-19 case within the household were independently and significantly associated with SARS-CoV-2 seropositivity.
Our study was conducted on March–April 2021 corresponding to the end of the second epidemic COVID-19 wave in Tunisia and the beginning of the third one. Hence and up to the study dates, the population has been exposed mainly to the wild original SARS-CoV-2 virus and to the alpha variant. Since then, the country was hit again by two additional much higher waves: the fourth wave on May–October 2021 mainly due to the circulation of the delta virus variant [6] (Figure 1) and the fifth wave starting in January 2022 due to the emergence of the Omicron virus variant.
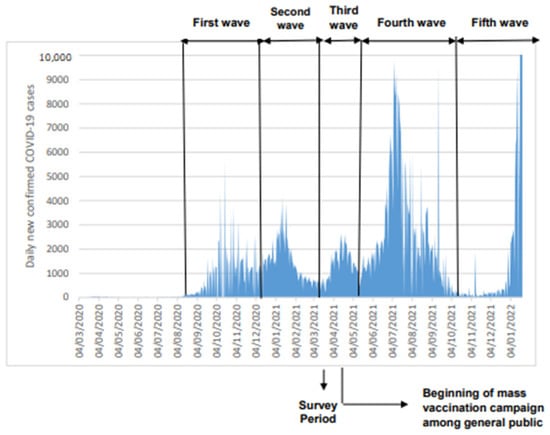
Figure 1.
Epidemic curve COVID-19 cases in Tunisia expressed as daily new confirmed cases (Data source: [16]).
Our results reveal that a large fraction (almost 40%) of the population of the study areas became infected after being exposed to just the second epidemic wave. In addition, the estimated seroprevalences were 34.5 and 14.8 times greater than the reported number of confirmed COVID-19 cases in “El Omrane” and “La Goulette”, respectively. These figures stress the key role played by asymptomatic infection in SARS-CoV-2 transmission and also illustrate the limits of case detection and contact tracing in the study areas. These shortcomings likely had severely jeopardized the impacts of individual preventive measures including isolation, in term of virus circulation. The prevalence figures also illustrate the high level of infection reached after an epidemic wave that was, all in all, relatively modest compared to the fourth and fifth waves that hit the country in the following 12 months.
Surprisingly, we found that the SARS-CoV-2 seroprevalence was higher in the area of “low to intermediate” COVID-19 incidence (“El Omrane”) compared to that in high COVID-19 incidence (“La Goulette”). One possible explanation could be that “La Goulette” is a seaside city and some Tunisians were living abroad while tourists arrived in the summer of 2021 after reopening of the borders, to spend holidays there. If they became infected in Tunisia during their temporary stay, they would have been registered as cases by the regional health directorates. Still, they were not included in our survey as we only considered permanent Tunisian residents.
Our results are in keeping with those reported at the global level in population surveys conducted among unvaccinated people and before the high circulation of the delta variant, which is known to be more contagious than previous variants [17]. Seropositivity rates reported worldwide [18,19,20,21,22,23,24,25,26,27], ranged from less than 2.6% in Sierra Leone [18] to 70.0% in Iquitos (Peru) [25] (Appendix C). Such heterogeneity likely reflects differences in survey designs, dates of epidemic onset, the adherence of exposed populations to social restrictions, and individual preventive measures applied in each country [28] and the type of laboratory test used.
Our results also corroborate previous studies mainly in the African continent in which a high underdetection and/or under-reporting of COVID-19 cases was noted [18,27,29,30,31]. This could be explained by the high percentage of COVID-19 asymptomatic cases. Indeed, we found that a large majority of seropositive participants (79.2%) did not develop any kind of COVID-19 related symptoms. Such high percentage of asymptomatic COVID-19 cases was also found in some other studies [32,33]. Nevertheless, a memory bias, which can lead to an overestimation of asymptomatic forms, cannot be eliminated in this survey. Indeed, participants were asked about their symptoms since the beginning of the COVID-19 pandemic in Tunisia. Such a large gap between the true number of SARS-CoV-2 infected persons and the declared cases of COVID-19 can also be explained by limited testing, fear of the disease, infection-related stigma and, in some cases, conviction that COVID-19 does not exist [8,33,34].This emphasizes the need of amplifying testing efforts, case finding and contact tracing [35], mainly with the circulation of the new Omicron variant characterized by a very high proportion of asymptomatic cases [36]. This is key to generating accurate data on SARS-CoV-2 in Tunisia and to implement evidence based public health measures to flatten the COVID-19 curve.
In this study, we found that age was independently associated with seropositivity. Indeed, children (age < 10) had the highest percentage of IgG antibodies and the same trend is observed in the next age range (10–20). According to literature, youth are more likely to have social contact than adults [37] and may be less adherent to barrier measures such as masking, hand hygiene and social distancing [38]. Contact in schools were also found to be more physical than those in the workplace [37]. Another explanation could be that children seem to have higher and more sustainable immune responses than adults [39]. However, the findings of the present study do not support most of previous research surveys that found either a lower seroprevalence among youngest participants [30,40,41,42] or a non-significant difference according to age [33,43]. Such differences in results may be explained by the fact that most of the aforementioned studies that found a lower seroprevalence among youngest participants were conducted during the first wave of the pandemic, when the majority of schools were closed, unlike during our study, which was conducted in spring 2021 after schools’ reopening. Previous longitudinal studies have found increased SARS-CoV-2 seropositivity among children along with the overall transmission of COVID-19 [44,45,46]. In addition, with the emergence of the Omicron variant, a rise in COVID-19 pediatric cases was noted [47]. This raises the concern of the potential influence of variants’ emergence on the transmission of SARS-CoV-2 among children.
We also found that none of the seropositive participants aged under 10 years were previously diagnosed with COVID-19, which shows that the spread of SARS-CoV-2 among children and adolescents is extremely underestimated in Tunisia. Public health measures to decrease SARS-CoV-2 transmission should thus include the entire population, and not only focus on adults [35]. Non-pharmaceutical intervention, including masking, hand hygiene and ventilation of indoor settings, should also be strengthened mainly in schools [48] since those under 18 years old are still not a priority target group for COVID-19 vaccination in Tunisia.
The seroprevalence to SARS-CoV-2 did not differ significantly according to sex, in line with results of previous studies [22,43,49,50]. Moreover, our study together with previous ones [22,28,40,43,51] found a higher prevalence of antibody seropositivity among participants who report a history of COVD-19 like symptoms. Consistent with other studies [43,51], previous diagnosis of COVID-19 infection and contact with a COVID-19 case within the household were also independently and significantly associated with a higher percentage of SARS-CoV-2 IgG antibodies. However, surprisingly, we found that current tobacco smokers had lower SARS-CoV-2 seroprevalence than non-smokers. A similar result was found by Alsuwaidi et al. and Paleiron et al. [22,52]. One hypothesis is that nicotine decreases the expression of the angiotensin converting enzyme 2 (ACE 2) which is a receptor of SARS-CoV-2. Another hypothesis is that SARS-CoV-2 and nicotine compete for binding to the nicotine acetylcholine receptor (nAChR) which is possibly involved in the physiopathology of COVID-19 infection [53]. However, our results should be interpreted with caution as we conducted an observational study. Also, participants may underreport their tobacco consumption introducing a social desirability bias to the survey. Indeed, a relationship between smoking and increased risk of COVID-19 infection was underlined by a British study that used mendelian randomization analysis [54]. In addition, evidence suggests that tobacco increases the risk of severe illness and deaths due to COVID-19 [55].
Finally, a non-significant association was found between seropositivity and the used means of transport. In accordance, an online survey conducted in France assessing factors associated with a higher risk to COVID-19 contagion, found that public transport was not associated with a higher risk of virus transmission unlike restaurants and bars [56].
Strengths and Limitations
This is the first study in Tunisia that reports the extent of the COVID-19 infection among both children and adults. As well, our study is, to the best of our knowledge, the first seroprevalence survey reported from countries in North Africa. Our study was conducted at the nadir of the second epidemic wave that peaked on 4 January 2021 and the start of the third epidemic wave that peaked on 15 April 2021 (Figure 1) [6]. Importantly it took place just before the beginning of the COVID-19 vaccination campaign in Tunisia and hence the detected antibodies could be unequivocally attributed to natural SARS-CoV-2 infection and not to vaccine administration. The results of the present study can guide policy makers in tailoring vaccination strategies and are also useful indicators/parameters (such as infection rate between age groups) that can be used for mathematical modeling in order to predict the spread of the COVID-19 pandemic, which represents a decision support tool to assist policy makers for the measures imposed and lifted. Also, the serum samples were tested using two in-house ELISA tests developed by Institut Pasteur in Tunis that detect with a high sensitivity and specificity anti-N and anti-S-RBD IgG antibodies, respectively. Indeed, anti-N IgG antibodies may appear before the anti-S-RBD [57] and the latter tends to wane at a slower rate than the anti-N antibodies [58]. In fact, Schoenhals et al. found a decrease of more than 10% in the percentage of anti-N IgG antibodies among seropositive blood donors during a three month follow up in Toamasina (Madagascar) [59]. The detection in our study of antibodies to the two viral proteins provides a better chance to detect more infected cases than when using only one antigen.
Our study has some limitations. The ELISA tests detect SARS-CoV-2 antibodies which are known to be evanescent after natural infection and vaccination [60] In fact, the antibody decay after natural infection [58] may have influenced he seroprevalence rates in our study population. Also, as we conducted a cross sectional study, the association between seropositivity and contact with a COVID-19 case within the household may be underestimated. Indeed, some contacts of infected individuals may have been contaminated, but they have not yet developed antibodies when the survey was conducted. Therefore, long term cohort longitudinal serological studies are warranted to assess the temporal dynamics of prevalence rates that integrate the opposing effects of natural antibody decay and the successive reinfections by different variants.
Our study was conducted only in the capital city Tunis. Larger populational serosurveys including other regions in Tunisia, would best describe the actual dynamics of the epidemics in the country according to the diversity of local conditions (i.e., in rural areas and in the various country eco-climatic stages, effect of transborder human movements, population density, etc.). Besides, assessing, in addition to serology, the protective virus neutralizing antibodies as well as the cellular immune responses to the COVID-19 infection, would certainly improve the estimation of the actual proportion of the population immune to SARS-CoV-2.
5. Conclusions
Almost 40% of participants to our serosurvey had antibodies against SARS-CoV-2 after the second epidemic wave of COVID-19 in Tunisia. This figure illustrates the true proportion of individuals who became infected and as expected, was much higher than the reported number of confirmed COVID-19 cases. Our study calls for future larger seroprevalence surveys to monitor the impact of the successive epidemic waves that hit the country as well as the effects of introducing mass vaccination to COVID-19. This will inform on changes in the fraction of immune population and its impact on the evolving SARS-CoV-2 strains.
Author Contributions
I.C.: Data curation, Methodology, Formal analysis, Writing—original draft; G.K.: Data curation, Methodology, Investigation, Writing—original draft; S.C.: Project management and coordination, Investigation, Data curation; R.Y.: Data curation, Investigation; M.D.: Data Curation, Investigation; M.A.S.: Data curation, investigation; S.S.: Data curation, Investigation; S.M.: Investigation, Validation; W.K.R.: Data curation, Investigation; S.R.: Data curation, Investigation; K.D.: Conception of the work, Resources, Funding acquisition, Supervision, Validation, Visualization, Writing—review & editing; M.R.B.: Validation, review & editing; C.B.: Supervision, Validation, Visualization, review & editing; M.B.A.: Supervision, Validation, Visualization, review & editing; J.B.: Conception of the work, Data curation, Formal analysis, Investigation, Methodology, Project administration, Resources, Supervision, Validation, Visualization, Writing—review & editing. All authors have read and agreed to the published version of the manuscript.
Funding
The study was conducted in the frame of the COVID Africa Repair Project, a multipartner research program of the Pasteur Network, grouping the 10 Institutes Pasteur established in Africa. Repair was funded by the French Ministry for Europe and Foreign Affairs (MEAE) grant number: SC/projet_REPAIR N°57/2020.ipt.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by the Biomedical Ethics Committee (CEBM) of Institut Pasteur de Tunis (IPT) (2021/02/I/LR16IPT), approval date: 10 March 2021.
Informed Consent Statement
A written informed consent was obtained from participants aged 18 years and over. For minors or illiterate, the consent was obtained from a legally acceptable representative.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Acknowledgments
We thank the Tunis Regional Directorate of health for field supervision and support provided. Special thanks to the residents who took part in the study.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Appendix A. Questionnaire
| Household Report Form |
Unique ID I____I - I_____I - I___I___I___I
Id. area Id. community Id. household
1. Data collector information
| First name–Last name | /______________________________________/ |
| Telephone number | /_____________________________________/ |
| Date of contact | ____/____/________ |
| Appointment date for the survey | ____/____/________ |
2. Household information
| Address | /__________________________________________ ___________________________________________/ | ||
| Dwelling type | □ Apartment □ Independent house | ||
| Number of rooms | _____ | ||
| Number of household members | _____ | ||
| First and last name of the household member | Sex | Age in years (months if <5 years) | Consent |
| □F □H | ____ years (____ months) | □Yes □ No | |
| □F □H | ____ years (____ months) | □Yes □ No | |
| □F □H | ____ years (____ months) | □Yes □ No | |
| □F □H | ____ years (____ months) | □Yes □ No | |
| □F □H | ____ years (____ months) | □Yes □ No | |
| □F □H | ____ years (____ months) | □Yes □ No | |
| □F □H | ____ years (____ months) | □Yes □ No | |
| □F □H | ____ years (____ months) | □Yes □ No | |
| □F □H | ____ years (____ months) | □Yes □ No | |
| □F □H | ____ years (____ months) | □Yes □ No | |
| □F □H | ____ years (____ months) | □Yes □ No | |
| □F □H | ____ years (____ months) | □Yes □ No | |
| □F □H | ____ years (____ months) | □Yes □ No | |
| Participant Report Form |
Unique ID I____I - I_____I - I___I___I___I - I___I___I
Id. area Id. community Id. household Id. participant
1. Data collector information
| First name–Last name | /______________________________________/ |
| Telephone number | /_____________________________________/ |
| Date of interview | ____/____/________ |
2. Participant information
| First name–Last name | I__I__I__I__I__I__I__I__I__I__I__I__I__I__I__I__I__I__I__I__I__I |
| Sex | □ Male □ Female |
| Date of birth | ____/____/________ |
| Age in years (months if <5 years) | ____ years ____ months |
| Nationality | /______________________________________/ |
| Telephone number | /______________________________________/ |
| Profession/activity | □ Without activities/Retired □ Student/ Kindergarten Name and location of the school, university or kindergarten: □ Having an activity Nature of the activity/profession:…………………… Location of the work/activity:…………………………… |
3. Comorbidities
| Morbid obesity (BMI> 40 kg/m2 if age ≥ 16 years) | □ Yes □ No □ Unknown |
| Cancer | □ Yes □ No □ Unknown |
| Diabetes | □ Yes □ No □ Unknown |
| HIV/other immune deficiency | □ Yes □ No □ Unknown |
| Heart disease | □ Yes □ No □ Unknown |
| Asthma | □ Yes □ No □ Unknown |
| Chronic respiratory disease (other than asthma) | □ Yes □ No □ Unknown |
| Chronic liver disease | □ Yes □ No □ Unknown |
| Blood disorder If yes, specify the disease: | □ Yes □ No □ Unknown |
| Chronic kidney disease | □ Yes □ No □ Unknown |
| Chronic neurological disorder | □ Yes □ No □ Unknown |
| Pregnancy | □ Yes □ No □ Unknown □ NA |
| If yes, specify:- Trimestre | □ First □ Second □ Third |
| - Estimated date of delivery | ____/___/________ |
| Other comorbidities | □ Yes □ No □ Unknown |
| If yes, specify: |
4. Tobacco smoking
| Current tobacco smoking? | □ Yes □ No |
5. Respect of barrier measures
| Do you wear a face mask in public places? | □ Always □ Often □ Occasionally □ Never |
| Are you following recommended hand hygiene practices (using soap and/or hydroalcoholic solutions)? | □ Always □ Often □ Occasionally □ Never |
| Are you following the recommended physical distancing measures? | □ Always □ Often □ Occasionally □ Never |
6. Risk factors (exposures)
| Since December 2019, have you traveled to a foreign country? | □ Yes □ No □ Unknown | |
| Since the begining of the COVID-19 pandemic (March 2020): | ||
| Seeking care in a health facility | □ Yes □ No □ Unknown | |
| What means of transportation do you usually use? | □Car □Public transport □Bicycle/motorcycle □Different means of transport □None | |
7. History of Covid-19 and previous diagnostic test(s) performed:
| A. Have you been tested for COVID-19? □ Yes □ No | |
| If No, have you presented any symptoms suggestive of Covid-19 since the diffusion of the coronavirus in Tunisia (mars 2020)? □ Yes□ No If yes, what symptoms? ………………………………..; If you answered Yes to question A, proceed to question B. If you answered No to question A, you do not have to answer the rest of the questions. | |
| B. Did you get a positive test result for COVID-19 □ Yes □ No If you answered No to question B, go to question C. If you answered Yes to question B, please answer questions: a, b, c, d. a. What was (were) the type(s) and result(s) of the diagnostic test(s) performed? □RT-PCR Result:…………………. □Rapid antigen test Result:………………… □Chest scan Result ………………… | |
| b. What was the date of COVID-19 diagnosis? c. What was the clinical form of COVID-19? | ____ / ____/ ________ □Symptomatic form □Asymptomatic form |
| d. If you had a symptomatic form, did you experience the following symptoms? | |
| -Fever (≥38 °C) □ Yes □ No □ Unknown | |
| -Sore throa □ Yes □ No □ Unknown | |
| -Cough □ Yes □ No □ Unknown | |
| -Runny nose □ Yes □ No □ Unknown | |
| -Shortness of breath □ Yes □ No □ Unknown | |
| -Sweating □ Yes □ No □ Unknown | |
| -Chills □ Yes □ No □ Unknown | |
| -Vomiting □ Yes □ No □ Unknown | |
| -Nausea □ Yes □ No □ Unknown | |
| -Diarrhea □ Yes □ No □ Unknown | |
| -Abdominal pain □ Yes □ No □ Unknown | |
| -Chest pain □ Yes □ No □ Unknown | |
| -Headache □ Yes □ No □ Unknown | |
| -Skin rash □ Yes □ No □ Unknown | |
| -Conjunctivitis □ Yes □ No □ Unknown | |
| -Muscle ache □ Yes □ No □ Unknown | |
| -Joint pain □ Yes □ No □ Unknown | |
| -Loss of appetite □ Yes □ No □ Unknown | |
| -Loss of taste □ Yes □ No □ Unknown | |
| -Loss of smell □ Yes □ No □ Unknown | |
| -Nosebleed □ Yes □ No □ Unknown | |
| -Tiredness □ Yes □ No □ Unknown | |
| -Convulsions □ Yes □ No □ Unknown | |
| -Loss of consciousness □ Yes □ No □ Unknown | |
| -Other symptoms □ Yes □ No □ Unknown. If yes, specify | |
| C. If you have had one or more diagnostic test(s) for COVID-19 and they were all negative, specify: | |
| a. Why did you do the test: □ Travel abroad □ Contact with a confirmed case of COVID-19 □ Presence of signs suggestive of COVID-19, if so, specify the symptoms …………………………………………………………□ Other, Specify:………………… b. Date of the last test: ___/___/_________ c. Type of the last test: □RT-PCR □Rapid antigen test □Chest scan | |
| Thank you for your participation | |
Appendix B. Distribution of Study Subjects According to the Risk Factors for Exposure to SARS-CoV-2, Tunis, March–April 2021
| Variables | N | % |
| Respect of preventive measures (n = 1663) | ||
| Frequently | 1138 | 68.4 |
| Occasionally/Never | 525 | 31.6 |
| Travelling abroad since December, 2019 (n = 1676) | ||
| Yes | 52 | 3.1 |
| No | 1624 | 96.9 |
| Contact with a COVID-19 case within the household (n = 1676) | ||
| Yes | 1198 | 71.5 |
| No | 478 | 28.5 |
| Seeking care in a health facility since the beginning of the COVID-19 pandemic in Tunisia (Mars 2020) (n = 1667) | ||
| Yes | 909 | 54.5 |
| No | 758 | 45.5 |
| Means of transport used (n = 1632) | ||
| Car | 607 | 37.2 |
| Public transport | 441 | 27.0 |
| Bicycle/motorcycle | 14 | 0.9 |
| Different means of transport | 176 | 10.8 |
| None | 394 | 24.1 |
Appendix C. Some Previous SARS-CoV-2 Seroprevalence Surveys among General Public before the Beginning of Mass Vaccination Campaigns
| Country | References | Population Size | Study Period | Antibodies | Antibody Detection Tests | Seroprevalence (%) |
| France | Le Vu et al. [21] | 2879 | May 2020 | IgG anti-S b IgG anti-N c pseudo-neutralizing antibodies | In-house test (LuLISA) | 4.9 |
| UAE a | Alsuwaidi et al. [22] | 8831 | July August 2020 | IgG anti-S | Commercial test (Diasorin) | 10.4 |
| Peru, Iquitos | Álvarez-Antonio et al. [25] | 716 | July 2020 | IgG/IgM | Rapid test | 70.0 |
| Colombia, Monteria | Mattar et al. [26] | 1368 | August 2020 | IgG/IgM/IgA | Commercial test (ELISA) | 55.3 |
| Israel | Reicher et al. [20] | 54,357 | June-Sept 2020 | IgG anti-S | Commercial test (Diasorin) | 4.6 |
| South Sudan (Juba) | Wiens et al. [27] | 2214 | August-September 2020 | IgG anti-S-RBD d | Commercial test (Quantitative ELISA) | 22.3 |
| Oman | Al-Abri et al. [23] | 4064 | November 2020 | IgG anti-S | Commercial test (Diasorin) | 22.0 |
| Sri Lanka | Jeewandara et al. [24] | 2547 | January 2021 | IgA, IgM, IgG anti-RBD | Commercial test (Wantai SARS-CoV-2 Ab ELISA) | 24.5 |
| Sierra Leone | Barrie et al. [18] | 1893 | March 2021 | IgG/IgM | Commercial Rapid test | 2.6 |
| Zimbabwe | Fryatt et al. [19] | 2340 | Feb-April 2021 | IgG anti-N b | Commercial test Roche Elecsys | 53.0 |
| a: United Arab Emirates; b: Immunoglobuline G antibodies to the the spike protein; c: Immunoglobuline G antibodies to the nucleocapsid protein; d: Immunoglobuline G antibodies to the receptor-binding domain of the spike protein. | ||||||
References
- World Health Organization. Listings of WHO’s Response to COVID-19. Available online: https://www.who.int/news/item/29-06-2020-covidtimeline (accessed on 30 November 2021).
- World Health Organization. Weekly Epidemiological Update on COVID-19—30 March 2021. Available online: https://www.who.int/publications/m/item/weekly-epidemiological-update-on-covid-19—31-march-2021 (accessed on 30 November 2021).
- WHO. WHO Coronavirus (COVID-19) Dashboard. 2021. Available online: https://covid19.who.int (accessed on 30 November 2021).
- National Observatory of New and Emerging Diseases. COVID-19 en Tunisie: Point de Situation a la Date du 07 Juin 2020. Available online: https://www.onmne.tn/?p=10229 (accessed on 30 November 2021).
- National Observatory of New and Emerging diseases. Point de Situation sur L’épidémie D’infections au Nouveau Coronavirus « COVID-19 » à la Date du 17 Mars 2020. Available online: https://www.onmne.tn/?p=10363 (accessed on 22 December 2021).
- National Observatory of New and Emerging Diseases. Bulletin de Veille COVID-19 a la Date du 12 Décembre 2021. Available online: https://www.onmne.tn/?p=15695 (accessed on 22 December 2021).
- Ministry of Health. Point de Situation en Tunisie—Coronavirus. Available online: http://coronavirus.rns.tn/point-de-situation-en-tunisie/ (accessed on 10 January 2022).
- McGowan, C.R.; Hellman, N.; Chowdhury, S.; Mannan, A.; Newell, K.; Cummings, R. COVID-19 testing acceptability and uptake amongst the Rohingya and host community in Camp 21, Teknaf, Bangladesh. Confl. Health 2020, 14, 74. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.; Song, Y.; Wang, Q.; Pu, J.; Sun, F.Y.; Zhang, Y.; Zhou, X.; Larson, H.J.; Hou, Z. Public Attitudes and Factors of COVID-19 Testing Hesitancy in the United Kingdom and China: Comparative Infodemiology Study. JMIR Infodemiol. 2021, 1, e26895. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Chen, Z.; Azman, A.S.; Deng, X.; Sun, R.; Zhao, Z.; Zheng, N.; Chen, X.; Lu, W.; Zhuang, T.; et al. Serological evidence of human infection with SARS-CoV-2: A systematic review and meta-analysis. Lancet Glob. Health 2021, 5, e598–e609. [Google Scholar] [CrossRef]
- World Health Organization. Population-Based Age-Stratified Seroepidemiological Investigation Protocol for COVID-19 Virus Infection; WHO: Geneva, Switzerland, 17 March 2020. [Google Scholar]
- National Institute of Statistics. Bulletin Mensuel de la Statistique, Septembre 2021. Available online: http://www.ins.tn/publication/bulletin-mensuel-de-la-statistique-septembre-2021 (accessed on 10 January 2022).
- Benabdessalem, C.; Marzouki, S.; Hamouda, W.B.; Trabelsi, K.; Boumaiza, M.; Hamouda, S.B.; Ouni, R.; Bchiri, S.; Chaaban, A.; Gdoura, M.; et al. COVID-19 in Tunisia (North Africa): IgG and IgG Subclass Antibody Responses to SARS-CoV-2 According to Disease Severity [Preprint]. 2022. Available online: https://www.medrxiv.org/content/10.1101/2022.03.01.22271696v1 (accessed on 5 March 2022).
- National Institute of Statistics. Recensement Général de la Population et de l’Habitat 2014. Available online: http://ins.tn/enquetes/recensement-general-de-la-population-et-de-lhabitat-2014 (accessed on 10 January 2022).
- Sempos, C.T.; Tian, L. Adjusting Coronavirus Prevalence Estimates for Laboratory Test Kit Error. Am. J. Epidemiol. 2020, 190, 109–115. [Google Scholar] [CrossRef]
- Ritchie, H.; Ortiz-Ospina, E.; Beltekian, D.; Mathieu, E.; Hasell, J.; Macdonald, B.; Giattino, C.; Appel, C.; Rodés-Guirao, L.; Roser, M. Coronavirus Pandemic (COVID-19). 2021. Available online: https://ourworldindata.org/coronavirus (accessed on 14 December 2021).
- Centers for Disease Control and Prevention. Delta Variant: What We Know about the Science. Available online: https://www.cdc.gov/coronavirus/2019-ncov/variants/delta-variant.html (accessed on 12 January 2022).
- Barrie, M.B.; Lakoh, S.; Kelly, J.D.; Kanu, J.S.; Squire, J.S.; Koroma, Z.; Bah, S.; Sankoh, O.; Brima, A.; Ansumana, R.; et al. SARS-CoV-2 antibody prevalence in Sierra Leone, March 2021: A cross-sectional, nationally representative, age-stratified serosurvey. BMJ Glob. Health 2021, 6, e007271. [Google Scholar] [CrossRef]
- Fryatt, A.; Simms, V.; Bandason, T.; Redzo, N.; Olaru, I.D.; Ndhlovu, C.E.; Mujuru, H.; Rusakaniko, S.; Hoelscher, M.; Rubio-Acero, R.; et al. Community SARS-CoV-2 seroprevalence before and after the second wave of SARS-CoV-2 infection in Harare, Zimbabwe. eClinicalMedicine 2021, 41, 101172. [Google Scholar] [CrossRef]
- Reicher, S.; Ratzon, R.; Ben-Sahar, S.; Hermoni-Alon, S.; Mossinson, D.; Shenhar, Y.; Friger, M.; Lustig, Y.; Alroy-Preis, S.; Anis, E.; et al. Nationwide seroprevalence of antibodies against SARS-CoV-2 in Israel. Eur. J. Epidemiol. 2021, 36, 727–734. [Google Scholar] [CrossRef]
- Le Vu, S.; Jones, G.; Anna, F.; Rose, T.; Richard, J.-B.; Bernard-Stoecklin, S.; Goyard, S.; Demeret, C.; Helynck, O.; Escriou, N.; et al. Prevalence of SARS-CoV-2 antibodies in France: Results from nationwide serological surveillance. Nat. Commun. 2021, 12, 3025. [Google Scholar] [CrossRef]
- Alsuwaidi, A.R.; I Al Hosani, F.; Al Memari, S.; Narchi, H.; Wareth, L.A.; Kamal, H.; Al Ketbi, M.; Al Baloushi, D.; Elfateh, A.; Khudair, A.; et al. Seroprevalence of COVID-19 infection in the Emirate of Abu Dhabi, United Arab Emirates: A population-based cross-sectional study. Int. J. Epidemiol. 2021, 50, 1077–1090. [Google Scholar] [CrossRef]
- Al-Abri, S.S.; Al-Wahaibi, A.; Al-Kindi, H.; Kurup, P.J.; Al-Maqbali, A.; Al-Mayahi, Z.; Al-Tobi, M.H.; Al-Katheri, S.H.; Albusaidi, S.; Al-Sukaiti, M.H.; et al. Seroprevalence of SARS-CoV-2 antibodies in the general population of Oman: Results from four successive nationwide sero-epidemiological surveys. Int. J. Infect. Dis. 2021, 112, 269–277. [Google Scholar] [CrossRef]
- Jeewandara, C.; Guruge, D.; Abyrathna, I.S.; Danasekara, S.; Gunasekera, B.; Pushpakumara, P.D.; Madhusanka, D.; Jayathilaka, D.; Ranasinghe, T.; Somathilake, G.; et al. Seroprevalence of SARS-CoV-2 Infection in the Colombo Municipality Region, Sri Lanka. Front. Public Health 2021, 9, 724398. [Google Scholar] [CrossRef] [PubMed]
- Álvarez-Antonio, C.; Meza-Sánchez, G.; Calampa, C.; Casanova, W.; Carey, C.; Alava, F.; Rodríguez-Ferrucci, H.; Quispe, A.M. Seroprevalence of anti-SARS-CoV-2 antibodies in Iquitos, Peru in July and August, 2020: A population-based study. Lancet Glob. Health 2021, 9, e925–e931. [Google Scholar] [CrossRef]
- Mattar, S.; Alvis-Guzman, N.; Garay, E.; Rivero, R.; García, A.; Botero, Y.; Miranda, J.; Galeano, K.; De La Hoz, F.; Martínez, C.; et al. Severe Acute Respiratory Syndrome Coronavirus 2 Seroprevalence Among Adults in a Tropical City of the Caribbean Area, Colombia: Are We Much Closer to Herd Immunity Than Developed Countries? Open Forum Infect. Dis. 2020, 7, ofaa550. [Google Scholar] [CrossRef] [PubMed]
- Wiens, K.E.; Mawien, P.N.; Rumunu, J.; Slater, D.; Jones, F.K.; Moheed, S.; Caflish, A.; Bior, B.K.; Jacob, I.A.; Lako, R.L.L.; et al. Seroprevalence of Anti-SARS-CoV-2 IgG Antibodies in Juba, South Sudan: A Population-Based Study. Emerg. Infect. Dis. 2021, 27, 1598–1606. [Google Scholar] [CrossRef] [PubMed]
- Poustchi, H.; Darvishian, M.; Mohammadi, Z.; Shayanrad, A.; Delavari, A.; Bahadorimonfared, A.; Eslami, S.; Javanmard, S.H.; Shakiba, E.; Somi, M.H.; et al. SARS-CoV-2 antibody seroprevalence in the general population and high-risk occupational groups across 18 cities in Iran: A population-based cross-sectional study. Lancet Infect. Dis. 2020, 21, 473–481. [Google Scholar] [CrossRef]
- Mulenga, L.B.; Hines, J.Z.; Fwoloshi, S.; Chirwa, L.; Siwingwa, M.; Yingst, S.; Wolkon, A.; Barradas, D.T.; Favaloro, J.; Zulu, J.E.; et al. Prevalence of SARS-CoV-2 in six districts in Zambia in July, 2020: A cross-sectional cluster sample survey. Lancet Glob. Health 2021, 9, e773–e781. [Google Scholar] [CrossRef]
- Nkuba, A.N.; Makiala, S.M.; Guichet, E.; Tshiminyi, P.M.; Bazitama, Y.M.; Yambayamba, M.K.; Kazenza, B.M.; Kabeya, T.M.; Matungulu, E.B.; Baketana, L.K.; et al. High Prevalence of Anti–Severe Acute Respiratory Syndrome Coronavirus 2 (Anti–SARS-CoV-2) Antibodies After the First Wave of Coronavirus Disease 2019 (COVID-19) in Kinshasa, Democratic Republic of the Congo: Results of a Cross-sectional Household-Based Survey. Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. 2021, 74, 882–890. [Google Scholar] [CrossRef]
- Mwananyanda, L.; Gill, C.J.; MacLeod, W.; Kwenda, G.; Pieciak, R.; Mupila, Z.; Lapidot, R.; Mupeta, F.; Forman, L.; Ziko, L.; et al. Covid-19 deaths in Africa: Prospective systematic postmortem surveillance study. BMJ 2021, 372, n334. [Google Scholar] [CrossRef]
- Ing, A.J.; Cocks, C.; Green, J.P. COVID-19: In the footsteps of Ernest Shackleton. Thorax 2020, 75, 693–694. [Google Scholar] [CrossRef]
- George, C.E.; Inbaraj, L.R.; Chandrasingh, S.; de Witte, L.P. High seroprevalence of COVID-19 infection in a large slum in South India; what does it tell us about managing a pandemic and beyond? Epidemiol. Infect. 2021, 149, 1–19. [Google Scholar] [CrossRef]
- McArthur, L.; Sakthivel, D.; Ataide, R.; Chan, F.; Richards, J.S.; Narh, C.A. Review of Burden, Clinical Definitions, and Management of COVID-19 Cases. Am. J. Trop. Med. Hyg. 2020, 103, 625–638. [Google Scholar] [CrossRef] [PubMed]
- Byambasuren, O.; Dobler, C.C.; Bell, K.; Rojas, D.P.; Clark, J.; McLaws, M.-L.; Glasziou, P. Comparison of seroprevalence of SARS-CoV-2 infections with cumulative and imputed COVID-19 cases: Systematic review. PLoS ONE 2021, 16, e0248946. [Google Scholar] [CrossRef] [PubMed]
- Murray, C.J.L. COVID-19 will continue but the end of the pandemic is near. Lancet 2022, 399, 417–419. [Google Scholar] [CrossRef]
- Mossong, J.; Hens, N.; Jit, M.; Beutels, P.; Auranen, K.; Mikolajczyk, R.; Massari, M.; Salmaso, S.; Tomba, G.S.; Wallinga, J.; et al. Social Contacts and Mixing Patterns Relevant to the Spread of Infectious Diseases. PLoS Med. 2008, 5, e74. [Google Scholar] [CrossRef] [PubMed]
- Rumain, B.; Schneiderman, M.; Geliebter, A. Prevalence of COVID-19 in adolescents and youth compared with older adults in states experiencing surges. PLoS ONE 2021, 16, e0242587. [Google Scholar] [CrossRef] [PubMed]
- Dowell, A.C.; Butler, M.S.; Jinks, E.; Tut, G.; Lancaster, T.; Sylla, P.; Begum, J.; Bruton, R.; Pearce, H.; Verma, K.; et al. Children develop robust and sustained cross-reactive spike-specific immune responses to SARS-CoV-2 infection. Nat. Immunol. 2021, 23, 40–49. [Google Scholar] [CrossRef]
- Shakiba, M.; Nazemipour, M.; Salari, A.; Mehrabian, F.; Nazari, S.S.H.; Rezvani, S.M.; Ghasempour, Z.; Heidarzadeh, A.; Mansournia, M.A. Seroprevalence of SARS-CoV-2 in Guilan Province, Iran, April 2020. Emerg. Infect. Dis. 2021, 27, 636–638. [Google Scholar] [CrossRef]
- Stringhini, S.; Wisniak, A.; Piumatti, G.; Azman, A.S.; Lauer, S.A.; Baysson, H.; De Ridder, D.; Petrovic, D.; Schrempft, S.; Marcus, K.; et al. Seroprevalence of anti-SARS-CoV-2 IgG antibodies in Geneva, Switzerland (SEROCoV-POP): A population-based study. Lancet 2020, 396, 313–319. [Google Scholar] [CrossRef]
- Pollán, M.; Pérez-Gómez, B.; Pastor-Barriuso, R.; Oteo, J.; Hernán, M.A.; Perez-Olmeda, M.; Sanmartín, J.L.; Fernández-García, A.; Cruz, I.; de Larrea, N.F.; et al. Prevalence of SARS-CoV-2 in Spain (ENE-COVID): A nationwide, population-based seroepidemiological study. Lancet 2020, 396, 535–544. [Google Scholar] [CrossRef]
- Murhekar, M.V.; Bhatnagar, T.; Thangaraj, J.W.V.; Saravanakumar, V.; Kumar, M.S.; Selvaraju, S.; Rade, K.; Kumar, C.G.; Sabarinathan, R.; Turuk, A.; et al. SARS-CoV-2 seroprevalence among the general population and healthcare workers in India, December 2020–January 2021. Int. J. Infect. Dis. 2021, 108, 145–155. [Google Scholar] [CrossRef]
- Indenbaum, V.; Lustig, Y.; Mendelson, E.; Hershkovitz, Y.; Glatman-Freedman, A.; Keinan-Boker, L.; Bassal, R. Under-diagnosis of SARS-CoV-2 infections among children aged 0–15 years, a nationwide seroprevalence study, Israel, January 2020 to March 2021. Eurosurveillance 2021, 26, 2101040. [Google Scholar] [CrossRef]
- Ulyte, A.; Radtke, T.; Abela, I.A.; Haile, S.R.; Berger, C.; Huber, M.; Schanz, M.; Schwarzmueller, M.; Trkola, A.; Fehr, J.; et al. Clustering and longitudinal change in SARS-CoV-2 seroprevalence in school children in the canton of Zurich, Switzerland: Prospective cohort study of 55 schools. BMJ 2021, 372, n616. [Google Scholar] [CrossRef] [PubMed]
- Bundle, N.; Dave, N.; Pharris, A.; Spiteri, G.; Deogan, C.; Suk, J.E. Study group members COVID-19 trends and severity among symptomatic children aged 0–17 years in 10 European Union countries, 3 August 2020 to 3 October 2021. Eurosurveillance 2021, 26, 2101098. [Google Scholar] [CrossRef] [PubMed]
- Cloete, J.; Kruger, A.; Masha, M.; Plessis, N.M.D.; Mawela, D.; Tshukudu, M.; Manyane, T.; Komane, L.; Venter, M.; Jassat, W.; et al. Rapid Rise in Paediatric COVID-19 Hospitalisations during the Early Stages of the Omicron Wave, Tshwane District, South Africa [Preprint]. 2021. Available online: https://www.medrxiv.org/content/10.1101/2021.12.21.21268108v1#:~:text=Background%20South%20Africa%20reported%20a,COVID%2D19%2Dassociated%20hospitalisations (accessed on 15 January 2022).
- World Health Organization. COVID-19 Disease in Children and Adolescents: Scientific Brief, 29 September 2021. Available online: https://www.who.int/publications-detail-redirect/WHO-2019-nCoV-Sci_Brief-Children_and_adolescents-2021.1 (accessed on 23 January 2022).
- Bobrovitz, N.; Arora, R.K.; Cao, C.; Boucher, E.; Liu, M.; Donnici, C.; Yanes-Lane, M.; Whelan, M.; Perlman-Arrow, S.; Chen, J.; et al. Global seroprevalence of SARS-CoV-2 antibodies: A systematic review and meta-analysis. PLoS ONE 2021, 16, e0252617. [Google Scholar] [CrossRef] [PubMed]
- Hallal, P.C.; Hartwig, F.P.; Horta, B.L.; Silveira, M.F.; Struchiner, C.J.; Vidaletti, L.P.; Neumann, N.A.; Pellanda, L.C.; Dellagostin, O.A.; Burattini, M.N.; et al. SARS-CoV-2 antibody prevalence in Brazil: Results from two successive nationwide serological household surveys. Lancet Glob. Health 2020, 8, e1390–e1398. [Google Scholar] [CrossRef]
- Tuells, J.; Egoavil, C.M.; Pena Pardo, M.A.; Montagud, A.C.; Montagud, E.; Caballero, P.; Zapater, P.; Puig-Barberá, J.; Hurtado-Sanchez, J.A. Seroprevalence Study and Cross-Sectional Survey on COVID-19 for a Plan to Reopen the University of Alicante (Spain). Int. J. Environ. Res. Public Health 2021, 18, 1908. [Google Scholar] [CrossRef]
- Paleiron, N.; Mayet, A.; Marbac, V.; Perisse, A.; Barazzutti, H.; Brocq, F.-X.; Janvier, F.; Dautzenberg, B.; Bylicki, O. Impact of Tobacco Smoking on the Risk of COVID-19: A Large Scale Retrospective Cohort Study. Nicotine Tob. Res. Off. J. Soc. Res. Nicotine Tob. 2021, 23, 1398–1404. [Google Scholar] [CrossRef]
- Changeux, J.-P.; Amoura, Z.; Rey, F.A.; Miyara, M. A nicotinic hypothesis for Covid-19 with preventive and therapeutic implications. Comptes Rendus. Biol. 2020, 343, 33–39. [Google Scholar] [CrossRef]
- Clift, A.K.; von Ende, A.; Tan, P.S.; Sallis, H.M.; Lindson, N.; Coupland, C.A.C.; Munafò, M.R.; Aveyard, P.; Hippisley-Cox, J.; Hopewell, J.C. Smoking and COVID-19 outcomes: An observational and Mendelian randomisation study using the UK Biobank cohort. Thorax 2021, 77, 65–73. [Google Scholar] [CrossRef]
- Patanavanich, R.; Glantz, S.A. Smoking is associated with worse outcomes of COVID-19 particularly among younger adults: A systematic review and meta-analysis. BMC Public Health 2021, 21, 1–9. [Google Scholar] [CrossRef]
- Tude ComCor sur Les Lieux de Contamination au SARS-CoV-2: Où Les Français S’infectent-Ils? Available online: https://www.pasteur.fr/fr/espace-presse/documents-presse/etude-comcor-lieux-contamination-au-sars-cov-2-ou-francais-s-infectent-ils (accessed on 19 January 2022).
- Burbelo, P.D.; Riedo, F.X.; Morishima, C.; Rawlings, S.; Smith, D.; Das, S.; Strich, J.R.; Chertow, D.S.; Davey, R.T.; Cohen, J.I. Sensitivity in Detection of Antibodies to Nucleocapsid and Spike Proteins of Severe Acute Respiratory Syndrome Coronavirus 2 in Patients With Coronavirus Disease 2019. J. Infect. Dis. 2020, 222, jiaa273. [Google Scholar] [CrossRef] [PubMed]
- Gallais, F.; Gantner, P.; Bruel, T.; Velay, A.; Planas, D.; Wendling, M.-J.; Bayer, S.; Solis, M.; Laugel, E.; Reix, N.; et al. Evolution of antibody responses up to 13 months after SARS-CoV-2 infection and risk of reinfection. EBioMedicine 2021, 71, 103561. [Google Scholar] [CrossRef] [PubMed]
- Schoenhals, M.; Rabenindrina, N.; Rakotondramanga, J.M.; Dussart, P.; Randremanana, R.; Heraud, J.-M.; Andriamandimby, S.F.; Sahondranirina, P.H.; Vololoniaina, M.C.A.; Randriatsarafara, F.M.; et al. SARS-CoV-2 antibody seroprevalence follow-up in Malagasy blood donors during the 2020 COVID-19 Epidemic. EBioMedicine 2021, 68, 103419. [Google Scholar] [CrossRef] [PubMed]
- Centers for Disease Control and Prevention. Science Brief: SARS-CoV-2 Infection-Induced and Vaccine-Induced Immunity. Available online: https://www.cdc.gov/coronavirus/2019-ncov/science/science-briefs/vaccine-induced-immunity.html (accessed on 22 January 2022).
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).