Predictive Variables for Interventional Angiography among Patients with Knee Hemarthrosis
Abstract
:1. Introduction
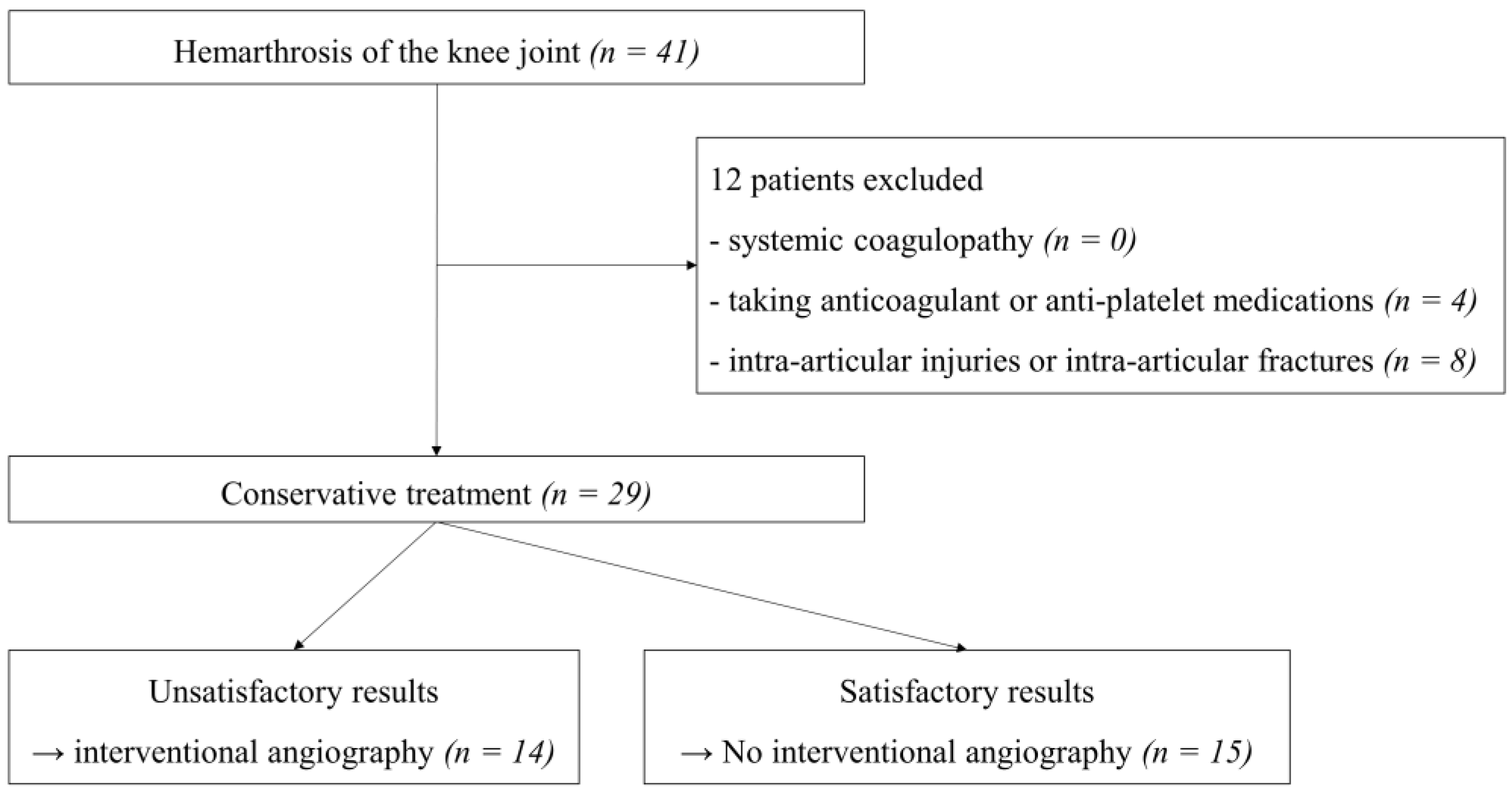
2. Materials and Methods
2.1. Study Population
2.2. Evaluation
2.3. Statistical Analysis
3. Results
3.1. Description of the Study Population and Their Surgical History
3.2. Comparison of Serologic Test Results and Synovial Fluid Analyses
3.3. Angiographic Findings and Follow-Up
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Given, M.F.; Smith, P.; Lyon, S.M.; Robertson, D.; Thomson, K.R. Embolization of spontaneous hemarthrosis post total knee replacement. Cardiovasc. Intervent. Radiol. 2008, 31, 986–988. [Google Scholar] [CrossRef] [PubMed]
- Saksena, J.; Platts, A.D.; Dowd, G.S. Recurrent haemarthrosis following total knee replacement. Knee 2010, 17, 7–14. [Google Scholar] [CrossRef] [PubMed]
- Karataglis, D.; Marlow, D.; Learmonth, D.J. Atraumatic haemarthrosis following total knee replacement treated with selective embolisation. Acta Orthop. Belg. 2006, 72, 375–377. [Google Scholar] [PubMed]
- Lombardi, M.; Cardenas, A.C. Hemarthrosis. [Updated 9 August 2021]. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2021. Available online: https://www.ncbi.nlm.nih.gov/books/NBK525999/ (accessed on 3 January 2022).
- Brown, J.S.; Frobell, R.B.; Isacsson, A.; Englund, M.; Olsson, O. Agreement Between Clinical Examination and Magnetic Resonance Imaging in Acute Knee Trauma With Hemarthrosis. Clin. J. Sport Med. 2021. [Google Scholar] [CrossRef] [PubMed]
- Lyons, L.P.; Weinberg, J.B.; Wittstein, J.R.; McNulty, A.L. Blood in the joint: Effects of hemarthrosis on meniscus health and repair techniques. Osteoarthr. Cartil. 2021, 29, 471–479. [Google Scholar] [CrossRef]
- Olsson, O.; Isacsson, A.; Englund, M.; Frobell, R.B. Epidemiology of intra- and peri-articular structural injuries in traumatic knee joint hemarthrosis—Data from 1145 consecutive knees with subacute MRI. Osteoarthr. Cartil. 2016, 24, 1890–1897. [Google Scholar] [CrossRef] [Green Version]
- Lim, H.A.; Song, E.K.; Seon, J.K.; Park, K.S.; Shin, Y.J.; Yang, H.Y. Causes of Aseptic Persistent Pain after Total Knee Arthroplasty. Clin. Orthop. Surg. 2017, 9, 50–56. [Google Scholar] [CrossRef]
- Rukavina, A.; Kerkhoffs, G.M.M.J.; Schneider, P.; Kuster, M.S. Recurrent hemarthrosis after total knee arthroplasty. Knee Surg. Sports Traumatol. Arthrosc. 2010, 18, 898–900. [Google Scholar] [CrossRef] [Green Version]
- Lee, K.B.; Song, S.Y.; Kwon, D.J.; Shin, J.; Paik, S.H. Pseudoaneurysm of the Medial Superior Genicular Artery after Arthroscopic Partial Meniscectomy. Clin. Orthop. Surg. 2009, 1, 173–175. [Google Scholar] [CrossRef] [Green Version]
- Yamagami, T.; Yoshimatsu, R.; Miura, H.; Arai, Y.; Terauchi, R.; Nakagawa, S.; Yamada, K. Selective arterial embolization with gelatin particles for refractory knee hemarthrosis. Diagn. Interv. Radiol. 2013, 19, 423–426. [Google Scholar] [CrossRef]
- Aldin, Z.; Obaid, H.; Abdelsalam, H.; Bolia, A. Case report: Spontaneous recurrent idiopathic knee haemarthrosis in a paediatric patient: Successful transcatheter embolization. Clin. Radiol. 2010, 65, 82–84. [Google Scholar] [CrossRef] [PubMed]
- Sasho, T.; Ogino, S.; Tsuruoka, H.; Nakagawa, K.; Ochiai, N.; Nagashima, R.; Moriya, H.; Watanabe, A.; Wada, Y.; Takahashi, K. Spontaneous recurrent hemarthrosis of the knee in the elderly: Arthroscopic treatment and etiology. Arthroscopy 2008, 24, 1027–1033. [Google Scholar] [CrossRef] [PubMed]
- Oni, J.K.; Cavallo, R.J. A rare case of diffuse pigmented villonodular synovitis after total knee arthroplasty. J. Arthroplast. 2011, 26, 978. [Google Scholar] [CrossRef] [PubMed]
- Ji, J.H.; Lee, Y.S.; Shafi, M. Spontaneous recurrent hemarthrosis of the knee joint in elderly patients with osteoarthritis: An infrequent presentation of synovial lipoma arborescens. Knee Surg. Sports Traumatol. Arthrosc. 2010, 18, 1352–1355. [Google Scholar] [CrossRef] [PubMed]
- Matsuzaki, T.; Akisue, T.; Kishimoto, K.; Kishimoto, S.; Imabori, M.; Hara, H.; Okada, Y.; Hitora, T.; Kuroda, R.; Kurosaka, M.; et al. Intra-articular nodular fasciitis of the knee: A rare cause of recurrent hemarthrosis. Rheumatol. Int. 2012, 32, 1691–1694. [Google Scholar] [CrossRef]
- Park, K.H.; Kim, D.H.; Jang, S.W.; Ryu, J.H.; Ko, K.Y. Treatment of Recurrent Hemarthrosis Following Total Knee Arthroplasty Using Surgical Interventions. Clin. Orthop. Surg. 2021, 13, 152–159. [Google Scholar] [CrossRef]
- Shaerf, D.; Banerjee, A. Assessment and management of posttraumatic haemarthrosis of the knee. Br. J. Hosp. Med. 2008, 69, 459–463. [Google Scholar] [CrossRef]
- Lee, K.W.; Song, Y.; Song, D.G.; Choy, W.S. Therapeutic Embolization for Spontaneous Recurrent Hemarthrosis of the Knee in the Elderly. J. Korean Orthop. Assoc. 2014, 49, 480–484. [Google Scholar] [CrossRef]
- Waldenberger, P.; Chemelli, A.; Hennerbichler, A.; Wick, M.; Freund, M.C.; Jaschke, W.; Thaler, M.; Chemelli-Steingruber, I.E. Transarterial embolization for the management of hemarthrosis of the knee. Eur. J. Radiol. 2012, 81, 2737–2740. [Google Scholar] [CrossRef]
- Bagla, S.; Rholl, K.S.; van Breda, A.; Sterling, K.M.; van Breda, A. Geniculate artery embolization in the management of spontaneous recurrent hemarthrosis of the knee: Case series. J. Vasc. Interv. Radiol. 2013, 24, 439–442. [Google Scholar] [CrossRef]
- Katsimihas, M.; Robinson, D.; Thornton, M.; Langkamer, V.G. Therapeutic embolization of the genicular arteries for recurrent hemarthrosis after total knee arthroplasty. J. Arthroplast. 2001, 16, 935–937. [Google Scholar] [CrossRef] [PubMed]
- Cipriano, C.A.; Brown, N.M.; Michael, A.M.; Moric, M.; Sporer, S.M.; Della Valle, C.J. Serum and synovial fluid analysis for diagnosing chronic periprosthetic infection in patients with inflammatory arthritis. J. Bone Jt. Surg. Am. 2012, 94, 594–600. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ghanem, E.; Parvizi, J.; Burnett, R.S.; Sharkey, P.F.; Keshavarzi, N.; Aggarwal, A.; Barrack, R.L. Cell count and differential of aspirated fluid in the diagnosis of infection at the site of total knee arthroplasty. J. Bone Jt. Surg. Am. 2008, 90, 1637–1643. [Google Scholar] [CrossRef] [PubMed]
- Hernandez, N.M.; Taunton, M.J.; Perry, K.I.; Mara, K.C.; Hanssen, A.D.; Abdel, M.P. The analysis of synovial fluid in total knee arthroplasties with flexion instability. Bone Jt. J. 2017, 99, 1477–1481. [Google Scholar] [CrossRef] [PubMed]
- Yoo, J.H.; Oh, H.C.; Park, S.H.; Lee, S.; Lee, Y.; Kim, S.H. Treatment of Recurrent Hemarthrosis after Total Knee Arthroplasty. Knee Surg. Relat. Res. 2018, 30, 147–152. [Google Scholar] [CrossRef] [PubMed]
- Pow, R.; Fritsch, B.; Waugh, R.; Rogan, C. Endovascular management of recurrent hemarthrosis of the knee: A case series. CVIR Endovasc. 2020, 3, 43. [Google Scholar] [CrossRef]
- Kolber, M.K.; Shukla, P.A.; Kumar, A.; Zybulewski, A.; Markowitz, T.; Silberzweig, J.E. Endovascular Management of Recurrent Spontaneous Hemarthrosis After Arthroplasty. Cardiovasc. Intervent. Radiol. 2017, 40, 216–222. [Google Scholar] [CrossRef]
- Dhondt, E.; Vanhoenacker, F.M.; D’ Archambeau, O.; Snoeckx, A.; Defreyne, L. Angiographic findings and therapeutic embolization of late hemarthrosis after total joint arthroplasty. Skelet. Radiol. 2009, 38, 31–36. [Google Scholar] [CrossRef]
- van Baardewijk, L.J.; Hoogeveen, Y.L.; van der Geest, I.; Schultze Kool, L.J. Embolization of the Geniculate Arteries Is an Effective Treatment of Recurrent Hemarthrosis Following Total Knee Arthroplasty That Can Be Safely Repeated. J. Arthroplast. 2018, 33, 1177–1180. [Google Scholar] [CrossRef] [Green Version]
- Guevara, C.J.; Lee, K.A.; Barrack, R.; Darcy, M.D. Technically Successful Geniculate Artery Embolization Does Not Equate Clinical Success for Treatment of Recurrent Knee Hemarthrosis after Knee Surgery. J. Vasc. Interv. Radiol. 2016, 27, 383–387. [Google Scholar] [CrossRef]

| Variables | Group A (n = 14) | Group B (n = 15) | p-Value |
|---|---|---|---|
| Mean (range) | |||
| Age (years) | 67.2 (52–85) | 68.5 (51–83) | 0.73 |
| Duration of conservative treatment (months) | 4.3 (2.4–7.5) | 3.9 (2.8–5.1) | 0.21 |
| Number of arthrocentesis procedures | 5.1 (4–6) | 3.3 (3–4) | <0.01 |
| Follow-up period (months) | 22.3 (12.6–38.2) | 21.2 (12.3–50.6) | 0.75 |
| Number | |||
| Sex (female:male) | 8:6 | 6:9 | 0.37 |
| Surgical history | |||
| None (native knee) | 5 | 7 | |
| TKA | 5 | 7 | |
| Revision TKA | 4 | 1 | |
| Variables | Group A (n = 14) | Group B (n = 15) | p-Value |
|---|---|---|---|
| Serum | |||
| Hb (g/dL) | 10.3 (5.3–14.9) | 10.7 (6.3–13.9) | 0.66 |
| Hct (%) | 31.0 (16.1–44.3) | 33.1 (18.7–43.1) | 0.45 |
| PT (s) | 12.5 (10.7–14.3) | 11.9 (10.3–13.4) | 0.14 |
| aPTT (s) | 30.9 (24.3–38.8) | 31.7 (24.9–36.7) | 0.57 |
| INR | 1.1 (0.9–1.5) | 1.0 (0.9–1.2) | 0.20 |
| ESR (mm/h) | 12.5 (2–20) | 14.7 (2–24) | 0.37 |
| CRP (mg/dL) | 0.5 (0.1–1.0) | 0.6 (0.1–1.1) | 0.19 |
| Synovial Fluid | |||
| RBC (cells/µL) | 1,905,857 (200,000–4,890,000) | 7730 (15–28,000) | <0.01 |
| WBC (cells/µL) | 1386.5 (4–4500) | 2230.0 (140–4160) | 0.15 |
| Neutrophil (%) | 39.6 (13–52) | 32.6 (10–69) | 0.25 |
| RBC (number/HPF) | 68.9 (25–100) | 3.2 (0.5–25) | <0.01 |
| Age (Years)/Sex | Surgical History | Feeding Artery | Findings |
|---|---|---|---|
| 75/Male | TKA | LSGA, DGA | Hypervascularization |
| 59/Male | TKA | LIGA | Hypervascularization, contrast blush |
| 67/Male | TKA | LIGA, anterior tibial recurrent artery, | Contrast blush |
| 53/Male | None | Medial DGA | Hypervascularization |
| 71/Male | None | LIGA, LSGA, Medial DGA | Hypervascularization, contrast blush |
| 52/Male | None | LIGA, LSGA | Hypervascularization |
| 72/Female | Revision TKA | LIGA | Hypervascularization, contrast blush |
| 78/Female | Revision TKA | LSGA, MSGA, muscular artery | Multiple pseudoaneurysm, contrast blush |
| 85/Female | Revision TKA | Medial DGA, anterior tibial recurrent artery | Hypervascularization, contrast blush |
| 74/Female | Revision TKA | Multiple genicular artery | Hypervascularization, contrast blush |
| 75/Male | TKA | Medial DGA, LSGA | Hypervascularization |
| 63/Female | None | Medial DGA | Hypervascularization |
| 60/Male | None | LIGA, LSGA | Hypervascularization |
| 57/Female | TKA | Medial DGA, LIGA | Hypervascularization, contrast blush |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ko, S.-H.; Jung, K.-H.; Cha, J.-R.; Park, K.-B. Predictive Variables for Interventional Angiography among Patients with Knee Hemarthrosis. Diagnostics 2022, 12, 976. https://doi.org/10.3390/diagnostics12040976
Ko S-H, Jung K-H, Cha J-R, Park K-B. Predictive Variables for Interventional Angiography among Patients with Knee Hemarthrosis. Diagnostics. 2022; 12(4):976. https://doi.org/10.3390/diagnostics12040976
Chicago/Turabian StyleKo, Sang-Hun, Kwang-Hwan Jung, Jae-Ryong Cha, and Ki-Bong Park. 2022. "Predictive Variables for Interventional Angiography among Patients with Knee Hemarthrosis" Diagnostics 12, no. 4: 976. https://doi.org/10.3390/diagnostics12040976
APA StyleKo, S.-H., Jung, K.-H., Cha, J.-R., & Park, K.-B. (2022). Predictive Variables for Interventional Angiography among Patients with Knee Hemarthrosis. Diagnostics, 12(4), 976. https://doi.org/10.3390/diagnostics12040976






