Current Evidence in SARS-CoV-2 mRNA Vaccines and Post-Vaccination Adverse Reports: Knowns and Unknowns
Abstract
:1. Introduction
2. mRNA Vaccination against COVID-19 and Diagnosis of Spontaneous Post-Vaccination Adverse Events
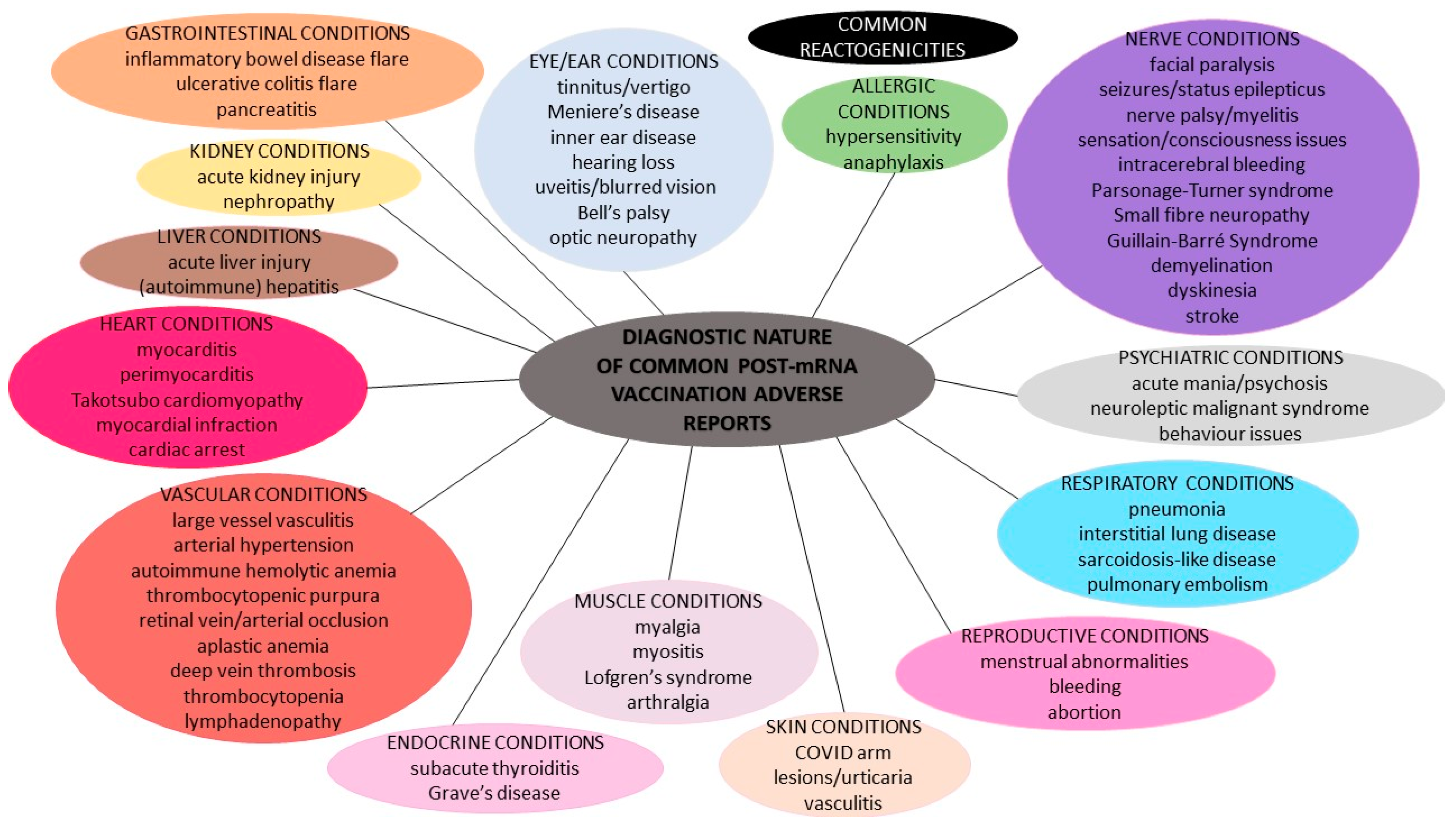
2.1. Common Reactogenicities
2.2. Spontaneous Adverse Reports after SARS-CoV-2 mRNA Vaccinations
2.2.1. Allergic Reactions
2.2.2. Skin Conditions
2.2.3. Vascular and Blood Conditions
2.2.4. Endocrine Conditions
2.2.5. Heart Conditions
2.2.6. Respiratory Conditions
2.2.7. Gastrointestinal Conditions
2.2.8. Hepatic Conditions
2.2.9. Kidney/Urinary Conditions
2.2.10. Reproductive and Pregnancy Conditions
2.2.11. Muscle and Tissue Conditions
2.2.12. Ear and Eye Conditions
2.2.13. Nervous System Conditions
2.2.14. Psychiatric Conditions
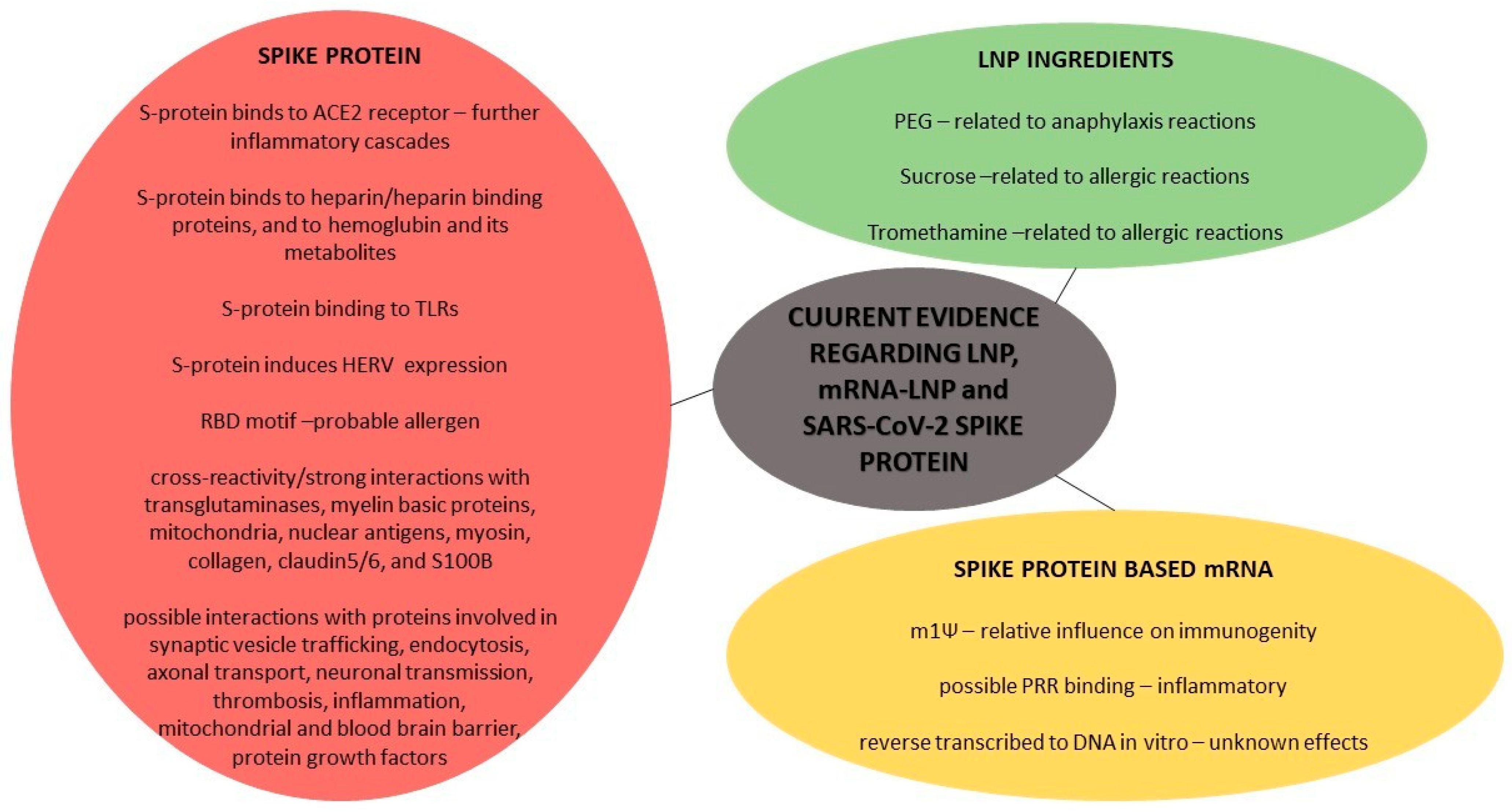
3. Evidence Regarding mRNA-LNP Based Strategies against COVID-19
- i.
- Generally, allergic reactions due to various vaccines have been revealed to occur at a rate of 1.31 cases per million vaccine doses, with no fatalities reported and no gender or age differences, despite the fact that slightly higher frequencies have been reported in women [173]. Hypersensitivity reactions after current vaccinations have been reported in approximately 1.5% of the recipients [149]. Generally, the confirmed unfavorable allergic reactions to vaccines are not attributed to the active bioingredients, but they are frequently related to the inactive ingredients/excipients. A pre-pandemic study on similar mRNA-LNP vaccines against H10N8 and H7N9 influenza viruses showed that the vaccine can distribute rather nonspecifically to several organs, such as the liver, spleen, heart, kidney, lung and brain, and, also, that the liver concentration is roughly 100 times lower than that of the intra-muscular injection site [174]. A study has shown that LNPs used in preclinical studies are highly inflammatory, by activating multiple inflammatory pathways and inducing IL-1β and IL-6, due to their ionizable lipid components [175]. Heretofore, no licensed vaccine includes the excipient Polyethylene Glycol (PEG), but this substance is non-toxic and was approved by the FDA in 1990, and it is found in a number of medications that have caused anaphylaxis, a potential fatal reaction. It has been proposed that people with pre-exposure to PEG and possible anti-PEG antibodies may be at risk for an allergic reaction due to current mRNA vaccines’ administrations. Literature data reveal that there is a wide variance in the measurements of pre-existing anti-PEG antibodies, ranging from 0.2% to 72% of healthy individuals [176]. Despite being thought of as safe and biologically inert, multiple studies have found that up to 70% of patients treated with PEGylated medicines generate anti-PEG IgG antibodies [177]. Nevertheless, some general reports on PEG reveal that the avidity of PEG-specific IgG from individuals with PEG anaphylaxis rose as the molecular weight of the PEG tested climbed from 1000 to above, with a clinical tolerance of PEG300 upon being challenged, implying that not all PEGs are equally dangerous at triggering responses; this may be attributed to various PEGs’ molecular weights [177]. While additional research is needed to understand the cause of the possible higher prevalence of allergy to mRNA vaccines compared to other vaccines, PEG 2000 is thought to be the most likely problem (before LNP enters into the cell, during it being inside the cell and after it is secreted by the cell) based on previous experience with PEG-conjugated biologics, yet it is labeled as a high-risk hidden allergen, as it is difficult to detect as a possible cause of allergic reaction [177,178]. IgE-mediated allergic reactions to PEGylated lipids have been reported, while Complement Activation-Related Pseudoallergy (CARPA) has also been seen in comparable liposomes; CARPA is partly attributed to the binding of pre-existing anti-PEG IgM to LNPs with a following complement activation [177,178]. Complement activation should occur in practically all vaccine recipients if LNP-based vaccines can elicit an immediate local complement activation, yet complement activation alone cannot explain anaphylactic episodes [178]. In addition, anaphylactic reactions to PEGylated nanomedicines such as pegnivacogin have been most common in people with high anti-PEG IgG titers, but not everyone with high levels of these antibodies had allergic reactions [179]. Moreover, mRNA vaccines contain sucrose, and not only PEG, as a stabilizing agent, and it is known that carbohydrate (sucrose) intolerance does not disturb the immune system but can cause common allergic reactions, namely gastrointestinal disturbances, as a stabilizer-induced anaphylaxis [179]. Additionally, the mRNA-1273 vaccine contains tromethamine (trometamol), which has been linked to allergy in people who have been exposed to gadolinium-based contrast media/iodinated contrast media, and, also, the excipient 1,2-distearoyl-sn-glycero-3- phosphocholine (DSPC) included in the LNPs of both mRNA vaccines could contribute to allergic reactions [34]. A non-related pre-pandemic study showed that that intravenously injected mRNA-LNPs can interact with phagocytic myeloid cells and neutrophils that can elevate cytokines—but determined that the PseudoU modification of mRNA does not reduce this immune response [180]. However, the extended biopersistence duration of such adjuvants or the ability of adjuvant particles to move and slowly accumulate in lymphoid organs or other tissues are major safety concerns. A non-related-to-COVID-19 study in mice showed that extracellular vesicles secreted after the endocytosis of that specific studied LNP-mRNA trigger inflammatory cytokines at lower rates than the original LNP, but the fate of the LNP-mRNA after endosome-engulfing and escape from the autophagy–lysosomal pathway is unclear [181].
- ii.
- Regarding the mRNA biomolecule, it has been said that extracellular RNA seems to predispose to endothelial damage, intercellular conjunctions relaxation and edema, increased viscosity, hyper-coagulation and thromboembolic events [182]. However, secreted RNases endowed with immuno-modulatory and antimicrobial properties can facilitate inflammation termination and tissue repair [183]. A pre-pandemic study showed that human fetal membranes respond to viral signatures in different ways, generating divergent inflammatory cytokine/chemokine profiles and antiviral responses [184]. This event, related to mRNA vaccination deriving and producing biomolecules, is currently unknown. The N1-methylpseudouridine reduces synthetic mRNA immunogenicity, but, concerning mRNA vaccinations against COVID-19, the relative influence of sequence engineering and m1Ψ incorporation of some specific immunogenic mechanisms into the cell remain to be reported [185]. Before translation, the mRNA could also bind to Pattern Recognition Receptors (PRRs) in endosomes or the cytosol prior in order to translate, and the final result is the activation of several pro-inflammatory cascades, such as inflammasome platforms, a type I interferon (IFN) response and the nuclear translocation of the transcription factor nuclear factor (NF)—kB [186]. The upregulation of these immunological pathways seems to be the basis for immune-mediated illnesses, particularly in genetically predisposed people who have an impaired clearance of nucleic acids, such as young women, due to the overexpression of X-linked genes because of the required antiviral response and the immune system’s stimulation by estrogens [186]. A recent study found that BNT162b2 is rapidly absorbed into the Huh7 human liver cell line in vitro, causing alterations in LINE-1 expression and distribution, and, after BNT162b2 exposure, BNT162b2 mRNA is reverse transcribed intracellularly into DNA in as little as 6 h; further research into the exact regulation of LINE-1 activity in response to BNT162b2 is needed [187]. Such findings raise questions on whether the vaccine-derived mRNA might be integrated to the human genome, resulting in potential genotoxic side effects, and, heretofore, it is unknown if DNA reversely transcribed from the mRNA vaccine is incorporated into the human cell genome in vivo [187].
- iii.
- Initially, the vaccine-derived mRNA is translated into proteins by ribosomes, used as an endogenous antigen and degraded by the proteasome into antigenic peptides, which are presented to CD8+ cytotoxic T cells via the MHC class I molecular pathway to activate cell-mediated immune responses; these products can be secreted into the extracellular space, thus entering the bloodstream, in which, they are uptaken by APCs, and antigenic peptides are delivered to CD4+ T lymphocytes through MHC class II molecules [188]. However, coronavirus infection was known for the massive production of viral proteins that can overload the folding capacities of the endoplasmic reticulum, and the possible misfolded antigens lead to final cell apoptosis, but this point is not studied regarding the vaccine-derived proteins [189]. A study showed that the circulating antigen was identified at detectable levels in the plasma of few mRNA vaccine recipients 1 day after vaccination. IgA levels were reported early, and IgG levels were reported 14 days post-vaccination; antigen levels declined and became undetectable by day 14 for most recipients, and no protein was detectable after the second vaccination, except for a case that was detected 1 day after but was undetected 2 days later [190]. On the contrary, a recent study revealed that mRNA vaccination stimulates robust germinal centers in lymph nodes containing vaccine mRNA and spike antigens up to 8 weeks post-vaccination in certain cases [191]. Some evidence indicates that the receptor-binding motif RBD, binding to human ACE2B (437–508), as well as to two fusion peptides, may be a possible allergen that may cause possible anaphylaxis, whereas the results from both tools point to the receptor-binding motif RBD of the spike protein as a probable allergen [192]. A study also assessing a mouse model injected with the S1 subunit spike protein concluded that the SARS-CoV-2 main pathology starts by ACE2 endothelial damage and that this pathology may be induced by the injected spike protein, and another study in mice revealed that the S1 protein crosses the Blood–Brain Barrier (BBB) [193,194]. The ACE2 receptors’ distribution in all tissues and women’s vulnerability due to chromosome X was evident from the very beginning of the COVID-19 pandemic; however, even if ACE2 mRNA is expressed homogeneously in all tissues, the same is not always the case for ACE2 protein expression [195]. A proteomic analysis revealed that SARS-CoV-2 proteins (including the spike protein) bind to hemoglobin and its metabolites, and that this—in extreme conditions—could even result in an integrated stress response and the global inhibition of mRNA translation, but, generally, in further medical conditions [196]. Furthermore, Human Endogenous Retroviruses (HERVs) or their viral products may enhance virus infection and penetration into human cells, or HERVs may create proteins that regulate the ribosome’s translation start, altering the pattern of COVID ORFs in various human hosts. In addition, a study showed that the spike protein induced in vitro human endogenous retroviruses’ HERV-W ENV expression, and, also, HERV-K was found to be elevated in COVID-19 patients [197]. The spike protein has also been linked with Alzheiner’s disease; like dementia and cognitive impairment, the Spike protein S1 RBD motif can bind to heparin and heparin-binding proteins, suggesting clues about neurodegeneration. In addition, the spike protein was found to have a significant binding to Toll-like Receptors (TLRs) in in silico studies—especially TLR4 [198,199,200]. Finally, two studies revealed a SARS-CoV-2 spike protein cross-reactivity and strong interactions with transglutaminases, myelin basic proteins, mitochondria, nuclear antigens, myosin, collagen, claudin5/6 and S100B, as well as possible interactions with many proteins, including proteins involved in synaptic vesicle trafficking, endocytosis, axonal transport, neuronal transmission, thrombosis, inflammation and the mitochondrial and blood–brain barrier, as well as protein growth factors [201,202].
4. Knowns and Unknowns: The Expert Opinion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Zhu, N.; Zhang, D.; Wang, W.; Li, X.; Yang, B.; Song, J.; Zhao, X.; Huang, B.; Shi, W.; Lu, R.; et al. A Novel Coronavirus from Patients with Pneumonia in China, 2019. N. Engl. J. Med. 2020, 382, 727–733. [Google Scholar] [CrossRef] [PubMed]
- Mouliou, D.S.; Pantazopoulos, I.; Gourgoulianis, K.I. COVID-19 Smart Diagnosis in the Emergency Department: All-in in Practice. Expert Rev. Respir. Med. 2022, 16, 263–272. [Google Scholar] [CrossRef] [PubMed]
- Mouliou, D.S.; Pantazopoulos, I.; Gourgoulianis, K. COVID-19 Diagnosis in the Emergency Department: Seeing the Tree but Losing the Forest. Emerg. Med. J. 2022, 39, 563–564. [Google Scholar] [CrossRef] [PubMed]
- Mouliou, D.S. Managing Viral Emerging Infectious Diseases via Current Molecular Diagnostics in the Emergency Department: The Tricky Cases. Expert Rev. Anti-Infect. Ther. 2022, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Mouliou, D.S.; Pantazopoulos, I.; Gourgoulianis, K.I. Societal Criticism towards COVID-19: Assessing the Theory of Self-Diagnosis Contrasted to Medical Diagnosis. Diagnostics 2021, 11, 1777. [Google Scholar] [CrossRef]
- Mouliou, D.S.; Gourgoulianis, K.I. False-Positive and False-Negative COVID-19 Cases: Respiratory Prevention and Management Strategies, Vaccination, and Further Perspectives. Expert Rev. Respir. Med. 2021, 15, 993–1002. [Google Scholar] [CrossRef]
- Mouliou, D.S.; Gourgoulianis, K.I. COVID-19 ‘Asymptomatic’ Patients: An Old Wives’ Tale. Expert Rev. Respir. Med. 2022, 16, 399–407. [Google Scholar] [CrossRef]
- Mouliou, D.S.; Pantazopoulos, I.; Gourgoulianis, K.I. Medical/Surgical, Cloth and FFP/(K)N95 Masks: Unmasking Preference, SARS-CoV-2 Transmissibility and Respiratory Side Effects. J. Pers. Med. 2022, 12, 325. [Google Scholar] [CrossRef]
- Le, T.T.; Cramer, J.P.; Chen, R.; Mayhew, S. Evolution of the COVID-19 vaccine development landscape. Nat. Rev. Drug Discov. 2020, 19, 667–668. [Google Scholar] [CrossRef]
- Accelerating a Safe and Effective COVID-19 Vaccine. Available online: https://www.who.int/emergencies/diseases/novel-coronavirus-2019/global-research-on-novel-coronavirus-2019-ncov/accelerating-a-safe-and-effective-covid-19-vaccine (accessed on 24 November 2021).
- Forni, G.; Mantovani, A. COVID-19 Commission of Accademia Nazionale dei Lincei, Rome COVID-19 vaccines: Where we stand and challenges ahead. Cell Death Differ. 2021, 28, 626–639. [Google Scholar] [CrossRef]
- CDC Different COVID-19 Vaccines. Available online: https://www.cdc.gov/coronavirus/2019-ncov/vaccines/different-vaccines.html (accessed on 28 February 2022).
- Nagy, A.; Alhatlani, B. An Overview of Current COVID-19 Vaccine Platforms. Comput. Struct. Biotechnol. J. 2021, 19, 2508–2517. [Google Scholar] [CrossRef] [PubMed]
- Mouliou, D.S.; Kotsiou, O.S.; Gourgoulianis, K.I. Estimates of COVID-19 Risk Factors among Social Strata and Predictors for a Vulnerability to the Infection. Int. J. Environ. Res. Public Health 2021, 18, 8701. [Google Scholar] [CrossRef] [PubMed]
- Ritchie, H.; Mathieu, E.; Rodés-Guirao, L.; Appel, C.; Giattino, C.; Ortiz-Ospina, E.; Hasell, J.; Macdonald, B.; Beltekian, D.; Roser, M. Coronavirus Pandemic (COVID-19). Our World Data. 2020. Available online: https://ourworldindata.org/covid-vaccinations (accessed on 21 March 2022).
- Mouliou, D.S.; Pantazopoulos, I.; Gourgoulianis, K.I. Social Response to the Vaccine against COVID-19: The Underrated Power of Influence. J. Pers. Med. 2022, 12, 15. [Google Scholar] [CrossRef] [PubMed]
- Polack, F.P.; Thomas, S.J.; Kitchin, N.; Absalon, J.; Gurtman, A.; Lockhart, S.; Perez, J.L.; Pérez Marc, G.; Moreira, E.D.; Zerbini, C.; et al. Safety and Efficacy of the BNT162b2 MRNA COVID-19 Vaccine. N. Engl. J. Med. 2020, 383, 2603–2615. [Google Scholar] [CrossRef] [PubMed]
- Voysey, M.; Clemens, S.A.C.; Madhi, S.A.; Weckx, L.Y.; Folegatti, P.M.; Aley, P.K.; Angus, B.; Baillie, V.L.; Barnabas, S.L.; Bhorat, Q.E.; et al. Safety and Efficacy of the ChAdOx1 NCoV-19 Vaccine (AZD1222) against SARS-CoV-2: An Interim Analysis of Four Randomised Controlled Trials in Brazil, South Africa, and the UK. Lancet 2021, 397, 99–111. [Google Scholar] [CrossRef]
- Baden, L.R.; Sahly, H.M.E.; Essink, B.; Kotloff, K.; Frey, S.; Novak, R.; Diemert, D.; Spector, S.A.; Rouphael, N.; Creech, C.B.; et al. Efficacy and Safety of the MRNA-1273 SARS-CoV-2 Vaccine. N. Engl. J. Med. 2020, 384, 403–416. [Google Scholar] [CrossRef]
- Meo, S.A.; Bukhari, I.A.; Akram, J.; Meo, A.S.; Klonoff, D.C. COVID-19 Vaccines: Comparison of Biological, Pharmacological Characteristics and Adverse Effects of Pfizer/BioNTech and Moderna Vaccines. Eur. Rev. Med. Pharmacol. Sci. 2021, 25, 1663–1669. [Google Scholar]
- CDC Information about the Pfizer-BioNTech COVID-19 Vaccine. Available online: https://www.cdc.gov/coronavirus/2019-ncov/vaccines/different-vaccines/Pfizer-BioNTech.html (accessed on 1 March 2022).
- CDC Information about the Moderna COVID-19 Vaccine. Available online: https://www.cdc.gov/coronavirus/2019-ncov/vaccines/different-vaccines/Moderna.html (accessed on 21 March 2022).
- Summary of Product Characteristics for Spikevax. Available online: https://www.gov.uk/government/publications/regulatory-approval-of-covid-19-vaccine-moderna/information-for-healthcare-professionals-on-covid-19-vaccine-moderna (accessed on 21 March 2022).
- Kadali, R.A.K.; Janagama, R.; Peruru, S.; Malayala, S.V. Side Effects of BNT162b2 MRNA COVID-19 Vaccine: A Randomized, Cross-Sectional Study with Detailed Self-Reported Symptoms from Healthcare Workers. Int. J. Infect. Dis. 2021, 106, 376–381. [Google Scholar] [CrossRef]
- Kadali, R.A.K.; Janagama, R.; Peruru, S.; Gajula, V.; Madathala, R.R.; Chennaiahgari, N.; Malayala, S.V. Non-Life-Threatening Adverse Effects with COVID-19 MRNA-1273 Vaccine: A Randomized, Cross-Sectional Study on Healthcare Workers with Detailed Self-Reported Symptoms. J. Med. Virol. 2021, 93, 4420–4429. [Google Scholar] [CrossRef]
- Frenck, R.W.; Klein, N.P.; Kitchin, N.; Gurtman, A.; Absalon, J.; Lockhart, S.; Perez, J.L.; Walter, E.B.; Senders, S.; Bailey, R.; et al. Safety, Immunogenicity, and Efficacy of the BNT162b2 COVID-19 Vaccine in Adolescents. N. Engl. J. Med. 2021, 385, 239–250. [Google Scholar] [CrossRef]
- Walter, E.B.; Talaat, K.R.; Sabharwal, C.; Gurtman, A.; Lockhart, S.; Paulsen, G.C.; Barnett, E.D.; Muñoz, F.M.; Maldonado, Y.; Pahud, B.A.; et al. Evaluation of the BNT162b2 COVID-19 Vaccine in Children 5 to 11 Years of Age. N. Engl. J. Med. 2022, 386, 35–46. [Google Scholar] [CrossRef] [PubMed]
- COVID-19 mRNA Pfizer-BioNTech Vaccine Analysis Print. Available online: https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/1069177/COVID-19_Pfizer-BioNTech_Vaccine_Analysis_Print_DLP_6.04.2022.pdf (accessed on 21 April 2022).
- CDC COVID-19 Vaccines and Severe Allergic Reactions. Available online: https://www.cdc.gov/coronavirus/2019-ncov/vaccines/safety/allergic-reaction.html (accessed on 17 March 2022).
- CDCMMWR. Allergic Reactions Including Anaphylaxis after Receipt of the First Dose of Pfizer-BioNTech COVID-19 Vaccine—United States, December 14–23, 2020. Morb. Mortal. Wkly. Rep. 2021, 70, 46–51. [Google Scholar] [CrossRef] [PubMed]
- Shimabukuro, T.; Nair, N. Allergic Reactions Including Anaphylaxis after Receipt of the First Dose of Pfizer-BioNTech COVID-19 Vaccine. JAMA 2021, 325, 780–781. [Google Scholar] [CrossRef] [PubMed]
- Sampath, V.; Rabinowitz, G.; Shah, M.; Jain, S.; Diamant, Z.; Jesenak, M.; Rabin, R.; Vieths, S.; Agache, I.; Akdis, M.; et al. Vaccines and Allergic Reactions: The Past, the Current COVID-19 Pandemic, and Future Perspectives. Allergy 2021, 76, 1640–1660. [Google Scholar] [CrossRef]
- Cabanillas, B.; Novak, N. Allergy to COVID-19 Vaccines: A Current Update. Allergol. Int. 2021, 70, 313–318. [Google Scholar] [CrossRef]
- Nittner-Marszalska, M.; Rosiek-Biegus, M.; Kopeć, A.; Pawłowicz, R.; Kosińska, M.; Łata, A.; Szenborn, L. Pfizer-BioNTech COVID-19 Vaccine Tolerance in Allergic versus Non-Allergic Individuals. Vaccines 2021, 9, 553. [Google Scholar] [CrossRef]
- Edler, C.; Klein, A.; Schröder, A.S.; Sperhake, J.-P.; Ondruschka, B. Deaths Associated with Newly Launched SARS-CoV-2 Vaccination (Comirnaty). Leg. Med. 2021, 51, 101895. [Google Scholar] [CrossRef]
- Ramos, C.L.; Kelso, J.M. “COVID Arm”: Very Delayed Large Injection Site Reactions to MRNA COVID-19 Vaccines. J. Allergy Clin. Immunol. Pract. 2021, 9, 2480–2481. [Google Scholar] [CrossRef]
- Wei, N.; Fishman, M.; Wattenberg, D.; Gordon, M.; Lebwohl, M. “COVID Arm”: A Reaction to the Moderna Vaccine. JAAD Case Rep. 2021, 10, 92–95. [Google Scholar] [CrossRef]
- Kempf, W.; Kettelhack, N.; Kind, F.; Courvoisier, S.; Galambos, J.; Pfaltz, K. ‘COVID Arm’—Histological Features of a Delayed-type Hypersensitivity Reaction to Moderna MRNA-1273 SARS-CoV-2 Vaccine. J. Eur. Acad. Dermatol. Venereol. 2021. [Google Scholar] [CrossRef]
- Fernandez-Nieto, D.; Hammerle, J.; Fernandez-Escribano, M.; Moreno-del Real, C.M.; Garcia-Abellas, P.; Carretero-Barrio, I.; Solano-Solares, E.; de-la-Hoz-Caballer, B.; Jimenez-Cauhe, J.; Ortega-Quijano, D.; et al. Skin Manifestations of the BNT162b2 mRNA COVID-19 Vaccine in Healthcare Workers. ‘COVID-arm’: A Clinical and Histological Characterization. J. Eur. Acad. Dermatol. Venereol. 2021, 35, e425–e427. [Google Scholar] [CrossRef]
- McMahon, D.E.; Amerson, E.; Rosenbach, M.; Lipoff, J.B.; Moustafa, D.; Tyagi, A.; Desai, S.R.; French, L.E.; Lim, H.W.; Thiers, B.H.; et al. Cutaneous Reactions Reported after Moderna and Pfizer COVID-19 Vaccination: A Registry-Based Study of 414 Cases. J. Am. Acad. Dermatol. 2021, 85, 46–55. [Google Scholar] [CrossRef] [PubMed]
- Poulas, K.; Farsalinos, K. Response to McMahon et al’s “Cutaneous Reactions Reported after Moderna and Pfizer COVID-19 Vaccination: A Registry-Based Study of Four Hundred Fourteen Cases. J. Am. Acad. Dermatol. 2022, 86, e163–e164. [Google Scholar] [CrossRef] [PubMed]
- Vassallo, C.; Boveri, E.; Brazzelli, V.; Rampino, T.; Bruno, R.; Bonometti, A.; Gregorini, M. Cutaneous Lymphocytic Vasculitis after Administration of COVID-19 MRNA Vaccine. Dermatol. Ther. 2021, e15076. [Google Scholar] [CrossRef] [PubMed]
- Gambichler, T.; Scholl, L.; Dickel, H.; Ocker, L.; Stranzenbach, R. Prompt Onset of Rowell’s Syndrome Following the First BNT162b2 SARS-CoV-2 Vaccination. J. Eur. Acad. Dermatol. Venereol. 2021, 35, e415. [Google Scholar] [CrossRef] [PubMed]
- Cohen, S.R.; Prussick, L.; Kahn, J.S.; Gao, D.X.; Radfar, A.; Rosmarin, D. Leukocytoclastic Vasculitis Flare Following the COVID-19 Vaccine. Int. J. Dermatol. 2021, 60, 1032–1033. [Google Scholar] [CrossRef]
- Terentes-Printzios, D.; Gardikioti, V.; Solomou, E.; Emmanouil, E.; Gourgouli, I.; Xydis, P.; Christopoulou, G.; Georgakopoulos, C.; Dima, I.; Miliou, A.; et al. The Effect of an mRNA Vaccine against COVID-19 on Endothelial Function and Arterial Stiffness. Hyperten. Res. 2022, 45, 846–855. [Google Scholar] [CrossRef]
- Anderegg, M.A.; Liu, M.; Saganas, C.; Montani, M.; Vogt, B.; Huynh-Do, U.; Fuster, D.G. De Novo Vasculitis after MRNA-1273 (Moderna) Vaccination. Kidney Int. 2021, 100, 474–476. [Google Scholar] [CrossRef]
- Schierz, J.-H.; Merkel, C.; Kittner, T.; Ali, F. Vasculitis and Bursitis on [18F]FDG-PET/CT Following COVID-19 MRNA Vaccine: Post Hoc Ergo Propter Hoc? Eur. J. Nucl. Med. Mol. Imaging 2022, 49, 1086–1087. [Google Scholar] [CrossRef]
- Athyros, V.G.; Doumas, M. A Possible Case of Hypertensive Crisis with Intracranial Haemorrhage after an mRNA Anti-COVID-19 Vaccine. Angiology 2022, 73, 87. [Google Scholar] [CrossRef]
- Matheny, M.; Maleque, N.; Channell, N.; Eisch, A.R.; Auld, S.C.; Banerji, A.; Druey, K.M. Severe Exacerbations of Systemic Capillary Leak Syndrome After COVID-19 Vaccination: A Case Series. Ann. Intern. Med. 2021, 174, 1476–1478. [Google Scholar] [CrossRef] [PubMed]
- Mahgoub, A.E.; Awuah, D.; Hussain, M.; Deliwala, S.; Bachuwa, G.; Younas, M. Development of Venous Thromboembolism After COVID-19 MRNA-1273 Vaccine Inoculation. Cureus 2022, 14, e22179. [Google Scholar] [CrossRef] [PubMed]
- Bhan, C.; Bheesham, N.; Shakuntulla, F.; Sharma, M.; Sun, C.; Weinstein, M. An Unusual Presentation of Acute Deep Vein Thrombosis after the Moderna COVID-19 Vaccine—A Case Report. Ann. Transl. Med. 2021, 9, 1605. [Google Scholar] [CrossRef] [PubMed]
- Carli, G.; Nichele, I.; Ruggeri, M.; Barra, S.; Tosetto, A. Deep Vein Thrombosis (DVT) Occurring Shortly after the Second Dose of MRNA SARS-CoV-2 Vaccine. Intern. Emerg. Med. 2021, 16, 803–804. [Google Scholar] [CrossRef]
- Waqar, S.H.B.; Khan, A.A.; Memon, S. Thrombotic Thrombocytopenic Purpura: A New Menace after COVID Bnt162b2 Vaccine. Int. J. Hematol. 2021, 114, 626–629. [Google Scholar] [CrossRef]
- Welsh, K.J.; Baumblatt, J.; Chege, W.; Goud, R.; Nair, N. Thrombocytopenia Including Immune Thrombocytopenia after Receipt of MRNA COVID-19 Vaccines Reported to the Vaccine Adverse Event Reporting System (VAERS). Vaccine 2021, 39, 3329–3332. [Google Scholar] [CrossRef]
- Bilotta, C.; Perrone, G.; Adelfio, V.; Spatola, G.F.; Uzzo, M.L.; Argo, A.; Zerbo, S. COVID-19 Vaccine-Related Thrombosis: A Systematic Review and Exploratory Analysis. Front. Immunol. 2021, 12, 729251. [Google Scholar] [CrossRef]
- Hippisley-Cox, J.; Patone, M.; Mei, X.W.; Saatci, D.; Dixon, S.; Khunti, K.; Zaccardi, F.; Watkinson, P.; Shankar-Hari, M.; Doidge, J.; et al. Risk of Thrombocytopenia and Thromboembolism after COVID-19 Vaccination and SARS-CoV-2 Positive Testing: Self-Controlled Case Series Study. BMJ 2021, 374, n1931. [Google Scholar] [CrossRef]
- Cines, D.B.; Bussel, J.B. SARS-CoV-2 Vaccine–Induced Immune Thrombotic Thrombocytopenia. N. Engl. J. Med. 2021, 384, 2254–2256. [Google Scholar] [CrossRef]
- Greinacher, A.; Selleng, K.; Mayerle, J.; Palankar, R.; Wesche, J.; Reiche, S.; Aebischer, A.; Warkentin, T.E.; Muenchhoff, M.; Hellmuth, J.C.; et al. Anti-Platelet Factor 4 Antibodies Causing VITT Do Not Cross-React with SARS-CoV-2 Spike Protein. Blood 2021, 138, 1269–1277. [Google Scholar] [CrossRef]
- Jaydev, F.; Kumar, V.; Khatri, J.; Shahani, S.; Beganovic, S. A Case of Autoimmune Hemolytic Anemia after the First Dose of COVID-19 MRNA-1273 Vaccine with Undetected Pernicious Anemia. Case Rep. Hematol. 2022, 2022, e2036460. [Google Scholar] [CrossRef] [PubMed]
- Okuno, S.; Hashimoto, K.; Shimizu, R.; Takagi, E.; Kajiguchi, T. Development of autoimmune hemolytic anemia after BNT162b2 mRNA COVID-19 vaccination. Rinsho Ketsueki 2021, 62, 1510–1514. [Google Scholar] [CrossRef] [PubMed]
- Tabata, S.; Hosoi, H.; Murata, S.; Takeda, S.; Mushino, T.; Sonoki, T. Severe Aplastic Anemia after COVID-19 MRNA Vaccination: Causality or Coincidence? J. Autoimmun. 2022, 126, 102782. [Google Scholar] [CrossRef] [PubMed]
- Pur, D.R.; Catherine Danielle Bursztyn, L.L.; Iordanous, Y. Branch Retinal Vein Occlusion in a Healthy Young Man Following MRNA COVID-19 Vaccination. Am. J. Ophthalmol. Case Rep. 2022, 26, 101445. [Google Scholar] [CrossRef] [PubMed]
- Soliman, D.S.; Al Battah, A.; Al Faridi, D.; Ibrahim, F. Acquired Hemophilia A Developed Post COVID-19 Vaccine: An Extremely Rare Complication. J. Med. Cases 2022, 13, 1–4. [Google Scholar] [CrossRef]
- Tu, W.; Gierada, D.S.; Joe, B.N. COVID-19 Vaccination–Related Lymphadenopathy: What To Be Aware Of. Radiol. Imaging Cancer 2021, 3, e210038. [Google Scholar] [CrossRef] [PubMed]
- Wolfson, S.; Kim, E.; Plaunova, A.; Bukhman, R.; Sarmiento, R.D.; Samreen, N.; Awal, D.; Sheth, M.M.; Toth, H.B.; Moy, L.; et al. Axillary adenopathy after COVID-19 vaccine: No reason to delay screening mammogram. Radiology 2022, 303, 297. [Google Scholar] [CrossRef] [PubMed]
- Pujol, A.; Gómez, L.-A.; Gallegos, C.; Nicolau, J.; Sanchís, P.; González-Freire, M.; López-González, Á.A.; Dotres, K.; Masmiquel, L. Thyroid as a Target of Adjuvant Autoimmunity/Inflammatory Syndrome Due to MRNA-Based SARS-CoV2 Vaccination: From Graves’ Disease to Silent Thyroiditis. J. Endocrinol. Investig. 2022, 45, 875–882. [Google Scholar] [CrossRef]
- Chee, Y.J.; Liew, H.; Hoi, W.H.; Lee, Y.; Lim, B.; Chin, H.X.; Lai, R.T.R.; Koh, Y.; Tham, M.; Seow, C.J.; et al. SARS-CoV-2 MRNA Vaccination and Graves’ Disease: A Report of 12 Cases and Review of the Literature. J. Clin. Endocrinol. Metab. 2022, 107, e2324–e2330. [Google Scholar] [CrossRef]
- Franquemont, S.; Galvez, J. Subacute Thyroiditis after MRNA Vaccine for COVID-19. J. Endocr. Soc. 2021, 5, A956–A957. [Google Scholar] [CrossRef]
- Pierman, G.; Delgrange, E.; Jonas, C. Recurrence of Graves’ Disease (a Th1-Type Cytokine Disease) Following SARS-CoV-2 MRNA Vaccine Administration: A Simple Coincidence? Eur. J. Case Rep. Intern. Med. 2021, 8, 002807. [Google Scholar] [CrossRef] [PubMed]
- Schimmel, J.; Alba, E.L.; Chen, A.; Russell, M.; Srinath, R. Letter to the Editor: Thyroiditis and Thyrotoxicosis after the SARS-CoV-2 MRNA Vaccine. Thyroid 2021, 31, 1440. [Google Scholar] [CrossRef] [PubMed]
- Xiong, X.; Wong, C.K.H.; Au, I.C.H.; Lai, F.T.T.; Li, X.; Wan, E.Y.F.; Chui, C.S.L.; Chan, E.W.Y.; Cheng, F.W.T.; Lau, K.T.K.; et al. Safety of Inactivated and MRNA COVID-19 Vaccination among Patients Treated for Hypothyroidism: A Population-Based Cohort Study. Thyroid 2022, 32, 505–514. [Google Scholar] [CrossRef]
- Kaur, R.J.; Dutta, S.; Charan, J.; Bhardwaj, P.; Tandon, A.; Yadav, D.; Islam, S.; Haque, M. Cardiovascular Adverse Events Reported from COVID-19 Vaccines: A Study Based on WHO Database. Int. J. Gen. Med. 2021, 14, 3909–3927. [Google Scholar] [CrossRef] [PubMed]
- Gargano, J.W.; Wallace, M.; Hadler, S.C.; Langley, G.; Su, J.R.; Oster, M.E.; Broder, K.R.; Gee, J.; Weintraub, E.; Shimabukuro, T.; et al. Use of MRNA COVID-19 Vaccine After Reports of Myocarditis Among Vaccine Recipients: Update from the Advisory Committee on Immunization Practices—United States, June 2021. Morb. Mortal. Wkly. Rep. 2021, 70, 977–982. [Google Scholar] [CrossRef]
- Bozkurt, B.; Kamat, I.; Hotez, P.J. Myocarditis with COVID-19 MRNA Vaccines. Circulation 2021, 144, 471–484. [Google Scholar] [CrossRef]
- Barda, N.; Dagan, N.; Ben-Shlomo, Y.; Kepten, E.; Waxman, J.; Ohana, R.; Hernán, M.A.; Lipsitch, M.; Kohane, I.; Netzer, D.; et al. Safety of the BNT162b2 MRNA COVID-19 Vaccine in a Nationwide Setting. N. Engl. J. Med. 2021, 385, 1078–1090. [Google Scholar] [CrossRef] [PubMed]
- Truong, D.T.; Dionne, A.; Muniz, J.C.; McHugh, K.E.; Portman, M.A.; Lambert, L.M.; Thacker, D.; Elias, M.D.; Li, J.S.; Toro-Salazar, O.H.; et al. Clinically Suspected Myocarditis Temporally Related to COVID-19 Vaccination in Adolescents and Young Adults: Suspected Myocarditis after COVID-19 Vaccination. Circulation 2022, 145, 345–356. [Google Scholar] [CrossRef]
- Choi, S.; Lee, S.; Seo, J.-W.; Kim, M.-J.; Jeon, Y.H.; Park, J.H.; Lee, J.K.; Yeo, N.S. Myocarditis-Induced Sudden Death after BNT162b2 MRNA COVID-19 Vaccination in Korea: Case Report Focusing on Histopathological Findings. J. Korean Med. Sci. 2021, 36, e286. [Google Scholar] [CrossRef]
- Mevorach, D.; Anis, E.; Cedar, N.; Bromberg, M.; Haas, E.J.; Nadir, E.; Olsha-Castell, S.; Arad, D.; Hasin, T.; Levi, N.; et al. Myocarditis after BNT162b2 MRNA Vaccine against COVID-19 in Israel. N. Engl. J. Med. 2021, 385, 2140–2149. [Google Scholar] [CrossRef]
- Woo, W.; Kim, A.Y.; Yon, D.K.; Lee, S.W.; Hwang, J.; Jacob, L.; Koyanagi, A.; Kim, M.S.; Moon, D.H.; Jung, J.W.; et al. Clinical Characteristics and Prognostic Factors of Myocarditis Associated with the MRNA COVID-19 Vaccine. J. Med. Virol. 2022, 94, 1566–1580. [Google Scholar] [CrossRef] [PubMed]
- Khogali, F.; Abdelrahman, R. Unusual Presentation of Acute Perimyocarditis Following SARS-CoV-2 MRNA-1237 Moderna Vaccination. Cureus 2021, 13, e16590. [Google Scholar] [CrossRef] [PubMed]
- Patone, M.; Mei, X.W.; Handunnetthi, L.; Dixon, S.; Zaccardi, F.; Shankar-Hari, M.; Watkinson, P.; Khunti, K.; Harnden, A.; Coupland, C.A.C.; et al. Risks of Myocarditis, Pericarditis, and Cardiac Arrhythmias Associated with COVID-19 Vaccination or SARS-CoV-2 Infection. Nat. Med. 2022, 28, 410–422. [Google Scholar] [CrossRef] [PubMed]
- Heymans, S.; Cooper, L.T. Myocarditis after COVID-19 MRNA Vaccination: Clinical Observations and Potential Mechanisms. Nat. Rev. Cardiol. 2022, 19, 75–77. [Google Scholar] [CrossRef]
- Tsilingiris, D.; Vallianou, N.G.; Karampela, I.; Liu, J.; Dalamaga, M. Potential Implications of Lipid Nanoparticles in the Pathogenesis of Myocarditis Associated with the Use of MRNA Vaccines against SARS-CoV-2. Metab. Open 2022, 13, 100159. [Google Scholar] [CrossRef]
- Boscolo Berto, M.; Spano, G.; Wagner, B.; Bernhard, B.; Häner, J.; Huber, A.T.; Gräni, C. Takotsubo Cardiomyopathy After MRNA COVID-19 Vaccination. Heart Lung Circ. 2021, 30, e119–e120. [Google Scholar] [CrossRef]
- Fearon, C.; Parwani, P.; Gow-Lee, B.; Abramov, D. Takotsubo Syndrome after Receiving the COVID-19 Vaccine. J. Cardiol. Cases 2021, 24, 223–226. [Google Scholar] [CrossRef]
- Gräni, C. Reply: Takotsubo Cardiomyopathy after Receiving MRNA COVID-19 Vaccination Is Very Rare. Heart Lung Circ. 2022, 31, e78–e79. [Google Scholar] [CrossRef]
- Jabagi, M.J.; Botton, J.; Bertrand, M.; Weill, A.; Farrington, P.; Zureik, M.; Dray-Spira, R. Myocardial Infarction, Stroke, and Pulmonary Embolism after BNT162b2 MRNA COVID-19 Vaccine in People Aged 75 Years or Older. JAMA 2022, 327, 80–82. [Google Scholar] [CrossRef]
- Amiya, S.; Fujimoto, J.; Matsumoto, K.; Yamamoto, M.; Yamamoto, Y.; Yoneda, M.; Kuge, T.; Miyake, K.; Shiroyama, T.; Hirata, H.; et al. Case Report: Acute Exacerbation of Interstitial Pneumonia Related to Messenger RNA COVID-19 Vaccination. Int. J. Infect. Dis. 2022, 116, 255–257. [Google Scholar] [CrossRef]
- Kono, A.; Yoshioka, R.; Hawk, P.; Iwashina, K.; Inoue, D.; Suzuki, M.; Narita, C.; Haruta, K.; Miyake, A.; Yoshida, H.; et al. A case of severe interstitial lung disease after COVID-19 vaccination. QJM Int. J. Med. 2021, 114, 805–806. [Google Scholar] [CrossRef] [PubMed]
- Colaneri, M.; De Filippo, M.; Licari, A.; Marseglia, A.; Maiocchi, L.; Ricciardi, A.; Corsico, A.; Marseglia, G.; Mondelli, M.U.; Bruno, R. COVID Vaccination and Asthma Exacerbation: Might There Be a Link? Int. J. Infect. 2021, 112, 243–246. [Google Scholar] [CrossRef] [PubMed]
- Klimek, L.; Jutel, M.; Akdis, C.A.; Bousquet, J.; Akdis, M.; Torres, M.J.; Agache, I.; Canonica, G.W.; Del Giacco, S.; O’Mahony, L.; et al. ARIA-EAACI Statement on Severe Allergic Reactions to COVID-19 Vaccines—An EAACI-ARIA Position Paper. Allergy 2021, 76, 1624–1628. [Google Scholar] [CrossRef] [PubMed]
- Al-Maqbali, J.S.; Rasbi, S.A.; Kashoub, M.S.; Hinaai, A.M.A.; Farhan, H.; Rawahi, B.A.; Al Alawi, A.M. A 59-Year-Old Woman with Extensive Deep Vein Thrombosis and Pulmonary Thromboembolism 7 Days Following a First Dose of the Pfizer-BioNTech BNT162b2 MRNA COVID-19 Vaccine. Am. J. Case Rep. 2021, 22, e932946-1–e932946-4. [Google Scholar] [CrossRef]
- Atoui, A.; Jarrah, K.; Al Mahmasani, L.; Bou-Fakhredin, R.; Taher, A.T. Deep Venous Thrombosis and Pulmonary Embolism after COVID-19 MRNA Vaccination. Ann. Hematol. 2022, 101, 1111–1113. [Google Scholar] [CrossRef]
- Kluger, N.; Klimenko, T.; Bosonnet, S. Herpes Simplex, Herpes Zoster and Periorbital Erythema Flares after SARS-CoV-2 Vaccination: 4 Cases. Ann. Dermatol. Venereol. 2022, 149, 58–60. [Google Scholar] [CrossRef]
- Matsuo, T.; Honda, H.; Tanaka, T.; Uraguchi, K.; Kawahara, M.; Hagiya, H. COVID-19 MRNA Vaccine–Associated Uveitis Leading to Diagnosis of Sarcoidosis: Case Report and Review of Literature. J. Investig. Med. High Impact Case Rep. 2022, 10, 232470962210864. [Google Scholar] [CrossRef]
- Kumar, R.; Botwin, G.J.; Appel, K.L.; Mujukian, A.; Boland, B.; Chiorean, M.; Cohen, E.R.; Fudman, D.; Hou, J.; Hwang, C.; et al. S904 Gastrointestinal Symptoms in Patients With Inflammatory Bowel Disease After SARS-CoV-2 mRNA Vaccination. Off. J. Am. Coll. Gastroenterol. 2021, 116, S427–S428. [Google Scholar] [CrossRef]
- D’Souza, S.M.; Yoo, B.S.; Smith, J.H.; Johnson, D.A. S2452 Two Cases of Ulcerative Colitis Flare Following SARS-CoV-2 mRNA Vaccination. Off. J. Am. Coll. Gastroenterol. 2021, 116, S1037. [Google Scholar] [CrossRef]
- Wang, Y.; Hsieh, T.-C.; Lee, G.Y.; Gorelick, S.; Maslak, D. S2431 Ulcerative Colitis Flare-Ups Following MRNA COVID-19 Vaccination. Off. J. Am. Coll. Gastroenterol. 2021, 116, S1029. [Google Scholar] [CrossRef]
- Mitchell, J.; Yue, Q.-Y. Appendicitis as a Possible Safety Signal for the COVID-19 Vaccines. Vaccine X 2021, 9, 100122. [Google Scholar] [CrossRef] [PubMed]
- Walter, T.; Connor, S.; Stedman, C.; Doogue, M. A Case of Acute Necrotising Pancreatitis Following the Second Dose of Pfizer-BioNTech COVID-19 MRNA Vaccine. Br. J. Clin. Pharmacol. 2021, 88, 1385–1386. [Google Scholar] [CrossRef]
- Parkash, O.; Sharko, A.; Farooqi, A.; Ying, G.W.; Sura, P. Acute Pancreatitis: A Possible Side Effect of COVID-19 Vaccine. Cureus 2021, 13, e14741. [Google Scholar] [CrossRef]
- Bircakova, B.; Bruha, R.; Lambert, L.; Grusova, G.; Michalek, P.; Burgetova, A. A Bimodal Pattern of the Onset of COVID-19 Related Acute Pancreatitis Supports Both the Cytotoxic and Immune-Related Pathogenesis—A Systematic Review. Scand. J. Gastroenterol. 2021, 56, 870–873. [Google Scholar] [CrossRef] [PubMed]
- Dumortier, J. Liver Injury after MRNA-Based SARS-CoV-2 Vaccination in a Liver Transplant Recipient. Clin. Res. Hepatol. Gastroenterol. 2022, 46, 101743. [Google Scholar] [CrossRef] [PubMed]
- Hines, A.; Shen, J.G.; Olazagasti, C.; Shams, S. Immune Thrombocytopenic Purpura and Acute Liver Injury after COVID-19 Vaccine. BMJ Case Rep. 2021, 14, e242678. [Google Scholar] [CrossRef]
- Ghielmetti, M.; Schaufelberger, H.D.; Mieli-Vergani, G.; Cerny, A.; Dayer, E.; Vergani, D.; Terziroli Beretta-Piccoli, B. Acute Autoimmune-like Hepatitis with Atypical Anti-Mitochondrial Antibody after MRNA COVID-19 Vaccination: A Novel Clinical Entity? J. Autoimmun. 2021, 123, 102706. [Google Scholar] [CrossRef] [PubMed]
- Shroff, H.; Satapathy, S.K.; Crawford, J.M.; Todd, N.J.; VanWagner, L.B. Liver Injury Following SARS-CoV-2 Vaccination: A Multicenter Case Series. J. Hepatol. 2022, 76, 211–214. [Google Scholar] [CrossRef]
- Lodato, F.; Larocca, A.; D’Errico, A.; Cennamo, V. An Unusual Case of Acute Cholestatic Hepatitis after M-RNABNT162b2 (Comirnaty) SARS-CoV-2 Vaccine: Coincidence, Autoimmunity or Drug-Related Liver Injury. J. Hepatol. 2021, 75, 1254–1256. [Google Scholar] [CrossRef]
- Plasse, R.; Nee, R.; Gao, S.; Olson, S. Acute Kidney Injury with Gross Hematuria and IgA Nephropathy after COVID-19 Vaccination. Kidney Int. 2021, 100, 944–945. [Google Scholar] [CrossRef]
- Kronbichler, A.; Jung, S.Y.; Kim, M.S.; Shin, J.I. Distinct Glomerular Disease Association after Vaccination with BNT162b2 and MRNA-1273: A VigiBase Analysis. Kidney Int. 2022, 101, 415–416. [Google Scholar] [CrossRef] [PubMed]
- Gueguen, L.; Loheac, C.; Saidani, N.; Khatchatourian, L. Membranous Nephropathy Following Anti–COVID-19 MRNA Vaccination. Kidney Int. 2021, 100, 1140–1141. [Google Scholar] [CrossRef] [PubMed]
- Schwotzer, N.; Kissling, S.; Fakhouri, F. Letter Regarding “Minimal Change Disease Relapse Following SARS-CoV-2 MRNA Vaccine. Kidney Int. 2021, 100, 458–459. [Google Scholar] [CrossRef]
- Sacker, A.; Kung, V.; Andeen, N. Anti-GBM Nephritis with Mesangial IgA Deposits after SARS-CoV-2 MRNA Vaccination. Kidney Int. 2021, 100, 471–472. [Google Scholar] [CrossRef]
- Masumoto, A.; Yamamoto, H.; Taniguchi, Y.; Kawai, H.; Takaya, T. Isolated Renal Arteritis with Infarction Identified after SARS-CoV-2 Vaccine. Circ. J. 2022, 86, 1144. [Google Scholar] [CrossRef]
- Sekar, A.; Campbell, R.; Tabbara, J.; Rastogi, P. ANCA Glomerulonephritis after the Moderna COVID-19 Vaccination. Kidney Int. 2021, 100, 473–474. [Google Scholar] [CrossRef] [PubMed]
- Oniszczuk, J.; Pagot, E.; Limal, N.; Hüe, S.; Audard, V.; Moktefi, A.; El Karoui, K. Scleroderma Renal Crisis Following MRNA Vaccination against SARS-CoV-2. Kidney Int. 2021, 100, 940–941. [Google Scholar] [CrossRef] [PubMed]
- Male, V. Menstrual Changes after COVID-19 Vaccination. BMJ 2021, 374, n2211. [Google Scholar] [CrossRef]
- Edelman, A.; Boniface, E.R.; Benhar, E.; Han, L.; Matteson, K.A.; Favaro, C.; Pearson, J.T.; Darney, B.G. Association between menstrual cycle length and coronavirus disease 2019 (COVID-19) vaccination: A US Cohort. Obstet. Gynecol. 2022, 139, 481–489. [Google Scholar] [CrossRef]
- Merchant, H. COVID-19 Post-Vaccine Menorrhagia, Metrorrhagia or Postmenopausal Bleeding and Potential Risk of Vaccine-Induced Thrombocytopenia in Women. BMJ 2021, 373, n958. [Google Scholar]
- Kuzumi, A.; Yoshizaki, A.; Chiba, K.; Mitsuo, S.; Matsuda, K.M.; Norimatsu, Y.; Nagai, K.; Omatsu, J.; Miyake, T.; Sato, S. Genital Necrosis with Cutaneous Thrombosis after COVID-19 MRNA Vaccination. Acad. Dermatol. Venereol. 2022, 36, e186. [Google Scholar] [CrossRef]
- Kazemi, S.N.; Hajikhani, B.; Didar, H.; Hosseini, S.S.; Haddadi, S.; Khalili, F.; Mirsaeidi, M.; Nasiri, M.J. COVID-19 and Cause of Pregnancy Loss during the Pandemic: A Systematic Review. PLoS ONE 2021, 16, e0255994. [Google Scholar] [CrossRef]
- Zauche, L.H.; Wallace, B.; Smoots, A.N.; Olson, C.K.; Oduyebo, T.; Kim, S.Y.; Petersen, E.E.; Ju, J.; Beauregard, J.; Wilcox, A.J.; et al. Receipt of MRNA COVID-19 Vaccines and Risk of Spontaneous Abortion. N. Engl. J. Med. 2021, 385, 1533–1535. [Google Scholar] [CrossRef] [PubMed]
- Mendoza, M.; Garcia-Ruiz, I.; Maiz, N.; Rodo, C.; Garcia-Manau, P.; Serrano, B.; Lopez-Martinez, R.; Balcells, J.; Fernandez-Hidalgo, N.; Carreras, E.; et al. Pre-eclampsia-like Syndrome Induced by Severe COVID-19: A Prospective Observational Study. BJOG Int. J. Obstet. Gynaecol. 2020, 127, 1374–1380. [Google Scholar] [CrossRef] [PubMed]
- Prasad, M.; Lin, J.L.; Gu, Y.; Gupta, R.; Macary, P.; Schwarz, H. No Crossreactivity of Anti-SARS-CoV-2 Spike Protein Antibodies with Syncytin-1. Cell Mol. Immunol. 2021, 18, 2566–2568. [Google Scholar] [CrossRef]
- Faissner, S.; Richter, D.; Ceylan, U.; Schneider-Gold, C.; Gold, R. COVID-19 MRNA Vaccine Induced Rhabdomyolysis and Fasciitis. J. Neurol. 2022, 269, 1774–1775. [Google Scholar] [CrossRef] [PubMed]
- Orbach, H.; Tanay, A. Vaccines as a Trigger for Myopathies. Lupus 2009, 18, 1213–1216. [Google Scholar] [CrossRef] [PubMed]
- Manzo, C.; Natale, M.; Castagna, A. Polymyalgia Rheumatica as Uncommon Adverse Event Following Immunization with COVID-19 Vaccine: A Case Report and Review of Literature. Aging Med. 2021, 4, 234–238. [Google Scholar] [CrossRef]
- Ottaviani, S.; Juge, P.-A.; Forien, M.; Ebstein, E.; Palazzo, E.; Dieudé, P. Polymyalgia Rheumatica Following COVID-19 Vaccination: A Case-Series of Ten Patients. Jt. Bone Spine 2022, 89, 105334. [Google Scholar] [CrossRef]
- Li, X.; Tong, X.; Yeung, W.W.Y.; Kuan, P.; Yum, S.H.H.; Chui, C.S.L.; Lai, F.T.T.; Wan, E.Y.F.; Wong, C.K.H.; Chan, E.W.Y.; et al. Two-Dose COVID-19 Vaccination and Possible Arthritis Flare among Patients with Rheumatoid Arthritis in Hong Kong. Ann. Rheum. Dis. 2022, 81, 564–568. [Google Scholar] [CrossRef]
- Terracina, K.A.; Tan, F.K. Flare of Rheumatoid Arthritis after COVID-19 Vaccination. Lancet Rheumatol. 2021, 3, e469–e470. [Google Scholar] [CrossRef]
- Rademacher, J.-G.; Tampe, B.; Korsten, P. First Report of Two Cases of Löfgren’s Syndrome after SARS-CoV-2 Vaccination-Coincidence or Causality? Vaccines 2021, 9, 1313. [Google Scholar] [CrossRef] [PubMed]
- Eslam, M.; Avila, A.; Lam, W. M039 RINGING SHOT: TINNITUS AS A POSSIBLE SIDE EFFECT TO THE NEW COVID19 MRNA VACCINE. Ann. Allergy Asthma Immunol. 2021, 127, S69. [Google Scholar] [CrossRef]
- Parrino, D.; Frosolini, A.; Gallo, C.; De Siati, R.D.; Spinato, G.; de Filippis, C. Tinnitus Following COVID-19 Vaccination: Report of Three Cases. Int. J. Audiol. 2022, 61, 526–529. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, S.H.; Waseem, S.; Shaikh, T.G.; Qadir, N.A.; Siddiqui, S.A.; Ullah, I.; Waris, A.; Yousaf, Z. SARS-CoV-2 Vaccine-Associated-Tinnitus: A Review. Ann. Med. Surg. 2022, 75, 103293. [Google Scholar] [CrossRef] [PubMed]
- Wichova, H.; Miller, M.E.; Derebery, M.J. Otologic Manifestations after COVID-19 Vaccination: The House Ear Clinic Experience. Otol. Neurotol. 2021, 42, e1213–e1218. [Google Scholar] [CrossRef] [PubMed]
- Jeong, J. Vestibular Neuritis after COVID-19 Vaccination. Hum. Vaccines Immunother. 2021, 17, 5126–5128. [Google Scholar] [CrossRef]
- Di Mauro, P.; La Mantia, I.; Cocuzza, S.; Sciancalepore, P.I.; Rasà, D.; Maniaci, A.; Ferlito, S.; Tundo, I.; Anzivino, R. Acute Vertigo after COVID-19 Vaccination: Case Series and Literature Review. Front. Med. 2022, 8, 2766. [Google Scholar] [CrossRef]
- Yanir, Y.; Doweck, I.; Shibli, R.; Najjar-Debbiny, R.; Saliba, W. Association between the BNT162b2 Messenger RNA COVID-19 Vaccine and the Risk of Sudden Sensorineural Hearing Loss. JAMA Otolaryngol. Head Neck Surg. 2022, 148, 299–306. [Google Scholar] [CrossRef]
- Formeister, E.J.; Chien, W.; Agrawal, Y.; Carey, J.P.; Stewart, C.M.; Sun, D.Q. Preliminary Analysis of Association Between COVID-19 Vaccination and Sudden Hearing Loss Using US Centers for Disease Control and Prevention Vaccine Adverse Events Reporting System Data. JAMA Otolaryngol. Head Neck Surg. 2021, 147, 674–676. [Google Scholar] [CrossRef]
- Rabinovitch, T.; Ben-Arie-Weintrob, Y.; Hareuveni-Blum, T.; Shaer, B.; Vishnevskia-Dai, V.; Shulman, S.; Newman, H.; Biadsy, M.; Masarwa, D.; Fischer, N.; et al. Uveitis after the Bnt162b2 Mrna Vaccination against SARS-CoV-2 Infection: A Possible Association. Retina 2021, 41, 2462–2471. [Google Scholar] [CrossRef]
- Sacconi, R.; Simona, F.; Forte, P.; Querques, G. Retinal Vein Occlusion Following Two Doses of MRNA-1237 (Moderna) Immunization for SARS-CoV-2: A Case Report. Ophthalmol. Ther. 2022, 11, 453–458. [Google Scholar] [CrossRef] [PubMed]
- Girbardt, C.; Busch, C.; Al-Sheikh, M.; Gunzinger, J.M.; Invernizzi, A.; Xhepa, A.; Unterlauft, J.D.; Rehak, M. Retinal Vascular Events after MRNA and Adenoviral-Vectored COVID-19 Vaccines—A Case Series. Vaccines 2021, 9, 1349. [Google Scholar] [CrossRef] [PubMed]
- Koong, L.R.; Chee, W.K.; Toh, Z.H.; Ng, X.L.; Agrawal, R.; Ho, S.L. Vogt-Koyanagi-Harada Disease Associated with COVID-19 MRNA Vaccine. Ocul. Immunol. Inflamm. 2021, 29, 1212–1215. [Google Scholar] [CrossRef]
- Goyal, M.; Murthy, S.I.; Annum, S. Bilateral Multifocal Choroiditis Following COVID-19 Vaccination. Ocul. Immunol. Inflamm. 2021, 29, 753–757. [Google Scholar] [CrossRef]
- Ng, X.L.; Betzler, B.K.; Testi, I.; Ho, S.L.; Tien, M.; Ngo, W.K.; Zierhut, M.; Chee, S.P.; Gupta, V.; Pavesio, C.E.; et al. Ocular Adverse Events After COVID-19 Vaccination. Ocul. Immunol. Inflamm. 2021, 29, 1216–1224. [Google Scholar] [CrossRef] [PubMed]
- Eleiwa, T.K.; Gaier, E.D.; Haseeb, A.; ElSheikh, R.H.; Sallam, A.B.; Elhusseiny, A.M. Adverse Ocular Events Following COVID-19 Vaccination. Inflamm. Res. 2021, 70, 1005–1009. [Google Scholar] [CrossRef]
- Kubota, T.; Hasegawa, T.; Ikeda, K.; Aoki, M. Case Report: Isolated, unilateral oculomotor palsy with anti-GQ1b antibody following COVID-19 vaccination [version 1; peer review: 1 approved, 1 approved with reservations]. F1000Research 2021, 10, 1142. [Google Scholar] [CrossRef]
- Maleki, A.; Look-Why, S.; Manhapra, A.; Foster, C.S. COVID-19 Recombinant MRNA Vaccines and Serious Ocular Inflammatory Side Effects: Real or Coincidence? J. Ophthalmic Vis. Res. 2021, 16, 490–501. [Google Scholar] [CrossRef]
- Dutta, S.; Kaur, R.; Charan, J.; Bhardwaj, P.; Ambwani, S.R.; Babu, S.; Goyal, J.P.; Haque, M. Analysis of Neurological Adverse Events Reported in VigiBase from COVID-19 Vaccines. Cureus 2022, 14, e21376. [Google Scholar] [CrossRef]
- Šín, R.; Štruncová, D. Status Epilepticus as a Complication after COVID-19 MRNA-1273 Vaccine: A Case Report. World J. Clin. Cases 2021, 9, 7218–7223. [Google Scholar] [CrossRef]
- Perez, B.; Sims, H.S.; Mularczyk, C. Vocal Cord Paresis: A Case Report on a Novel Complication of the Mode RNA COVID-19 Vaccine. Otolaryngol. Head Neck Surg. 2021, 165, 242. [Google Scholar]
- Renoud, L.; Khouri, C.; Revol, B.; Lepelley, M.; Perez, J.; Roustit, M.; Cracowski, J.-L. Association of Facial Paralysis with MRNA COVID-19 Vaccines: A Disproportionality Analysis Using the World Health Organization Pharmacovigilance Database. JAMA Intern. Med. 2021, 181, 1243–1245. [Google Scholar] [CrossRef]
- Finsterer, J.; Korn, M. Aphasia Seven Days after Second Dose of an MRNA-Based SARS-CoV-2 Vaccine. Brain Hemorrhages 2021, 2, 165–167. [Google Scholar] [CrossRef] [PubMed]
- Schulz, J.B.; Berlit, P.; Diener, H.; Gerloff, C.; Greinacher, A.; Klein, C.; Petzold, G.C.; Piccininni, M.; Poli, S.; Röhrig, R.; et al. COVID -19 Vaccine-Associated Cerebral Venous Thrombosis in Germany. Ann. Neurol. 2021, 90, 627–639. [Google Scholar] [CrossRef] [PubMed]
- Yagi, Y.; Asami, Y.; Kyoya, M.; Yokota, T. Cerebral Venous Sinus Thrombosis after MRNA-Based COVID-19 Vaccination. Neurol. Sci. 2022, 43, 41–43. [Google Scholar] [CrossRef]
- Garg, R.K.; Paliwal, V.K. Spectrum of Neurological Complications Following COVID-19 Vaccination. Neurol. Sci. 2022, 43, 3–40. [Google Scholar] [CrossRef]
- Torrealba-Acosta, G.; Martin, J.C.; Huttenbach, Y.; Garcia, C.R.; Sohail, M.R.; Agarwal, S.K.; Wasko, C.; Bershad, E.M.; Hirzallah, M.I. Acute Encephalitis, Myoclonus and Sweet Syndrome after MRNA-1273 Vaccine. BMJ Case Rep. 2021, 14, e243173. [Google Scholar] [CrossRef] [PubMed]
- Luca, A.; Squillaci, R.; Terravecchia, C.; Contrafatto, F.; Reggio, E.; Nicoletti, A.; Zappia, M. Pure Sensitive Chronic Inflammatory Axonal Polyneuropathy Following Pfizer COVID-19 Vaccine. Neurol. Sci. 2022, 43, 1431–1433. [Google Scholar] [CrossRef]
- Khayat-Khoei, M.; Bhattacharyya, S.; Katz, J.; Harrison, D.; Tauhid, S.; Bruso, P.; Houtchens, M.K.; Edwards, K.R.; Bakshi, R. COVID-19 MRNA Vaccination Leading to CNS Inflammation: A Case Series. J. Neurol. 2022, 269, 1093–1106. [Google Scholar] [CrossRef]
- Kataria, S.; Rogers, S.; Bilal, U.; Baktashi, H.; Singh, R. Multiple Sclerosis Relapse Following COVID-19 Vaccination: A Case Report and Literature Review. Cureus 2022, 14, e21374. [Google Scholar] [CrossRef]
- Filippo, M.D.; Cordioli, C.; Malucchi, S.; Annovazzi, P.; Cavalla, P.; Clerici, V.T.; Ragonese, P.; Nociti, V.; Radaelli, M.; Laroni, A.; et al. MRNA COVID-19 Vaccines Do Not Increase the Short-Term Risk of Clinical Relapses in Multiple Sclerosis. J. Neurol. Neurosurg. Psychiatry 2022, 93, 448–450. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, Y.; Ohyama, A.; Kubota, T.; Ikeda, K.; Kaneko, K.; Takai, Y.; Warita, H.; Takahashi, T.; Misu, T.; Aoki, M. MOG Antibody-Associated Disorders Following SARS-CoV-2 Vaccination: A Case Report and Literature Review. Front. Neurol. 2022, 13, 845755. [Google Scholar] [CrossRef] [PubMed]
- Razok, A.; Shams, A.; Almeer, A.; Zahid, M. Post-COVID-19 Vaccine Guillain-Barré Syndrome; First Reported Case from Qatar. Ann. Med. Surg. 2021, 67, 102540. [Google Scholar] [CrossRef]
- Contaldi, E.; Comi, C.; Cantello, R.; Magistrelli, L. Motor and Non-Motor Symptom Improvement after MRNA-1273 Vaccine in a Parkinson’s Disease Patient. Neurol. Sci. 2022, 43, 1447–1448. [Google Scholar] [CrossRef] [PubMed]
- Chavez, A.; Pougnier, C. A Case of COVID-19 Vaccine Associated New Diagnosis Myasthenia Gravis. J. Prim. Care Community Health 2021, 12, 215013272110519. [Google Scholar] [CrossRef] [PubMed]
- Kang, H.S.; Kim, J.E.; Yoo, J.R.; Oh, H.; Kim, M.; Kim, Y.R.; Heo, S.T. Aseptic Meningitis Following Second Dose of an MRNA Coronavirus Disease 2019 Vaccine in a Healthy Male: Case Report and Literature Review. Infect. Chemother. 2022, 54, 189. [Google Scholar] [CrossRef]
- Nagamine, T. Neuroleptic Malignant Syndrome Associated with COVID-19 Vaccination. Can. J. Emerg. Med. 2021, 24, 349–350. [Google Scholar] [CrossRef]
- Yesilkaya, U.H.; Sen, M.; Tasdemir, B.G. A Novel Adverse Effect of the BNT162b2 MRNA Vaccine: First Episode of Acute Mania with Psychotic Features. Brain Behav. Immun. Health 2021, 18, 100363. [Google Scholar] [CrossRef]
- Reinfeld, S.; Cáceda, R.; Gil, R.; Strom, H.; Chacko, M. Can New Onset Psychosis Occur after MRNA Based COVID-19 Vaccine Administration? A Case Report. Psychiatry Res. 2021, 304, 114165. [Google Scholar] [CrossRef]
- Mariette, C.; Lavaud, J.; Descamps, V. Stress Induced by Messenger Ribonucleic Acid (RNA) Vaccination May Reveal Acute Adrenal Insufficiency. Endocrine 2022, 75, 659–660. [Google Scholar] [CrossRef]
- Al-Mashdali, A.F.; Ata, Y.M.; Sadik, N. Post-COVID-19 vaccine acute hyperactive encephalopathy with dramatic response to methylprednisolone: A case report. Ann. Med. Surg. 2021, 69, 102803. [Google Scholar] [CrossRef] [PubMed]
- Zavala-Jonguitud, L.F.; Pérez-García, C.C. Delirium Triggered by COVID-19 Vaccine in an Elderly Patient. Geriatr. Gerontol. Int. 2021, 21, 540. [Google Scholar] [CrossRef] [PubMed]
- MODULE 3. Adverse Events Following Immunization. Available online: https://www.who.int/vaccine_safety/initiative/tech_support/Part-3.pdf?ua=1 (accessed on 31 March 2022).
- McNeil, M.M.; Weintraub, E.S.; Duffy, J.; Sukumaran, L.; Jacobsen, S.J.; Klein, N.P.; Hambidge, S.J.; Lee, G.M.; Jackson, L.A.; Irving, S.A.; et al. Risk of Anaphylaxis after Vaccination in Children and Adults. J. Allergy Clin. Immunol. 2016, 137, 868–878. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bahl, K.; Senn, J.J.; Yuzhakov, O.; Bulychev, A.; Brito, L.A.; Hassett, K.J.; Laska, M.E.; Smith, M.; Almarsson, O.; Thompson, J.; et al. Preclinical and Clinical Demonstration of Immunogenicity by mRNA Vaccines against H10N8 and H7N9 Influenza Viruses. Mol. Ther. 2017, 25, 1316–1327. [Google Scholar] [CrossRef] [Green Version]
- Ndeupen, S.; Qin, Z.; Jacobsen, S.; Bouteau, A.; Estanbouli, H.; Igyártó, B.Z. The MRNA-LNP Platform’s Lipid Nanoparticle Component Used in Preclinical Vaccine Studies Is Highly Inflammatory. iScience 2021, 24, 103479. [Google Scholar] [CrossRef]
- Hong, L.; Wang, Z.; Wei, X.; Shi, J.; Li, C. Antibodies against Polyethylene Glycol in Human Blood: A Literature Review. J. Pharm. Toxicol. Methods 2020, 102, 106678. [Google Scholar] [CrossRef]
- Banerji, A.; Wickner, P.G.; Saff, R.; Stone, C.A.; Robinson, L.B.; Long, A.A.; Wolfson, A.R.; Williams, P.; Khan, D.A.; Phillips, E.; et al. MRNA Vaccines to Prevent COVID-19 Disease and Reported Allergic Reactions: Current Evidence and Suggested Approach. J. Allergy Clin. Immunol. Pract. 2021, 9, 1423–1437. [Google Scholar] [CrossRef]
- Klimek, L.; Novak, N.; Cabanillas, B.; Jutel, M.; Bousquet, J.; Akdis, C.A. Allergenic Components of the MRNA-1273 Vaccine for COVID-19: Possible Involvement of Polyethylene Glycol and IgG-Mediated Complement Activation. Allergy 2021, 76, 3307–3313. [Google Scholar] [CrossRef]
- Moghimi, S.M. Allergic Reactions and Anaphylaxis to LNP-Based COVID-19 Vaccines. Mol. Ther. 2021, 29, 898–900. [Google Scholar] [CrossRef] [PubMed]
- Kauffman, K.J.; Mir, F.F.; Jhunjhunwala, S.; Kaczmarek, J.C.; Hurtado, J.E.; Yang, J.H.; Webber, M.J.; Kowalski, P.S.; Heartlein, M.W.; DeRosa, F.; et al. Efficacy and Immunogenicity of Unmodified and Pseudouridine-Modified MRNA Delivered Systemically with Lipid Nanoparticles in Vivo. Biomaterials 2016, 109, 78–87. [Google Scholar] [CrossRef] [Green Version]
- Maugeri, M.; Nawaz, M.; Papadimitriou, A.; Angerfors, A.; Camponeschi, A.; Na, M.; Hölttä, M.; Skantze, P.; Johansson, S.; Sundqvist, M.; et al. Linkage between Endosomal Escape of LNP-MRNA and Loading into EVs for Transport to Other Cells. Nat. Commun. 2019, 10, 4333. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Doulberis, M.; Papaefthymiou, A.; Kotronis, G.; Gialamprinou, D.; Soteriades, E.S.; Kyriakopoulos, A.; Chatzimichael, E.; Kafafyllidou, K.; Liatsos, C.; Chatzistefanou, I.; et al. Does COVID-19 Vaccination Warrant the Classical Principle “Ofelein i Mi Vlaptin”? Medicina 2021, 57, 253. [Google Scholar] [CrossRef] [PubMed]
- Lu, L.; Li, J.; Moussaoui, M.; Boix, E. Immune Modulation by Human Secreted RNases at the Extracellular Space. Front. Immunol. 2018, 9, 1012. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bakaysa, S.L.; Potter, J.A.; Hoang, M.; Han, C.S.; Guller, S.; Norwitz, E.R.; Abrahams, V.M. Single- and Double-Stranded Viral RNA Generate Distinct Cytokine and Antiviral Responses in Human Fetal Membranes. Mol. Hum. Reprod. 2014, 20, 701–708. [Google Scholar] [CrossRef]
- Nance, K.D.; Meier, J.L. Modifications in an Emergency: The Role of N1-Methylpseudouridine in COVID-19 Vaccines. ACS Cent. Sci. 2021, 7, 748–756. [Google Scholar] [CrossRef]
- Talotta, R. Do COVID-19 RNA-Based Vaccines Put at Risk of Immune-Mediated Diseases? In Reply to “Potential Antigenic Cross-Reactivity between SARS-CoV-2 and Human Tissue with a Possible Link to an Increase in Autoimmune Diseases”. Clin. Immunol. 2021, 224, 108665. [Google Scholar] [CrossRef]
- Aldén, M.; Olofsson Falla, F.; Yang, D.; Barghouth, M.; Luan, C.; Rasmussen, M.; De Marinis, Y. Intracellular Reverse Transcription of Pfizer BioNTech COVID-19 MRNA Vaccine BNT162b2 In Vitro in Human Liver Cell Line. Curr. Issues Mol. Biol. 2022, 44, 1115–1126. [Google Scholar] [CrossRef]
- Fang, E.; Liu, X.; Li, M.; Zhang, Z.; Song, L.; Zhu, B.; Wu, X.; Liu, J.; Zhao, D.; Li, Y. Advances in COVID-19 MRNA Vaccine Development. Signal Transduct. Target Ther. 2022, 7, 1–31. [Google Scholar] [CrossRef]
- Fung, T.S.; Liu, D.X. Coronavirus Infection, ER Stress, Apoptosis and Innate Immunity. Front. Microbiol. 2014, 5, 296. [Google Scholar] [CrossRef] [Green Version]
- Ogata, A.F.; Cheng, C.-A.; Desjardins, M.; Senussi, Y.; Sherman, A.C.; Powell, M.; Novack, L.; Von, S.; Li, X.; Baden, L.R.; et al. Circulating Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) Vaccine Antigen Detected in the Plasma of MRNA-1273 Vaccine Recipients. Clin. Infect. Dis. 2022, 74, 715–718. [Google Scholar] [CrossRef]
- Röltgen, K.; Nielsen, S.C.A.; Silva, O.; Younes, S.F.; Zaslavsky, M.; Costales, C.; Yang, F.; Wirz, O.F.; Solis, D.; Hoh, R.A.; et al. Immune Imprinting, Breadth of Variant Recognition, and Germinal Center Response in Human SARS-CoV-2 Infection and Vaccination. Cell 2022, 185, 1025–1040.e14. [Google Scholar] [CrossRef] [PubMed]
- Selvaraj, G.; Kaliamurthi, S.; Peslherbe, G.H.; Wei, D.-Q. Are the Allergic Reactions of COVID-19 Vaccines Caused by MRNA Constructs or Nanocarriers? Immunological Insights. Interdiscip. Sci. Comput. Life Sci. 2021, 13, 344–347. [Google Scholar] [CrossRef]
- Nuovo, G.J.; Magro, C.; Shaffer, T.; Awad, H.; Suster, D.; Mikhail, S.; He, B.; Michaille, J.-J.; Liechty, B.; Tili, E. Endothelial Cell Damage Is the Central Part of COVID-19 and a Mouse Model Induced by Injection of the S1 Subunit of the Spike Protein. Ann. Diagn. Pathol. 2021, 51, 151682. [Google Scholar] [CrossRef]
- Rhea, E.M.; Logsdon, A.F.; Hansen, K.M.; Williams, L.M.; Reed, M.J.; Baumann, K.K.; Holden, S.J.; Raber, J.; Banks, W.A.; Erickson, M.A. The S1 protein of SARS-CoV-2 crosses the blood-brain barrier in mice. Nat. Neurosci. 2021, 24, 368–378. [Google Scholar] [CrossRef]
- Salamanna, F.; Maglio, M.; Landini, M.P.; Fini, M. Body Localization of ACE-2: On the Trail of the Keyhole of SARS-CoV-2. Front. Med. 2020, 7, 594495. [Google Scholar] [CrossRef]
- Lechuga, G.C.; Souza-Silva, F.; Sacramento, C.Q.; Trugilho, M.R.O.; Valente, R.H.; Napoleão-Pêgo, P.; Dias, S.S.G.; Fintelman-Rodrigues, N.; Temerozo, J.R.; Carels, N.; et al. SARS-CoV-2 Proteins Bind to Hemoglobin and Its Metabolites. Int. J. Mol. Sci. 2021, 22, 9035. [Google Scholar] [CrossRef] [PubMed]
- Balestrieri, E.; Minutolo, A.; Petrone, V.; Fanelli, M.; Iannetta, M.; Malagnino, V.; Zordan, M.; Vitale, P.; Charvet, B.; Horvat, B.; et al. Evidence of the Pathogenic HERV-W Envelope Expression in T Lymphocytes in Association with the Respiratory Outcome of COVID-19 Patients. eBioMedicine 2021, 66, 103341. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Xu, J.; Hou, Y.; Leverenz, J.B.; Kallianpur, A.; Mehra, R.; Liu, Y.; Yu, H.; Pieper, A.A.; Jehi, L.; et al. Network Medicine Links SARS-CoV-2/COVID-19 Infection to Brain Microvascular Injury and Neuroinflammation in Dementia-like Cognitive Impairment. Alzheimer’s Res. Ther. 2021, 13, 110. [Google Scholar] [CrossRef]
- Idrees, D.; Kumar, V. SARS-CoV-2 Spike Protein Interactions with Amyloidogenic Proteins: Potential Clues to Neurodegeneration. Biochem. Biophy. Res. Commun. 2021, 554, 94–98. [Google Scholar] [CrossRef]
- Choudhury, A.; Mukherjee, S. In Silico Studies on the Comparative Characterization of the Interactions of SARS-CoV-2 Spike Glycoprotein with ACE-2 Receptor Homologs and Human TLRs. J. Med. Virol. 2020, 92, 2105–2113. [Google Scholar] [CrossRef] [PubMed]
- Vojdani, A.; Kharrazian, D. Potential Antigenic Cross-Reactivity between SARS-CoV-2 and Human Tissue with a Possible Link to an Increase in Autoimmune Diseases. Clin. Immunol. 2020, 217, 108480. [Google Scholar] [CrossRef] [PubMed]
- Yapici-Eser, H.; Koroglu, Y.E.; Oztop-Cakmak, O.; Keskin, O.; Gursoy, A.; Gursoy-Ozdemir, Y. Neuropsychiatric Symptoms of COVID-19 Explained by SARS-CoV-2 Proteins’ Mimicry of Human Protein Interactions. Front. Hum. Neurosci. 2021, 15, 656313. [Google Scholar] [CrossRef]
- Schneider, J.; Sottmann, L.; Greinacher, A.; Hagen, M.; Kasper, H.-U.; Kuhnen, C.; Schlepper, S.; Schmidt, S.; Schulz, R.; Thiele, T.; et al. Postmortem Investigation of Fatalities Following Vaccination with COVID-19 Vaccines. Int. J. Leg. Med. 2021, 135, 2335–2345. [Google Scholar] [CrossRef] [PubMed]
- Guruprasad, K. Mutations in Human SARS-CoV-2 Spike Proteins, Potential Drug Binding and Epitope Sites for COVID-19 Therapeutics Development. Curr. Res. Struct. Biol. 2022, 4, 41–50. [Google Scholar] [CrossRef]
- Xu, L.; Ma, Z.; Li, Y.; Pang, Z.; Xiao, S. Antibody Dependent Enhancement: Unavoidable Problems in Vaccine Development. Adv. Immunol. 2021, 151, 99–133. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mouliou, D.S.; Dardiotis, E. Current Evidence in SARS-CoV-2 mRNA Vaccines and Post-Vaccination Adverse Reports: Knowns and Unknowns. Diagnostics 2022, 12, 1555. https://doi.org/10.3390/diagnostics12071555
Mouliou DS, Dardiotis E. Current Evidence in SARS-CoV-2 mRNA Vaccines and Post-Vaccination Adverse Reports: Knowns and Unknowns. Diagnostics. 2022; 12(7):1555. https://doi.org/10.3390/diagnostics12071555
Chicago/Turabian StyleMouliou, Dimitra S., and Efthimios Dardiotis. 2022. "Current Evidence in SARS-CoV-2 mRNA Vaccines and Post-Vaccination Adverse Reports: Knowns and Unknowns" Diagnostics 12, no. 7: 1555. https://doi.org/10.3390/diagnostics12071555
APA StyleMouliou, D. S., & Dardiotis, E. (2022). Current Evidence in SARS-CoV-2 mRNA Vaccines and Post-Vaccination Adverse Reports: Knowns and Unknowns. Diagnostics, 12(7), 1555. https://doi.org/10.3390/diagnostics12071555






