A Race against the Clock: A Case Report and Literature Review Concerning the Importance of ADAMTS13 Testing in Diagnosis and Management of Thrombotic Thrombocytopenic Purpura during Pregnancy
Abstract
:1. Introduction
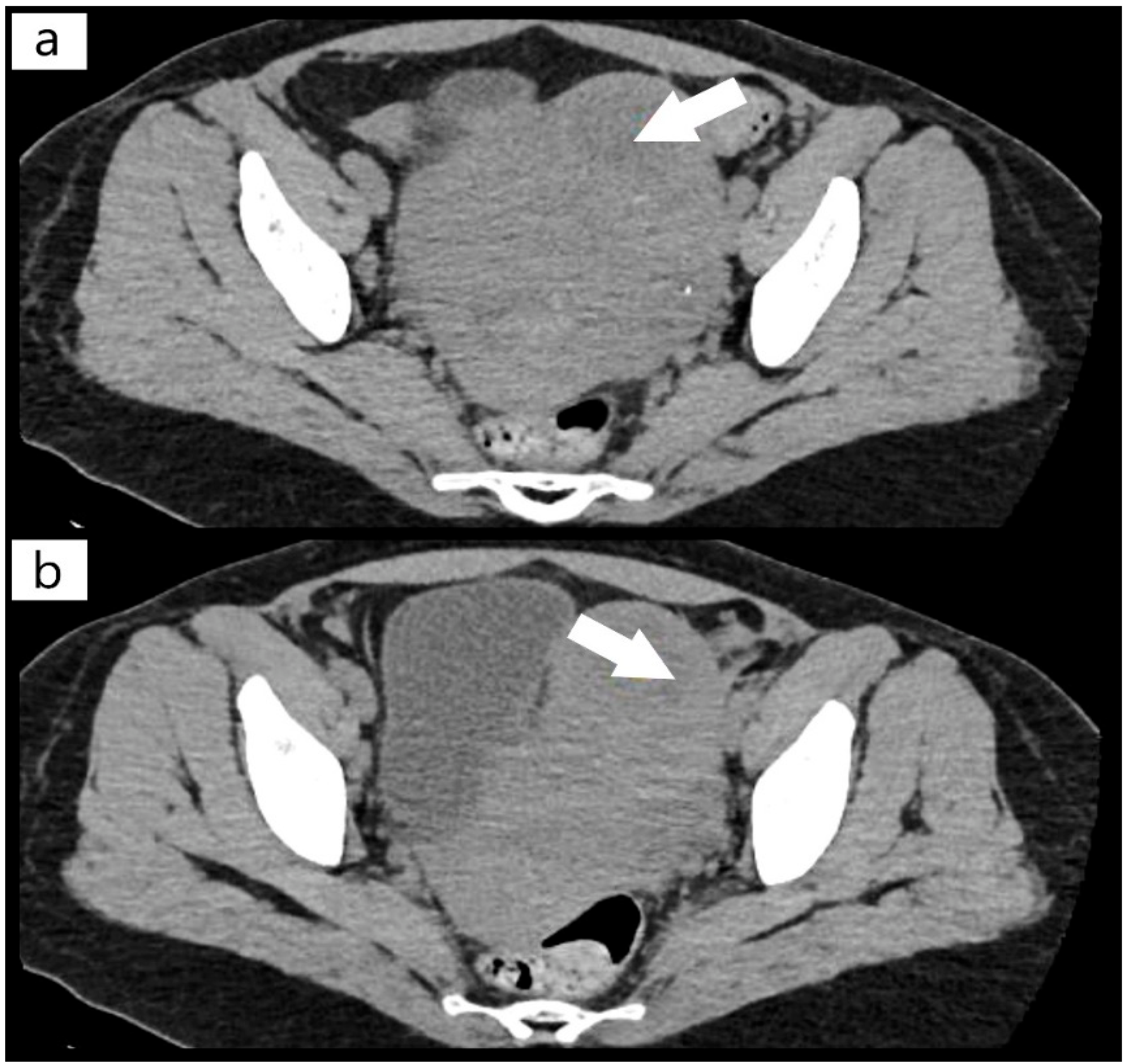
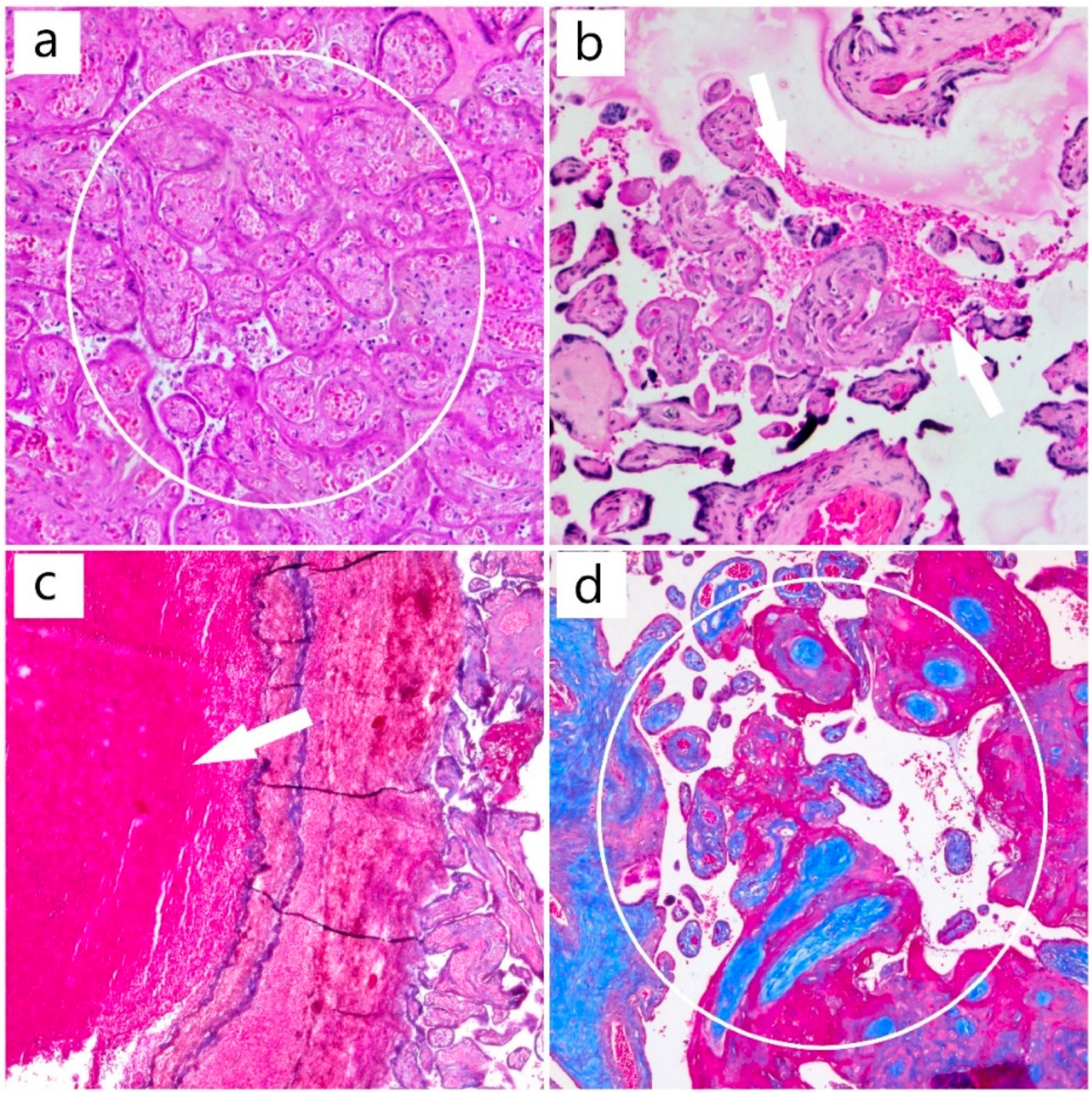
2. Case Report
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Sakai, K.; Fujimura, Y.; Nagata, Y.; Higasa, S.; Moriyama, M.; Isonishi, A.; Konno, M.; Kajiwara, M.; Ogawa, Y.; Kaburagi, S.; et al. Success and limitations of plasma treatment in pregnant women with congenital thrombotic thrombocytopenic purpura. J. Thromb. Haemost. 2020, 18, 2929–2941. [Google Scholar] [CrossRef] [PubMed]
- Al-Husban, N.; Al-Kuran, O. Post-Partum Thrombotic Thrombocytopenic Purpura (TTP) in a Patient with known Idiopathic (Immune) Thrombocytopenic Purpura: A case report and review of the literature. J. Med. Case Rep. 2018, 12, 147. [Google Scholar] [CrossRef]
- Romão de Souza, V.; Beatriz Cavalcante de Oliveira, A.; Maria Vanderlei, A.; Queiroz da Mota Silveira Aroucha, A.; Pontes Duarte, B.; Nunes Machado, A.; Netto Chaer, L.; Wanderley de Barros Correia, C.; da Conceição de Barros Correia, M.; Freire Hazin Costa, M. Inherited thrombotic thrombocytopenic purpura mimicking immune thrombocytopenic purpura during pregnancy: A case report. J. Med. Case Rep. 2018, 12, 15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roose, E.; Tersteeg, C.; Demeersseman, R.; Schelpe, A.S.; Deforche, L.; Pareyn, I.; Vandenbulcke, A.; Vandeputte, N.; Dierickx, D.; Voorberg, J.; et al. Anti-ADAMTS13 Antibodies and a Novel Heterozygous p.R1177Q Mutation in a Case of Pregnancy-Onset Immune-Mediated Thrombotic Thrombocytopenic Purpura. TH Open 2018, 2, e8–e15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nonaka, T.; Yamaguchi, M.; Nishijima, K.; Moriyama, M.; Takakuwa, K.; Enomoto, T. A successfully treated case of an acute presentation of congenital thrombotic thrombocytopenic purpura (Upshaw-Schulman syndrome) with decreased ADAMTS13 during late stage of pregnancy. J. Obstet. Gynaecol. Res. 2021, 47, 1892–1897. [Google Scholar] [CrossRef]
- Perez Botero, J.; Reese, J.A.; George, J.N.; McIntosh, J.J. Severe thrombocytopenia and microangiopathic hemolytic anemia in pregnancy: A guide for the consulting hematologist. Am. J. Hematol. 2021, 96, 1655–1665. [Google Scholar] [CrossRef]
- Scully, M. Thrombotic Thrombocytopenic Purpura and Atypical Hemolytic Uremic Syndrome Microangiopathy in Pregnancy. Semin. Thromb. Hemost. 2016, 42, 774–779. [Google Scholar] [CrossRef]
- Ramadan, M.K.; Badr, D.A.; Hubeish, M.; Itani, S.; Hijazi, H.; Mogharbil, A. HELLP Syndrome, Thrombotic Thrombocytopenic Purpura or Both: Appraising the Complex Association and Proposing a Stepwise Practical Plan for Differential Diagnosis. J. Hematol. 2018, 7, 32–37. [Google Scholar] [CrossRef]
- Marins, L.R.; da Rocha Oppermann, M.L. Thrombotic thrombocytopenic purpura and acquired immunodeficiency syndrome diagnosed in pregnancy: Case report. J. Obstet. Gynaecol. Res. 2021, 47, 1898–1902. [Google Scholar] [CrossRef]
- Umemura, A.; Sasaki, A.; Nitta, H.; Obuchi, T.; Baba, S.; Wakabayashi, G. Laparoscopic splenectomy for the treatment of refractory thrombotic thrombocytopenic purpura. Clin. J. Gastroenterol. 2013, 6, 420–423. [Google Scholar] [CrossRef]
- Ferrari, B.; Maino, A.; Lotta, L.A.; Artoni, A.; Pontiggia, S.; Trisolini, S.M.; Malato, A.; Rosendaal, F.R.; Peyvandi, F. Pregnancy complications in acquired thrombotic thrombocytopenic purpura: A case-control study. Orphanet J. Rare Dis. 2014, 9, 193. [Google Scholar] [CrossRef] [Green Version]
- Zhao, C.; Qu, Y.; Sui, R.; Feng, J.; Gao, J.; Ma, J.; Jiang, R.; Li, H. Delayed visual recovery in pregnancy-associated thrombotic thrombocytopenic purpura with bilateral serous retinal detachment. Doc. Ophthalmol. 2013, 126, 163–169. [Google Scholar] [CrossRef] [PubMed]
- Mariotte, E.; Azoulay, E.; Galicier, L.; Rondeau, E.; Zouiti, F.; Boisseau, P.; Poullin, P.; de Maistre, E.; Provôt, F.; Delmas, Y.; et al. Epidemiology and pathophysiology of adulthood-onset thrombotic microangiopathy with severe ADAMTS13 deficiency (thrombotic thrombocytopenic purpura): A cross-sectional analysis of the French national registry for thrombotic microangiopathy. Lancet Haematol 2016, 3, e237–e245. [Google Scholar] [CrossRef]
- Ferrari, B.; Peyvandi, F. How I treat thrombotic thrombocytopenic purpura in pregnancy. Blood 2020, 136, 2125–2132. [Google Scholar] [CrossRef] [PubMed]
- Lucania, G.; Camiolo, E.; Carmina, M.G.; Fiandaca, T.; Indovina, A.; Malato, A.; Messina, R.; Fabbiano, F.; Marcenò, R. Multidisciplinary approach in pregnancy-associated thrombotic thrombocytopenic purpura: A case report. Blood Transfus. 2014, 12 (Suppl. 1), s137–s140. [Google Scholar] [CrossRef] [PubMed]
- Kaneda, Y.; Kitagawa, J.; Yamaguchi, K.; Matsumoto, T.; Nakamura, N.; Nakamura, H.; Ninomiya, S.; Kanemura, N.; Kasahara, S.; Hara, T.; et al. Thrombotic thrombocytopenic purpura during pregnancy refractory to plasma exchange and rituximab. Rinsho Ketsueki 2019, 60, 209–212. [Google Scholar] [CrossRef]
- González-Mesa, E.; Narbona, I.; Blasco, M.; Cohen, I. Unfavorable course in pregnancy-associated thrombotic thrombocytopenic purpura necessitating a perimortem Cesarean section: A case report. J. Med. Case Rep. 2013, 7, 119. [Google Scholar] [CrossRef] [Green Version]
- Sukumar, S.; Lämmle, B.; Cataland, S.R. Thrombotic Thrombocytopenic Purpura: Pathophysiology, Diagnosis, and Management. J. Clin. Med. 2021, 10, 536. [Google Scholar] [CrossRef]
- Von Krogh, A.S.; Kremer Hovinga, J.A.; Tjønnfjord, G.E.; Ringen, I.M.; Lämmle, B.; Waage, A.; Quist-Paulsen, P. The impact of congenital thrombotic thrombocytopenic purpura on pregnancy complications. Thromb. Haemost. 2014, 111, 1180–1183. [Google Scholar] [CrossRef] [Green Version]
- Maayan, H.; Kirgner, I.; Gutwein, O.; Herzog-Tzarfati, K.; Rahimi-Levene, N.; Koren-Michowitz, M.; Blickstein, D. Acquired thrombotic thrombocytopenic purpura: A rare disease associated with BNT162b2 vaccine. J. Thromb. Haemost. 2021, 19, 2314–2317. [Google Scholar] [CrossRef]
- Aminimoghaddam, S.; Afrooz, N.; Nasiri, S.; Motaghi Nejad, O.; Mahmoudzadeh, F. A COVID-19 pregnant patient with thrombotic thrombocytopenic purpura: A case report. J. Med. Case Rep. 2021, 15, 104. [Google Scholar] [CrossRef] [PubMed]
- Nikolaou, M.; Karakantza, M.; Adonakis, G.; Theodorou, G.; Zoumbos, N.; Decavalas, G. A case of severe ADAMTS13 deficiency presenting as thrombotic thrombocytopenic purpura in pregnancy. Med. Pregl. 2012, 65, 436–439. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Williams, L.A.; Marques, M.B. Pathology Consultation on the Diagnosis and Treatment of Thrombotic Microangiopathies (TMAs). Am. J. Clin. Pathol. 2016, 145, 158–165. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moatti-Cohen, M.; Garrec, C.; Wolf, M.; Boisseau, P.; Galicier, L.; Azoulay, E.; Stepanian, A.; Delmas, Y.; Rondeau, E.; Bezieau, S.; et al. Unexpected frequency of Upshaw-Schulman syndrome in pregnancy-onset thrombotic thrombocytopenic purpura. Blood 2012, 119, 5888–5897. [Google Scholar] [CrossRef]
- Xu, J.; Yu, S.; Zhang, F. Frequent recurrence of pregnancy-triggered congenital thrombotic thrombocytopenic purpura: A rare case report. Clin. Hemorheol. Microcirc. 2021, 77, 195–200. [Google Scholar] [CrossRef]
- Fakhouri, F.; Scully, M.; Provôt, F.; Blasco, M.; Coppo, P.; Noris, M.; Paizis, K.; Kavanagh, D.; Pène, F.; Quezada, S.; et al. Management of thrombotic microangiopathy in pregnancy and postpartum: Report from an international working group. Blood 2020, 136, 2103–2117. [Google Scholar] [CrossRef]
- George, J.N. The remarkable diversity of thrombotic thrombocytopenic purpura: A perspective. Blood Adv. 2018, 2, 1510–1516. [Google Scholar] [CrossRef]
- Cines, D.B.; Levine, L.D. Thrombocytopenia in pregnancy. Blood 2017, 130, 2271–2277. [Google Scholar] [CrossRef] [Green Version]
- Gasparri, M.L.; Bellati, F.; Brunelli, R.; Perrone, G.; Pecorini, F.; Papadia, A.; Meloni, G.; Trisolini, S.M.; Gozzer, M.; Domenici, L.; et al. Thrombotic thrombocytopenic purpura during pregnancy versus imitator of preeclampsia. Transfusion 2015, 55, 2516–2518. [Google Scholar] [CrossRef]
- Zheng, X.L.; Vesely, S.K.; Cataland, S.R.; Coppo, P.; Geldziler, B.; Iorio, A.; Matsumoto, M.; Mustafa, R.A.; Pai, M.; Rock, G.; et al. ISTH guidelines for treatment of thrombotic thrombocytopenic purpura. J. Thromb. Haemost. 2020, 18, 2496–2502. [Google Scholar] [CrossRef]
- Miesbach, W.; Menne, J.; Bommer, M.; Schönermarck, U.; Feldkamp, T.; Nitschke, M.; Westhoff, T.H.; Seibert, F.S.; Woitas, R.; Sousa, R.; et al. Incidence of acquired thrombotic thrombocytopenic purpura in Germany: A hospital level study. Orphanet J. Rare Dis. 2019, 14, 260. [Google Scholar] [CrossRef] [PubMed]


| Differential Diagnosis | ||
|---|---|---|
| Diagnostic | Characteristics | |
| TTP |
| |
| DIC |
| |
| Disseminated Lupus Erythematosus (Vasculitis from Antiphospholipid syndrome) |
| |
| HELLP Syndrome and Preeclampsia |
| |
| not more than in PTT | ||
| Hemolytic Uremic Syndrome |
| |
| Viral Infection Acute Sepsis COVID-19, Vaccination with Pfizer BioNTech (BNT2b2) |
| |
| Acute Fatty Liver |
| |
| Time of Admission | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| 19 April 2021 | 20 April 2021 | 21 April 2021 | 22 April 2021 | ||||||
| Tests | Result 1 | Result 2 | Result 1 | Result 2 | Result 1 | Result 2 | Result 1 | Result 2 | Normal Value |
| Leukocytes (WBC) | 14.7 × 109/L | 16.2 × 109/L | 14.2 × 109/L | - | 14.8 × 109/L | 17.4 × 109/L | 15.8 × 109/L | - | 4.00–10.00 × 109/L |
| Hemoglobin (HGB) | 5.3 g/dL | 5.5 g/dL | 6.9 g/dL | - | 6.1 g/dL | 8.0 g/dL | 7.7 g/dL | - | 11.5–16 g/dL |
| Hematocrit (HCT) | 17.2% | 17.4% | 23% | - | 20.7% | 26.5% | 25.5 | - | 35–48% |
| Platelets (PLT) | 13 × 109/L | 11 × 109/L | 18 × 109/L | - | 22 × 109/L | 32 × 109/L | 26 × 109/L | 150–450 × 109/L | |
| INR (International Normalized Ratio) | 1.05 | - | 1.09 | - | 1.1 | - | 1.04 | - | 0.8–1.2 |
| APTT | 29.1 | - | 25.1 | - | 25.5 | - | 26.5 | 25–38 s | |
| Fibrinogen | 410 mg/dL | - | 453 mg/dL | - | 387 mg/dL | - | 415 mg/dL | - | 180–450 mg/dL |
| Creatinine | 1.36 mg/dL | 1.38 mg/dL | 1.54 mg/dL | - | 1.48 mg/dL | 1.62 mg/dL | 1.56 mg/dL | - | 0.50–0.90 mg/dL |
| Urea | 53 mg/dL | 50.47 mg/dL | 51.03 mg/dL | - | 60.06 mg/dL | 58.86 mg/dL | 69.96 mg/dL | - | 16–43 mg/dL |
| Uric Acid | 6.94 mg/dL | 6.97 mg/dL | - | - | - | - | - | - | 2.3–6.10 mg/dL |
| Total Bilirubin | - | - | 1.30 mg/dL | - | 1.66 mg/dL | - | 1.39 mg/dL | - | 0.30–1.10 mg/dL |
| Direct Bilirubin | - | - | 0.37 mg/dL | - | 0.31 mg/dL | - | 0.49 mg/dL | - | 0.10–0.40 mg/dL |
| C Reactive Protein (CRP) | 75.5 mg/L | 79.04 mg/L | 85.47 mg/L | - | 84.5 mg/L | - | 0.00–5.00 mg/L | ||
| Lactate dehydrogenase (LDH) | - | - | - | - | 1374 U/L | 1582 U/L | 1764 U/L | - | 0.00–247.00 U/L |
| Total Protein | - | - | 5.6 g/dL | - | 5.26 g/dL | 5.71 g/dL | 5.84 g/dL | - | 6.60–8.30 g/dL |
| Peripheral Blood Smear | Frequent target erythrocytes and schizocytes, aspect that is consistent | ||||||||
| Reticulocytes | Reticulocytes 66 per 1000 red blood cells are found (normal is 5–15 per 1000 red blood cells) | ||||||||
| Troponin I | 7.11 ng/mL | <0.5 ng/mL | |||||||
| D-dimers | >3000 ng/mL | <100 ng/mL | |||||||
| COVID19 (PCR) | Negative | ||||||||
| Cultures | All cultures were negative | ||||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mitranovici, M.I.; Pușcașiu, L.; Oală, I.E.; Petre, I.; Craina, M.L.; Mager, A.R.; Vasile, K.; Chiorean, D.M.; Sabău, A.-H.; Turdean, S.G.; et al. A Race against the Clock: A Case Report and Literature Review Concerning the Importance of ADAMTS13 Testing in Diagnosis and Management of Thrombotic Thrombocytopenic Purpura during Pregnancy. Diagnostics 2022, 12, 1559. https://doi.org/10.3390/diagnostics12071559
Mitranovici MI, Pușcașiu L, Oală IE, Petre I, Craina ML, Mager AR, Vasile K, Chiorean DM, Sabău A-H, Turdean SG, et al. A Race against the Clock: A Case Report and Literature Review Concerning the Importance of ADAMTS13 Testing in Diagnosis and Management of Thrombotic Thrombocytopenic Purpura during Pregnancy. Diagnostics. 2022; 12(7):1559. https://doi.org/10.3390/diagnostics12071559
Chicago/Turabian StyleMitranovici, Melinda Ildiko, Lucian Pușcașiu, Ioan Emilian Oală, Izabella Petre, Marius Lucian Craina, Antonia Rebeka Mager, Kinga Vasile, Diana Maria Chiorean, Adrian-Horațiu Sabău, Sabin Gligore Turdean, and et al. 2022. "A Race against the Clock: A Case Report and Literature Review Concerning the Importance of ADAMTS13 Testing in Diagnosis and Management of Thrombotic Thrombocytopenic Purpura during Pregnancy" Diagnostics 12, no. 7: 1559. https://doi.org/10.3390/diagnostics12071559








