Pharmacogenomics of Methotrexate Pathway in Rheumatoid Arthritis Patients: Approach toward Personalized Medicine
Abstract
1. Background
2. Subjects and Methods
2.1. Study Design and Population
2.2. Clinical Assessment
2.3. Molecular Analysis
2.3.1. DNA Extraction
2.3.2. SNP Selection and Genotyping via Real-Time PCR
2.4. Statistical Analysis
2.5. Ethical Considerations
3. Results
3.1. The Study Population Baseline Characteristics
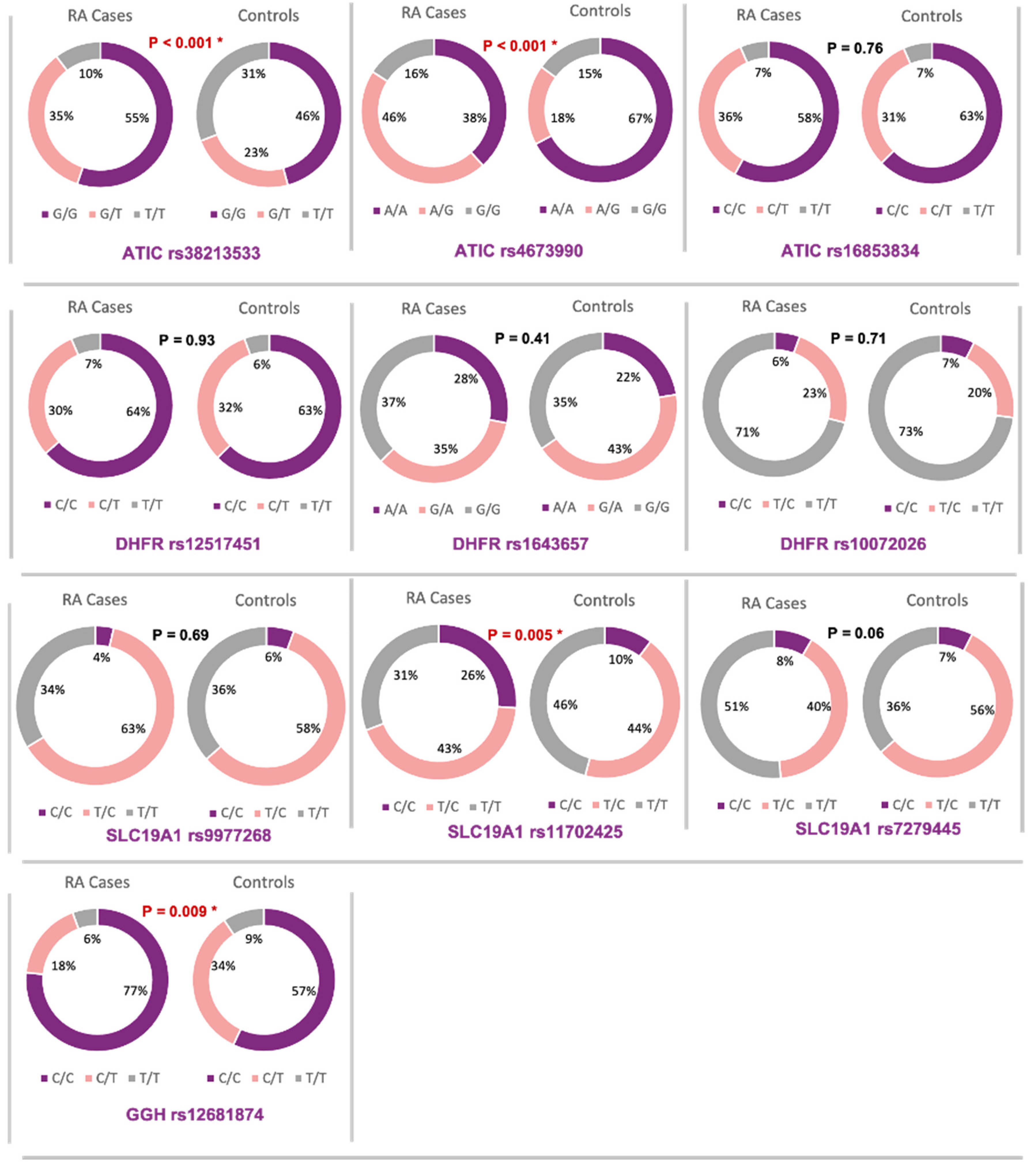
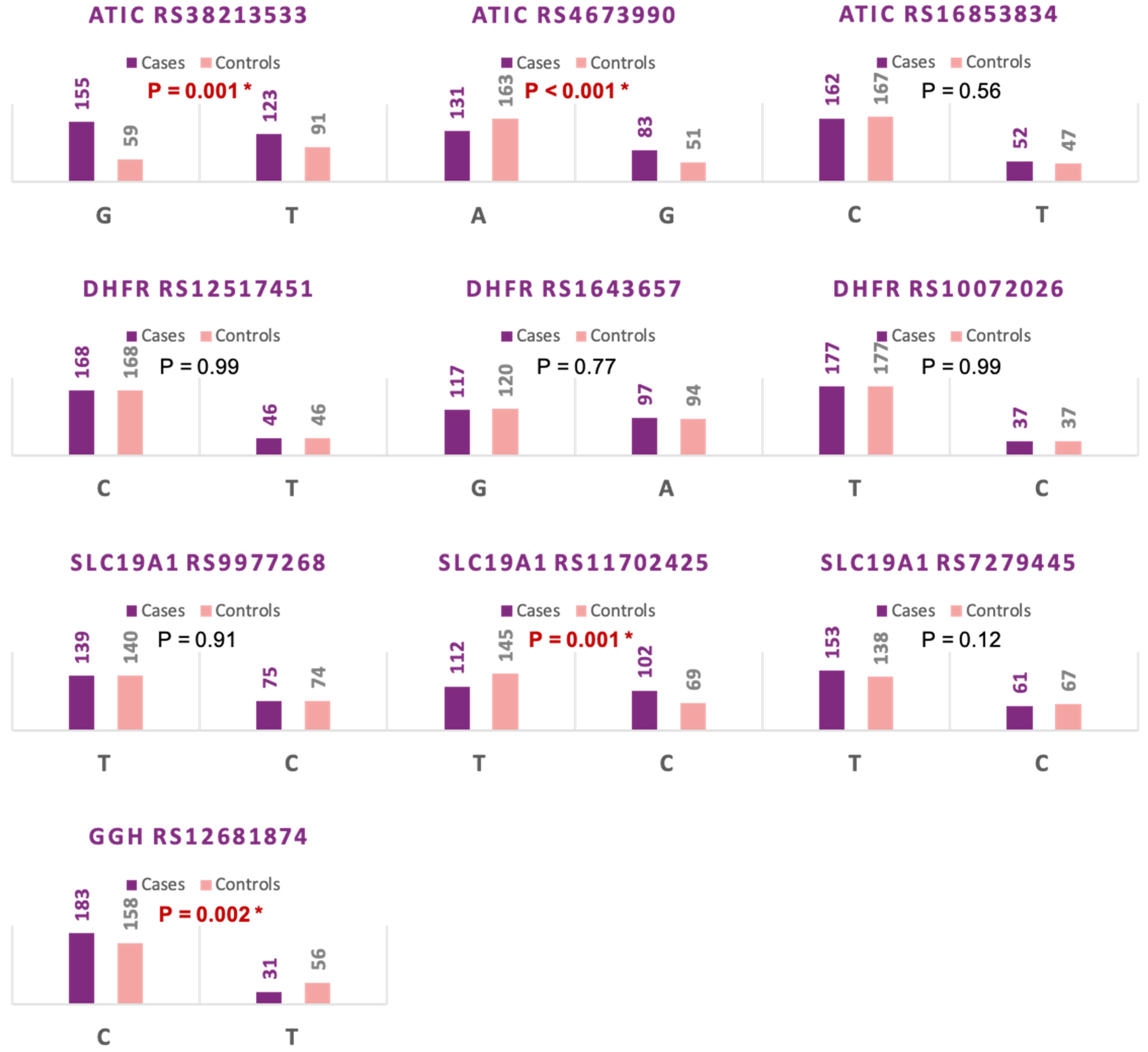
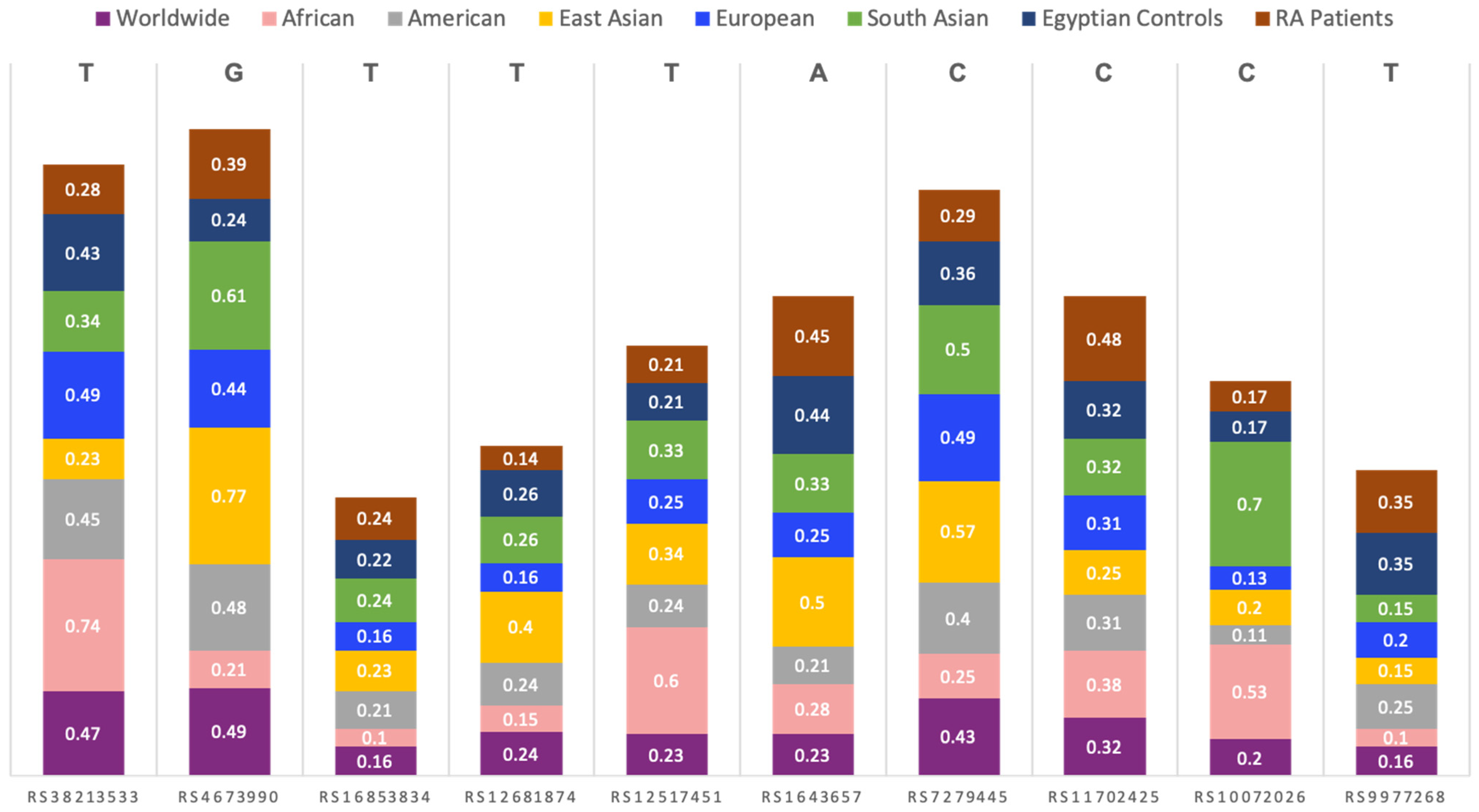
3.2. Allelic Discrimination Analysis
3.3. Association of Methotrexate Pathway Genes Variants with Disease Risk
3.3.1. ATIC Gene rs3821353
3.3.2. ATIC Gene rs4673990
3.3.3. SLC19A1 Gene rs11702425
3.3.4. SLC19A1 Gene rs7279445
3.3.5. GGH Gene rs12681874
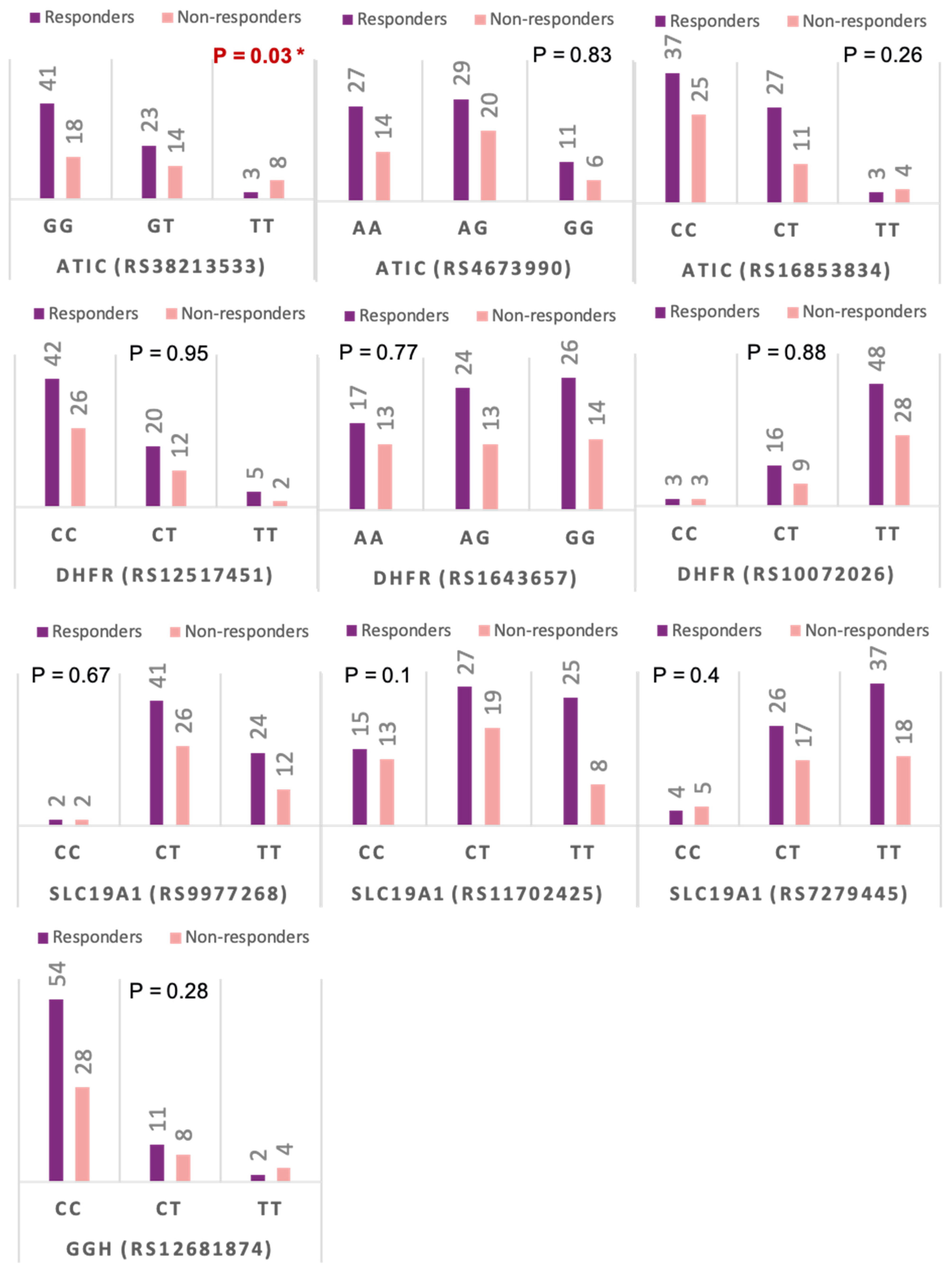
3.4. Association of MTX Pathway Gene Variants and the Treatment Response to MTX
3.4.1. ATIC Gene rs3821353 and SLC19A1 Gene rs7279445
3.4.2. DHFR Gene rs10072026
3.5. Haplotypes of Methotrexate Pathway Gene Variants and the Clinical Response to MTX
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gervasini, G.; Benitez, J.; Carrillo, J.A. Pharmacogenetic testing and therapeutic drug monitoring are complementary tools for optimal individualization of drug therapy. Eur. J. Clin. Pharmacol. 2010, 66, 755–774. [Google Scholar] [CrossRef]
- Haga, S.B.; Burke, W. Using pharmacogenetics to improve drug safety and efficacy. JAMA 2004, 291, 2869–2871. [Google Scholar] [CrossRef]
- Klareskog, L.; van der Heijde, D.; de Jager, J.P.; Gough, A.; Kalden, J.; Malaise, M.; Mola, E.M.; Pavelka, K.; Sany, J.; Settas, L.; et al. Therapeutic effect of the combination of etanercept and methotrexate compared with each treatment alone in patients with rheumatoid arthritis: Double-blind randomised controlled trial. Lancet 2004, 363, 675–681. [Google Scholar] [CrossRef]
- Lima, A.; Monteiro, J.; Bernardes, M.; Sousa, H.; Azevedo, R.; Seabra, V.; Medeiros, R. Prediction of Methotrexate Clinical Response in Portuguese Rheumatoid Arthritis Patients: Implication ofMTHFRrs1801133 andATICrs4673993 Polymorphisms. BioMed Res. Int. 2014, 2014, 368681. [Google Scholar] [CrossRef]
- Guo, Q.; Wang, Y.; Xu, D.; Nossent, J.; Pavlos, N.; Xu, J. Rheumatoid arthritis: Pathological mechanisms and modern pharmacologic therapies. Bone Res. 2018, 6, 15. [Google Scholar] [CrossRef]
- England, B.R.; Mikuls, T.R. Epidemiology of, Risk Factors for, and Possible Causes of Rheumatoid Arthritis. UpToDate. 2021. Available online: https://www.uptodate.com/contents/epidemiology-of-risk-factors-for-and-possible-causes-of-rheumatoid-arthritis (accessed on 7 September 2021).
- Almoallim, H.; Al Saleh, J.; Badsha, H.; Ahmed, H.M.; Habjoka, S.; Menassa, J.A.; El-Garf, A. A Review of the Prevalence and Unmet Needs in the Management of Rheumatoid Arthritis in Africa and the Middle East. Rheumatol. Ther. 2021, 8, 1–16. [Google Scholar] [CrossRef]
- Adelowo, O.; Mody, G.M.; Tikly, M.; Oyoo, O.; Slimani, S. Rheumatic diseases in Africa. Nat. Rev. Rheumatol. 2021, 17, 363–374. [Google Scholar] [CrossRef]
- Dowman, B.; Campbell, R.M.; Zgaga, L.; Adeloye, D.; Chan, K.Y. Estimating the burden of rheumatoid arthritis in Africa: A systematic analysis. J. Glob. Health 2012, 2, 020406. [Google Scholar] [CrossRef]
- Porter, C.K.; Riddle, M.S.; Laird, R.M.; Loza, M.; Cole, S.; Gariepy, C.; Alcala, A.; Gutierréz, R.; Baribaud, F.; Rao, N.L.; et al. Cohort profile of a US military population for evaluating pre-disease and disease serological biomarkers in rheumatoid and reactive arthritis: Rationale, organization, design, and baseline characteristics. Contemp. Clin. Trials Commun. 2020, 17, 100522. [Google Scholar] [CrossRef]
- Bullock, J.; Rizvi, S.A.A.; Saleh, A.M.; Ahmed, S.S.; Do, D.P.; Ansari, R.A.; Ahmed, J. Rheumatoid Arthritis: A Brief Overview of the Treatment. Med. Princ. Pract. 2018, 27, 501–507. [Google Scholar] [CrossRef]
- Bedoui, Y.; Guillot, X.; Sélambarom, J.; Guiraud, P.; Giry, C.; Jaffar-Bandjee, M.C.; Ralandison, S.; Gasque, P. Methotrexate an Old Drug with New Tricks. Int. J. Mol. Sci. 2019, 20, 5023. [Google Scholar] [CrossRef]
- Cronstein, B.N.; Aune, T.M. Methotrexate and its mechanisms of action in inflammatory arthritis. Nat. Rev. Rheumatol. 2020, 16, 145–154. [Google Scholar] [CrossRef]
- Owen, S.A.; Lunt, M.; Bowes, J.; Hider, S.L.; Bruce, I.N.; Thomson, W.; Barton, A. MTHFR gene polymorphisms and outcome of methotrexate treatment in patients with rheumatoid arthritis: Analysis of key polymorphisms and meta-analysis of C677T and A1298C polymorphisms. Pharmacogenom. J. 2013, 13, 137–147. [Google Scholar] [CrossRef]
- McWilliams, D.F.; Kiely, P.D.W.; Young, A.; Joharatnam, N.; Wilson, D.; Walsh, D.A. Interpretation of DAS28 and its components in the assessment of inflammatory and non-inflammatory aspects of rheumatoid arthritis. BMC Rheumatol. 2018, 2, 8. [Google Scholar] [CrossRef]
- Drozdzik, M.; Rudas, T.; Pawlik, A.; Kurzawski, M.; Czerny, B.; Gornik, W.; Herczynska, M. The effect of 3435C > T MDR1 gene polymorphism on rheumatoid arthritis treatment with disease-modifying antirheumatic drugs. Eur. J. Clin. Pharmacol. 2006, 62, 933–937. [Google Scholar] [CrossRef]
- Muralidharan, N.; Antony, P.T.; Jain, V.K.; Mariaselvam, C.M.; Negi, V.S. Multidrug resistance 1 (MDR1) 3435C > T gene polymorphism influences the clinical phenotype and methotrexate-induced adverse events in South Indian Tamil rheumatoid arthritis. Eur. J. Clin. Pharmacol. 2015, 71, 959–965. [Google Scholar] [CrossRef]
- Kato, T.; Hamada, A.; Mori, S.; Saito, H. Genetic Polymorphisms in Metabolic and Cellular Transport Pathway of Methotrexate Impact Clinical Outcome of Methotrexate Monotherapy in Japanese Patients with Rheumatoid Arthritis. Drug Metab. Pharmacokinet. 2012, 27, 192–199. [Google Scholar] [CrossRef]
- Samara, S.A.; Irshaid, Y.M.; Mustafa, K.N. Association of MDR1 C3435T and RFC1 G80A polymorphisms with methotrexate toxicity and response in Jordanian rheumatoid arthritis patients. Int. J. Clin. Pharmacol. Ther. 2014, 52, 746–755. [Google Scholar] [CrossRef]
- Drozdzik, M.; Rudas, T.; Pawlik, A.; Gornik, W.; Kurzawski, M.; Herczynska, M. Reduced folate carrier-1 80G>A polymorphism affects methotrexate treatment outcome in rheumatoid arthritis. Pharmacogenom. J. 2007, 7, 404–407. [Google Scholar] [CrossRef]
- Owen, S.A.; Hider, S.L.; Martin, P.; Bruce, I.N.; Barton, A.; Thomson, W. Genetic polymorphisms in key methotrexate pathway genes are associated with response to treatment in rheumatoid arthritis patients. Pharmacogenom. J. 2013, 13, 227–234. [Google Scholar] [CrossRef]
- López-Rodríguez, R.; Ferreiro-Iglesias, A.; Lima, A.; Bernardes, M.; Pawlik, A.; Paradowska-Gorycka, A.; Świerkot, J.; Slezak, R.; Dolžan, V.; González-Álvaro, I.; et al. Replication study of polymorphisms associated with response to methotrexate in patients with rheumatoid arthritis. Sci. Rep. 2018, 8, 7342. [Google Scholar] [CrossRef]
- Aletaha, D.; Neogi, T.; Silman, A.J.; Funovits, J.; Felson, D.T.; Bingham, C.O., III; Birnbaum, N.S.; Burmester, G.R.; Bykerk, V.P.; Cohen, M.D.; et al. 2010 Rheumatoid arthritis classification criteria: An American College of Rheumatology/European League Against Rheumatism collaborative initiative. Ann. Rheum. Dis. 2010, 69, 1580–1588. [Google Scholar] [CrossRef]
- Prevoo, M.L.L.; van’t Hof, M.A.; Kuper, H.H.; Van Leeuwen, M.A.; Van De Putte, L.B.A.; Van Riel, P.L.C.M. Modified disease activity scores that include twenty-eight-joint counts development and validation in a prospective longitudinal study of patients with rheumatoid arthritis. Arthritis Rheum. 1995, 38, 44–48. [Google Scholar] [CrossRef]
- Whirl-Carrillo, M.; Huddart, R.; Gong, L.; Sangkuhl, K.; Thorn, C.F.; Whaley, R.; Klein, T.E. An evidence-based framework for evaluating pharmacogenomics knowledge for personalized medicine. Clin. Pharmacol. Ther. 2021, 110, 563–572. [Google Scholar] [CrossRef]
- Wadelius, M.; Chen, L.Y.; Downes, K.; Ghori, J.; Hunt, S.; Eriksson, N.; Wallerman, O.; Melhus, H.; Bentley, D.; Deloukas, P.; et al. Common VKORC1 and GGCX polymorphisms associated with warfarin dose. Pharmacogenom. J. 2005, 5, 262–270. [Google Scholar] [CrossRef]
- Eichelbaum, M.; Ingelman-Sundberg, M.; Evans, W.E. Pharmacogenomics and individualized drug therapy. Annu. Rev. Med. 2006, 57, 119–137. [Google Scholar] [CrossRef]
- Cooper, G.M.; Johnson, J.A.; Langaee, T.Y.; Feng, H.; Stanaway, I.B.; Schwarz, U.I.; Ritchie, M.D.; Stein, C.M.; Roden, D.M.; Smith, J.D.; et al. A genome-wide scan for common genetic variants with a large influence on warfarin maintenance dose. Blood 2008, 112, 1022–1027. [Google Scholar] [CrossRef]
- Takeuchi, F.; McGinnis, R.; Bourgeois, S.; Barnes, C.; Eriksson, N.; Soranzo, N.; Whittaker, P.; Ranganath, V.; Kumanduri, V.; McLaren, W.; et al. A Genome-Wide Association Study Confirms VKORC1, CYP2C9, and CYP4F2 as Principal Genetic Determinants of Warfarin Dose. PLoS Genet. 2009, 5, e1000433. [Google Scholar] [CrossRef]
- Restrepo, L.F.; Giraldo, R.; Londoño, J.; Pinzón, C.; Cortes, A.; Ballesteros, G.; Santos, A.M. Farmacogenética del metotrexato en artritis reumatoide: Revisión sistemática. Rev. Colomb. Reumatol. 2016, 23, 102–114. [Google Scholar] [CrossRef]
- Askari, B.S.; Krajinovic, M. Dihydrofolate Reductase Gene Variations in Susceptibility to Disease and Treatment Outcomes. Curr. Genom. 2010, 11, 578–583. [Google Scholar] [CrossRef]
- Grabar, P.B.; Leandro-García, L.J.; Inglada-Pérez, L.; Logar, D.; Rodríguez-Antona, C.; Dolžan, V. Genetic variation in the SLC19A1 gene and methotrexate toxicity in rheumatoid arthritis patients. Pharmacogenomics 2012, 13, 1583–1594. [Google Scholar] [CrossRef] [PubMed]
- Takano, M.; Yumoto, R.; Murakami, T. Expression and function of efflux drug transporters in the intestine. Pharmacol. Ther. 2006, 109, 137–161. [Google Scholar] [CrossRef] [PubMed]
- Grabar, P.B.; Logar, D.; Lestan, B.; Dolžan, V. Genetic determinants of methotrexate toxicity in rheumatoid arthritis patients: A study of polymorphisms affecting methotrexate transport and folate metabolism. Eur. J. Clin. Pharmacol. 2008, 64, 1057–1068. [Google Scholar] [CrossRef] [PubMed]
- Dervieux, T.; Furst, D.; Lein, D.O.; Capps, R.; Smith, K.; Walsh, M.; Kremer, J. Polyglutamation of methotrexate with common polymorphisms in reduced folate carrier, aminoimidazole carboxamide ribonucleotide transformylase, and thymidylate synthase are associated with methotrexate effects in rheumatoid arthritis. Arthritis. Rheum. 2004, 50, 2766–2774. [Google Scholar] [CrossRef]
- Weisman, M.H.; Furst, D.E.; Park, G.S.; Kremer, J.M.; Smith, K.M.; Wallace, D.J.; Caldwell, J.R.; Dervieux, T. Risk genotypes in folate-dependent enzymes and their association with methotrexate-related side effects in rheumatoid arthritis. Arthritis. Rheum. 2006, 54, 607–612. [Google Scholar] [CrossRef]
- Dervieux, T.; Furst, D.; Lein, D.O.; Capps, R.; Smith, K.; Caldwell, J.; Kremer, J. Pharmacogenetic and metabolite measurements are associated with clinical status in patients with rheumatoid arthritis treated with methotrexate: Results of a multicentred cross sectional observational study. Ann. Rheum. Dis. 2005, 64, 1180–1185. [Google Scholar] [CrossRef]
- Sharma, S.; Das, M.; Kumar, A.; Marwaha, V.; Shankar, S.; Aneja, R.; Grover, R.; Arya, V.; Dhir, V.; Gupta, R.; et al. Interaction of genes from influx-metabolism-efflux pathway and their influence on methotrexate efficacy in rheumatoid arthritis patients among Indians. Pharmacogenet. Genom. 2008, 18, 1041–1049. [Google Scholar] [CrossRef]
- Kooloos, W.; Huizinga, T.; Guchelaar, H.-J.; Wessels, J. Pharmacogenetics in Treatment of Rheumatoid Arthritis. Curr. Pharm. Des. 2010, 16, 164–175. [Google Scholar] [CrossRef]
- Zhu, H.; Deng, F.-Y.; Mo, X.-B.; Qiu, Y.-H.; Lei, S.-F. Pharmacogenetics and pharmacogenomics for rheumatoid arthritis responsiveness to methotrexate treatment: The 2013 update. Pharmacogenomics 2014, 15, 551–566. [Google Scholar] [CrossRef]
- Inoue, K.; Yuasa, H. Molecular Basis for Pharmacokinetics and Pharmacodynamics of Methotrexate in Rheumatoid Arthritis Therapy. Drug Metab. Pharmacokinet. 2014, 29, 12–19. [Google Scholar] [CrossRef]
- Szekanecz, Z.; Meskó, B.; Poliska, S.; Váncsa, A.; Szamosi, S.; Végh, E.; Simkovics, E.; Laki, J.; Kurkó, J.; Besenyei, T.; et al. Pharmacogenetics and pharmacogenomics in rheumatology. Immunol. Res. 2013, 56, 325–333. [Google Scholar] [CrossRef]
- Zhang, L.L.; Yang, S.; Wei, W.; Zhang, X.J. Genetic polymorphisms affect efficacy and adverse drug reactions of DMARDs in rheumatoid arthritis. Pharmacogenet. Genom. 2014, 24, 531–538. [Google Scholar] [CrossRef]
- James, H.M.; Gillis, D.; Hissaria, P.; Lester, S.; Somogyi, A.A.; Cleland, L.G.; Proudman, S.M. Common polymorphisms in the folate pathway predict efficacy of combination regimens containing methotrexate and sulfasalazine in early rheumatoid arthritis. J. Rheumatol. 2008, 35, 562–571. [Google Scholar]
- Sharma, S.; Das, M.; Kumar, A.; Marwaha, V.; Shankar, S.; Singh, P.; Raghu, P.; Aneja, R.; Grover, R.; Arya, V.; et al. Purine biosynthetic pathway genes and methotrexate response in rheumatoid arthritis patients among north Indians. Pharmacogenet. Genom. 2009, 19, 823–828. [Google Scholar] [CrossRef]
- Wessels, J.A.M.; De Vries-Bouwstra, J.K.; Heijmans, B.T.; Slagboom, P.E.; Goekoop-Ruiterman, Y.P.M.; Allaart, C.F.; Kerstens, P.J.S.M.; Van Zeben, D.; Breedveld, F.C.; Dijkmans, B.A.C.; et al. Efficacy and toxicity of methotrexate in early rheumatoid arthritis are associated with single-nucleotide polymorphisms in genes coding for folate pathway enzymes. Arthritis Rheum. 2006, 54, 1087–1095. [Google Scholar] [CrossRef]
- Hayashi, H.; Fujimaki, C.; Daimon, T.; Tsuboi, S.; Matsuyama, T.; Itoh, K.; Msc, H.H.; Bs, C.F.; Bs, T.M. Genetic polymorphisms in folate pathway enzymes as a possible marker for predicting the outcome of methotrexate therapy in Japanese patients with rheumatoid arthritis. J. Clin. Pharm. Ther. 2009, 34, 355–361. [Google Scholar] [CrossRef]
- Takatori, R.; Takahashi, K.A.; Tokunaga, D.; Hojo, T.; Fujioka, M.; Asano, T.; Hirata, T.; Kawahito, T.; Satomi, Y.; Nishino, H.; et al. ABCB1 C3435T polymorphism influences metho-trexate sensitivity in rheumatoid arthritis patients. Clin. Exp. Rheumatol. 2006, 24, 546–554. [Google Scholar]
- Fukino, K.; Kawashima, T.; Suzuki, M.; Ueno, K. Methylenetetrahydrofolate reductase and reduced folate carrier-1 genotypes and methotrexate serum concentrations in patients with rheumatoid arthritis. J. Toxicol. Sci. 2007, 32, 449–452. [Google Scholar] [CrossRef][Green Version]
- Chatzikyriakidou, A.; Georgiou, I.; Voulgari, P.V.; Papadopoulos, C.G.; Tzavaras, T.; Drosos, A.A. Transcription regulatory polymorphism −43T > C in the 5′-flanking region of SLC19A1 gene could affect rheumatoid arthritis patient response to methotrexate therapy. Rheumatol. Int. 2007, 27, 1057–1061. [Google Scholar] [CrossRef]
| Gene | SNP | Genomic Location | Alleles | RA Response and Toxicity (Association Information) |
|---|---|---|---|---|
| ATIC | rs4673990 | Introns | A > G | Allele A is linked to lesser response with MTX |
| rs16853834 | Introns | C > T | Allele T is linked with better MTX therapy response than allele G | |
| rs3821353 | Introns | G > T | Failure to respond to MTX is associated with allele T | |
| SLC19A1 | rs11702425 | Synonymous | T > A T > C T > G | Allele C is associated with lesser MTX treatment response |
| rs9977268 | Introns | C > T | Allele T is associated with lesser MTX treatment response | |
| rs7279445 | Introns | C > T | MTX resistance has been linked to the presence of allele T | |
| GGH | rs12681874 | Introns | C > T | RA patients with allele C show lesser MTX treatment response |
| DHFR | rs12517451 | 5′ Flanking region | C > A | RA patients are more likely to experience side effects from MTX if they have allele T |
| C > G | ||||
| C > T | ||||
| rs10072026 | Introns | T > C | RA patients are less likely to experience MTX side effects if they have allele C | |
| rs1643657 | Introns | T > C | RA patients with allele C are less likely to experience MTX side effects |
| Gene | SNP rs# | SNP ID | Context Sequence [VIC/FAM] |
|---|---|---|---|
| ATIC | rs4673990 | C_28017839_30 | TAGATTCCATGGTACCATGTTGAGA[A/G]TTGTGCCTAGCTACTGAGAGTCTTT |
| rs16853834 | C_33295734_10 | ATATTACACTTCTTTCCTAGTGTCC[C/T]GAGCCTCAGAATACAAGATGGAGCT | |
| rs3821353 | C_362253_20 | TATCAAAGTGATATCAAGCAGAACA[G/T]AGAGAAAGAGTGAGTCAGTAATGAA | |
| SLC19A1 | rs11702425 | C_2176981_10 | AGGGACCTCCCGGCCTGCCGGGACT[C/T]AAGGTCAGTGACGGATATGTCTGGG |
| rs9977268 | C_25766978_10 | AAGCATGTCCCACCCTCCTCTCGGG[C/T]AGTGCCACCCCAGGGAGGGGTCCTT | |
| rs7279445 | C_31153754_20 | AAGCATGTCCCACCCTCCTCTCGGG[C/T]AGTGCCACCCCAGGGAGGGGTCCTT | |
| GGH | rs12681874 | C_11852315_10 | TTAAGAGTAATGGTGAATATTTTTT[C/T]CCAATTTACCTGAAAAAAAAAATCA |
| DHFR | rs12517451 | C_32167960_10 | GCTTTATTCCCCTTTATCCCTGTGA[C/T]GGCGGGGGCCTGTAATAATATCTTG |
| rs10072026 | C_3103314_10 | AATAGCTCCTTTTATACAATTTCAT[C/T]TTATCATATACTGATCTCCACTATG | |
| rs1643657 | C_3103233_10 | TAGTCACATATTTCACTGCTGAATT[C/T]CTTTCCTATAATTATTTTAAACCAA |
| Variable | Responders (n = 67) mean ± SD | Non-Responders (n = 40) mean ± SD | p-Value |
|---|---|---|---|
| RA duration (Yrs.) | 4.75 ± 4.12 | 8.01 ± 6.56 | <0.01 ** |
| MTX duration (Yrs.) | 3.23 ± 2.33 | 4.34 ± 4.26 | 0.11 |
| Current smoking—no (%) Past smoking—no (%) Non-smoking—no (%) | 6 (9.0) 10 (14.9) 51 (76.1) | 2 (5.0) 10 (25.0) 28 (70.0) | 0.37 † |
| Tender-joint count | 4.65 ± 3.33 | 14.67 ± 8.48 | <0.01 ** |
| Swollen-joint count | 0.35 ± 0.81 | 4.51 ± 4.35 | <0.01 ** |
| VAS for pain (cm) | 5.67 ± 1.89 | 8.71 ± 1.36 | <0.01 ** |
| DAS-28 | 2.50 ± 0.53 | 5.59 ± 1.17 | <0.01 ** |
| Hemoglobin level (g/dL) | 11.94 ± 1.5 | 11.44 ± 1.3 | 0.11 |
| Platelet count (×10/mm3) | 278.23 ± 62.5 | 288.23 ± 80.5 | 0.53 |
| Total leucocytic count (×10/mm3) | 8.21 ± 2.33 | 7.77 ± 2.35 | 0.39 |
| ALT (IU/L) | 19.51 ± 17.1 | 19.03 ± 8.7 | 0.87 |
| AST (IU/L) | 20.02 ± 9.7 | 19.28 ± 7.1 | 0.69 |
| Serum creatinine (mg/dL) | 0.78 ± 0.4 | 0.67 ± 0.1 | 0.10 |
| ESR (mm/hr) | 45.19 ± 24.4 | 59.19 ± 24.2 | <0.01 ** |
| CRP (mg/dL) | 9.64 ± 6.50 | 27.21 ± 19.10 | <0.01 ** |
| Rheumatoid factor (median, (IQR)) | 20 (11–32) | 20 (20–67) | 0.96 |
| Anti-CCP (median, (IQR)) | 30 (21.25–64.13) | 30 (25–40) | 0.78 |
| SNP | Model | Genotype | RA Patients | Controls | Odds Ratio (95% CI) | p-Value |
|---|---|---|---|---|---|---|
| ATIC Gene rs3821353 | Codominant | G/G | 59 | 49 | Reference | |
| G/T | 37 | 25 | 1.23 (0.65–2.32) | 0.52 | ||
| T/T | 11 | 33 | 0.28 (0.13–0.60) | 0.001 * | ||
| Dominant | G/G | 59 | 49 | Reference | ||
| G/T-T/T | 48 | 58 | 0.69 (0.40–1.18) | 0.17 | ||
| Recessive | G/G-G/T | 96 | 74 | Reference | ||
| T/T | 11 | 33 | 0.26 (0.12–0.54) | 0.000 * | ||
| Over-dominant | G/G-T/T | 70 | 82 | Reference | ||
| G/T | 37 | 25 | 1.7 (0.95–3.16) | 0.07 | ||
| Allelic Model | T | 59 | 91 | Reference | ||
| G | 155 | 123 | 1.94 (1.3–2.9) | 0.001 * | ||
| ATIC Gene rs4673990 | Codominant | A/A | 41 | 72 | Reference | |
| A/G | 49 | 19 | 4.5 (2.35–8.7) | 0.000 * | ||
| G/G | 17 | 16 | 1.86 (0.85–4.08) | 0.12 | ||
| Dominant | A/A | 59 | 49 | Reference | ||
| A/G-G/G | 66 | 35 | 1.56 (0.9–2.7) | 0.11 | ||
| Recessive | A/A-A/G | 90 | 91 | Reference | ||
| G/G | 11 | 33 | 0.34 (0.16–0.7) | 0.004 * | ||
| Over-dominant | A/A-G/G | 58 | 88 | Reference | ||
| A/G | 37 | 25 | 2.24 (1.22–4.11) | 0.008 * | ||
| Allelic Model | A | 131 | 163 | Reference | ||
| G | 83 | 51 | 2 (1.33–3.07) | 0.000 * | ||
| ATIC Gene rs16853834 | Codominant | C/C | 62 | 67 | Reference | |
| C/T | 38 | 33 | 1.24 (0.69–2.22) | 0.46 | ||
| T/T | 7 | 7 | 1.08 (0.36–3.25) | 0.89 | ||
| Dominant | C/C | 62 | 67 | Reference | ||
| C/T-T/T | 45 | 40 | 1.2 (0.7–2.1) | 0.48 | ||
| Recessive | C/C-C/T | 100 | 100 | Reference | ||
| T/T | 7 | 7 | 1.00 (0.3–2.9) | 1.00 | ||
| Over-dominant | C/C-T/T | 69 | 74 | Reference | ||
| C/T | 38 | 33 | 1.23 (0.7–2.1) | 0.47 | ||
| Allelic Model | C | 162 | 167 | Reference | ||
| T | 52 | 47 | 1.14 (0.73–1.79) | 0.56 | ||
| DHFR Gene rs12517451 | Codominant | C/C | 68 | 67 | Reference | |
| C/T | 32 | 34 | 0.92 (0.5–1.67) | 0.8 | ||
| T/T | 7 | 6 | 1.15 (0.37–3.6) | 0.81 | ||
| Dominant | C/C | 68 | 67 | Reference | ||
| C/T-T/T | 39 | 40 | 0.96 (0.55–1.67) | 0.88 | ||
| Recessive | C/C-C/T | 100 | 101 | Reference | ||
| T/T | 7 | 6 | 1.18 (0.38–3.63) | 0.77 | ||
| Over-dominant | C/C-T/T | 75 | 73 | Reference | ||
| C/T | 32 | 34 | 0.9 (0.51–1.63) | 0.76 | ||
| Allelic Model | C | 168 | 168 | Reference | ||
| T | 46 | 46 | 1.00 (0.63–1.6) | 1.0 | ||
| DHFR Gene rs1643657 | Codominant | A/A | 30 | 24 | Reference | |
| A/G | 37 | 46 | 0.64 (0.32–1.28) | 0.21 | ||
| G/G | 40 | 37 | 0.86 (0.43–1.73) | 0.68 | ||
| Dominant | A/A | 30 | 24 | Reference | ||
| A/G-G/G | 77 | 83 | 0.74 (0.4–1.38) | 0.34 | ||
| Recessive | A/A-A/G | 67 | 70 | Reference | ||
| G/G | 40 | 37 | 1.12 (0.64–1.97) | 0.67 | ||
| Over-dominant | A/A-G/G | 70 | 61 | Reference | ||
| A/G | 37 | 46 | 0.92 (0.53–1.6) | 0.77 | ||
| Allelic Model | A | 97 | 94 | Reference | ||
| G | 117 | 120 | 0.94 (0.64–1.38) | 0.77 | ||
| DHFR Gene rs10072026 | Codominant | C/C | 6 | 8 | Reference | |
| C/T | 25 | 21 | 1.58 (0.47–5.3) | 0.45 | ||
| T/T | 76 | 78 | 1.3 (0.43–3.9) | 0.64 | ||
| Dominant | C/C | 6 | 8 | Reference | ||
| C/T-T/T | 101 | 99 | 1.36 (0.45–4.06) | 0.58 | ||
| Recessive | C/C-C/T | 31 | 29 | Reference | ||
| T/T | 76 | 78 | 0.91 (0.50–1.65) | 0.76 | ||
| Over-dominant | C/C-T/T | 82 | 86 | Reference | ||
| C/T | 25 | 21 | 1.25 (0.65–2.4) | 0.5 | ||
| Allelic Model | C | 37 | 37 | Reference | ||
| T | 177 | 177 | 1.00 (0.6–1.6) | 1.00 | ||
| SLC19A1 Gene rs9977268 | Codominant | C/C | 4 | 6 | Reference | |
| C/T | 67 | 62 | 1.62 (0.43–6.01) | 0.47 | ||
| T/T | 36 | 39 | 1.38 (0.36–5.3) | 0.63 | ||
| Dominant | C/C | 4 | 6 | Reference | ||
| C/T-T/T | 103 | 101 | 1.52 (0.42–5.58) | 0.52 | ||
| Recessive | C/C-C/T | 71 | 68 | Reference | ||
| T/T | 36 | 39 | 0.88 (0.5–1.5) | 0.66 | ||
| Over-dominant | C/C-T/T | 40 | 45 | Reference | ||
| C/T | 67 | 62 | 1.2 (0.7– 2.1) | 0.48 | ||
| Allelic Model | C | 75 | 74 | Reference | ||
| T | 139 | 140 | 0.98 (0.68–1.46) | 0.91 | ||
| SLC19A1 Gene rs11702425 | Codominant | C/C | 28 | 11 | Reference | |
| C/T | 46 | 47 | 0.38 (0.17–0.86) | 0.02 * | ||
| T/T | 33 | 49 | 0.26 (0.11–0.6) | 0.001 * | ||
| Dominant | C/C | 28 | 11 | Reference | ||
| C/T-T/T | 79 | 96 | 0.32 (0.15–0.69) | 0.003 * | ||
| Recessive | C/C-C/T | 74 | 58 | Reference | ||
| T/T | 33 | 49 | 0.52 (0.3– 0.92) | 0.02 * | ||
| Over-dominant | C/C-T/T | 61 | 60 | Reference | ||
| C/T | 46 | 47 | 0.96 (0.56–1.65) | 0.89 | ||
| Allelic Model | C | 102 | 69 | Reference | ||
| T | 112 | 145 | 0.52 (0.35–0.77) | 0.001 * | ||
| SLC19A1 Gene rs7279445 | Codominant | C/C | 9 | 8 | Reference | |
| C/T | 43 | 60 | 0.63 (0.22–1.78) | 0.39 | ||
| T/T | 55 | 39 | 1.25 (0.44–3.53) | 0.67 | ||
| Dominant | C/C | 9 | 8 | Reference | ||
| C/T-T/T | 98 | 99 | 0.88 (0.32–2.37) | 0.8 | ||
| Recessive | C/C-C/T | 52 | 68 | Reference | ||
| T/T | 55 | 39 | 1.84 (1.06–3.18) | 0.02 * | ||
| Over-dominant | C/C-T/T | 64 | 47 | Reference | ||
| C/T | 43 | 60 | 0.82 (0.49–1.38) | 0.47 | ||
| Allelic Model | C | 61 | 76 | Reference | ||
| T | 153 | 138 | 1.38 (0.91–2.07) | 0.12 | ||
| GGH Gene rs12681874 | Codominant | C/C | 82 | 61 | Reference | |
| C/T | 19 | 36 | 0.4 (0.2–0.75) | 0.004 * | ||
| T/T | 6 | 10 | 0.45 (0.15–1.29) | 0.14 | ||
| Dominant | C/C | 82 | 61 | Reference | ||
| C/T-T/T | 25 | 46 | 0.4 (0.22–0.73) | 0.002 * | ||
| Recessive | C/C-C/T | 101 | 97 | Reference | ||
| T/T | 6 | 10 | 0.57 (0.20–1.64) | 0.3 | ||
| Over-dominant | C/C-T/T | 88 | 71 | Reference | ||
| C/T | 19 | 36 | 0.42 (0.22–0.8) | 0.008 * | ||
| Allelic Model | C | 183 | 158 | Reference | ||
| T | 31 | 56 | 0.48 (0.3–0.78) | 0.003 * |
| SNP | Model | Genotype | MTX Respondent | MTX Non-Respondent | Odds Ratio (95% CI) | p-Value |
|---|---|---|---|---|---|---|
| ATIC Gene rs3821353 | Codominant | G/G | 14 | 45 | Reference | - |
| G/T | 7 | 30 | 0.33 (0.12–0.9) | 0.034 * | ||
| T/T | 2 | 9 | 1.4 (0.27–7.2) | 0.68 | ||
| Dominant | G/G | 14 | 45 | Reference | - | |
| G/T-T/T | 9 | 39 | 1.35 (0.53–3.45) | 0.62 | ||
| Recessive | G/G-G/T | 21 | 75 | Reference | - | |
| T/T | 2 | 9 | 1.26 (0.25–6.28) | 0.78 | ||
| Over-dominant | G/G-T/T | 16 | 54 | Reference | - | |
| G/T | 7 | 30 | 1.2 (0.47–3.4) | 0.64 | ||
| Allelic Model | T | 48 | 11 | Reference | - | |
| G | 120 | 35 | 1.27 (0.6–2.7) | 0.53 | ||
| ATIC Gene rs4673990 | Codominant | A/A | 10 | 31 | Reference | - |
| A/G | 9 | 40 | 1.43 (0.52–3.96) | 0.49 | ||
| G/G | 4 | 13 | 1.05 (0.28–3.96) | 0.94 | ||
| Dominant | A/A | 10 | 31 | Reference | - | |
| A/G-G/G | 13 | 53 | 1.3 (0.5–3.35) | 0.56 | ||
| Recessive | A/A-A/G | 19 | 71 | Reference | - | |
| G/G | 4 | 13 | 0.9 (0.25–2.97) | 0.82 | ||
| Over-dominant | A/A-G/G | 14 | 44 | Reference | - | |
| A/G | 9 | 40 | 1.4 (0.55–3.62) | 0.47 | ||
| Allelic Model | A | 29 | 102 | Reference | - | |
| G | 17 | 66 | 1.1 (0.56–2.16) | 0.77 | ||
| ATIC Gene rs16853834 | Codominant | C/C | 12 | 50 | Reference | - |
| C/T | 9 | 29 | 0.77 (0.29–2.05) | 0.6 | ||
| T/T | 2 | 5 | 0.6 (0.1–3.48) | 0.57 | ||
| Dominant | C/C | 12 | 50 | Reference | - | |
| C/T-T/T | 11 | 34 | 0.74 (0.29–1.87) | 0.53 | ||
| Recessive | C/C-C/T | 21 | 79 | Reference | - | |
| T/T | 2 | 5 | 0.66 (0.12–3.67) | 0.64 | ||
| Over-dominant | C/C-T/T | 14 | 55 | Reference | - | |
| C/T | 9 | 29 | 0.82 (0.32–2.12) | 0.68 | ||
| Allelic Model | C | 33 | 129 | Reference | - | |
| T | 13 | 39 | 0.77 (0.37–1.6) | 0.48 | ||
| DHFR Gene rs12517451 | Codominant | C/C | 14 | 54 | Reference | - |
| C/T | 7 | 25 | 0.9 (0.3–2.6) | 0.88 | ||
| T/T | 2 | 5 | 0.6 (0.1–3.7) | 0.62 | ||
| Dominant | C/C | 14 | 54 | Reference | - | |
| C/T-T/T | 9 | 30 | 0.86 (0.3–2.23) | 0.76 | ||
| Recessive | C/C-C/T | 21 | 79 | Reference | - | |
| T/T | 2 | 5 | 0.66 (0.12–3.67) | 0.63 | ||
| Over-dominant | C/C-T/T | 16 | 59 | Reference | - | |
| C/T | 7 | 25 | 0.97 (0.35–2.64) | 0.95 | ||
| Allelic Model | C | 35 | 133 | Reference | - | |
| T | 11 | 35 | 0.83 (0.39–1.81) | 0.65 | ||
| DHFR Gene rs1643657 | Codominant | A/A | 5 | 25 | Reference | - |
| A/G | 10 | 27 | 0.54 (0.16–1.8) | 0.3 | ||
| G/G | 8 | 32 | 0.8 (0.23–2.74) | 0.72 | ||
| Dominant | A/A | 5 | 25 | Reference | - | |
| A/G-G/G | 18 | 59 | 0.65 (0.2–1.96) | 0.45 | ||
| Recessive | A/A-A/G | 15 | 52 | Reference | - | |
| G/G | 8 | 32 | 1.15 (0.44–3.02) | 0.77 | ||
| Over-dominant | A/A-G/G | 13 | 57 | Reference | - | |
| A/G | 10 | 27 | 0.61(0.24–1.58) | 0.31 | ||
| Allelic Model | A | 20 | 77 | Reference | - | |
| G | 26 | 91 | 0.9 (0.47–1.75) | 0.78 | ||
| DHFR Gene rs10072026 | Codominant | T/T | 18 | 58 | Reference | - |
| C/T | 5 | 20 | 1.24 (0.41–3.78) | 0.7 | ||
| C/C | 0 | 6 | 4.1 (0.22–76.55) | 0.34 | ||
| Dominant | T/T | 18 | 58 | Reference | - | |
| C/T-C/C | 26 | 5 | 0.05 (0.02–0.17) | 0.0001 * | ||
| Recessive | T/T-C/T | 78 | 23 | Reference | - | |
| C/C | 6 | 0 | 0.25 (0.01–4.7) | 0.36 | ||
| Over-dominant | C/C-T/T | 18 | 64 | Reference | - | |
| C/T | 5 | 20 | 1.1 (0.4–3.4) | 0.84 | ||
| Allelic Model | C | 5 | 32 | Reference | - | |
| T | 41 | 136 | 0.5 (0.19–1.4) | 0.2 | ||
| SLC19A1 Gene rs9977268 | Codominant | T/T | 7 | 29 | Reference | - |
| C/T | 15 | 52 | 0.84 (0.3–2.3) | 0.72 | ||
| C/C | 1 | 3 | 0.72 (0.06–8.05) | 0.8 | ||
| Dominant | T/T | 7 | 29 | Reference | - | |
| C/T-C/C | 16 | 55 | 1.23 (0.12–12.4) | 0.86 | ||
| Recessive | T/T-C/T | 22 | 81 | Reference | - | |
| C/C | 1 | 3 | 0.82 (0.30–2.25) | 0.71 | ||
| Over-dominant | C/C-T/T | 8 | 32 | Reference | - | |
| C/T | 15 | 52 | 0.87 (0.33–2.27) | 0.77 | ||
| Allelic Model | C | 17 | 58 | Reference | - | |
| T | 29 | 110 | 1.11 (0.56–2.19) | 0.76 | ||
| SLC19A1 Gene rs11702425 | Codominant | C/C | 5 | 23 | Reference | - |
| C/T | 9 | 37 | 0.89 (2.66–3) | 0.86 | ||
| T/T | 9 | 24 | 0.58 (0.17–2) | 0.39 | ||
| Dominant | C/C | 5 | 23 | Reference | - | |
| C/T-T/T | 18 | 61 | 0.74 (0.25–2.22) | 0.59 | ||
| Recessive | C/C-C/T | 14 | 60 | Reference | - | |
| T/T | 9 | 24 | 0.62 (0.24–1.63) | 0.33 | ||
| Over-dominant | C/C-T/T | 14 | 47 | Reference | - | |
| C/T | 9 | 37 | 1.22 (0.48–3.14) | 0.67 | ||
| Allelic Model | C | 19 | 83 | Reference | - | |
| T | 27 | 85 | 0.72 (0.37–1.4) | 0.33 | ||
| SLC19A1 Gene rs7279445 | Codominant | T/T | 12 | 43 | Reference | - |
| C/T | 10 | 33 | 0.92 (0.35–2.4) | 0.87 | ||
| C/C | 1 | 8 | 2.23 (0.25–19.65) | 0.47 | ||
| Dominant | T/T | 12 | 8 | Reference | - | |
| C/T-C/C | 11 | 43 | 5.86 (1.92–17.84) | 0.0018 * | ||
| Recessive | T/T-C/T | 11 | 41 | Reference | - | |
| C/C | 12 | 43 | 0.96 (0.38–2.42) | 0.93 | ||
| Over-dominant | C/C-T/T | 13 | 51 | Reference | - | |
| C/T | 10 | 33 | 0.84 (0.33–2.14) | 0.72 | ||
| Allelic Model | C | 12 | 49 | Reference | - | |
| T | 34 | 119 | 0.86 (0.41–1.79) | 0.68 | ||
| GGH Gene rs12681874 | Codominant | C/C | 16 | 66 | Reference | - |
| C/T | 6 | 13 | 0.52 (0.17–1.6) | 0.26 | ||
| T/T | 1 | 5 | 1.2 (0.13–11.1) | 0.86 | ||
| Dominant | C/C | 66 | 16 | Reference | - | |
| C/T-T/T | 18 | 7 | 1.6 (0.57–4.5) | 0.37 | ||
| Recessive | C/C-C/T | 79 | 22 | Reference | - | |
| T/T | 5 | 1 | 0.7 (0.08–6.5) | 0.77 | ||
| Over-dominant | C/C-T/T | 71 | 17 | Reference | - | |
| C/T | 13 | 6 | 2.09 (0.7–6.3) | 0.19 | ||
| Allelic Model | C | 38 | 145 | Reference | - | |
| T | 8 | 23 | 0.75 (0.31–1.81) | 0.53 |
| Haplotype | Frequency | OR (95% CI) | p-Value | ||
|---|---|---|---|---|---|
| DHFR Gene Variants | |||||
| (rs10072026) | (rs12517451) | (rs1643657) | |||
| T | C | A | 0.327 | Reference | - |
| T | C | G | 0.3052 | 0.83 (0.36–1.92) | 0.66 |
| C | C | G | 0.1183 | 0.87 (0.31–2.49) | 0.8 |
| T | T | G | 0.1037 | 1.76 (0.64–4.85) | 0.27 |
| T | T | A | 0.0912 | 0.75 (0.18–3.20) | 0.7 |
| ATIC Gene Variants | |||||
| (rs3821353) | (rs4673990) | (rs16853834) | |||
| G | A | C | 0.3275 | Reference | - |
| G | G | C | 0.2387 | 0.89 (0.32–2.46) | 0.82 |
| G | A | T | 0.1505 | 1.09 (0.35–3.37) | 0.88 |
| T | G | C | 0.1044 | 1.09 (0.31–3.79) | 0.89 |
| T | A | C | 0.0865 | 0.58 (0.12–2.89) | 0.51 |
| T | A | T | 0.0477 | 3.11 (0.46–20.81) | 0.24 |
| SLC19A1 Gene Variants | |||||
| (rs7279445) | (rs11702425) | (rs9977268) | |||
| T | C | T | 0.2962 | Reference | - |
| T | T | T | 0.1851 | 1.53 (0.51–4.57) | 0.45 |
| T | T | C | 0.1475 | 2.15 (0.57–8.10) | 0.26 |
| C | T | T | 0.1333 | 2.28 (0.64–8.10) | 0.21 |
| T | C | C | 0.0862 | 1.68 (0.30–9.51) | 0.56 |
| C | C | C | 0.0593 | 2.80 (0.41–18.92) | 0.29 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abdallah, H.Y.; Ibrahim, M.E.; Abd El-Fadeal, N.M.; Ali, D.A.; Elsehrawy, G.G.; Badr, R.E.; Hassoba, H.M. Pharmacogenomics of Methotrexate Pathway in Rheumatoid Arthritis Patients: Approach toward Personalized Medicine. Diagnostics 2022, 12, 1560. https://doi.org/10.3390/diagnostics12071560
Abdallah HY, Ibrahim ME, Abd El-Fadeal NM, Ali DA, Elsehrawy GG, Badr RE, Hassoba HM. Pharmacogenomics of Methotrexate Pathway in Rheumatoid Arthritis Patients: Approach toward Personalized Medicine. Diagnostics. 2022; 12(7):1560. https://doi.org/10.3390/diagnostics12071560
Chicago/Turabian StyleAbdallah, Hoda Y., Maha E. Ibrahim, Noha M. Abd El-Fadeal, Dina A. Ali, Gehad G. Elsehrawy, Rasha E. Badr, and Howayda M. Hassoba. 2022. "Pharmacogenomics of Methotrexate Pathway in Rheumatoid Arthritis Patients: Approach toward Personalized Medicine" Diagnostics 12, no. 7: 1560. https://doi.org/10.3390/diagnostics12071560
APA StyleAbdallah, H. Y., Ibrahim, M. E., Abd El-Fadeal, N. M., Ali, D. A., Elsehrawy, G. G., Badr, R. E., & Hassoba, H. M. (2022). Pharmacogenomics of Methotrexate Pathway in Rheumatoid Arthritis Patients: Approach toward Personalized Medicine. Diagnostics, 12(7), 1560. https://doi.org/10.3390/diagnostics12071560







