Abstract
Background: Antenatal depression (AND) and post-partum depression (PPD) are long-term debilitating psychiatric disorders that significantly influence the composition of the gut flora of mothers and infants that starts from the intrauterine life. Not only does bacterial ratio shift impact the immune system, but it also increases the risk of potentially life-threatening disorders. Material and Methods: Therefore, we conducted a narrative mini-review aiming to gather all evidence published between 2018–2022 regarding microflora changes in all three stages of pregnancy. Results: We initially identified 47 potentially eligible studies, from which only 7 strictly report translocations; 3 were conducted on rodent models and 4 on human patients. The remaining studies were divided based on their topic, precisely focused on how probiotics, breastfeeding, diet, antidepressants, exogenous stressors, and plant-derived compounds modulate in a bidirectional way upon behavior and microbiota. Almost imperatively, dysbacteriosis cause cognitive impairments, reflected by abnormal temperament and personality traits that last up until 2 years old. Thankfully, a distinct technique that involves fecal matter transfer between individuals has been perfected over the years and was successfully translated into clinical practice. It proved to be a reliable approach in diminishing functional non- and gastrointestinal deficiencies, but a clear link between depressive women’s gastrointestinal/vaginal microbiota and clinical outcomes following reproductive procedures is yet to be established. Another gut-dysbiosis-driving factor is antibiotics, known for their potential to trigger inflammation. Fortunately, the studies conducted on mice that lack microbiota offer, without a shadow of a doubt, insight. Conclusions: It can be concluded that the microbiota is a powerful organ, and its optimum functionality is crucial, likely being the missing puzzle piece in the etiopathogenesis of psychiatric disorders.
1. Introduction
AND, also known as prenatal or perinatal depression alongside postnatal or PPD, reunited under perinatal mood and anxiety disorders (PMADs), is an umbrella that comprises psychiatric episodes, with an overall prevalence of 17% in PPD [1] and up to 20% in AND [2]. However, the interval between the undergoing pregnancy and the first month follow-up could represent the transition to a major depressive disorder (MDD), classified as peripartum depression according to the DSM 5 [3,4].
The associated phenotype in advanced stages exert antagonistic effects on the patient’s psychological and physiological profile. Precisely, suicidal ideation is a significant co-founder of mortality among women [5]. Left untreated, it negatively impacts the cognitive and socio-emotional development of the newborn [6,7]. Unfortunately, no curative strategy involving prevention, diagnosis, and treatment proved reliable [8].
Beside these aspects stands a shorter breastfeeding duration [9], but prior to this is the intrauterine environment and subsequent risk of compromising the infant’s immune system [10]. Thus, researchers emphasize the possible role of the women’s microbiota as a biological factor in the pregnancy progress [11].
There are numerous shaping factors responsible for the loss of eubiosis. Among the plethora of them are delivery methods, antibiotics, antidepressants, and child nutrition, which are definitive [12]. Breastfeeding promotes shifts in bacterial ratio and ensures the proliferation of the so-called “beneficial” microorganisms, even in small amounts [11].
Considering the multifaceted and branched involvement of the microscopic entities harbored by almost every constitutive site of the human body, the present narrative mini-review aims to offer an update on the current discoveries surrounding microflora and behavioral changes in women suffering from AND PPD.
2. Methodology
This narrative mini-review respects the standard protocol described by Green et al. [13].
2.1. Database Search Strategy
The literature databases explored for information until inception (May 2022) were PubMed/Medline, ISI Web of Knowledge, ScienceDirect, and Scopus. Several keywords, including “microbiota/microflora” in combination with “antenatal depression”, “perinatal depression“, “prenatal depression”, “postnatal depression”, and “postpartum depression”, were employed during database tracking.
2.2. Inclusion Criteria
We included only articles that reported experience on human patients or experimental models in mice, rats, and zebrafish (Danio rerio).
2.3. Exclusion Criteria
Case report(s)/series, meta-analyses, review(s), standard or systematic, articles written in another language than English, letters to the editor, conference posters, work protocols, preprints, and computational simulations were not considered suitable.
2.4. Study Selection
Four independent authors (O.-D.I., R.D., D.H., I.S.) screened the titles and abstracts of the retrieved entries. We completed the assignment of all literature based on title, abstract, and full content. Any discrepancy was solved by consent with the remaining two authors (B.D., C.I.).
3. Results
A total of 1558 articles were returned in the interval 2018–2022. Per database searched, we identified 56 in PubMed/Medline, 59 in Scopus, 83 in ISI Web of Knowledge, and 1360 in ScienceDirect. Divided in chronological order, n = 17, n = 17, n = 29, and n = 332 were conducted concerning PPD, while n = 39, n = 42, n = 54, and n = 1028 for AND. A total of n = 47 articles met the eligibility criteria.
Table 1 contains a stratification that strictly analyzes the changes occurred in microflora composition. From n = 10 studies that sought to demonstrate a link between different stressors and subsequent development along all three stages of pregnancy, only n = 7 brought evidence into microflora translocations.

Table 1.
Stratification of studies in which are strictly reported changes that occurred in the gut flora.
In order to offer a comprehensive overview, we found it suitable to mark each Section from this mini-review with “*”, “**”, or “***”, indicating either experiments conducted strictly on humans, from a combined perspective (humans + experimental models), or strictly on experimental models. To maintain continuity with a logical argument, we summarize studies marked with “**” in a table that contains the key observations made by the authors (Table 2).
3.1. Microbiota Shaping Factors in Non- and Pregnant Females **
Prenatal stress inhibits the growth and development of neurons [14,15,21]. Thus, behind cytokine elevation and neurotransmitters depletion might be the involvement of the tryptophan pathway [16,22,23]. There is an interconnection between bacterial shifts [17,18,19,20] and how microglia respond to environmental stressors in a time- and sex-dependent manner [24,25]. CD4T initiates transcriptional maturation in microglia, and in its absence, it triggers a defective synaptic behavior [26]. Kang et al. [27,28] discussed in two reports an irrespective link between breastfeeding and secretory immunoglobulin A (sIgA) in infants born of mothers with antenatal depression. The concentrations of anti-inflammatory cytokines IL-1β and IL-10 are higher in Caesarean (C)-section-born individuals by comparison with those born vaginally [29], while maternal precarity and maternal immune activation (MIA) has low diversity and high relative abundance of Enterobacteriaceae and Streptococcaceae next to the low relative abundance of Bifidobacterium and Lachnospiraceae [30,31].
3.1.1. Probiotics **
Probiotics are the most efficient vectors used to re-establish disruption of the gut–brain axis (GBA), mitigating lipopolysaccharide (LPS) programming in a sex-dependent manner the hypothalamic–pituitary–adrenal (HPA) axis [32]. Early exposure causes a decrease in Lactobacillus genera, with L. reuteri replenishment countering bacterial endotoxins action [33]. Even though the first signs of improvements arose approximately half a day post-administration, the effect in females is limited in contrast to their counterparts [34]. Lactobacillus paracasei DTA 83 [35], Lactobacillus murinus HU-1 [36], Lactobacillus rhamnosus [37], and Lactobacillus casei [38] reverse atypical neurodevelopmental abnormalities in offspring and carriers. Maternal Bimuno® galacto-oligosaccharides (B-GOS) increase short-chain fatty acids (SCFAs) levels [39], but there were situations where probiotics or other supplements such as n-3 long-chain polyunsaturated fatty acids (n-3 LC-PUFAs) and ecologic barrier had modest effects [40,41]. The symbiotic microorganisms within Tibetan kefir grains (TKGs) may offer robust gastrointestinal (GI) immunity due to enriched Lactobacillus kefiranofaciens and Lactobacillus helveticus [42]. Another reliable approach to reconstructing GI flora represents the synergic effect with psychobiotics ADR-159 [43] or with pre- (α-lactalbumin) and postbiotics (sodium butyrate) [44]. There are two registered clinical trials (NCT04741971, NCT04472065) underway. They are estimated to be completed in 2024. Hopefully, they will offer insight into diminishing psychiatric symptoms. Unfortunately, the estimated number of people enrolled is quite small (n = 76, n = 220), currently being limited to a single center (where the case is being studied).
3.1.2. Breastfeeding **
The human milk incorporates a multitude of bioactive structures, especially Lactobacillus. Interestingly, psychosocial distress does not influence the bacterial ratio in the first trimester post-delivery but rather the milk content [45], with recent evidence suggesting changes in the milk metabolome [46]. Early limited formula feeding followed by breastfeeding reduces the risk of infant mortality and the likelihood of readmission [47]. For example, milk fat globule membrane (MFGM) modestly secures the persistence of homeostasis in rats, particularly visceral hypersensitivity [48].
3.1.3. Diet ***
The ketogenic diet (KD) influences brain volume in a non-selective manner [49]. A high-fiber diet could overcome obesity-induced cognitive and metabolic changes following a hypercaloric diet [50,51]. An optimum intake upregulates neurotransmitters expression and prevents neuroinflammation. Operational taxonomic units (OTUs) that belong to Bacteroidales_S24-7 and Lactobacillus enhance neuronal plasticity and SCFAs levels [52].

Table 2.
Comprehensive overview of the studies performed in the Section 3.1 based on the studied model.
Table 2.
Comprehensive overview of the studies performed in the Section 3.1 based on the studied model.
| Key Observations | Reference |
|---|---|
| Section 3.1. | |
| Rats | |
| [16] |
| [21] |
| Mice | |
| [14] |
| [15] |
| [24] |
| [25] |
| [26] |
| [31] |
| Humans | |
| [17] |
| [18] |
| [19] |
| [20] |
| [22] |
| [23] |
| [27] |
| [28] |
| [29] |
| [30] |
| Section 3.1.1. | |
| Rats | |
| [38] |
| Mice | |
| [32] |
| [33] |
| [34] |
| [35] |
| [36] |
| [37] |
| [39] |
| [43] |
| [44] |
| Humans | |
| [40] |
| [41] |
| Section 3.1.2. | |
| Rats | |
| [48] |
| Humans | |
| [45] |
| [46] |
| [47] |
| Section 3.1.4. | |
| Humans | |
| [53] |
| Rats | |
| [54] |
| Mice | |
| [55] |
5-HT, serotonin; Kyn, kynurenine; Trp, tryptophan; IDO, indoleamine 2,3-dioxygenase; TPH, tryptophan hydroxylase; MR, mineralocorticoid receptor; BDNF, brain-derived neurotrophic factor; IGF1R, insulin-like growth factor 1 receptor; GR, glucocorticoid receptor; NEUN, neuronal nuclei; GFAP, glial fibrillary acidic protein; CRHR1, corticotropin-releasing hormone receptor 1; GABAB2, gamma-aminobutyric acid B receptor 2; CORT, corticosterone; OXT, oxytocin; V1AR, vasopressin receptor 1a; IPV, intimate partner violence; FDR, false-discovery rate; SCL, Symptom Checklist-90; PRAQ-R2, Pregnancy-Related Anxiety Questionnaire—Revised 2; HCC, hair cortisol concentration; UDCA, ursodeoxycholic acid; C-section, Cesarean section; NR1, N-methyl-D-aspartic acid receptor 1; ERK1/2, extracellular signal-regulated kinase 1/2; PVN, paraventricular nucleus of the hypothalamus; PCF, prefrontal cortex; TLR-4, toll-like receptor 4; GLU, glutamate; FST, forced swimming test; PA, passive avoidance; NOR, novel object recognition; FO, fish oil; MS, maternal separation; MFGM, milk fat globule membrane; ELF, early limited formula; OPCML, opioid binding protein/cell adhesion molecule like.
3.1.4. Antidepressants **
Prenatal serotonin/norepinephrine reuptake inhibitors (SSRIs) are antidepressants whose usage amplifies the risk of GI disorders in young individuals [53]. Pretreatment based on antibiotics in combination with fluoxetine alters the microbial profile. The joint effect was already ascertained in relative abundance of elemental beneficial entities and transcriptional responses [54,55].
3.1.5. Exogenous Stressors ***
The widespread use of pesticides, organic compounds, and other pollutants has increased dramatically. It was shown that chlorpyrifos (CPF) [56], bisphenol A (BPA) [57], fluorescent nanosized polystyrene particles [58], polybrominated diphenyl ethers (PBDEs) [59], and triclosan (TCS) [60] are potent toxicant agents. The consequences are long-lasting even in small doses. Regardless of sex, these compounds have a neurotoxic profile dependent on the exposure period.
3.1.6. Conventional and Alternative Methods ***
Neurotransmitters modulate the neuroendocrine network, as reflected by the peculiarities promoted following the food intake [61], whereas a lack of vitamins and minerals intensifies the GI permeability [62]. Cumulatively, this might further offer data on the underlying mechanism of gut dysbiosis [63]. Considering the constantly growing need to find a suitable adjuvant, some authors tested the potential of naturally derived compounds obtained from plants, even traditional Chinese medicine [64,65,66,67,68].
3.2. Microflora and Behavioral Changing Factors *
Even though the microbiota is directly involved in the host’s eubiosis, it is also susceptible to a plethora of stressors. As anticipated, they have a pronounced influence on both newborn and mother’s homeostasis. This argument is strengthened by the studies conducted on infants (n = 2028) [69,70,71,72,73,74,75,76,77] and mothers (n = 255) [78,79]. Astsinki et al. [71] revealed using a clustering strategy that Bacteroidetes are associated with lower self-regulation capacity in contrast to a Bifidobacterium/Enterobacteriaceae cluster. They aimed at examining GI flora at 2.5 months of age and how it influences temperament at 6 months considering that Bifidobacterium and Streptococcus can shape positive emotionality. Loughman et al. [69] applied a different strategy by assessing the microbial associations on three occasions at 1, 6, and 12 months. Prevotella was significantly lower at 12 months compared with the other analyzed groups at 1 and 6 months. It is worth mentioning that this genus is practical as a predictor of antibiotic exposure. Similarly, Carlson et al. [73] analyzed GI microflora in full-term healthy individuals at 1 year and correlated them with Mullen Scale of Early Learning (MSEL) scores and brain anatomy (MRI) at 1 and 2 years of age. Of all the clusters, individuals populated by Bacteroidetes had more promising outcomes in terms of receptivity and expressive language than individuals reuniting Faecalibacterium and Ruminococcaceae groups. These performances were attributed to breastfeeding and vaginal delivery. There was a negative correlation between the α-diversity at 1 year and MSEL scores at 2 years, which was further probed in the study of Aatsinki but concentrated on opposing emotionality and fear reactivity. Xie et al. [70] confirmed the results of Carlson, revealing that Faecalibacterium is negatively related to emotional traits such as high-intensity pleasure and surgency but Bifidobacterium with perceptual sensitivity also at the age of 2. However, there was no association between gender and age linked to temperament and GI flora as reported through the Infant Behavior Questionnaire—Revised (IBQ-R) and Early Childhood Behavior Questionnaire (ECBQ). Kelsey et al. [74] found that abundance of Bacteroides and Lachnospiraceae are accountable for the connectivity of the neural network of the brain. The presence of Slackia isoflavonivonvertens could contribute to a longer gestational age, as recently demonstrated by Gough et al. [78], and Rikenellaceae, and Dialister abundance was linked with cortisol response to stress and negatively with Bacteroides [79]. Babies from families that have a low socioeconomic status (SES) displayed a higher α-diversity and, more precisely, a greater abundance of Bifidobacterium and Megasphaera. As highlighted above, an exogenous intake of probiotics reduces the risk of hospitalization due to Staphylococcus aureus [75]. Analyzing separate lines of research, maternal smoking has proven to be dangerous and interacts with cognitive functioning as measured with the Wechsler Preschool and Primary Scale of Intelligence (WPPSI-III). Presumably, this has to do with the relative abundance of Enterobacter asburiae [76]. The meconium microbiota is significantly influenced by anxiety, especially by a low level of Enterococcaceae [72], subsequently revealing that Akkermansia, Lactococcus, and Oscillospira ratio are positively associated with higher sociality scores [77].
3.3. Fecal Microbiota Transplantation as a Preventive Measure *,**
Per normative disseminated by the World Health Organization (WHO), fecal microbiota transplantation (FMT) and microbial transfer therapy (MTT) are techniques dedicated to reconstructing the gut flora, implying that the transfer of the fecal samples from a healthy donor are instilled in a patient. FMT is usually applied to treat recurrent Clostridium difficile (rCDI) infections, while MTT focuses on metabolic deficiencies [80,81]. Presently, the overall efficiency of FMT in the setting is >90%, but it is dependent on the working protocols. The success rate is higher if multiple infusions with fecal quantities (>50 g) are scheduled. On the other hand, it strongly fluctuates based on the route of delivery, particularly after colonoscopy [82]. As another parameter that may be a contributor to rCDI treatment, it has been also successfully applied in a wide of other GI manifestations: irritable bowel syndrome (IBS), inflammatory bowel disease (IBD), metabolic and hepatic illnesses, graft and host disorders, disturbance of the GBA, and even multi-drug-resistant colonization [83,84].
Xu et al. [85] observed that constantly increasing concentrations of alcohol drove anxious and depressive behaviors in rodents. To consolidate the utility of this protocol, the authors subjected the models to prolonged FMT by oral gavage from healthy individuals. Sustaining the inflammasome hypothesis of depression, Zhang et al. [86] reported that the NLR family pyrin domain containing 3 (NLRP3) knock-out mice exhibits a significantly greater locomotor activity and increased Lachnospiraceae, Ruminococcaceae, and Prevotellaceae abundance to the detriment of Bacteroides. A three-day FMT reverse stress-induced dysbiosis alleviated both anxiety- and depression-like symptoms through the attenuation of the astrocytes-driven inflammation. Schmidt et al. [87] conducted an incomplete unilateral cervical spinal cord injury (SCI) in injured rats. Congruent with previous observations, FMT from healthy rats rescues anxious behavior and SCI-driven dysbacteriosis. Faecalibacterium rodentium exacerbates depressive-like symptoms in mice subjected to FTM [88] and in human patients as well [89]. Moreover, GI microbiota transfer from depressed humans induces a specific depressive-like phenotype in rats pretreated with antibiotics [90]. Tangent results were seen in germ-free mice (GFM) [91], which comprises the topic of a small sub-section later in this manuscript.
The role of FMT in restoring eubiosis in human patients has also been a subject of great interest. Mazzawi et al. [92] reported an improvement for 28 weeks in 13 IBS patients after single FMT through gastro-duodenoscopy in a small placebo-controlled study. Using Eysenck Personality Questionnaire-Neuroticism and Hospital Anxiety and Depression (HAD) tests, they observed only an improvement in HAD scores at three weeks post-intervention. Kurokawa et al. [93] conducted a small observational study seeking to examine 17 adult patients who underwent FMT via colonoscopy. They followed the psychiatric symptoms trend using Hamilton Rating Scale (HAM) for Anxiety (HAM-A) and Depression (HAM–D). Twelve patients had HAM-D scores at baseline ≥ 8 but in a subset of six patients normalized after one month. Five patients had HAM-A scores ≥14, but the scores of three patients decreased to normal. A Japanese open-label study of 10 IBS patients conducted by Mizuno et al. [94] revealed that donor material rich in Bifidobacterium stimulates the growth and expansion of strains. A good responder is one that achieves type 3 or 4 in the Bristol Stool Form Scale. Unfortunately, the amelioration of depressive symptoms lasted until the 12th week in comparison with the non-significant difference but slight progress in anxiety in week 4. In a similar manner, Lahtinen et al. [95] applied the Beck Depression Inventory (BDI), Beck Anxiety Inventory (BAI), IBS Quality of Life (IBS-QOL) questionnaires, and 15D in 49 patients enrolled for allogenic FMT via colonoscopy. Interestingly, the scores registered by patients were not statistically significant in total depression or anxiety. Likewise, Huang et al. [96] carried out a study by performing FMT from healthy donors in a group of 30 IBS refractory patients. They used IBS-QOL, HAM-D, and HAM-A to assess the evolution of non-gastrointestinal aspects at 1, 3, and 6 months after FMT. Noteworthy, FMT indeed contributed significantly to the IBS-QOL at 1 and 3 months but not after half a year. HAM-A and HAM-D scores indicate a significant amelioration only at 1 and 3 months. Among those 83 patients that participated in the study of Johnsen et al. [97] that aimed to evaluate the impact of FMT via the lower route in fatigue and quality of life of patients in IBS non-C, most of them experienced minimal improvements in IBS-QOL. The researchers used Fatigue Impact Scale (FIS) at 3, 6, and 12 months and IBS-QOL at 6 and 12 months, respectively. The sub-domains with the higher responsivity were interference with activity, body image, and relationships. Amelioration in the FIS score from baseline was seen at half a year in the FMT recipients post-intervention. However, the lack of presence of other functional disorders was used as predictor of profound prolonged response to 12 months. Another small case series brought to light the beneficial effect of FMT on depressed patients diagnosed with IBS via colonoscopy. It is still questionable whether decreased symptomatology was due to FMT or improvement of IBS [98].
3.4. Microflora Involvement in Recurrent Implantation Failure
In contrast women who naturally achieve a pregnancy, those found in the pursuit of assisted reproductive technology (ART) are at higher risk of depression [99]. The fertility QOL of recurrent implantation failure (RIF) patients is dependent on numerous parameters [100], but the patterns are not related to the outcome of assisted pregnancy [101]. Patients experience symptoms of depression at one week post-intervention in the case of negative in vitro fertilization (IVF) [102]. Depression rates are high in women undergoing infertility treatment and even higher in those with IVF cycle failure [103]. As already documented, the vaginal tract is populated by the Lactobacillus genus, particularly with four strains, namely Lactobacillus crispatus, Lactobacillus gasseri, Lactobacillus iners, and Lactobacillus jensenii, which may counter the antagonistic activity of opportunistic bacteria. These microorganisms fulfill key roles and possess a eubiotic and immunological effect. These implications are centered on countering the proliferation of pathogens through SCFAs [104,105], antimicrobial peptides [106], and infections [107,108,109,110]. A possible dysbacteriosis causes consequences linked with different RIF stages [105,111], but there still are controversies regarding microbial translocations. It is known that endometrial fluid harbors Lactobacillus spp. and represents approximately 90% of all vaginal strains [112]. Subsequently, these data were contradicted and revealed the contrary [113]: Lactobacillus depletion causes repercussions reflected by poor clinical outcomes [112,113]. Two Lactobacillus species (crispatus and iners) are presently employed to categorize women with high chances of pregnancy [104].
3.5. Antibiotics as a Vehicle of Cytokine Elevation and Gut Dysbacteriosis ***
Nyangahu et al. [114] suggested that perturbations of the maternal microbiome dictate neonatal adaptive immunity in pups. Vancomycin alters the α-diversity, leading to a pro-inflammatory cascade by triggering. Besides the elevation of IgG and IgM in breast milk, it also impacts lymphocyte numbers in pups, particularly CD4+ and follicular B cells. Ceftriaxone is another potent drug that kills most of the normal flora but, in combination with vancomycin, causes morphological villi changes, an increase in IgE, and a decrease of Ki67-/Muc2-positive cells as argued by Cheng et al. [115]. Not only did the Proteobacteria ascend as the dominant phylum, but the abundance of Bacteroidetes, Firmicutes, Actinobacteria, and Deferribacteres demonstrated a downward trend. This argument is supported by the recent data concerning ceftriaxone influences on α-diversity, as indicated twice by Cheng et al. [115,116]. Bacteroidetes almost entirely disappear from the feces following exposure to ceftriaxone as per Miao et al. [117], which is consistent with data disseminated by Cheng et al. [118]. Maternal antibiotic treatment (MAT) lowered the relative abundance of Bacteroidetes and Firmicutes in parallel with a proliferation of Proteobacteria. Consequently, it inflicted major phenotypic changes, notably significant intestinal injury and cytokine levels. More than that, MAT decreased the expression of vascular endothelial growth factor (VEGF), proliferating cell nuclear antigen positive-(PCNA), goblet cells, and tight junction proteins, as demonstrated by Chen et al. [118]. Additionally, Tormo-Badia et al. [119] noted a high balance of CD8+ T cells in the mesenteric lymph nodes (MLN) of pups from non-obese diabetic (NOD) females exposed to antibiotics during pregnancy in contrast to control pups. Continuing with this context, pups born to females that received oral antibiotics during gestation and postpartum displayed a high susceptibility risk towards vaccinia virus due to reduced interferon (IFN)-γ production by CD8+ T cells, according to Gonzalez-Perez and the co-authors [120]. The possible explanation for CD8+ T-cell disturbance might be the altered activation and expression of the T-cell receptor (TCR) that sustains cytokine production, as indicated by Gonzalez-Perez et al. [121]. Four markers identified by Benner et al. [122] seem to coordinate the robustness of the immune system after antibiotics administration: splenic T helper 17 cells and CD5+, CD4+ T cells in mesenteric lymph nodes as well as RORγT mRNA in the placenta.
3.6. Germ-Free Nodels as a Novel Approach to Study AND and PDD ***
Among the first experimentations that aimed to offer a novel standpoint on how microorganisms inhabit the GI tract was the study of Sudo et al. [123]. The authors showed an elevated level of plasma CORT, an adrenocorticotropic hormone (ACTH), followed by a regressed expression in BNDF expression levels in GFM in both the cortex and hippocampus by comparison with the specific pathogen-free (SPF). Bifidobacterium infantis and depletion of Escherichia coli may correct HPA exaggerated stress response. Pessa-Morikawa et al. [124] outlined a range of gut microbial metabolites that cross the placenta into the fetal compartment to regulate the prenatal development process. Escherichia coli had an antagonistic role in maternal behavior maturation rather than directly impacting the infant. The presence of this pathogen interferes with IGF-1 signaling. Thus, Lee et al. [125] claimed that IGF-1 impairment resulted in malnourishment and consequential stunted growth. The conclusions of Sudo et al. [123] on the mechanism behind the transfer of feces were tested effective with “infant-type” Bifidobacterium strains. Luk et al. [126] generated a few years ago a simplified model community comprising human Bifidobacterium species displaying sex-dependent colonization reversible only in female GFM. The same authors again showed that a consortium of human-derived lactic acid bacteria strains belonging to Bifidobacterium (longum subspecies infantis, bifidum, breve, and dentium) are essential for microglial cell development. Otherwise, it might cause an elevation in synapse-promoting genes, markers of reactive microglia, and synaptic density in the hippocampus of GFM. Conclusively, Bifidobacterium-treated mice exhibit optimum synaptic density and neuronal activity, according to Luck et al. [127]. Martínez et al. [128] elegantly prioritize the effects of historical contingencies after they inoculated GFM in sequence with two contrasting microbial associations, with the mapping of communities six weeks later being related to colonization history. Koren [129], Ridaura [130], and Goodrich et al. [131] advanced how GFM inoculated with human feces from women in the first trimester resulted in several metabolic changes, such as weight gain and a slight reduction of insulin sensitivity [129], which are similar to the observations of inoculation from non-pregnant obese donors [130] but blocked by transplantation of Christensenella to prevent weight gain [131].
4. Conclusions
Due to the paucity of data, we were unable to conduct a quantitative meta-analysis of the studies that met the eligibility criteria. This is why we employed the best evidence to identify the key results and disentangle the possible interconnection between depression in all three stages and the disturbance of the gastrointestinal (GI)–vaginal microflora. Despite this impediment, we can argue that we have managed to provide an up-to-date comprehensive picture of the changes that might occur in the microbiota per searches and data identified following the centralization of the current evidence. Fortunately, this field of research benefits from an increasing interest reflected by the present mini-review, but future studies from an interdisciplinary approach are compulsory. We consider this manuscript to be the launching pad of the phase for deciphering the full potential behind the role of microbiota in depression and how this neuropsychiatric disorder can be modulated or triggered depending on the study design.
It can be concluded based on all aspects presented throughout this manuscript that depression during all three stages of pregnancy significantly influences the composition of GI microflora. Even though a possible dysbiosis may be corrected through numerous therapeutic approaches exclusively designed to date, there also exist hazardous factors that disrupt maternal–fetal interaction and implicitly the intrauterine life. In this context, the cognitive development of the infant is influenced, subjected to temperament and personality traits changes later in life. Thankfully, this field is evolving fast and has brought to life an alternative that proved its reliability in diminishing both anxiety- and depressive-like symptoms. However, depression is linked with associated with implantation failure, which could subsequently disturb microflora and diminish pregnancy chances. However, additional studies from our point of view are mandatory to establish links beyond theoretical stages. We can conclude that the studies contained in this mini-review are complementary, with the authors confirming/refuting the existing data. Although a transition to human patient experiences is being attempted, those performed on animal models proved to be groundbreaking. In order to create a defining parallel, the current field of research is evolving in tandem in terms of animal and human studies. A summary diagram can be found as seen below (Figure 1).
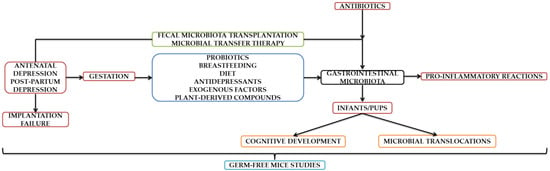
Figure 1.
Schematic representation of the aspects contained in the Section 3.1, Section 3.2, Section 3.3, Section 3.4, Section 3.5 and Section 3.6.
Author Contributions
B.D., O.-D.I., R.D., D.H. and I.S., conceptualization, data curation, investigation, formal analysis, methodology, and writing—original draft; B.D. and C.I., conceptualization, methodology, writing—review and editing, validation, and project administration. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The datasets used and analyzed during the current study are available from the corresponding author on reasonable request.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Wang, Z.; Liu, J.; Shuai, H.; Cai, Z.; Fu, X.; Liu, Y.; Xiao, X.; Zhang, W.; Krabbendam, E.; Liu, S.; et al. Mapping global prevalence of depression among postpartum women. Transl. Psychiatry 2021, 11, 543. [Google Scholar] [CrossRef] [PubMed]
- Yin, X.; Sun, N.; Jiang, N.; Xu, X.; Gan, Y.; Zhang, J.; Qiu, L.; Yang, C.; Shi, X.; Chang, J.; et al. Prevalence and associated factors of antenatal depression: Systematic reviews and meta-analyses. Clin. Psychol. Rev. 2021, 83, 101932. [Google Scholar] [CrossRef] [PubMed]
- American Psychiatric Association (Ed.) Diagnostic and Statistical Manual of Mental Disorders: DSM-5; American Psychiatric Association: Arlington, VA, USA, 2013; ISBN 089042554X. [Google Scholar]
- Gaynes, B.N.; Gavin, N.; Meltzer-Brody, S.; Lohr, K.N.; Swinson, T.; Gartlehner, G.; Brody, S.; Miller, W.C. Perinatal depression: Prevalence, screening accuracy, and screening outcomes. In AHRQ Evidence Report Summaries; Agency for Healthcare Research and Quality: Rockville, MD, USA, 2005; pp. 1–8. [Google Scholar]
- Gentile, S. Suicidal mothers. J. Inj. Violence Res. 2010, 3, 90–97. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rollè, L.; Giordano, M.; Santoniccolo, F.; Trombetta, T. Prenatal Attachment and Perinatal Depression: A Systematic Review. Int. J. Environ. Res. Public Health 2020, 17, 2644. [Google Scholar] [CrossRef] [Green Version]
- Oyetunji, A.; Chandra, P. Postpartum stress and infant outcome: A review of current literature. Psychiatry Res. 2020, 284, 112769. [Google Scholar] [CrossRef]
- Rolfes, J.J.; Paulsen, M. Protecting the infant-parent relationship: Special emphasis on perinatal mood and anxiety disorder screening and treatment in neonatal intensive care unit parents. J. Perinatol. 2021, 42, 815–818. [Google Scholar] [CrossRef]
- Dias, C.C.; Figueiredo, B. Breastfeeding and depression: A systematic review of the literature. J. Affect. Disord. 2015, 171, 142–154. [Google Scholar] [CrossRef] [Green Version]
- Lewis, A.J.; Austin, E.; Knapp, R.; Vaiano, T.; Galbally, M. Perinatal Maternal Mental Health, Fetal Programming and Child Development. Healthcare 2015, 3, 1212–1227. [Google Scholar] [CrossRef] [Green Version]
- Robertson, R.C.; Manges, A.R.; Finlay, B.B.; Prendergast, A.J. The Human Microbiome and Child Growth—First 1000 Days and Beyond. Trends Microbiol. 2019, 27, 131–147. [Google Scholar] [CrossRef] [Green Version]
- Christian, M.; Sabrina, D.; Francesca, B.; Eoghan, C.; Francesca, T.; Jennifer, M.; Clara, B.; Susana, D.P.; Silvia, A.M.; Leonardo, M.; et al. The First Microbial Colonizers of the Human Gut: Composition, Activities, and Health Implications of the Infant Gut Microbiota. Microbiol. Mol. Biol. Rev. 2022, 81, e00036-17. [Google Scholar]
- Green, B.N.; Johnson, C.D.; Adams, A. Writing narrative literature reviews for peer-reviewed journals: Secrets of the trade. J. Chiropr. Med. 2006, 5, 101–117. [Google Scholar] [CrossRef] [Green Version]
- Gur, T.L.; Palkar, A.V.; Rajasekera, T.; Allen, J.; Niraula, A.; Godbout, J.; Bailey, M.T. Prenatal stress disrupts social behavior, cortical neurobiology and commensal microbes in adult male offspring. Behav. Brain Res. 2019, 359, 886–894. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Li, N.; Chen, R.; Lee, T.; Gao, Y.; Yuan, Z.; Nie, Y.; Sun, T. Prenatal stress leads to deficits in brain development, mood related behaviors and gut microbiota in offspring. Neurobiol. Stress 2021, 15, 100333. [Google Scholar] [CrossRef] [PubMed]
- Zhao, R.; Zhou, Y.; Shi, H.; Ye, W.; Lyu, Y.; Wen, Z.; Li, R.; Xu, Y. Effect of Gestational Diabetes on Postpartum Depression-like Behavior in Rats and Its Mechanism. Nutrients 2022, 14, 1229. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Chen, C.; Yu, H.; Yang, Z. Fecal Microbiota Changes in Patients With Postpartum Depressive Disorder. Front. Cell. Infect. Microbiol. 2020, 10, 567268. [Google Scholar] [CrossRef] [PubMed]
- Naudé, P.J.W.; Claassen-Weitz, S.; Gardner-Lubbe, S.; Botha, G.; Kaba, M.; Zar, H.J.; Nicol, M.P.; Stein, D.J. Association of maternal prenatal psychological stressors and distress with maternal and early infant faecal bacterial profile. Acta Neuropsychiatr. 2020, 32, 32–42. [Google Scholar] [CrossRef]
- Aatsinki, A.-K.; Keskitalo, A.; Laitinen, V.; Munukka, E.; Uusitupa, H.-M.; Lahti, L.; Kortesluoma, S.; Mustonen, P.; Rodrigues, A.J.; Coimbra, B.; et al. Maternal prenatal psychological distress and hair cortisol levels associate with infant fecal microbiota composition at 2.5 months of age. Psychoneuroendocrinology 2020, 119, 104754. [Google Scholar] [CrossRef]
- Dawson, S.L.; O’Hely, M.; Jacka, F.N.; Ponsonby, A.-L.; Symeonides, C.; Loughman, A.; Collier, F.; Moreno-Betancur, M.; Sly, P.; Burgner, D.; et al. Maternal prenatal gut microbiota composition predicts child behaviour. eBioMedicine 2021, 68, 103400. [Google Scholar] [CrossRef]
- Lian, S.; Xu, B.; Wang, D.; Wang, L.; Li, W.; Yao, R.; Ji, H.; Wang, J.; Guo, J.; Li, S.; et al. Possible mechanisms of prenatal cold stress induced-anxiety-like behavior depression in offspring rats. Behav. Brain Res. 2019, 359, 304–311. [Google Scholar] [CrossRef]
- Nazzari, S.; Molteni, M.; Valtorta, F.; Comai, S.; Frigerio, A. Prenatal IL-6 levels and activation of the tryptophan to kynurenine pathway are associated with depressive but not anxiety symptoms across the perinatal and the post-partum period in a low-risk sample. Brain. Behav. Immun. 2020, 89, 175–183. [Google Scholar] [CrossRef]
- Kimmel, M.; Jin, W.; Xia, K.; Lun, K.; Azcarate-Peril, A.; Plantinga, A.; Wu, M.; Ataei, S.; Rackers, H.; Carroll, I.; et al. Metabolite trajectories across the perinatal period and mental health: A preliminary study of tryptophan-related metabolites, bile acids and microbial composition. Behav. Brain Res. 2022, 418, 113635. [Google Scholar] [CrossRef] [PubMed]
- Rincel, M.; Aubert, P.; Chevalier, J.; Grohard, P.-A.; Basso, L.; de Oliveira, C.M.; Helbling, J.C.; Lévy, É.; Chevalier, G.; Leboyer, M.; et al. Multi-hit early life adversity affects gut microbiota, brain and behavior in a sex-dependent manner. Brain Behav. Immun. 2019, 80, 179–192. [Google Scholar] [CrossRef] [PubMed]
- Thion, M.S.; Low, D.; Silvin, A.; Chen, J.; Grisel, P.; Schulte-Schrepping, J.; Blecher, R.; Ulas, T.; Squarzoni, P.; Hoeffel, G.; et al. Microbiome Influences Prenatal and Adult Microglia in a Sex-Specific Manner. Cell 2018, 172, 500–516.e16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pasciuto, E.; Burton, O.T.; Roca, C.P.; Lagou, V.; Rajan, W.D.; Theys, T.; Mancuso, R.; Tito, R.Y.; Kouser, L.; Callaerts-Vegh, Z.; et al. Microglia Require CD4 T Cells to Complete the Fetal-to-Adult Transition. Cell 2020, 182, 625–640.e24. [Google Scholar] [CrossRef] [PubMed]
- Kang, L.J.; Koleva, P.T.; Field, C.J.; Giesbrecht, G.F.; Wine, E.; Becker, A.B.; Mandhane, P.J.; Turvey, S.E.; Subbarao, P.; Sears, M.R.; et al. Maternal depressive symptoms linked to reduced fecal Immunoglobulin A concentrations in infants. Brain Behav. Immun. 2018, 68, 123–131. [Google Scholar] [CrossRef]
- Kang, L.J.; Vu, K.N.; Koleva, P.T.; Field, C.J.; Chow, A.; Azad, M.B.; Becker, A.B.; Mandhane, P.J.; Moraes, T.J.; Sears, M.R.; et al. Maternal psychological distress before birth influences gut immunity in mid-infancy. Clin. Exp. Allergy 2020, 50, 178–188. [Google Scholar] [CrossRef]
- Dinan, T.G.; Kennedy, P.J.; Morais, L.H.; Murphy, A.; Long-Smith, C.M.; Moloney, G.M.; Bastiaanssen, T.F.S.; Allen, A.P.; Collery, A.; Mullins, D.; et al. Altered stress responses in adults born by Caesarean section. Neurobiol. Stress 2022, 16, 100425. [Google Scholar] [CrossRef]
- Jahnke, J.R.; Roach, J.; Azcarate-Peril, M.A.; Thompson, A.L. Maternal precarity and HPA axis functioning shape infant gut microbiota and HPA axis development in humans. PLoS ONE 2021, 16, e0251782. [Google Scholar] [CrossRef]
- Morais, L.H.; Felice, D.; Golubeva, A.V.; Moloney, G.; Dinan, T.G.; Cryan, J.F. Strain differences in the susceptibility to the gut–brain axis and neurobehavioural alterations induced by maternal immune activation in mice. Behav. Pharmacol. 2018, 29, 181–198. [Google Scholar] [CrossRef]
- Smith, K.B.; Murray, E.; Gregory, J.G.; Liang, J.; Ismail, N. Pubertal probiotics mitigate lipopolysaccharide-induced programming of the hypothalamic-pituitary-adrenal axis in male mice only. Brain Res. Bull. 2021, 177, 111–118. [Google Scholar] [CrossRef]
- Murray, E.; Smith, K.B.; Stoby, K.S.; Thomas, B.J.; Swenson, M.J.; Arber, L.A.; Frenette, E.; Ismail, N. Pubertal probiotic blocks LPS-induced anxiety and the associated neurochemical and microbial outcomes, in a sex dependent manner. Psychoneuroendocrinology 2020, 112, 104481. [Google Scholar] [CrossRef] [PubMed]
- Murray, E.; Sharma, R.; Smith, K.B.; Mar, K.D.; Barve, R.; Lukasik, M.; Pirwani, A.F.; Malette-Guyon, E.; Lamba, S.; Thomas, B.J.; et al. Probiotic consumption during puberty mitigates LPS-induced immune responses and protects against stress-induced depression- and anxiety-like behaviors in adulthood in a sex-specific manner. Brain Behav. Immun. 2019, 81, 198–212. [Google Scholar] [CrossRef] [PubMed]
- Laureano-Melo, R.; Caldeira, R.; Guerra, A.; da Conceição, R.; Sena de Souza, J.; Giannocco, G.; Marinho, B.; Luchese, R.; Cortes, W. Maternal Supplementation with Lactobacillus paracasei DTA 83 Alters Emotional Behavior in Swiss Mice Offspring. PharmaNutrition 2019, 8, 100148. [Google Scholar] [CrossRef]
- Lebovitz, Y.; Kowalski, E.A.; Wang, X.; Kelly, C.; Lee, M.; McDonald, V.; Ward, R.; Creasey, M.; Mills, W.; Gudenschwager Basso, E.K.; et al. Lactobacillus rescues postnatal neurobehavioral and microglial dysfunction in a model of maternal microbiome dysbiosis. Brain. Behav. Immun. 2019, 81, 617–629. [Google Scholar] [CrossRef]
- Liu, Y.; Sanderson, D.; Mian, M.F.; Neufeld, K.-A.M.; Forsythe, P. Loss of vagal integrity disrupts immune components of the microbiota-gut-brain axis and inhibits the effect of Lactobacillus rhamnosus on behavior and the corticosterone stress response. Neuropharmacology 2021, 195, 108682. [Google Scholar] [CrossRef]
- Yang, Y.; Zhao, S.; Yang, X.; Li, W.; Si, J.; Yang, X. The antidepressant potential of lactobacillus casei in the postpartum depression rat model mediated by the microbiota-gut-brain axis. Neurosci. Lett. 2022, 774, 136474. [Google Scholar] [CrossRef]
- Hebert, J.C.; Radford-Smith, D.E.; Probert, F.; Ilott, N.; Chan, K.W.; Anthony, D.C.; Burnet, P.W.J. Mom’s diet matters: Maternal prebiotic intake in mice reduces anxiety and alters brain gene expression and the fecal microbiome in offspring. Brain Behav. Immun. 2021, 91, 230–244. [Google Scholar] [CrossRef]
- Hulkkonen, P.; Kataja, E.-L.; Vahlberg, T.; Koivuniemi, E.; Houttu, N.; Pellonperä, O.; Mokkala, K.; Karlsson, H.; Laitinen, K. The efficacy of probiotics and/or n-3 long-chain polyunsaturated fatty acids intervention on maternal prenatal and postnatal depressive and anxiety symptoms among overweight and obese women. J. Affect. Disord. 2021, 289, 21–30. [Google Scholar] [CrossRef]
- Browne, P.D.; Bolte, A.C.; Besseling-van der Vaart, I.; Claassen, E.; de Weerth, C. Probiotics as a treatment for prenatal maternal anxiety and depression: A double-blind randomized pilot trial. Sci. Rep. 2021, 11, 3051. [Google Scholar] [CrossRef]
- Zeng, X.; Wang, Y.; Jia, H.; Wang, Z.; Gao, Z.; Luo, Y.; Sheng, Q.; Yuan, Y.; Yue, T. Metagenomic analysis of microflora structure and functional capacity in probiotic Tibetan kefir grains. Food Res. Int. 2022, 151, 110849. [Google Scholar] [CrossRef]
- Warda, A.K.; Rea, K.; Fitzgerald, P.; Hueston, C.; Gonzalez-Tortuero, E.; Dinan, T.G.; Hill, C. Heat-killed lactobacilli alter both microbiota composition and behaviour. Behav. Brain Res. 2019, 362, 213–223. [Google Scholar] [CrossRef]
- Leo, A.; De Caro, C.; Mainardi, P.; Tallarico, M.; Nesci, V.; Marascio, N.; Striano, P.; Russo, E.; Constanti, A.; De Sarro, G.; et al. Increased efficacy of combining prebiotic and postbiotic in mouse models relevant to autism and depression. Neuropharmacology 2021, 198, 108782. [Google Scholar] [CrossRef]
- Browne, P.D.; Aparicio, M.; Alba, C.; Hechler, C.; Beijers, R.; Rodríguez, J.M.; Fernández, L.; de Weerth, C. Human Milk Microbiome and Maternal Postnatal Psychosocial Distress. Front. Microbiol. 2019, 10, 2333. [Google Scholar] [CrossRef] [PubMed]
- Kortesniemi, M.; Slupsky, C.M.; Aatsinki, A.-K.; Sinkkonen, J.; Karlsson, L.; Linderborg, K.M.; Yang, B.; Karlsson, H.; Kailanto, H.-M. Human milk metabolome is associated with symptoms of maternal psychological distress and milk cortisol. Food Chem. 2021, 356, 129628. [Google Scholar] [CrossRef]
- Flaherman, V.J.; Narayan, N.R.; Hartigan-O’Connor, D.; Cabana, M.D.; McCulloch, C.E.; Paul, I.M. The Effect of Early Limited Formula on Breastfeeding, Readmission, and Intestinal Microbiota: A Randomized Clinical Trial. J. Pediatr. 2018, 196, 84–90.e1. [Google Scholar] [CrossRef] [PubMed]
- Collins, J.M.; Caputi, V.; Manurung, S.; Gross, G.; Fitzgerald, P.; Golubeva, A.V.; Popov, J.; Deady, C.; Dinan, T.G.; Cryan, J.F.; et al. Supplementation with milk fat globule membrane from early life reduces maternal separation-induced visceral pain independent of enteric nervous system or intestinal permeability changes in the rat. Neuropharmacology 2022, 210, 109026. [Google Scholar] [CrossRef] [PubMed]
- Mayengbam, S.; Ellegood, J.; Kesler, M.; Reimer, R.A.; Shearer, J.; Murari, K.; Rho, J.M.; Lerch, J.P.; Cheng, N. A ketogenic diet affects brain volume and metabolome in juvenile mice. Neuroimage 2021, 244, 118542. [Google Scholar] [CrossRef]
- Liu, X.; Li, X.; Xia, B.; Jin, X.; Zou, Q.; Zeng, Z.; Zhao, W.; Yan, S.; Li, L.; Yuan, S.; et al. High-fiber diet mitigates maternal obesity-induced cognitive and social dysfunction in the offspring via gut-brain axis. Cell Metab. 2021, 33, 923–938.e6. [Google Scholar] [CrossRef]
- Júnior, R.E.M.; de Carvalho, L.M.; dos Reis, D.C.; Cassali, G.D.; Faria, A.M.C.; Maioli, T.U.; Brunialti-Godard, A.L. Diet-induced obesity leads to alterations in behavior and gut microbiota composition in mice. J. Nutr. Biochem. 2021, 92, 108622. [Google Scholar] [CrossRef]
- Liu, Z.; Li, L.; Ma, S.; Ye, J.; Zhang, H.; Li, Y.; Sair, A.T.; Pan, J.; Liu, X.; Li, X.; et al. High-Dietary Fiber Intake Alleviates Antenatal Obesity-Induced Postpartum Depression: Roles of Gut Microbiota and Microbial Metabolite Short-chain Fatty Acid Involved. J. Agric. Food Chem. 2020, 68, 13697–13710. [Google Scholar] [CrossRef]
- Salisbury, A.L.; Papandonatos, G.D.; Stroud, L.R.; Smith, A.K.; Brennan, P.A. Prenatal antidepressant exposures and gastrointestinal complaints in childhood: A gut–brain axis connection? Dev. Psychobiol. 2020, 62, 816–828. [Google Scholar] [CrossRef] [PubMed]
- Ramsteijn, A.S.; Jašarević, E.; Houwing, D.J.; Bale, T.L.; Olivier, J.D.A. Antidepressant treatment with fluoxetine during pregnancy and lactation modulates the gut microbiome and metabolome in a rat model relevant to depression. Gut Microbes 2020, 11, 735–753. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vuong, H.E.; Coley, E.J.L.; Kazantsev, M.; Cooke, M.E.; Rendon, T.K.; Paramo, J.; Hsiao, E.Y. Interactions between maternal fluoxetine exposure, the maternal gut microbiome and fetal neurodevelopment in mice. Behav. Brain Res. 2021, 410, 113353. [Google Scholar] [CrossRef] [PubMed]
- Perez-Fernandez, C.; Morales-Navas, M.; Guardia-Escote, L.; Garrido-Cárdenas, J.A.; Colomina, M.T.; Giménez, E.; Sánchez-Santed, F. Long-term effects of low doses of Chlorpyrifos exposure at the preweaning developmental stage: A locomotor, pharmacological, brain gene expression and gut microbiome analysis. Food Chem. Toxicol. 2020, 135, 110865. [Google Scholar] [CrossRef]
- Ni, Y.; Hu, L.; Yang, S.; Ni, L.; Ma, L.; Zhao, Y.; Zheng, A.; Jin, Y.; Fu, Z. Bisphenol A impairs cognitive function and 5-HT metabolism in adult male mice by modulating the microbiota-gut-brain axis. Chemosphere 2021, 282, 130952. [Google Scholar] [CrossRef]
- Nikolic, S.; Gazdic-Jankovic, M.; Rosic, G.; Miletic-Kovacevic, M.; Jovicic, N.; Nestorovic, N.; Stojkovic, P.; Filipovic, N.; Milosevic-Djordjevic, O.; Selakovic, D.; et al. Orally administered fluorescent nanosized polystyrene particles affect cell viability, hormonal and inflammatory profile, and behavior in treated mice. Environ. Pollut. 2022, 305, 119206. [Google Scholar] [CrossRef]
- Qiu, H.; Gao, H.; Yu, F.; Xiao, B.; Li, X.; Cai, B.; Ge, L.; Lu, Y.; Wan, Z.; Wang, Y.; et al. Perinatal exposure to low-level PBDE-47 programs gut microbiota, host metabolism and neurobehavior in adult rats: An integrated analysis. Sci. Total Environ. 2022, 825, 154150. [Google Scholar] [CrossRef]
- Hao, Y.; Meng, L.; Zhang, Y.; Chen, A.; Zhao, Y.; Lian, K.; Guo, X.; Wang, X.; Du, Y.; Wang, X.; et al. Effects of chronic triclosan exposure on social behaviors in adult mice. J. Hazard. Mater. 2022, 424, 127562. [Google Scholar] [CrossRef]
- Angoa-Pérez, M.; Zagorac, B.; Francescutti, D.M.; Theis, K.R.; Kuhn, D.M. Responses to chronic corticosterone on brain glucocorticoid receptors, adrenal gland, and gut microbiota in mice lacking neuronal serotonin. Brain Res. 2021, 1751, 147190. [Google Scholar] [CrossRef]
- Sauer, A.K.; Grabrucker, A.M. Zinc Deficiency During Pregnancy Leads to Altered Microbiome and Elevated Inflammatory Markers in Mice. Front. Neurosci. 2019, 13, 1295. [Google Scholar] [CrossRef]
- Liu, L.; Wang, H.; Rao, X.; Yu, Y.; Li, W.; Zheng, P.; Zhao, L.; Zhou, C.; Pu, J.; Yang, D.; et al. Comprehensive analysis of the lysine acetylome and succinylome in the hippocampus of gut microbiota-dysbiosis mice. J. Adv. Res. 2021, 30, 27–38. [Google Scholar] [CrossRef] [PubMed]
- Khadrawy, Y.A.; Hosny, E.N.; Magdy, M.; Mohammed, H.S. Antidepressant effects of curcumin-coated iron oxide nanoparticles in a rat model of depression. Eur. J. Pharmacol. 2021, 908, 174384. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Q.; Shu, R.; Wu, C.; Tong, Y.; Xiong, Z.; Zhou, J.; Yu, C.; Xie, X.; Fu, Z. Crocin-I alleviates the depression-like behaviors probably via modulating “microbiota-gut-brain” axis in mice exposed to chronic restraint stress. J. Affect. Disord. 2020, 276, 476–486. [Google Scholar] [CrossRef] [PubMed]
- Zhao, F.; Guan, S.; Fu, Y.; Wang, K.; Liu, Z.; Ng, T.B. Lycium barbarum polysaccharide attenuates emotional injury of offspring elicited by prenatal chronic stress in rats via regulation of gut microbiota. Biomed. Pharmacother. 2021, 143, 112087. [Google Scholar] [CrossRef] [PubMed]
- Krishna, G. Oral supplements of inulin during gestation offsets rotenone-induced oxidative impairments and neurotoxicity in maternal and prenatal rat brain. Biomed. Pharmacother. 2018, 104, 751–762. [Google Scholar] [CrossRef]
- Tian, X.-Y.; Xing, J.-W.; Zheng, Q.-Q.; Gao, P.-F. 919 Syrup Alleviates Postpartum Depression by Modulating the Structure and Metabolism of Gut Microbes and Affecting the Function of the Hippocampal GABA/Glutamate System. Front. Cell. Infect. Microbiol. 2021, 11, 694443. [Google Scholar] [CrossRef]
- Loughman, A.; Ponsonby, A.-L.; O’Hely, M.; Symeonides, C.; Collier, F.; Tang, M.L.K.; Carlin, J.; Ranganathan, S.; Allen, K.; Pezic, A.; et al. Gut microbiota composition during infancy and subsequent behavioural outcomes. EBioMedicine 2020, 52, 102640. [Google Scholar] [CrossRef] [Green Version]
- Xie, T.; Wang, Y.; Zou, Z.; Wu, Y.; Fan, X.; Dai, J.; Liu, Y.; Bai, J. Relationship between the gut microbiota and temperament in children 1–2 years old in Chinese birth cohort. J. Psychiatr. Res. 2022, 148, 52–60. [Google Scholar] [CrossRef]
- Aatsinki, A.-K.; Lahti, L.; Uusitupa, H.-M.; Munukka, E.; Keskitalo, A.; Nolvi, S.; O’Mahony, S.; Pietilä, S.; Elo, L.L.; Eerola, E.; et al. Gut microbiota composition is associated with temperament traits in infants. Brain. Behav. Immun. 2019, 80, 849–858. [Google Scholar] [CrossRef]
- Hu, J.; Ly, J.; Zhang, W.; Huang, Y.; Glover, V.; Peter, I.; Hurd, Y.L.; Nomura, Y. Microbiota of newborn meconium is associated with maternal anxiety experienced during pregnancy. Dev. Psychobiol. 2019, 61, 640–649. [Google Scholar] [CrossRef]
- Carlson, A.L.; Xia, K.; Azcarate-Peril, M.A.; Goldman, B.D.; Ahn, M.; Styner, M.A.; Thompson, A.L.; Geng, X.; Gilmore, J.H.; Knickmeyer, R.C. Infant Gut Microbiome Associated with Cognitive Development. Biol. Psychiatry 2018, 83, 148–159. [Google Scholar] [CrossRef] [PubMed]
- Kelsey, C.M.; Prescott, S.; McCulloch, J.A.; Trinchieri, G.; Valladares, T.L.; Dreisbach, C.; Alhusen, J.; Grossmann, T. Gut microbiota composition is associated with newborn functional brain connectivity and behavioral temperament. Brain. Behav. Immun. 2021, 91, 472–486. [Google Scholar] [CrossRef] [PubMed]
- Chong, C.Y.L.; Vatanen, T.; Alexander, T.; Bloomfield, F.H.; O’Sullivan, J.M. Factors Associated with the Microbiome in Moderate-Late Preterm Babies: A Cohort Study From the DIAMOND Randomized Controlled Trial. Front. Cell. Infect. Microbiol. 2021, 11, 595323. [Google Scholar] [CrossRef] [PubMed]
- Streit, F.; Prandovszky, E.; Send, T.; Zillich, L.; Frank, J.; Sabunciyan, S.; Foo, J.; Sirignano, L.; Lange, B.; Bardtke, S.; et al. Microbiome profiles are associated with cognitive functioning in 45-month-old children. Brain. Behav. Immun. 2021, 98, 151–160. [Google Scholar] [CrossRef] [PubMed]
- Johnson, K.V.-A. Gut microbiome composition and diversity are related to human personality traits. Hum. Microbiome J. 2020, 15, 100069. [Google Scholar] [CrossRef]
- Gough, E.K.; Edens, T.J.; Geum, H.M.; Baharmand, I.; Gill, S.K.; Robertson, R.C.; Mutasa, K.; Ntozini, R.; Smith, L.E.; Chasekwa, B.; et al. Maternal fecal microbiome predicts gestational age, birth weight and neonatal growth in rural Zimbabwe. eBioMedicine 2021, 68, 103421. [Google Scholar] [CrossRef]
- Hantsoo, L.; Jašarević, E.; Criniti, S.; McGeehan, B.; Tanes, C.; Sammel, M.D.; Elovitz, M.A.; Compher, C.; Wu, G.; Epperson, C.N. Childhood adversity impact on gut microbiota and inflammatory response to stress during pregnancy. Brain. Behav. Immun. 2019, 75, 240–250. [Google Scholar] [CrossRef]
- Aroniadis, O.C.; Brandt, L.J. Fecal microbiota transplantation: Past, present and future. Curr. Opin. Gastroenterol. 2013, 29, 79–84. [Google Scholar] [CrossRef]
- Rossen, N.G.; MacDonald, J.K.; de Vries, E.M.; D’Haens, G.R.; de Vos, W.M.; Zoetendal, E.G.; Ponsioen, C.Y. Fecal microbiota transplantation as novel therapy in gastroenterology: A systematic review. World J. Gastroenterol. 2015, 21, 5359–5371. [Google Scholar] [CrossRef]
- Ianiro, G.; Maida, M.; Burisch, J.; Simonelli, C.; Hold, G.; Ventimiglia, M.; Gasbarrini, A.; Cammarota, G. Efficacy of different faecal microbiota transplantation protocols for Clostridium difficile infection: A systematic review and meta-analysis. United Eur. Gastroenterol. J. 2018, 6, 1232–1244. [Google Scholar] [CrossRef] [Green Version]
- Bibbò, S.; Settanni, C.R.; Porcari, S.; Bocchino, E.; Ianiro, G.; Cammarota, G.; Gasbarrini, A. Fecal Microbiota Transplantation: Screening and Selection to Choose the Optimal Donor. J. Clin. Med. 2020, 9, 1757. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.; Allen-Vercoe, E.; Petrof, E.O. Fecal microbiota transplantation: In perspective. Therap. Adv. Gastroenterol. 2015, 9, 229–239. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, Z.; Liu, Z.; Dong, X.; Hu, T.; Wang, L.; Li, J.; Liu, X.; Sun, J. Fecal Microbiota Transplantation from Healthy Donors Reduced Alcohol-induced Anxiety and Depression in an Animal Model of Chronic Alcohol Exposure. Chin. J. Physiol. 2018, 61, 360–371. [Google Scholar] [PubMed]
- Zhang, Y.; Huang, R.; Cheng, M.; Wang, L.; Chao, J.; Li, J.; Zheng, P.; Xie, P.; Zhang, Z.; Yao, H. Gut microbiota from NLRP3-deficient mice ameliorates depressive-like behaviors by regulating astrocyte dysfunction via circHIPK2. Microbiome 2019, 7, 116. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, E.K.A.; Torres-Espin, A.; Raposo, P.J.F.; Madsen, K.L.; Kigerl, K.A.; Popovich, P.G.; Fenrich, K.K.; Fouad, K. Fecal transplant prevents gut dysbiosis and anxiety-like behaviour after spinal cord injury in rats. PLoS ONE 2020, 15, e0226128. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, S.; Ishima, T.; Qu, Y.; Shan, J.; Chang, L.; Wei, Y.; Zhang, J.; Pu, Y.; Fujita, Y.; Tan, Y.; et al. Ingestion of Faecalibaculum rodentium causes depression-like phenotypes in resilient Ephx2 knock-out mice: A role of brain–gut–microbiota axis via the subdiaphragmatic vagus nerve. J. Affect. Disord. 2021, 292, 565–573. [Google Scholar] [CrossRef]
- Jiang, H.; Ling, Z.; Zhang, Y.; Mao, H.; Ma, Z.; Yin, Y.; Wang, W.; Tang, W.; Tan, Z.; Shi, J.; et al. Altered fecal microbiota composition in patients with major depressive disorder. Brain. Behav. Immun. 2015, 48, 186–194. [Google Scholar] [CrossRef] [Green Version]
- Kelly, J.R.; Borre, Y.; O’ Brien, C.; Patterson, E.; El Aidy, S.; Deane, J.; Kennedy, P.J.; Beers, S.; Scott, K.; Moloney, G.; et al. Transferring the blues: Depression-associated gut microbiota induces neurobehavioural changes in the rat. J. Psychiatr. Res. 2016, 82, 109–118. [Google Scholar] [CrossRef]
- Zheng, P.; Zeng, B.; Zhou, C.; Liu, M.; Fang, Z.; Xu, X.; Zeng, L.; Chen, J.; Fan, S.; Du, X.; et al. Gut microbiome remodeling induces depressive-like behaviors through a pathway mediated by the host’s metabolism. Mol. Psychiatry 2016, 21, 786–796. [Google Scholar] [CrossRef]
- Mazzawi, T.; Lied, G.A.; Sangnes, D.A.; El-Salhy, M.; Hov, J.R.; Gilja, O.H.; Hatlebakk, J.G.; Hausken, T. The kinetics of gut microbial community composition in patients with irritable bowel syndrome following fecal microbiota transplantation. PLoS ONE 2018, 13, e0194904. [Google Scholar] [CrossRef] [Green Version]
- Kurokawa, S.; Kishimoto, T.; Mizuno, S.; Masaoka, T.; Naganuma, M.; Liang, K.; Kitazawa, M.; Nakashima, M.; Shindo, C.; Suda, W.; et al. The effect of fecal microbiota transplantation on psychiatric symptoms among patients with irritable bowel syndrome, functional diarrhea and functional constipation: An open-label observational study. J. Affect. Disord. 2018, 235, 506–512. [Google Scholar] [CrossRef]
- Mizuno, S.; Masaoka, T.; Naganuma, M.; Kishimoto, T.; Kitazawa, M.; Kurokawa, S.; Nakashima, M.; Takeshita, K.; Suda, W.; Mimura, M.; et al. Bifidobacterium-Rich Fecal Donor May Be a Positive Predictor for Successful Fecal Microbiota Transplantation in Patients with Irritable Bowel Syndrome. Digestion 2017, 96, 29–38. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lahtinen, P.; Jalanka, J.; Hartikainen, A.; Mattila, E.; Hillilä, M.; Punkkinen, J.; Koskenpato, J.; Anttila, V.-J.; Tillonen, J.; Satokari, R.; et al. Randomised clinical trial: Faecal microbiota transplantation versus autologous placebo administered via colonoscopy in irritable bowel syndrome. Aliment. Pharmacol. Ther. 2020, 51, 1321–1331. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.L.; Chen, H.T.; Luo, Q.L.; Xu, H.M.; He, J.; Li, Y.Q.; Zhou, Y.L.; Yao, F.; Nie, Y.Q.; Zhou, Y.J. Relief of irritable bowel syndrome by fecal microbiota transplantation is associated with changes in diversity and composition of the gut microbiota. J. Dig. Dis. 2019, 20, 401–408. [Google Scholar] [CrossRef]
- Johnsen, P.H.; Hilpüsch, F.; Valle, P.C.; Goll, R. The effect of fecal microbiota transplantation on IBS related quality of life and fatigue in moderate to severe non-constipated irritable bowel: Secondary endpoints of a double blind, randomized, placebo-controlled trial. eBioMedicine 2020, 51, 102562. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Collyer, R.; Clancy, A.; Borody, T. Faecal microbiota transplantation alleviates symptoms of depression in individuals with irritable bowel syndrome: A case series. Med. Microecol. 2020, 6, 100029. [Google Scholar] [CrossRef]
- Aimagambetova, G.; Issanov, A.; Terzic, S.; Bapayeva, G.; Ukybassova, T.; Baikoshkarova, S.; Aldiyarova, A.; Shauyen, F.; Terzic, M. The effect of psychological distress on IVF outcomes: Reality or speculations? PLoS ONE 2020, 15, e0242024. [Google Scholar]
- Ni, Y.; Tong, C.; Huang, L.; Zhou, W.; Zhang, A. The analysis of fertility quality of life and the influencing factors of patients with repeated implantation failure. Health Qual. Life Outcomes 2021, 19, 32. [Google Scholar] [CrossRef]
- Liu, Y.-F.; Fu, Z.; Chen, S.-W.; He, X.-P.; Fan, L.-Y. The Analysis of Anxiety and Depression in Different Stages of in vitro Fertilization-Embryo Transfer in Couples in China. Neuropsychiatr. Dis. Treat. 2021, 17, 649–657. [Google Scholar] [CrossRef]
- de Klerk, C.; Macklon, N.S.; Heijnen, E.M.E.W.; Eijkemans, M.J.C.; Fauser, B.C.J.M.; Passchier, J.; Hunfeld, J.A.M. The psychological impact of IVF failure after two or more cycles of IVF with a mild versus standard treatment strategy. Hum. Reprod. 2007, 22, 2554–2558. [Google Scholar] [CrossRef] [Green Version]
- Holley, S.R.; Passoni, M.R.; Nachtigall, R.D.; Bleil, M.E.; Adler, N.E.; Pasch, L.A. Rates of major depression following IVF failure. Fertil. Steril. 2012, 98, S234. [Google Scholar] [CrossRef]
- Schoenmakers, S.; Laven, J. The vaginal microbiome as a tool to predict IVF success. Curr. Opin. Obstet. Gynecol. 2020, 32, 169–178. [Google Scholar] [CrossRef] [PubMed]
- Min, F.; Xiaowei, Z.; Yiheng, L.; Shouren, L.; Weiping, Q.; Shangrong, F.; Xiaorong, L. Alterations in Vaginal Microbiota and Associated Metabolome in Women with Recurrent Implantation Failure. MBio 2022, 11, e03242-19. [Google Scholar]
- Suh, E.-K.; Yang, A.; Kettenbach, A.; Bamberger, C.; Michaelis, A.H.; Zhu, Z.; Elvin, J.A.; Bronson, R.T.; Crum, C.P.; McKeon, F. p63 protects the female germ line during meiotic arrest. Nature 2006, 444, 624–628. [Google Scholar] [CrossRef] [PubMed]
- Gong, Z.; Luna, Y.; Yu, P.; Fan, H. Lactobacilli Inactivate Chlamydia trachomatis through Lactic Acid but Not H2O2. PLoS ONE 2014, 9, e107758. [Google Scholar] [CrossRef] [Green Version]
- Cadieux, P.; Burton, J.; Devillard, E.; Reid, G. Lactobacillus by-products inhibit the growth and virulence of uropathogenic Escherichia coli. J. Physiol. Pharmacol. 2009, 60 (Suppl. S6), 13–18. [Google Scholar]
- Alakomi, H.-L.; Skyttä, E.; Saarela, M.; Mattila-Sandholm, T.; Latva-Kala, K.; Helander, I.M. Lactic Acid Permeabilizes Gram-Negative Bacteria by Disrupting the Outer Membrane. Appl. Environ. Microbiol. 2000, 66, 2001–2005. [Google Scholar] [CrossRef] [Green Version]
- Aldunate, M.; Tyssen, D.; Johnson, A.; Zakir, T.; Sonza, S.; Moench, T.; Cone, R.; Tachedjian, G. Vaginal concentrations of lactic acid potently inactivate HIV. J. Antimicrob. Chemother. 2013, 68, 2015–2025. [Google Scholar] [CrossRef]
- Hyman, R.W.; Herndon, C.N.; Jiang, H.; Palm, C.; Fukushima, M.; Bernstein, D.; Vo, K.C.; Zelenko, Z.; Davis, R.W.; Giudice, L.C. The dynamics of the vaginal microbiome during infertility therapy with in vitro fertilization-embryo transfer. J. Assist. Reprod. Genet. 2012, 29, 105–115. [Google Scholar] [CrossRef] [Green Version]
- Moreno, I.; Codoñer, F.M.; Vilella, F.; Valbuena, D.; Martinez-Blanch, J.F.; Jimenez-Almazán, J.; Alonso, R.; Alamá, P.; Remohí, J.; Pellicer, A.; et al. Evidence that the endometrial microbiota has an effect on implantation success or failure. Am. J. Obstet. Gynecol. 2016, 215, 684–703. [Google Scholar] [CrossRef] [Green Version]
- Moreno, I.; Simon, C. Relevance of assessing the uterine microbiota in infertility. Fertil. Steril. 2018, 110, 337–343. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nyangahu, D.D.; Lennard, K.S.; Brown, B.P.; Darby, M.G.; Wendoh, J.M.; Havyarimana, E.; Smith, P.; Butcher, J.; Stintzi, A.; Mulder, N.; et al. Disruption of maternal gut microbiota during gestation alters offspring microbiota and immunity. Microbiome 2018, 6, 124. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cheng, R.Y.; Li, M.; Li, S.S.; He, M.; Yu, X.H.; Shi, L.; He, F. Vancomycin and ceftriaxone can damage intestinal microbiota and affect the development of the intestinal tract and immune system to different degrees in neonatal mice. Pathog. Dis. 2017, 75, ftx104. [Google Scholar] [CrossRef]
- Cheng, R.; Guo, J.; Pu, F.; Wan, C.; Shi, L.; Li, H.; Yang, Y.; Huang, C.; Li, M.; He, F. Loading ceftriaxone, vancomycin, and Bifidobacteria bifidum TMC3115 to neonatal mice could differently and consequently affect intestinal microbiota and immunity in adulthood. Sci. Rep. 2019, 9, 3254. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miao, Z.; Cheng, R.; Zhang, Y.; Liang, H.; Jiang, F.; Shen, X.; Chen, G.; Zhang, Q.; He, F.; Li, M. Antibiotics can cause weight loss by impairing gut microbiota in mice and the potent benefits of lactobacilli. Biosci. Biotechnol. Biochem. 2020, 84, 411–420. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.-M.; Chou, H.-C.; Yang, Y.-C.S.H. Maternal Antibiotic Treatment Disrupts the Intestinal Microbiota and Intestinal Development in Neonatal Mice. Front. Microbiol. 2021, 12, 684233. [Google Scholar] [CrossRef] [PubMed]
- Tormo-Badia, N.; Håkansson, Å.; Vasudevan, K.; Molin, G.; Ahrné, S.; Cilio, C.M. Antibiotic Treatment of Pregnant Non-Obese Diabetic Mice Leads to Altered Gut Microbiota and Intestinal Immunological Changes in the Offspring. Scand. J. Immunol. 2014, 80, 250–260. [Google Scholar] [CrossRef]
- Gonzalez-Perez, G.; Hicks, A.L.; Tekieli, T.M.; Radens, C.M.; Williams, B.L.; Lamousé-Smith, E.S.N. Maternal Antibiotic Treatment Impacts Development of the Neonatal Intestinal Microbiome and Antiviral Immunity. J. Immunol. 2016, 196, 3768–3779. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gonzalez-Perez, G.; Lamousé-Smith, E.S.N. Gastrointestinal Microbiome Dysbiosis in Infant Mice Alters Peripheral CD8+ T Cell Receptor Signaling. Front. Immunol. 2017, 8, 265. [Google Scholar] [CrossRef] [Green Version]
- Benner, M.; Lopez-Rincon, A.; Thijssen, S.; Garssen, J.; Ferwerda, G.; Joosten, I.; van der Molen, R.G.; Hogenkamp, A. Antibiotic Intervention Affects Maternal Immunity During Gestation in Mice. Front. Immunol. 2021, 12, 685742. [Google Scholar] [CrossRef]
- Sudo, N.; Chida, Y.; Aiba, Y.; Sonoda, J.; Oyama, N.; Yu, X.-N.; Kubo, C.; Koga, Y. Postnatal microbial colonization programs the hypothalamic–pituitary–adrenal system for stress response in mice. J. Physiol. 2004, 558, 263–275. [Google Scholar] [CrossRef] [PubMed]
- Pessa-Morikawa, T.; Husso, A.; Kärkkäinen, O.; Koistinen, V.; Hanhineva, K.; Iivanainen, A.; Niku, M. Maternal microbiota-derived metabolic profile in fetal murine intestine, brain and placenta. BMC Microbiol. 2022, 22, 46. [Google Scholar] [CrossRef]
- Lee, Y.M.; Mu, A.; Wallace, M.; Gengatharan, J.M.; Furst, A.J.; Bode, L.; Metallo, C.M.; Ayres, J.S. Microbiota control of maternal behavior regulates early postnatal growth of offspring. Sci. Adv. 2022, 7, eabe6563. [Google Scholar] [CrossRef] [PubMed]
- Luk, B.; Veeraragavan, S.; Engevik, M.; Balderas, M.; Major, A.; Runge, J.; Luna, R.A.; Versalovic, J. Postnatal colonization with human “infant-type” Bifidobacterium species alters behavior of adult gnotobiotic mice. PLoS ONE 2018, 13, e0196510. [Google Scholar] [CrossRef]
- Luck, B.; Engevik, M.A.; Ganesh, B.P.; Lackey, E.P.; Lin, T.; Balderas, M.; Major, A.; Runge, J.; Luna, R.A.; Sillitoe, R.V.; et al. Bifidobacteria shape host neural circuits during postnatal development by promoting synapse formation and microglial function. Sci. Rep. 2020, 10, 7737. [Google Scholar] [CrossRef] [PubMed]
- Martínez, I.; Maldonado-Gomez, M.X.; Gomes-Neto, J.C.; Kittana, H.; Ding, H.; Schmaltz, R.; Joglekar, P.; Cardona, R.J.; Marsteller, N.L.; Kembel, S.W.; et al. Experimental evaluation of the importance of colonization history in early-life gut microbiota assembly. Elife 2018, 7, e36521. [Google Scholar] [CrossRef]
- Koren, O.; Goodrich, J.K.; Cullender, T.C.; Spor, A.; Laitinen, K.; Bäckhed, H.K.; Gonzalez, A.; Werner, J.J.; Angenent, L.T.; Knight, R.; et al. Host remodeling of the gut microbiome and metabolic changes during pregnancy. Cell 2012, 150, 470–480. [Google Scholar] [CrossRef] [Green Version]
- Ridaura, V.K.; Faith, J.J.; Rey, F.E.; Cheng, J.; Duncan, A.E.; Kau, A.L.; Griffin, N.W.; Lombard, V.; Henrissat, B.; Bain, J.R.; et al. Gut Microbiota from Twins Discordant for Obesity Modulate Metabolism in Mice. Science 2013, 341, 1241214. [Google Scholar] [CrossRef] [Green Version]
- Goodrich, J.K.; Waters, J.L.; Poole, A.C.; Sutter, J.L.; Koren, O.; Blekhman, R.; Beaumont, M.; Van Treuren, W.; Knight, R.; Bell, J.T.; et al. Human Genetics Shape the Gut Microbiome. Cell 2014, 159, 789–799. [Google Scholar] [CrossRef] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).