Predictive Value of Ultrasound Characteristics for Disease-Free Survival in Breast Cancer
Abstract
:1. Introduction
2. Materials and Methods
2.1. Ethical Approval
2.2. Patients
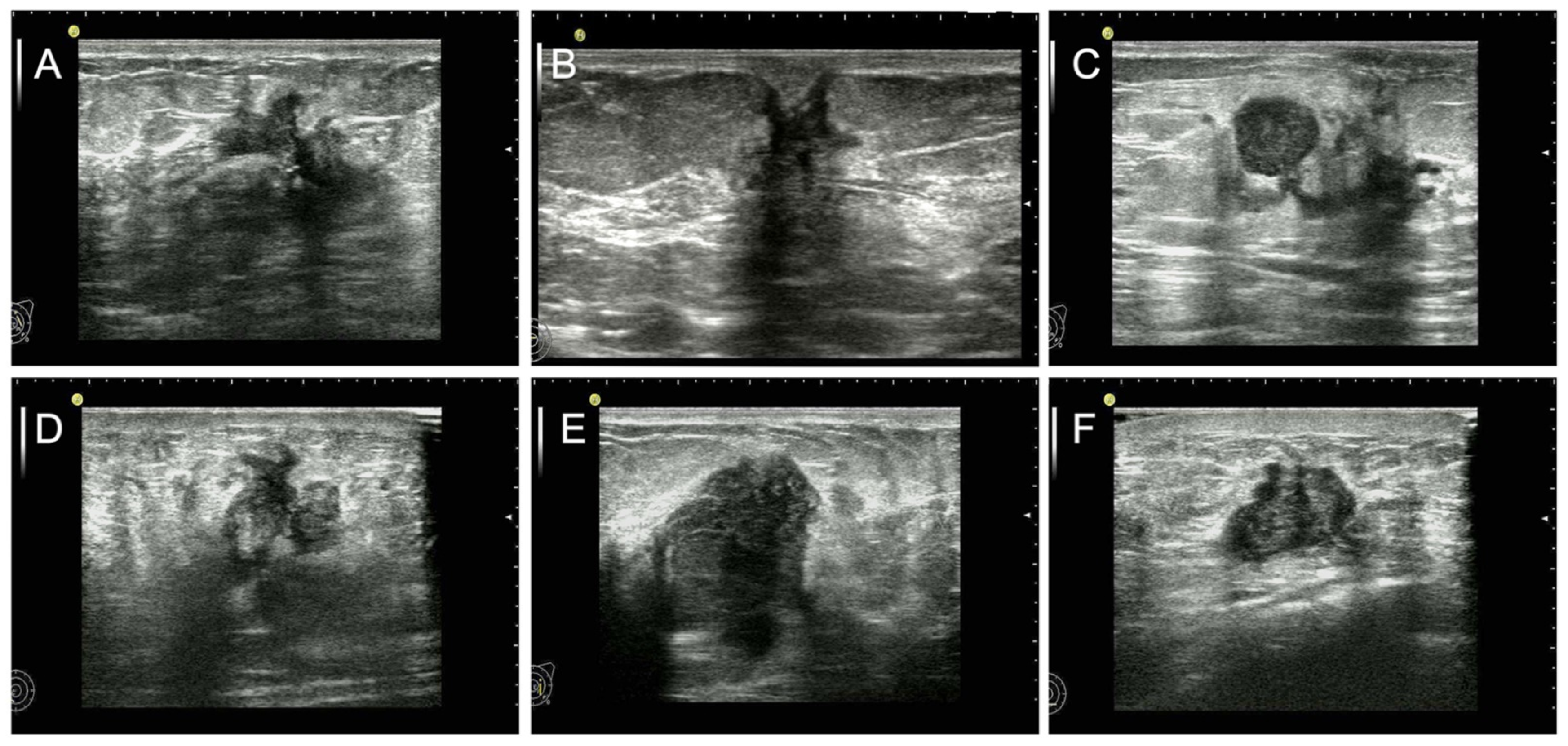
2.3. Data and Image Analysis
2.4. Clinicopathology and Laboratory Examinations
2.5. Statistical Analysis
3. Results
3.1. Clinical Characteristics
3.2. Multiple Logistic Regression Analysis
3.3. Survival Analysis for DFS
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2019. CA A Cancer J. Clin. 2019, 69, 7–34. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Corso, G. Aso author reflections: Clinical implication of nomograms in the breast oncology field. Ann. Surg. Oncol. 2020, 27, 1875–1876. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer statistics, 2022. CA A Cancer J. Clin. 2022, 72, 7–33. [Google Scholar] [CrossRef] [PubMed]
- Sughayer, M.; Alaaraj, R.; Alsughayer, A. Applying new magee equations for predicting the oncotype dx recurrence score. Breast Cancer 2018, 25, 597–604. [Google Scholar] [CrossRef]
- Turner, B.M.; Skinner, K.A.; Tang, P.; Jackson, M.C.; Soukiazian, N.; Shayne, M.; Huston, A.; Ling, M.; Hicks, D.G. Use of modified magee equations and histologic criteria to predict the oncotype dx recurrence score. Mod. Pathol. 2015, 28, 921–931. [Google Scholar] [CrossRef] [Green Version]
- Haybittle, J.L.; Blamey, R.W.; Elston, C.W.; Johnson, J.; Doyle, P.J.; Campbell, F.C.; Nicholson, R.I.; Griffiths, K. A prognostic index in primary breast cancer. Br. J. Cancer 1982, 45, 361–366. [Google Scholar] [CrossRef] [Green Version]
- Dietzel, M.; Schulz-Wendtland, R.; Ellmann, S.; Zoubi, R.; Wenkel, E.; Hammon, M.; Clauser, P.; Uder, M.; Runnebaum, I.B.; Baltzer, P.A.T. Automated volumetric radiomic analysis of breast cancer vascularization improves survival prediction in primary breast cancer. Sci. Rep. 2020, 10, 3664. [Google Scholar] [CrossRef]
- Dowsett, M.; Sestak, I.; Buus, R.; Lopez-Knowles, E.; Mallon, E.; Howell, A.; Forbes, J.F.; Buzdar, A.; Cuzick, J. Estrogen receptor expression in 21-gene recurrence score predicts increased late recurrence for estrogen-positive/her2-negative breast cancer. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2015, 21, 2763–2770. [Google Scholar] [CrossRef] [Green Version]
- Engelhardt, E.G.; van den Broek, A.J.; Linn, S.C.; Wishart, G.C.; Rutgers, E.J.T.; van de Velde, A.O.; Smit, V.; Voogd, A.C.; Siesling, S.; Brinkhuis, M.; et al. Accuracy of the online prognostication tools predict and adjuvant! For early-stage breast cancer patients younger than 50 years. Eur. J. Cancer 2017, 78, 37–44. [Google Scholar] [CrossRef]
- Saghatchian, M.; Mook, S.; Pruneri, G.; Viale, G.; Glas, A.M.; Guerin, S.; Cardoso, F.; Piccart, M.; Tursz, T.; Delaloge, S.; et al. Additional prognostic value of the 70-gene signature (mammaprint((r))) among breast cancer patients with 4-9 positive lymph nodes. Breast 2013, 22, 682–690. [Google Scholar] [CrossRef]
- Li, Z.; Tian, J.; Wang, X.; Wang, Y.; Wang, Z.; Zhang, L.; Jing, H.; Wu, T. Differences in multi-modal ultrasound imaging between triple negative and non-triple negative breast cancer. Ultrasound Med. Biol. 2016, 42, 882–890. [Google Scholar] [CrossRef]
- Zhang, L.; Li, J.; Xiao, Y.; Cui, H.; Du, G.; Wang, Y.; Li, Z.; Wu, T.; Li, X.; Tian, J. Identifying ultrasound and clinical features of breast cancer molecular subtypes by ensemble decision. Sci. Rep. 2015, 5, 11085. [Google Scholar] [CrossRef]
- Guo, Q.; Dong, Z.; Zhang, L.; Ning, C.; Li, Z.; Wang, D.; Liu, C.; Zhao, M.; Tian, J. Ultrasound features of breast cancer for predicting axillary lymph node metastasis. J. Ultrasound Med. 2018, 37, 1354–1353. [Google Scholar] [CrossRef] [Green Version]
- Guo, Q.; Zhang, L.; Di, Z.; Ning, C.; Dong, Z.; Li, Z.; Wang, D.; Liu, C.; Zhao, M.; Tian, J. Assessing risk category of breast cancer by ultrasound imaging characteristics. Ultrasound Med. Biol. 2018, 44, 815–824. [Google Scholar] [CrossRef]
- Sedgwick, E. The breast ultrasound lexicon: Breast imaging reporting and data system (bi-rads). Semin. Roentgenol. 2011, 46, 245–251. [Google Scholar] [CrossRef]
- Adler, D.D.; Carson, P.L.; Rubin, J.M.; Quinn-Reid, D. Doppler ultrasound color flow imaging in the study of breast cancer: Preliminary findings. Ultrasound Med. Biol. 1990, 16, 553–559. [Google Scholar] [CrossRef]
- Singletary, S.E.; Allred, C.; Ashley, P.; Bassett, L.W.; Berry, D.; Bland, K.I.; Borgen, P.I.; Clark, G.; Edge, S.B.; Hayes, D.F.; et al. Revision of the american joint committee on cancer staging system for breast cancer. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2002, 20, 3628–3636. [Google Scholar] [CrossRef]
- Falcone, V.; Reiser, E.; Grula, L.; Bago-Horvath, Z.; Stolz, M.; Catic, A.; Deutschmann, C.; Singer, C.; Pfeiler, G. Correlation between preoperative radiological and postoperative pathological tumor size in patients with her2(+) breast cancer after neoadjuvant chemotherapy plus trastuzumab and pertuzumab. Clin. Breast Cancer 2022, 22, 149–160. [Google Scholar] [CrossRef]
- You, K.Y.; Zou, W.L.; Ding, L.; Bi, Z.F.; Yao, H.R. Large tumor size is an indicator for the timely administration of adjuvant radiotherapy in luminal breast cancer with positive lymph node. Cancer Manag. Res. 2021, 13, 1325–1332. [Google Scholar] [CrossRef]
- Min, S.K.; Lee, S.K.; Woo, J.; Jung, S.M.; Ryu, J.M.; Yu, J.; Lee, J.E.; Kim, S.W.; Chae, B.J.; Nam, S.J. Relation between tumor size and lymph node metastasis according to subtypes of breast cancer. J. Breast Cancer 2021, 24, 75–84. [Google Scholar] [CrossRef]
- Foulkes, W.D.; Reis-Filho, J.S.; Narod, S.A. Tumor size and survival in breast cancer--a reappraisal. Nat. Rev. Clin. Oncol. 2010, 7, 348–353. [Google Scholar] [CrossRef] [PubMed]
- Malherbe, K.; Bresser, P. Association between ultrasound morphologic features and histopathological findings of lobular carcinoma. J. Med. Radiat. Sci. 2019, 66, 177–183. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stavros, A.T.; Thickman, D.; Rapp, C.L.; Dennis, M.A.; Parker, S.H.; Sisney, G.A. Solid breast nodules: Use of sonography to distinguish between benign and malignant lesions. Radiology 1995, 196, 123–134. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Z.; Ren, M.; Tian, J.; Jiang, S.; Liu, Y.; Zhang, L.; Wang, Z.; Song, Q.; Liu, C.; Wu, T. The differences in ultrasound and clinicopathological features between basal-like and normal-like subtypes of triple negative breast cancer. PLoS ONE 2015, 10, e0114820. [Google Scholar] [CrossRef]
- Choi, B. Comparison of ultrasound features with maximum standardized uptake value assessed by 18f-fluorodeoxyglucose-positron emission tomography/computed tomography for prognosis of estrogen receptor+/human epithelial growth factor receptor 2- breast cancer. Ultrasound Q. 2021, 38, 18–24. [Google Scholar] [CrossRef]
- Mercado, C.L. Bi-rads update. Radiol. Clin. N. Am. 2014, 52, 481–487. [Google Scholar] [CrossRef]
- Heimann, R.; Ferguson, D.; Gray, S.; Hellman, S. Assessment of intratumoral vascularization (angiogenesis) in breast cancer prognosis. Breast Cancer Res. Treat. 1998, 52, 147–158. [Google Scholar] [CrossRef]
- Wang, H.; Yao, J.; Zhu, Y.; Zhan, W.; Chen, X.; Shen, K. Association of sonographic features and molecular subtypes in predicting breast cancer disease outcomes. Cancer Med. 2020, 9, 6173–6185. [Google Scholar] [CrossRef]
- Wang, H.; Zhan, W.; Chen, W.; Li, Y.; Chen, X.; Shen, K. Sonography with vertical orientation feature predicts worse disease outcome in triple negative breast cancer. Breast 2020, 49, 33–40. [Google Scholar] [CrossRef] [Green Version]
- Zhu, S.; Li, Y.; Chen, W.; Fei, X.; Shen, K.; Chen, X. Molecular subtype may be more associated with prognosis and chemotherapy benefit than tumor size in t1n0 breast cancer patients: An analysis of 2168 patients for possible de-escalation treatment. Front. Oncol. 2021, 11, 636266. [Google Scholar] [CrossRef]
- Bae, S.Y.; Lee, J.H.; Bae, J.W.; Jung, S.P. Differences in prognosis by p53 expression after neoadjuvant chemotherapy in triple-negative breast cancer. Ann. Surg. Treat. Res. 2020, 98, 291–298. [Google Scholar] [CrossRef]
- Hamy, A.S.; Lam, G.T.; Laas, E.; Darrigues, L.; Balezeau, T.; Guerin, J.; Livartowski, A.; Sadacca, B.; Pierga, J.Y.; Vincent-Salomon, A.; et al. Lymphovascular invasion after neoadjuvant chemotherapy is strongly associated with poor prognosis in breast carcinoma. Breast Cancer Res. Treat. 2018, 169, 295–304. [Google Scholar] [CrossRef]
- Sudhir, R.; Koppula, V.C.; Rao, T.S.; Sannapareddy, K.; Rajappa, S.J.; Murthy, S.S. Accuracy of digital mammography, ultrasound and mri in predicting the pathological complete response and residual tumor size of breast cancer after completion of neoadjuvant chemotherapy. Indian J. Cancer 2021. [Google Scholar] [CrossRef]


| Variables | Disease-Free Survival | p Value | Odds Ratio (95% CI) | |
|---|---|---|---|---|
| <Ten Years n = 174, (%) | ≥Ten Years n = 200, (%) | |||
| Age (years) | 0.405 | 1.21 (0.81–1.82) | ||
| <50 | 90 (51.7) | 113 (56.5) | ||
| ≥50 | 84 (48.3) | 87 (43.5) | ||
| BMI (kg/m2) | 0.123 | 1.43 (0.93–2.20) | ||
| <25 | 109 (62.6) | 141 (70.5) | ||
| ≥25 | 65 (37.4) | 59 (29.5) | ||
| Pathological type | 0.062 | 0.67 (0.45–1.01) | ||
| IDC | 100 (57.5) | 95 (47.5) | ||
| Others | 74 (42.5) | 105 (52.5 | ||
| Nuclear grade | 0.009 | 1.77 (1.15–2.71) | ||
| Low/Intermediate | 101 (58.0) | 142 (71.0) | ||
| High | 73 (42.0) | 58 (29.0) | ||
| Size on US (mm) | 0.001 | 2.09 (1.38–3.17) | ||
| <20 | 65 (37.4) | 111 (55.5) | ||
| ≥20 | 109 (62.6) | 89 (44.5) | ||
| ALN | < 0.001 | 0.23 (0.15–0.36) | ||
| Positive | 96 (55.2) | 44 (22.0) | ||
| Negative | 78 (44.8) | 156 (78.0) | ||
| Mass shape | 0.001 | 2.03 (1.34–3.06) | ||
| Round, oval | 74 (42.5) | 120 (60.0) | ||
| Irregular | 100 (57.5) | 80 (40.0) | ||
| Mass orientation | 0.001 | 2.06 (1.36–3.12) | ||
| Parallel | 77 (44.3) | 124 (62.0) | ||
| Not-parallel | 97 (55.7) | 76 (38.0) | ||
| Mass margin | 0.334 | 1.25 (0.82–1.90) | ||
| Circumscribed | 59 (33.9) | 78 (39.0) | ||
| Not-circumscribed | 115 (66.1) | 122 (61.0) | ||
| Shadowing | 0.144 | 0.73 (0.49–1.11) | ||
| Yes | 82 (47.1) | 79 (39.5) | ||
| No | 92 (52.9) | 121 (60.5) | ||
| Hypoecho surround | 0.524 | 1.16 (0.768–1.77) | ||
| Yes | 65 (37.4) | 82 (41.0) | ||
| No | 109 (62.6) | 118 (59.0) | ||
| Calcifications on US | 0.078 | 0.69 (0.46–1.04) | ||
| Yes | 90 (51.7) | 85 (42.5) | ||
| No | 84 (48.3) | 115 (57.5) | ||
| Echo pattern | 0.412 | 0.82 (0.51–1.30) | ||
| Hypoechoic | 132 (75.9) | 144 (72.0) | ||
| Others | 42 (24.1) | 56 (28) | ||
| CDFI | 0.002 | 2.15 (1.33–3.48) | ||
| No flow, Minimal | 33 (19.0) | 67 (33.5) | ||
| Moderate, Marked | 141 (81.0) | 133 (66.5) | ||
| ER | 0.178 | 1.34 (0.89–2.02) | ||
| Positive | 82 (47.1) | 109 (54.5) | ||
| Negative | 92 (52.9) | 91 (45.5) | ||
| PR | 0.014 | 1.73 (1.14–2.63) | ||
| Positive | 95 (54.6) | 135 (67.5) | ||
| Negative | 79 (45.4) | 65 (32.5) | ||
| HER2 | 0.159 | 0.73 (0.48–1.13) | ||
| Positive | 67 (38.5) | 63 (31.5) | ||
| Negative | 107 (61.5) | 137 (68.5) | ||
| KI67 | 0.063 | 0.65 (0.41–1.02) | ||
| Positive | 55 (31.6) | 46 (23.0) | ||
| Negative | 119 (68.4) | 154 (77.0) | ||
| Molecular subtypes | <0.001 | |||
| Luminal A | 55 (31.6) | 94 (47.0) | ||
| Luminal B | 21 (12.1) | 40 (20.0) | 0.755 * | 1.11 (0.60–2.08) |
| HER2-enriched | 40 (23.0) | 48 (24.0) | 0.218 ** | 0.70 (0.41–1.20) |
| TN | 58 (33.3) | 18 (9.0) | < 0.001 *** | 0.18 (0.10–0.34) |
| Variable | Β | SE | Wals | p Value | Odds Ratio (95% CI) |
|---|---|---|---|---|---|
| Size on US (mm) | |||||
| <20 VS. ≥20 | 0.657 | 0.239 | 7.581 | 0.006 | 1.930 (1.209–3.082) |
| ALN | |||||
| Positive VS. Negative | −1.466 | 0.248 | 34.919 | <0.001 | 0.231 (0.142–0.375) |
| Mass shape | |||||
| Round, oval VS. Irregular | 0.719 | 0.239 | 9.017 | 0.003 | 2.052 (1.284–3.280) |
| Mass orientation | |||||
| Parallel VS. Not-parallel | 0.453 | 0.193 | 5.492 | 0.019 | 1.573 (1.077–2.297) |
| Molecular subtypes | |||||
| Luminal A VS. TN | 0.512 | 0.103 | 24.733 | <0.001 | 1.669 (1.364–2.042) |
| Constant | −1.773 | 0.789 | 5.037 | 0.025 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, Q.; Dong, Z.; Jiang, L.; Zhang, L.; Li, Z.; Wang, D. Predictive Value of Ultrasound Characteristics for Disease-Free Survival in Breast Cancer. Diagnostics 2022, 12, 1587. https://doi.org/10.3390/diagnostics12071587
Guo Q, Dong Z, Jiang L, Zhang L, Li Z, Wang D. Predictive Value of Ultrasound Characteristics for Disease-Free Survival in Breast Cancer. Diagnostics. 2022; 12(7):1587. https://doi.org/10.3390/diagnostics12071587
Chicago/Turabian StyleGuo, Qiang, Zhiwu Dong, Lixin Jiang, Lei Zhang, Ziyao Li, and Dongmo Wang. 2022. "Predictive Value of Ultrasound Characteristics for Disease-Free Survival in Breast Cancer" Diagnostics 12, no. 7: 1587. https://doi.org/10.3390/diagnostics12071587








