Therapy Follows Diagnosis: Old and New Approaches for the Treatment of Acute Porphyrias, What We Know and What We Should Know
Abstract
:1. General Aspects (Introduction)
1.1. Heme—Its Role in Human Metabolism
1.2. Heme Biosynthesis and Degradation
1.3. Regulation of Heme Biosynthesis and Degradation (“Hemeostasis”)
1.4. The Acute Porphyrias: Disorders of Dysregulation of Heme Biosynthesis
1.5. The Acute Attack: Pathophysiology and Diagnosis
1.6. Natural History of the Disease: Severe and Non-Severe Patients, the “Chronic” Patient
1.7. Translational Projects
2. Therapy
2.1. General Aspects and Rationale
2.2. Glucose Therapy-Pros and Cons
2.2.1. Relationship of Heme and Energy Metabolism
2.2.2. Translational Projects
2.3. Heme/Hemin Therapy-Pro and Cons
2.3.1. Heme/Hemin for Treatment of Acute Attacks
2.3.2. Prophylactic Heme/Hemin Therapy
2.3.3. Economical Aspects of Prophylactic Heme Therapy
2.3.4. Translational Projects
2.4. Givosiran Therapy-Pros and Cons
2.4.1. Givosiran Therapy in Acute Porphyrias
2.4.2. Economical Aspects
2.4.3. Translational Projects
3. New Therapeutical Approaches
4. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhang, L. Heme Biology: Heme Acts as a Versatile Signaling Molecule Regulating Diverse Biological Processes, 2nd ed.; World Scientific: Hackensack, NJ, USA, 2020. [Google Scholar]
- Shimizu, T.; Lengalova, A.; Martínek, V.; Martínková, M. Heme: Emergent roles of heme in signal transduction, functional regulation and as catalytic centres. Chem. Soc. Rev. 2019, 48, 5624–5657. [Google Scholar] [CrossRef] [PubMed]
- Kühl, T.; Imhof, D. Regulatory Fe II/III Heme: The reconstruction of a molecule´s biography. ChemBioChem 2014, 15, 2024–2035. [Google Scholar] [CrossRef] [PubMed]
- Taoka, S.; Lepore, B.W.; Kabil, O.; Ojha, S.; Ringe, D.; Banerjee, R. Human cystathionine beta-synthase is a heme sensor protein. Evidence that the redox sensor is heme and not the vicinal cysteines in the CXXC motif seen in the crystal structure of the truncated enzyme. Biochemistry 2002, 41, 10454–10461. [Google Scholar]
- Tolosano, E.; Fagoonee, S.; Morello, N.; Vinchi, F.; Fiorito, V. Heme scavenging and the other facets of hemopexin. Antioxid. Redox Signal. 2010, 12, 305–320. [Google Scholar] [CrossRef] [PubMed]
- Owen, O.E.; Kalhan, S.C.; Hanson, R.W. The key role of anaplerosis and cataplerosis for citric acid cycle function. J. Biol. Chem. 2002, 277, 30409–30412. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ryter, S.W. Heme Oxgenase-1, a Cardinal Modulator of Regulated Cell Death and Inflammation. Cells 2021, 10, 515. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, Y.; Ashino, T.; Kobayashi, Y. Chemical-induced and reciprocal changes in heme metabolism, cytochrome P450 synthesis and others in the liver of human and rodents. J. Toxicol. Sci. 2016, 41, SP89–SP103. [Google Scholar] [CrossRef] [Green Version]
- Philipps, J.D. Heme biosynthesis and the porphyrias. Mol. Gen. Metab. 2019, 128, 164–177. [Google Scholar] [CrossRef] [PubMed]
- Hahn, D.; Shin, S.H.; Bae, J.S. Natural Antioxidant and Anti-Inflammatory Compounds in Foodstuff or Medicinal Herbs Inducing Heme Oxygenase-1 Expression. Antioxidants 2020, 9, 1191. [Google Scholar] [CrossRef]
- Yasuda, M.; Chen, B.; Desnick, R.J. Recent advances on Porphyria genetics: Penetrance & molecular heterogeneity, including new modifying/causative genes. Mol. Gen. Metab. 2019, 128, 320–331. [Google Scholar]
- To-Figueras, J.; Lopez, R.M.; Deulofeu, R.; Herrero, C. Preliminary report: Hyperhomocysteinemia in patients with acute intermittent porphyria. Metabolism 2010, 59, 1809–1810. [Google Scholar] [CrossRef] [PubMed]
- Ventura, P.; Corradini, E.; Di Pierro, E.; Marchini, S.; Marcacci, M.; Cuoghi, C.; Buzzetti, E.; Pietrangelo, A. Hyperhomocysteinemia in patients with acute porphyrias: A potentially dangerous metabolic crossroad? Eur. J. Intern. Med. 2020, 79, 101–107. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Gomez, A.; Aguilera, P.; Langohr, K.; Casals, G.; Pavon, C.; Marcos, J.; To-Figueras, J.; Pozo, O.J. Evaluation of Metabolic Changes in Acute Intermittent Porphyria Patients by Targeted Metabolomics. Int. J. Mol. Sci. 2022, 23, 3219. [Google Scholar] [CrossRef] [PubMed]
- Bronisch, O.; Stauch, T.; Haverkamp, T.; Beykirch, M.K.; Petrides, P.E. Acute porphyrias: A German monocentric study of the biochemical, molecular genetic, and clinical data of 62 families. Ann. Hematol. 2019, 98, 2683–2691. [Google Scholar] [CrossRef]
- Zainuddin, N.M.; Sthnashwar, P.; DB Vethakkan, S.R. Acute intermittent porphyria: A rare cause of hyponatremia. Malays. J. Pathol. 2019, 41, 369–372. [Google Scholar]
- Ricci, A.; Di Pierro, E.; Marcacci, M.; Ventura, P. Mechanisms of Neuronal Damage in Acute Hepatic Porphyrias. Diagnostics 2021, 11, 2205. [Google Scholar] [CrossRef]
- Mustajoki, P.; Timonen, K.; Gorchein, A.; Seppäläinen, A.M.; Matikainen, E.; Tenhunen, R. Sustained high plasma 5-aminolaevulinic acid concentration in a volunteer: No porphyric symptoms. Eur. J. Clin. Investig. 1992, 22, 407–411. [Google Scholar] [CrossRef]
- Edwards, S.; Jackson, D.; Reynoldson, J.; Shanley, B. Neuropharmacology of δ-aminolaevulinic acid. II. Effect of chronic administration in mice. Neurosci. Lett. 1984, 50, 169–173. [Google Scholar]
- Cozzens, J.W.; Lokaitis, B.C.; Moore, B.E.; Amin, D.V.; Espinosa, J.A.; MacGregor, M.; Michael, A.P.; Jones, B.A. A Phase 1 Dose-Escalation Study of Oral 5-Aminolevulinic Acid in Adult Patients Undergoing Resection of a Newly Diagnosed or Recurrent High-Grade Glioma. Neurosurgery 2017, 81, 46–55. [Google Scholar] [CrossRef]
- Higashikawa, F.; Noda, M.; Awaya, T.; Tanaka, T.; Sugiyama, M. 5-aminolevulinic acid, a precursor of heme, reduces both fasting and postprandial glucose levels in mildly hyperglycemic subjects. Nutrition 2013, 29, 1030–1036. [Google Scholar] [CrossRef]
- Nakamura, Y.; Haraguchi, A.; Shigeno, R.; Ito, A.; Horie, I.; Kawakami, A.; Abiru, N. A single-arm, open-label, intervention study to investigate the improvement of glucose tolerance after administration of the 5-aminolevulinic acid (5-ALA) in the patients with mitochondrial diabetes mellitus. Medicine 2021, 100, e25100. [Google Scholar] [CrossRef]
- Higashikawa, F.; Kanno, K.; Ogata, A.; Sugiyama, M. Reduction of fatigue and anger-hostility by the oral administration of 5-aminolevulinic acid phosphate: A randomized, double-blind, placebo-controlled, parallel study. Sci. Rep. 2020, 10, 16004. [Google Scholar] [CrossRef] [PubMed]
- Bonkowsky, H.L.; Tschudy, D.P.; Collins, A.; Doherty, J.; Bossenmaier, I.; Cardinal, R.; Watson, C.J. Repression of the overproduction of porphyrin precursors in acute intermittent porphyria by intravenous infusions of hematin. Proc. Natl. Acad. Sci. USA 1971, 68, 2725–2729. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bechara, E.J.H.; Ramos, L.D.; Stevani, C.V. 5-Aminolevulinic acid: A matter of life and caveats. J. Photochem. Photobiol. 2021, 7, 100036. [Google Scholar] [CrossRef]
- Bechara, E.J.H.; Dutra, F.; Cardoso, V.E.S.; Sartori, A.; Olympio, K.P.K.; Penatti, C.A.A.; Adhikari, A.; Assunção, N.A. The dual face of endogenous alpha-aminoketones: Pro-oxidizing metabolic weapons. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2007, 146, 88–110. [Google Scholar] [CrossRef]
- Gouya, L.; Ventura, P.; Balwani, M.; Bissell, D.M.; Rees, D.C.; Stölzel, U.; Phillips, J.D.; Kauppinen, R.; Langendonk, J.G.; Desnick, R.J.; et al. Explore: A Prospective, Multinational, Natural History Study of Patients with Acute Hepatic Porphyria with Recurrent Attacks. Hepatology 2020, 71, 1546–1558. [Google Scholar] [CrossRef] [Green Version]
- Naik, H.; Stoecker, M.; Sanderson, S.C.; Balwani, M.; Desnick, R.J. Experiences and concerns of patients with recurrent attacks of acute hepatic porphyria: A qualitative study. Mol. Genet. Metab. 2016, 119, 278–283. [Google Scholar] [CrossRef] [Green Version]
- Karczewski, K.; Snyder, M. Integrative omics for health and disease. Nat. Rev. Genet. 2018, 19, 299–310. [Google Scholar] [CrossRef]
- Goncharova, M.; Pshenichnikova, O.; Luchinina, Y.; Pustovoit, Y.; Karpova, I.; Surin, V. Molecular genetic study of acute intermittent porphyria in Russia: HMBS gene mutation spectrum and problem of penetrance. Clin. Genet. 2019, 96, 91–97. [Google Scholar] [CrossRef]
- Lenglet, H.; Schmitt, C.; Grange, T.; Manceau, H.; Karboul, N.; Bouchet-Crivat, F.; Robreau, A.M.; Nicolas, G.; Lamoril, J.; Simonin, S.; et al. From a dominant to an oligogenic model of inheritance with environmental modifiers in acute intermittent porphyria. Hum. Mol. Genet. 2018, 27, 1164–1173. [Google Scholar] [CrossRef] [Green Version]
- Welland, F.H.; Hellman, E.S.; Gaddis, E.M.; Collins, G.; Hunter, G.W., Jr.; Tschudy, D.P. Factors affecting the excretion of porphyrin precursors by patients with acute intermittent porphyria. I. The effect of diet. Metabolism 1964, 13, 232–250. [Google Scholar] [CrossRef]
- Storjord, E.; Dahl, J.A.; Landsem, A.; Ludviksen, J.K.; Karlsen, M.B.; Karlsen, B.O.; Brekke, O.L. Lifestyle factors including diet and biochemical biomarkers in acute intermittent porphyria: Results from a case-control study in northern Norway. Mol. Genet. Metab. 2019, 128, 254–270. [Google Scholar] [CrossRef] [PubMed]
- Sixel-Dietrich, F.; Verspohl, F.; Doss, M. Hyperinsul.linemia in acute intermittent porphyria. Horm. Metab. Res. 1985, 17, 375–376. [Google Scholar] [CrossRef] [PubMed]
- Yalouris, A.G.; Raptis, S.A. Effect of diabetes on porphyric attacks. BMJ 1987, 295, 1237–1238. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lithner, F. Beneficial Effect of Diabetes on Acute Intermittent Porphyria. Diabetes Care 2002, 25, 797–798. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Andersson, C.; Bylesjö, I.; Lithner, F. Effects of diabetes mellitus on patients with acute intermittent porphyria. J. Intern. Med. 1999, 245, 193–197. [Google Scholar] [CrossRef] [PubMed]
- Bitar, M.; Weiner, M. Diabetes induced alterations in heme synthesis and degradation and various heme containing enzymes in female rats. Diabetes 1984, 33, 37–44. [Google Scholar] [CrossRef]
- Doss, M.; Sixel-Dietrich, F.; Verspohl, F. “Glucose effect” and rate limiting function of uroporphyrinogen synthase on porphyrin metabolism in hepatocyte culture: Relationship with human acute hepatic porphyrias. Clin. Chem. Lab. Med. 1981, 23, 505–513. [Google Scholar] [CrossRef] [Green Version]
- Sardh, E.; Harper, P.; Andersson, D.E.; Floderus, Y. Plasma porphobilinogen as a sensitive biomarker to monitor the clinical and therapeutic course of acute intermittent porphyria attacks. Eur. J. Intern. Med. 2009, 20, 201–207. [Google Scholar] [CrossRef]
- Brodie, M.D.; Moore, M.R.; Thompson, G.G.; Goldberg, A. The treatment of acute intermittent porphyria with laevulose. Clin. Sci. Mol. Med. 1977, 53, 365–371. [Google Scholar] [CrossRef]
- Bonkowsky, H.L.; Magnussen, C.R.; Collins, A.R.; Doherty, J.M.; Hess, R.A.; Tschudy, D.P. Comparative effects of glycerol and dextrose on porphyrin precursor excretion in acute intermittent porphyria. Metabolism 1976, 25, 405–414. [Google Scholar] [CrossRef]
- Di Pierro, E.; Granata, F. Nutrients and Porphyria: An intriguing crosstalk. Int. J. Mol. Sci. 2020, 21, 3462. [Google Scholar] [CrossRef] [PubMed]
- Liang, H.; Ward, W.F. PGC-1alpha: A key regulator of energy metabolism. Adv. Physiol. Educ. 2006, 30, 145–151. [Google Scholar] [CrossRef] [PubMed]
- Handschin, C.; Spiegelman, B.M. Peroxisome proliferator-activated receptor gamma coactivator 1 coactivators, energy homeostasis, and metabolism. Endocrine. Rev. 2006, 27, 728–735. [Google Scholar] [CrossRef]
- Handschin, C.; Lin, J.; Rhee, J.; Peyer, A.K.; Chin, S.; Wu, P.H.; Meyer, U.A.; Spiegelman, B.M. Nutritional regulation of hepatic heme biosynthesis and porphyria through PGC-1alpha. Cell 2005, 122, 505–515. [Google Scholar] [CrossRef] [Green Version]
- Li, D. PGC-1alpha: Looking behind the sweet treat for porphyria. Cell 2005, 122, 487–489. [Google Scholar] [CrossRef] [Green Version]
- Simcox, J.A.; Mitchell, T.C.; Gao, Y.; Just, S.F.; Cooksey, R.; Cox, J.; Ajioka, R.; Jones., D.; Lee, S.H.; King, D.; et al. Dietary iron controls circadian hepatic glucose metabolism through heme synthesis. Diabetes 2015, 64, 1108–1119. [Google Scholar] [CrossRef] [Green Version]
- Yin, L.; Wu, N.; Curtin, J.C.; Qatanani, M.; Szwergold, N.R.; Reid, R.A.; Waitt, G.M.; Parks, D.J.; Pearce, K.H.; Wisely, G.B.; et al. Rev-erbalpha, a heme sensor that coordinates metabolic and circadian pathways. Science 2007, 318, 1786–1789. [Google Scholar] [CrossRef]
- Solares, I.; Izquierdo-Sánchez, L.; Morales-Conejo, M.; Jericó, D.; Castelbón, F.J.; Córdoba, K.M.; Sampedro, A.; Lumbreras, C.; Moreno-Aliaga, M.J.; Enríquez De Salamanca, R.; et al. High Prevalence of Insulin Resistance in Asymptomatic Patients with Acute Intermittent Porphyria and Liver-Targeted Insulin as a Novel Therapeutic Approach. Biomedicines 2021, 9, 255. [Google Scholar] [CrossRef]
- Herrick, A.L.; Fisher, B.M.; Moore, M.R.; Cathcart, S.; Mccoll, K.E.; Goldberg, A. Elevation of blood lactate and pyruvate levels in acute intermittent porphyria—A reflection of haem deficiency? Clin. Chim. Acta 1990, 190, 157–162. [Google Scholar] [CrossRef]
- Delaby, C.; To-Figueras, J.; Deybach, J.C.; Casamitjana, R.; Puy, H.; Herrero, C. Role of two nutritional hepatic markers (insulin-like growth factor 1 and transthyretin) in the clinical assessment and follow-up of acute intermittent porphyria patients. J. Intern. Med. 2009, 266, 277–285. [Google Scholar] [CrossRef] [PubMed]
- Matkovic, L.B.; D’andrea, F.; Fornes, D.; San Martín De Viale, L.C.; Mazzetti, M.B. How porphyrinogenic drugs modeling acute porphyria impair the hormonal status that regulates glucose metabolism. Their relevance in the onset of this disease. Toxicology 2011, 290, 22–30. [Google Scholar] [PubMed]
- Collantes, M.; Serrano-Mendioroz, I.; Benito, M.; Molinet-Dronda, F.; Delgado, M.; Vinaixa, M.; Sampedro, A.; Enríquez de Salamanca, R.; Prieto, E.; Pozo, M.A.; et al. Glucose metabolism during fasting is altered in experimental porphobilinogen deaminase deficiency. Hum. Mol. Genet. 2016, 25, 1318–1327. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carichon, M.; Pallet, N.; Schmitt, C.; Lefebvre, T.; Gouya, L.; Talbi, N.; Deybach, J.C.; Beaune, P.; Vasos, P.; Puy, H.; et al. Urinary metabolic fingerprint of acute intermittent porphyria analyzed by (1)H NMR spectroscopy. Anal. Chem. 2014, 86, 2166–2174. [Google Scholar] [CrossRef]
- Luck, M.; Schmitt, C.; Talbi, N.; Gouya, L.; Caradeuc, C.; Puy, H.; Bertho, G.; Pallet, N. Urinary metabolic profiling of asymptomatic acute intermittent porphyria using a rule-mining-based algorithm. Metabolomics 2018, 14, 10. [Google Scholar] [CrossRef] [Green Version]
- Lin, C.; Shiao, M.; Cheng, M.; Chen, C.; Kuo, H. Profiling of Serum Metabolites of Acute Intermittent Porphyria and Asymptomatic HMBS Mutation Carriers. Cells 2021, 10, 2579. [Google Scholar] [CrossRef]
- Dixon, N.; Li, T.; Marion, B.; Faust, D.; Dozier, S.; Molina, A.; Rudnick, S.; Bonkovsky, H.L. Pilot study of mitochondrial bioenergetics in subjects with acute porphyrias. Mol. Genet. Metab. 2019, 128, 228–235. [Google Scholar] [CrossRef]
- Chacko, B.; Culp, M.L.; Bloomer, J.; Phillips, J.; Kuo, Y.; Darley-Usmar, V.; Singal, A.K. Feasibility of cellular bioenergetics as a biomarker in porphyria patients. Mol. Genet. Metab. Rep. 2019, 19, 100451. [Google Scholar] [CrossRef]
- Longo, M.; Paolini, E.; Meroni, M.; Duca, L.; Motta, I.; Fracanzani, A.L.; Di PIierro, E.; Dongiovanni, P. α-Lipoic Acid Improves Hepatic Metabolic Dysfunctions in Acute Intermittent Porphyria: A Proof-of-Concept Study. Diagnostics 2021, 11, 1628. [Google Scholar] [CrossRef]
- Storjord, E.; Dahl, J.A.; Landsem, A.; Fure, H.; Ludviksen, J.K.; Goldbeck-Wood, S.; Karlsen, B.O.; Berg, K.S.; Mollnes, T.E.; Nielsen, E.W.; et al. Systemic inflammation in acute intermittent porphyria: A case-control study. Clin. Exp. Immunol. 2017, 187, 466–479. [Google Scholar] [CrossRef] [Green Version]
- Saitoh, S.; Okano, S.; Nohara, H.; Nakano, H.; Shirasawa, N.; Naito, A.; Yamamoto, M.; Kelly, V.P.; Takahashi, K.; Tanaka, T.; et al. 5-aminolevulinic acid (ALA) deficiency causes impaired glucose tolerance and insulin resistance coincident with an attenuation of mitochondrial function in aged mice. PLoS ONE 2018, 13, e0189593. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Solares, I.; Tejedor, M.; Jerico, D.; Molares-Conejo, M.; de Salamanca, R.E.; Fontanellas, A. Management of hyponatremia associated with porphyria-proposal for the use of tolvaptan. Ann. Transl. Med. 2020, 8, 1098. [Google Scholar] [CrossRef] [PubMed]
- Bonkovsky, H.L.; Healey, J.F.; Lourie, A.N.; Gerron, G.G. Intravenous heme-albumin in acute intermittent porphyria: Evidence for repletion of hepatic hemoproteins and regulatory heme pools. Am. J. Gastroenterol. 1991, 86, 1050–1056. [Google Scholar] [PubMed]
- Anderson, K.E.; Bonkovsky, H.L.; Bloomer, J.R.; Shedlofsky, S.I. Reconstitution of hematin for intravenous infusion. Ann. Intern. Med. 2006, 144, 537–538. [Google Scholar] [CrossRef] [Green Version]
- Goetsch, C.A.; Bissell, D.M. Instability of hematin used in the treatment of acute hepatic porphyria. N. Engl. J. Med. 1986, 315, 235–238. [Google Scholar] [CrossRef]
- Pass, I.J.; Schwartz, S.; Watson, C.J. The conversion of hematin to bilirubin following intravenous administration in human subjects. J. Clin. Investig. 1945, 24, 283–291. [Google Scholar] [CrossRef]
- Dhar, G.J.; Bossenmaier, I.; Petryka, Z.J.; Cardinal, R.; Watson, C.J. Effects of hematin in hepatic porphyria. Further studies. Ann. Intern. Med. 1975, 83, 20–30. [Google Scholar] [CrossRef]
- Peterson, A.; Bossenmaier, I.; Cardinal, R.; Watson, C.J. Hematin treatment of acute porphyria. Early remission of an almost fatal relapse. JAMA 1976, 235, 520–522. [Google Scholar] [CrossRef]
- Watson, C.J.; Pierach, C.A.; Bossenmaier, I.; Cardinal, R. Postulated deficiency of hepatic heme and repair by hematin infusions in the “inducible” hepatic porphyrias. Proc. Natl. Acad. Sci. USA 1977, 74, 2118–2120. [Google Scholar] [CrossRef] [Green Version]
- Watson, C.J.; Pierach, C.A.; Bossenmaier, I.; Cardinal, R. Use of hematin in the acute attack of the “inducible” hepatic prophyrias. Adv. Intern. Med. 1978, 23, 265–286. [Google Scholar]
- Lamon, J.M.; Frykholm, B.C.; Hess, R.A.; Tschudy, D.P. Hematin therapy for acute porphyria. Medicine 1979, 58, 252–269. [Google Scholar] [CrossRef] [PubMed]
- McColl, K.E.; Moore, M.R.; Thompson, G.G.; Goldberg, A. Treatment with haematin in acute hepatic porphyria. QJM 1981, 50, 161–174. [Google Scholar] [PubMed]
- Pierach, C.A.; Bossenmaier, I.; Cardinal, R.; Weimer, M.; Watson, C.J. Hematin therapy in porphyric attacks. Klin. Wochenschr. 1980, 58, 829–832. [Google Scholar] [CrossRef] [PubMed]
- Pierach, C.A. Hematin therapy for the porphyric attack. Semin. Liver Dis. 1982, 2, 125–131. [Google Scholar] [CrossRef]
- Kuo, H.C.; Lee, M.J.; Chuang, W.L.; Huang, C.C. Acute intermittent porphyria with peripheral neuropathy: A follow-up study after hematin treatment. J. Neurol. Sci. 2007, 260, 231–235. [Google Scholar] [CrossRef]
- Attarian, S.; Yu, C.; Anderson, K.E.; Friedman, E.W. Effects of hemin and hemodialysis in a patient with acute intermittent porphyria and renal failure. Blood Adv. 2017, 1, 915–917. [Google Scholar] [CrossRef] [Green Version]
- Mustajoki, P.; Tenhunen, R.; Tokola, O.; Gothoni, G. Haem arginate in the treatment of acute hepatic porphyrias. BMJ 1986, 293, 538–539. [Google Scholar] [CrossRef] [Green Version]
- Herrick, A.; McColl, K.E.; Mclellan, A.; Moore, M.R.; Brodie, M.J.; Goldberg, A. Effect of haem arginate therapy on porphyrin metabolism and mixed function oxygenase activity in acute hepatic porphyria. Lancet 1987, 2, 1178–1179. [Google Scholar] [CrossRef]
- Tokola, O.; Mustajoki, P.; Himberg, J.J. Haem arginate improves hepatic oxidative metabolism in variegate porphyria. Br. J. Clin. Pharmacol. 1988, 26, 753–757. [Google Scholar] [CrossRef] [Green Version]
- Volin, L.; Rasi, V.; Vahtera, E.; Tenhunen, R. Heme arginate: Effects on hemostasis. Blood 1988, 71, 625–628. [Google Scholar] [CrossRef] [Green Version]
- Herrick, A.L.; McColl, K.E.; Moore, M.R.; Cook, A.; Goldberg, A. Controlled trial of haem arginate in acute hepatic porphyria. Lancet 1989, 1, 1295–1297. [Google Scholar] [CrossRef]
- Kostrzewska, E.; Gregor, A.; Tarczyńska-Nosal, S. Heme arginate (Normosang) in the treatment of attacks of acute hepatic porphyrias. Mater. Med. Pol. Pol. J. Med. Pharm. 1991, 23, 259–262. [Google Scholar]
- Mustajoki, P.; Nordmann, Y. Early administration of heme arginate for acute porphyric attacks. Arch. Intern. Med. 1993, 153, 2004–2008. [Google Scholar] [CrossRef]
- Muthane, U.B.; Vengamma, B.; Bharathi, K.C.; Mamatha, P. Porphyric neuropathy: Prevention of progression using haeme-arginate. J. Intern. Med. 1993, 234, 611–613. [Google Scholar] [CrossRef] [PubMed]
- Ma, E.; Mar, V.; Varigos, G.; Nicoll, A.; Ross, G. Haem arginate as effective maintenance therapy for hereditary coproporphyria. Australas. J. Dermatol. 2011, 52, 135–138. [Google Scholar] [CrossRef]
- Lahiji, A.P.; Anderson, K.E.; Chan, A.; Simon, A.; Desnick, R.J.; Ramanujam, V.M.S. 5-Aminolevulinate dehydratase porphyria: Update on hepatic 5-aminolevulinic acid synthase induction and long-term response to hemin. Mol. Genet. Metab. 2020, 131, 418–423. [Google Scholar] [CrossRef]
- Chapman, E.; Leal, D.; Matijasevic, E.; García, E. Desensitization in patients with hypersensitivity to haem arginate: A case report. World Allergy Organ. J. 2019, 12, 100002. [Google Scholar] [CrossRef] [Green Version]
- Dover, S.B.; Graham, A.; Fitzsimons, E.; Moore, M.R.; Mccoll, K.E.L. Haem-arginate plus tin-protoporphyrin for acute hepatic porphyria. Lancet 1991, 338, 263. [Google Scholar] [CrossRef]
- Isenschmid, M.; König, C.; Fässli, C.; Haenel, A.; Hänggi, W.; Schneider, H. Akute intermittierende Porphyrie in der Schwangerschaft: Therapie mit Glukose oder Hämatin? Acute intermittent porphyria in pregnancy: Glucose or hematin therapy? Schweiz. Med. Wochenschr. 1992, 122, 1741–1745. [Google Scholar]
- Kuo, H.; Lin, C.; Tang, Y. Prophylactic Heme Arginate Infusion for Acute Intermittent Porphyria. Front. Pharmacol. 2021, 12, 712305. [Google Scholar] [CrossRef]
- Yarra, P.; Faust, D.; Bennett, M.; Rudnick, S.; Bonkovsky, H.L. Benefits of prophylactic heme therapy in severe acute intermittent porphyria. Mol. Genet. Metab. Rep. 2019, 19, 100450. [Google Scholar] [CrossRef] [PubMed]
- Anderson, K.E.; Collins, S. Open-label study of hemin for acute porphyria: Clinical practice implications. Am. J. Med. 2006, 119, 801.e1–801.e6. [Google Scholar] [CrossRef] [PubMed]
- Bonkovsky, H.L.; Maddukuri, V.C.; Yazici, C.; Anderson, K.E.; Bissell, D.M.; Bloomer, J.R.; Phillips, J.D.; Naik, H.; Peter, I.; Baillargeon, G.; et al. Acute porphyrias in the USA: Features of 108 subjects from porphyrias consortium. Am. J. Med. 2014, 127, 1233–1241. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marsden, J.T.; Guppy, S.; Stein, P.; Cox, T.M.; Badminton, M.; Gardiner, T.; Barth, J.H.; Stewart, M.F.; Rees, D.C. Audit of the Use of Regular Haem Arginate Infusions in Patients with Acute Porphyria to Prevent Recurrent Symptoms. JIMD Rep. 2015, 22, 57–65. [Google Scholar]
- Schmitt, C.; Lenglet, H.; Yu, A.; Delaby, C.; Benecke, A.; Lefebvre, T.; Letteron, P.; Paradis, V.; Wahlin, S.; Sandberg, S.; et al. Recurrent attacks of acute hepatic porphyria: Major role of the chronic inflammatory response in the liver. J. Intern. Med. 2018, 284, 78–91. [Google Scholar] [CrossRef] [Green Version]
- Willandt, B.; Langendonk, J.G.; Biermann, K.; Meersseman, W.; D’Heygere, F.; George, C.; Verslype, C.; Monbaliu, D.; Cassiman, D. Liver Fibrosis Associated with Iron Accumulation Due to Long-Term Heme-Arginate Treatment in Acute Intermittent Porphyria: A Case Series. JIMD Rep. 2016, 25, 77–81. [Google Scholar]
- Connolly, M.P.; Kotsopoulos, N.; Vermeersch, S.; Patris, J.; Cassiman, D. Estimating the broader fiscal consequences of acute hepatic porphyria (AHP) with recurrent attacks in Belgium using a public economic analytic framework. Orphanet J. Rare Dis. 2021, 16, 346. [Google Scholar] [CrossRef]
- Neeleman, R.A.; Wagenmakers, M.; Koole-Lesuis, R.H.; Mijnhout, G.S.; Wilson, J.; Friesema, E.; Langendonk, J.G. Medical and financial burden of acute intermittent porphyria. J. Inherit. Metab. Dis. 2018, 41, 809–817. [Google Scholar] [CrossRef] [Green Version]
- Blaylock, B.; Epstein, J.; Stickler, P. Real-world annualized healthcare utilization and expenditures among insured US patients with acute intermittent porphyria (AIP) treated with hemin. J. Med. Econ. 2020, 23, 537–545. [Google Scholar] [CrossRef] [Green Version]
- Bonkowsky, H.L.; Sinclair, P.R.; Sinclair, J.F. Hepatic heme metabolism and its control. Yale J. Biol. Med. 1979, 52, 13–37. [Google Scholar]
- Waxman, A.D.; Collins, A.; Tschudy, D.P. Oscillations of hepatic δ-aminolevulinic acid synthetase produced in, vivo by heme. Biochem. Biophys. Res. Commun. 1966, 24, 675–683. [Google Scholar] [CrossRef]
- Elbashir, S.M.; Harborth, J.; Lendeckel, W.; Yalcin, A.; Weber, K.; Tuschl, T. Duplexes of 21-nucleotide RNAs mediate RNA interference in cultured mammalian cells. Nature 2001, 411, 494–498. [Google Scholar] [CrossRef] [PubMed]
- Yasuda, M.; Gan, L.; Chen, B.; Kadirvel, S.; Yu, C.; Phillips, J.D.; New, M.I.; Liebow, A.; Fitzgerald, K.; Querbes, W.; et al. RNAi-mediated silencing of hepatic Alas1 effectively prevents and treats the induced acute attacks in acute intermittent porphyria mice. Proc. Natl. Acad. Sci. USA 2021, 111, 7777–7782. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vassiliou, D.; Sardh, E.; Harper, P.; Simon, A.R.; Clausen, V.A.; Najafian, N.; Robbie, G.J.; Agarwal, S. A Drug-Drug Interaction Study Evaluating the Effect of Givosiran, a Small Interfering Ribonucleic Acid, on Cytochrome P450 Activity in the Liver. Clin. Pharmacol. Ther. 2021, 110, 1250–1260. [Google Scholar] [CrossRef]
- Balwani, M.; Sardh, E.; Ventura, P.; Peiró, P.A.; Rees, D.C.; Stölzel, U.; Bissell, D.M.; Bonkovsky, H.L.; Windyga, J.; Anderson, K.E.; et al. Phase 3 Trial of RNAi Therapeutic Givosiran for Acute Intermittent Porphyria. New Engl. J. Med. 2020, 382, 2289–2301. [Google Scholar] [CrossRef]
- Ventura, P.; Bonkovsky, H.L.; Gouya, L.; Aguilera-Peiró, P.; Montgomery Bissell, D.; Stein, P.E.; Balwani, M.; Anderson, D.; Parker, C.; Kuter, D.J.; et al. Envision Investigators. Efficacy and safety of givosiran for acute hepatic porphyria: 24-month interim analysis of the randomized phase 3 ENVISION study. Liver Int. 2022, 42, 161–172. [Google Scholar] [CrossRef]
- Lazareth, H.; Poli, A.; Bignon, Y.; Mirmiran, A.; Rabant, M.; Cohen, R.; Schmitt, C.; Puy, H.; Karras, A.; Gouya, L.; et al. Renal Function Decline With Small Interfering RNA Silencing Aminolevulinic Acid Synthase 1 (ALAS1). Kidney Int. Rep. 2021, 6, 1904–1911. [Google Scholar] [CrossRef]
- Petrides, P.E.; Klein, M.; Schuhmann, E.; Torkler, H.; Molitor, B.; Loehr, C.; Obermeier, Z.; Beykirch, M.K. Severe homocysteinemia in two givosiran-treated porphyria patients: Is free heme deficiency the culprit? Ann. Hematol. 2021, 100, 1685–1693. [Google Scholar] [CrossRef]
- To-Figueras, J.; Wijngaard, R.; García-Villoria, J.; Aarsand, A.K.; Aguilera, P.; Deulofeu, R.; Brunet, M.; Gómez-Gómez, À.; Pozo, O.J.; Sandberg, S. Dysregulation of homocysteine homeostasis in acute intermittent porphyria patients receiving heme arginate or givosiran. J. Inherit. Metab. Dis. 2021, 44, 961–971. [Google Scholar] [CrossRef]
- Ricci, A.; Marcacci, M.; Cuoghi, C.; Pietrangelo, A.; Ventura, P. Hyperhomocysteinemia in patients with acute porphyrias: A possible effect of ALAS1 modulation by siRNAm therapy and its control by vitamin supplementation. Eur. J. Intern. Med. 2021, 92, 121–123. [Google Scholar] [CrossRef]
- Fontanellas, A.; Ávila, M.A.; Arranz, E.; Enríquez de Salamanca, R.; Morales-Conejo, M. Acute intermittent porphyria, givosiran, and homocysteine. J. Inherit. Metab. Dis. 2021, 44, 790–791. [Google Scholar] [CrossRef] [PubMed]
- Vassiliou, D.; Sardh, E. Homocysteine elevation in givosiran treatment: Suggested ALAS1 siRNA effect on cystathionine beta-synthase. J. Intern. Med. 2021, 290, 928–930. [Google Scholar] [CrossRef] [PubMed]
- Ventura, P.; Sardh, E.; Longo, N.; Balwani, M.; Plutsky, J.; Gouya, L.; Philipps, J.; Rhyee, S.; Fanelli, M.-J.; Sweetser, M.T.; et al. Hyperhomocysteinemia in acute hepatic porphyria (AHP) and implications for treatment with givosiran. 2022; Revised manuscript submitted. [Google Scholar]
- Poli, A.; Schmitt, C.; Moulouel, B.; Mirmiran, A.; Talbi, N.; Rivière, S.; Cerutti, D.; Bouchoule, I.; Faivre, A.; Grobost, V.; et al. Givosiran in acute intermittent porphyria: A personalized medicine approach. Mol. Genet. Metab. 2022, 135, 206–214. [Google Scholar] [CrossRef] [PubMed]
- Hausdorf, G.; Roggenbuck, D.; Feist, E.; Büttner, T.; Jungblut, P.R.; Conrad, K.; Berg, C.; Klein, R. Autoantibodies to asialoglycoprotein receptor (ASGPR) measured by a novel ELISA—Revival of a disease-activity marker in autoimmune hepatitis. Clin. Chim. Acta 2009, 408, 19–24. [Google Scholar] [CrossRef]
- Massachi, S.; Epstein, J.; Hurd, J.; Bonkovsky, H.L. Cost savings with hemin versus givosiran for the treatment of patients with acute intermittent porphyria (AIP). J. Med. Econ. 2020, 23, 1441–1449. [Google Scholar] [CrossRef]
- Sardh, E.; Rejkjaer, L.; Andersson, D.E.; Harper, P. Safety, pharmacokinetics and pharmocodynamics of recombinant human porphobilinogen deaminase in healthy subjects and asymptomatic carriers of the acute intermittent porphyria gene who have increased porphyrin precursor excretion. Clin. Pharmacokinet. 2007, 46, 335–349. [Google Scholar] [CrossRef]
- Córdoba, K.M.; Serrano-Mendioroz, I.; Jericó, D.; Merino, M.; Jiang, L.; Sampedro, A.; Alegre, M.; Corrales, F.; Garrido, M.J.; Martini, P.; et al. Recombinant porphobilinogen deaminase targeted to the liver corrects enzymopenia in a mouse model of acute intermittent porphyria. Sci. Transl. Med. 2022, 14, eabc0700. [Google Scholar] [CrossRef]
- Jiang, L.; Berraondo, P.; Jericó, D.; Guey, L.T.; Sampedro, A.; Frassetto, A.; Benenato, K.E.; Burke, K.; Santamaría, E.; Alegre, M.; et al. Systemic messenger RNA as an etiological treatment for acute intermittent porphyria. Nat. Med. 2018, 24, 1899–1909. [Google Scholar] [CrossRef]
- Bustad, H.J.; Kallio, J.P.; Vorland, M.; Fiorentino, V.; Sandberg, S.; Schmitt, C.; Aarsand, A.K.; Martinez, A. Acute Intermittent Porphyria: An Overview of Therapy Developments and Future Perspectives Focusing on Stabilisation of HMBS and Proteostasis Regulators. Int. J. Mol. Sci. 2021, 22, 675. [Google Scholar] [CrossRef]
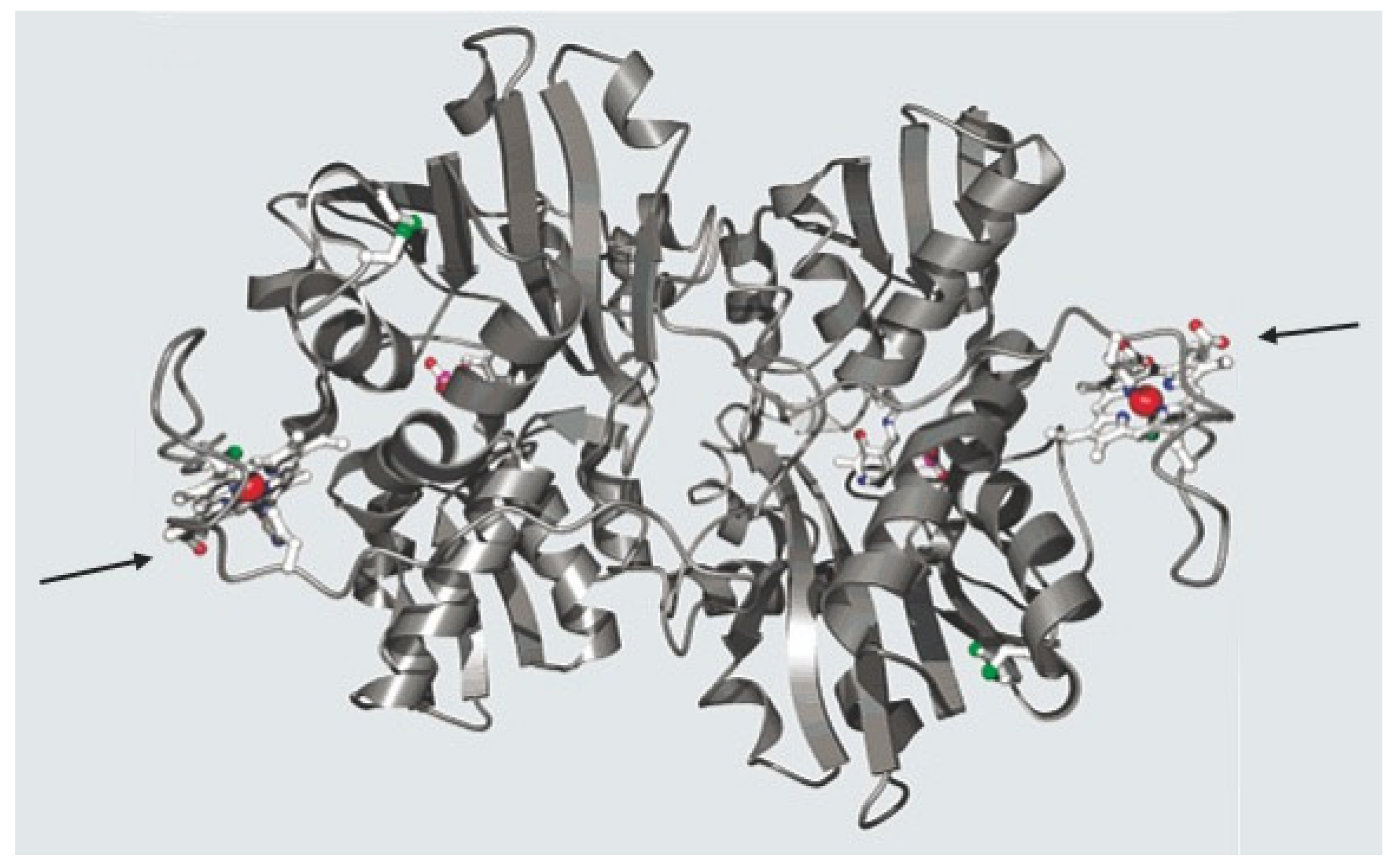
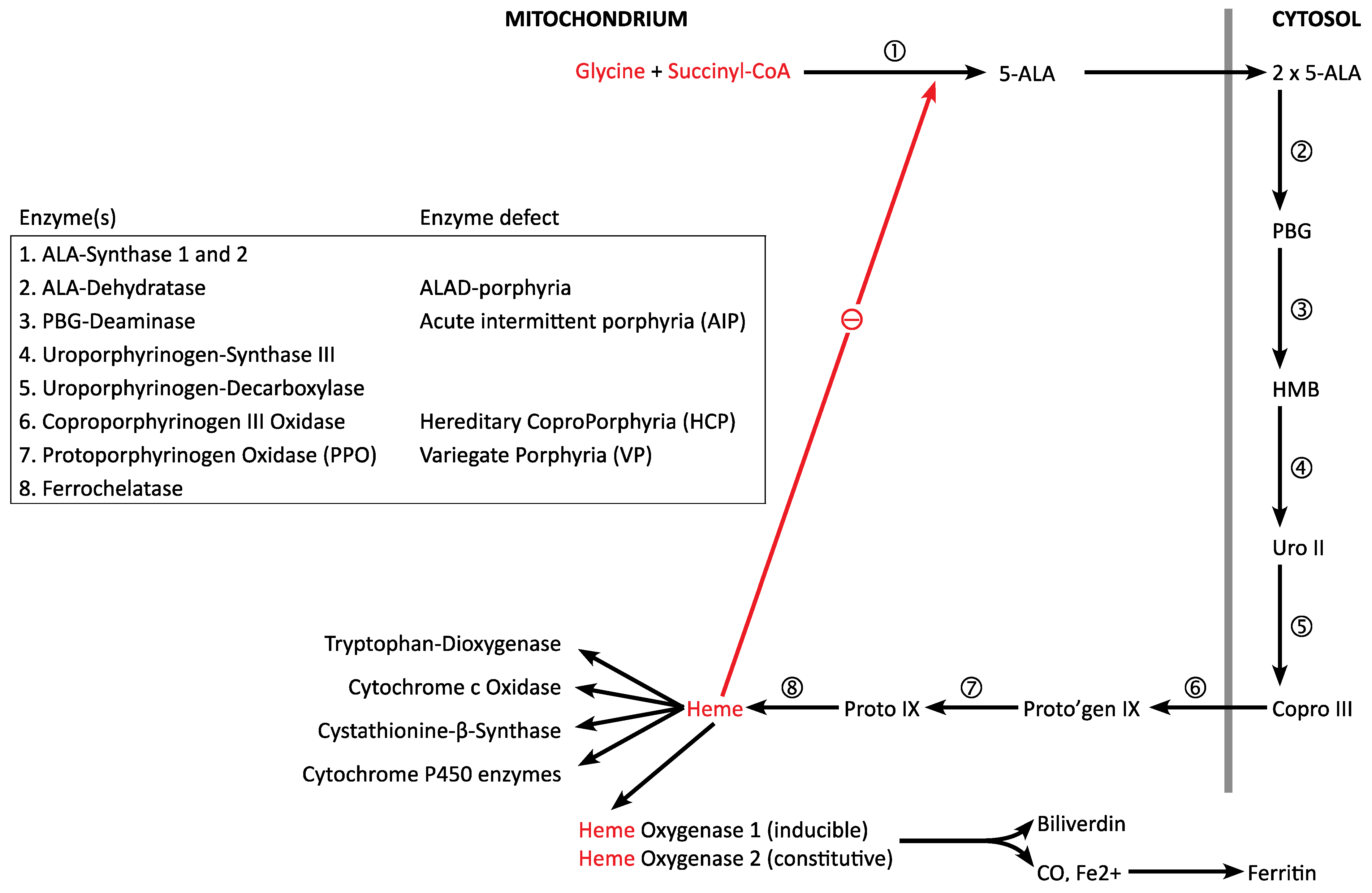

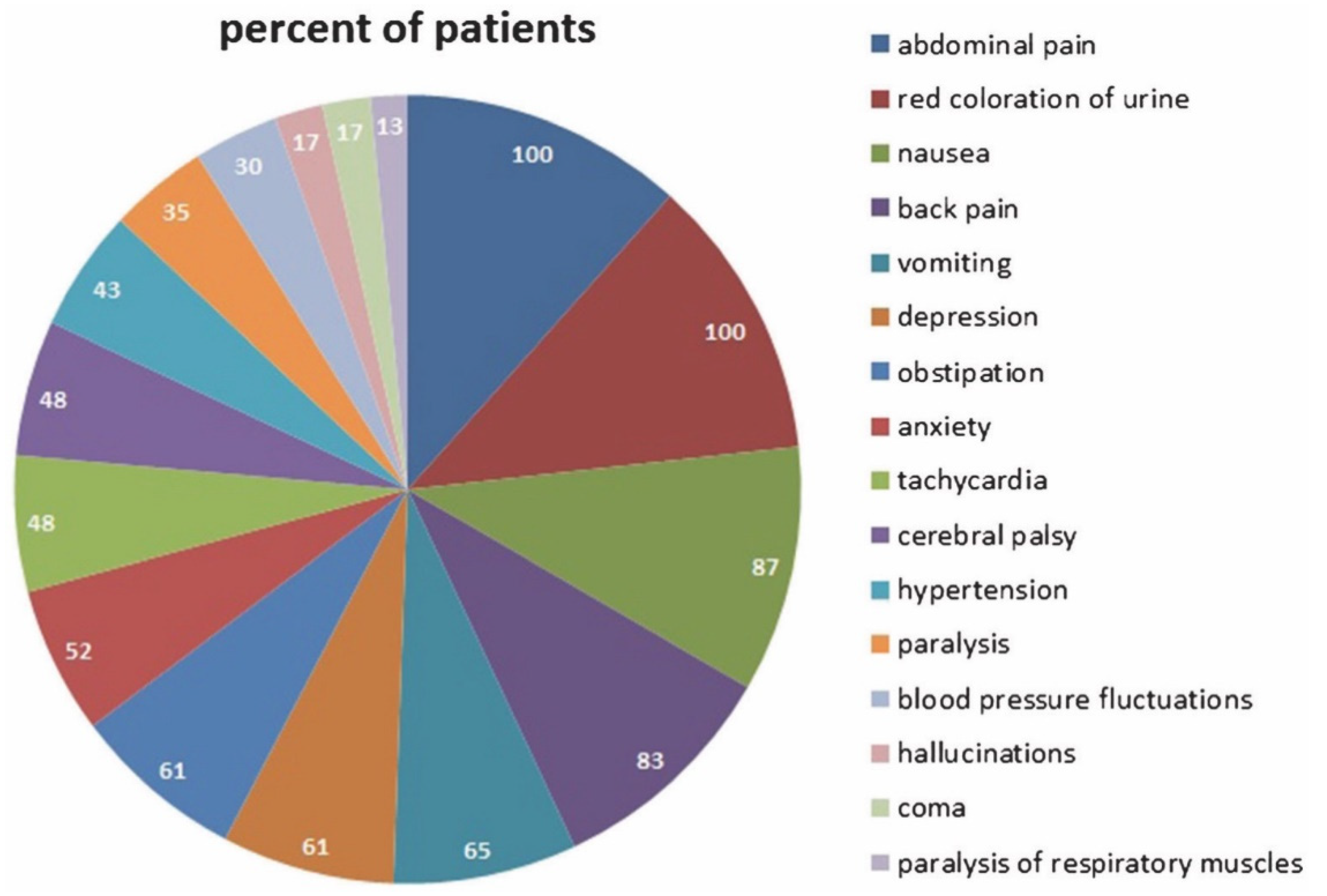
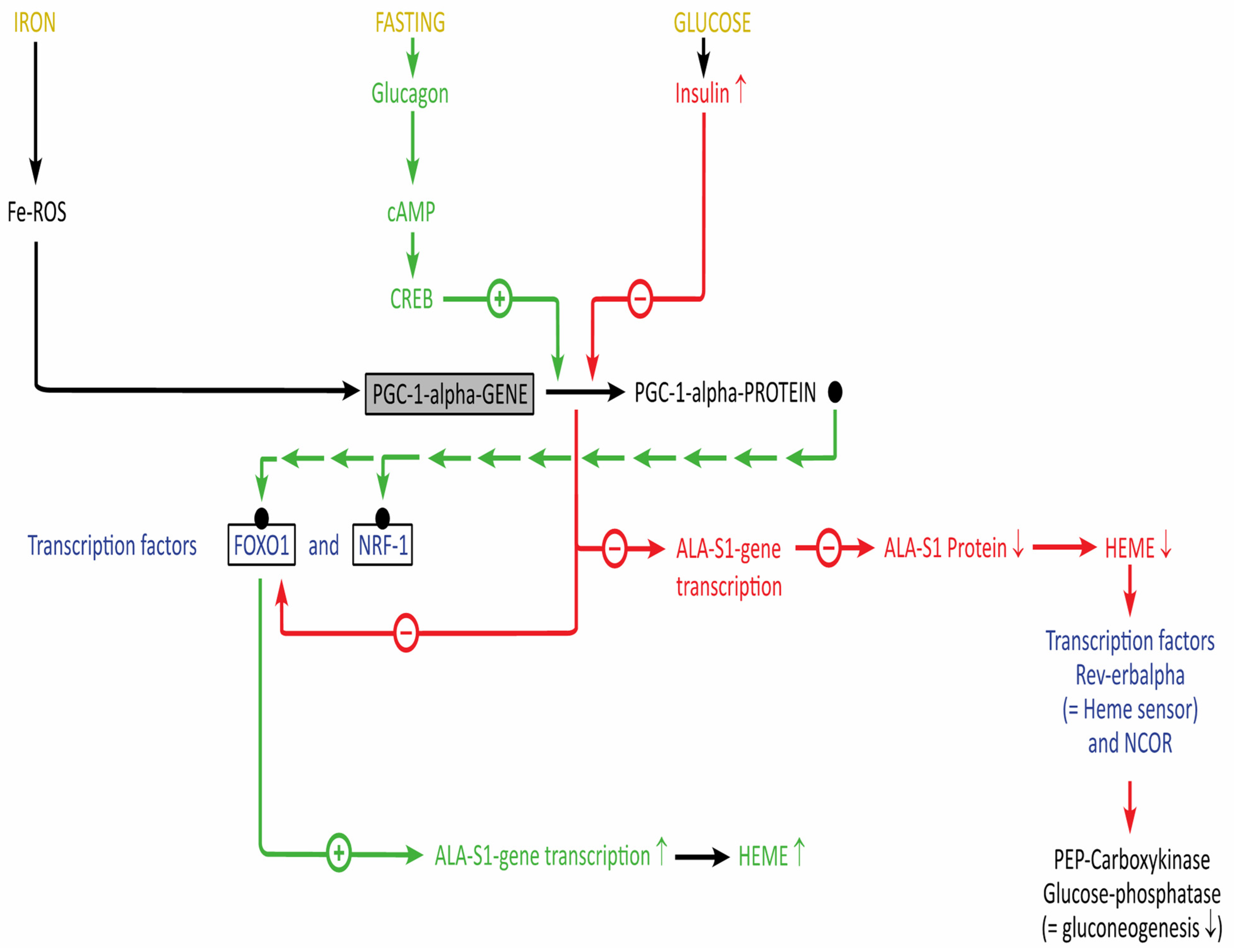
| Heme Protein | Function |
|---|---|
| Cytochrome c | electron transport |
| Hemoglobin | oxygen transport |
| Myo- und Neuroglobin | oxygen storage |
| Cytochrome P450 | metabolism of drugs and steroids |
| 5-ALA-Synthase 1 | regulation of enzyme activity |
| Transcription factor BACH 1 | regulation of heme oxygenase |
| ferritin and ferroportin | |
| Cystathionine-ß-Synthase | degradation of homocysteine |
| Myeloperoxidase Nitric oxid synthases | formation of HOCL in neutrophils production of NO from arginine |
| Rev-Erb-alpha/ß | transcription factor (circadian rhythm) |
| K+-channels | ion transporters |
| Panhematin® | Normosang® | |
|---|---|---|
| Preparation | lyophilized powder | 10 mL concentrated solution |
| Heme content per vial | 350 mg | 250 mg |
| Additional ingredients | 240 mg sodium carbonate 335 mg sorbitol | propylenglykol 96% ethanol (1 g/10 mL) |
| Arginine | ||
| Storage temperature | 20–25 °C | 2–8 °C |
| Maximum Duration of stability | 2 years | |
| Recommended dosage | 1–4 mg/kg/day for 4 days | 3 mg/kg/day for 3–14 days |
| Use of sterile filter/glass bottle recommended | yes/yes | yes/no |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Petrides, P.E. Therapy Follows Diagnosis: Old and New Approaches for the Treatment of Acute Porphyrias, What We Know and What We Should Know. Diagnostics 2022, 12, 1618. https://doi.org/10.3390/diagnostics12071618
Petrides PE. Therapy Follows Diagnosis: Old and New Approaches for the Treatment of Acute Porphyrias, What We Know and What We Should Know. Diagnostics. 2022; 12(7):1618. https://doi.org/10.3390/diagnostics12071618
Chicago/Turabian StylePetrides, Petro E. 2022. "Therapy Follows Diagnosis: Old and New Approaches for the Treatment of Acute Porphyrias, What We Know and What We Should Know" Diagnostics 12, no. 7: 1618. https://doi.org/10.3390/diagnostics12071618






