Optimization of Patient Management in the Gynecology Emergency Department Using Point-of-Care Beta hCG
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Study Protocol
2.3. Verification of the AQT90 FLEX βhCG Analytical Performances
2.4. Endpoints
2.5. Statistical Analysis
3. Results
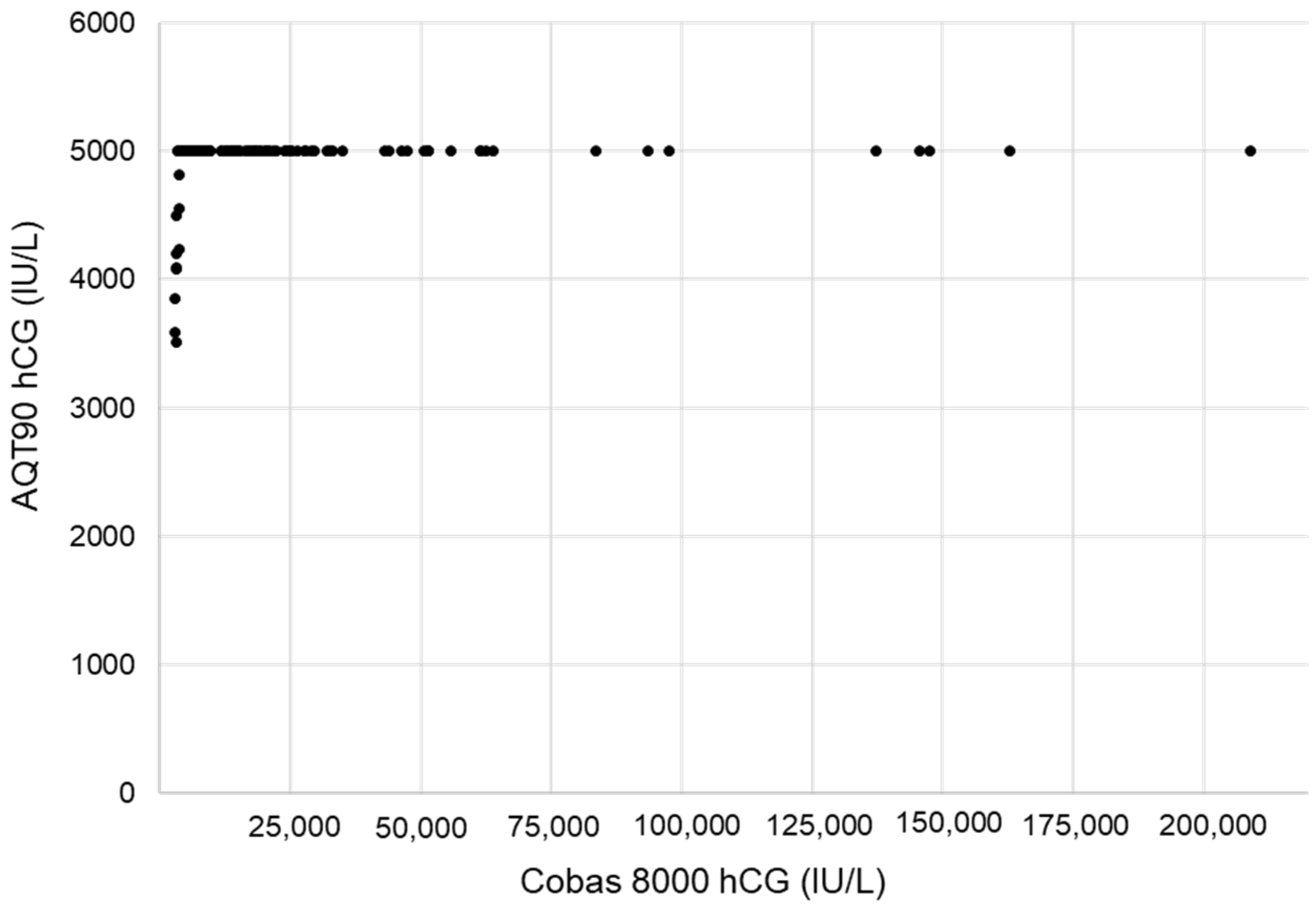
3.1. Verification of the AQT90 FLEX βhCG Performance
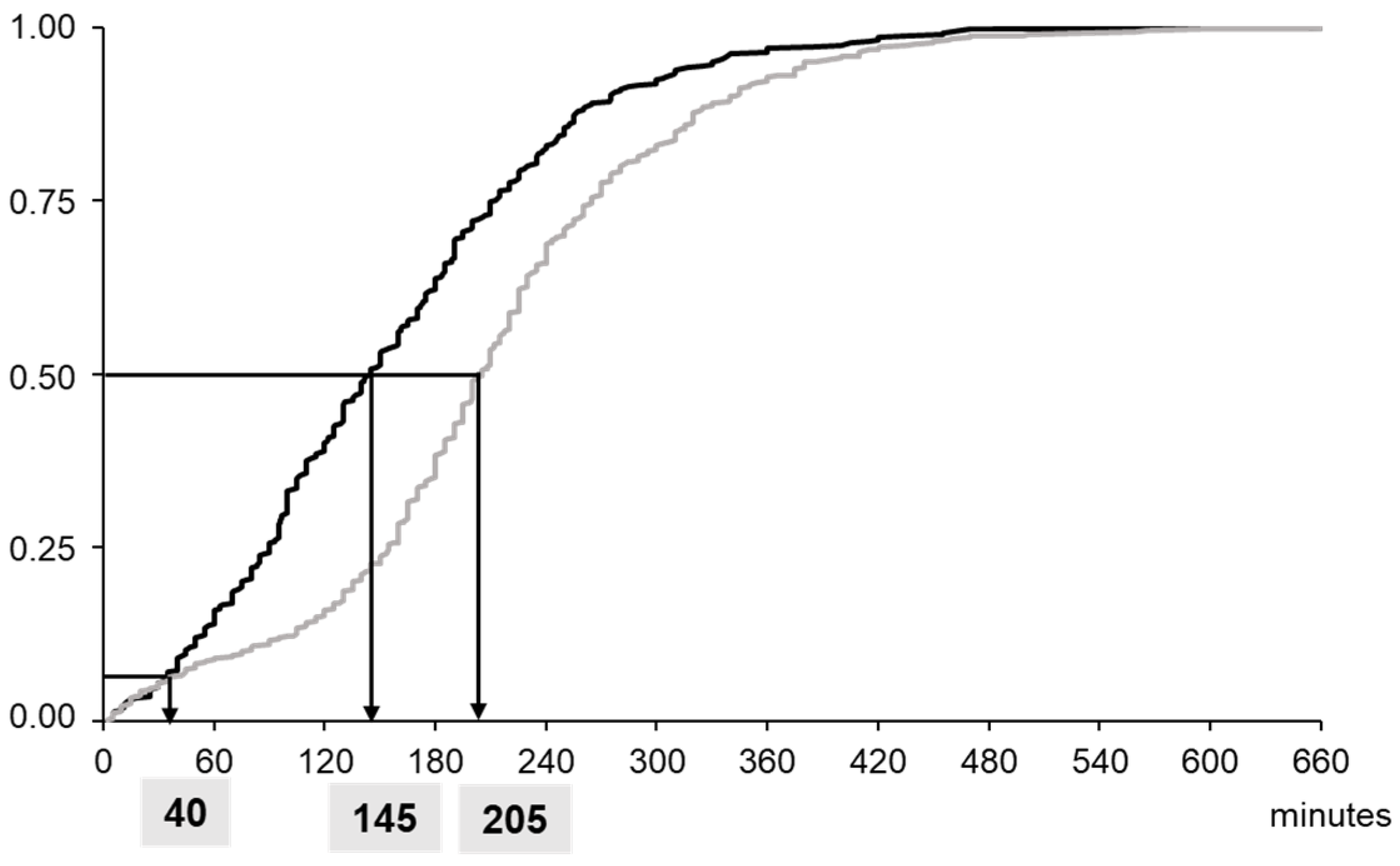
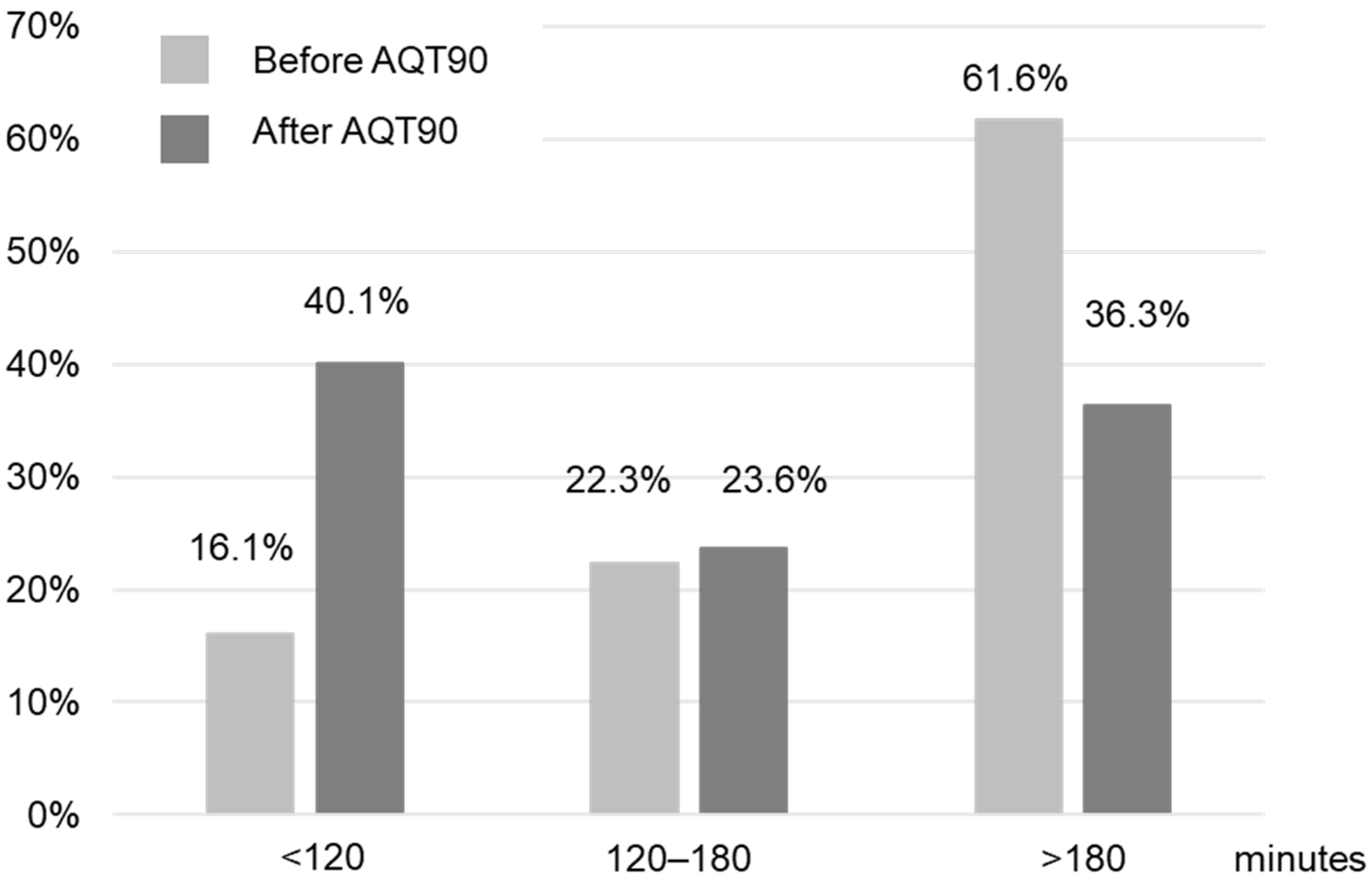
3.2. Evaluation of POCT Implementation in the Emergency Department
4. Discussion
4.1. Analytical Performances
4.2. Clinical Endpoints
4.3. Advantages and Disadvantages of the POC
4.4. Follow-Up and Routine Practice
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- McKenna, P.; Heslin, S.M.; Viccellio, P.; Mallon, W.K.; Hernandez, C.; Morley, E.J. Emergency department and hospital crowding: Causes, consequences, and cures. Clin. Exp. Emerg. Med. 2019, 6, 189–195. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rooney, K.D.; Schilling, U.M. Point-of-care testing in the overcrowded emergency department--can it make a difference? Crit. Care 2014, 18, 692. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rajsic, S.; Breitkopf, R.; Bachler, M.; Treml, B. Diagnostic Modalities in Critical Care: Point-of-Care Approach. Diagnostics 2021, 11, 2202. [Google Scholar] [CrossRef] [PubMed]
- Oredsson, S.; Jonsson, H.; Rognes, J.; Lind, L.; Göransson, K.E.; Ehrenberg, A.; Asplund, K.; Castrén, M.; Farrohknia, N. A systematic review of triage-related interventions to improve patient flow in emergency departments. Scand. J. Trauma. Resusc. Emerg. Med. 2011, 19, 19–43. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jarvis, P.R. Improving emergency department patient flow. Clin. Exp. Emerg. Med. 2016, 3, 63–68. [Google Scholar] [CrossRef]
- Alter, D.N. Point-of-Care Testing for the Emergency Department Patient: Quantity and Quality of the Available Evidence. Arch. Pathol. Lab. Med. 2021, 145, 308–319. [Google Scholar] [CrossRef] [PubMed]
- Goyder, C.; Tan, P.S.; Verbakel, J.; Ananthakumar, T.; Lee, J.J.; Hayward, G.; Turner, P.J.; Van Den Bruel, A. Impact of point-of-care panel tests in ambulatory care: A systematic review and meta-analysis. BMJ Open 2020, 10, e032132. [Google Scholar] [CrossRef] [Green Version]
- Boran, G.; O’Gorman, P.; Jackson, B.; O’Kelly, R.; O’Shea, P.; Keogan, M. Guidelines for Safe and Effective Near-Patient Testing (NPT); Group NN-PTNC, Ed.; Academy of Clinical Science and Laboratory Medicine: Dublin, Ireland, 2020. [Google Scholar]
- Greene, D.N.; Grenache, D.G. Education Committee of the Academy of Clinical Laboratory Physicians and Scientist. Pathology consultation on human chorionic gonadotropin testing for pregnancy assessment. Am. J. Clin. Pathol. 2015, 144, 830–836. [Google Scholar] [CrossRef] [Green Version]
- Legoupil, C.; Enderle, I.; Le Baccon, F.A.; Bendavid, C.; Peltier, L.; Bauville, E.; Leveque, J.; Lavoue, V.; Le Lous, M. Performance of a quick pregnancy test on whole blood in early pregnancy units: A prospective cohort study. Eur. J. Emerg. Med. 2019, 26, 105–111. [Google Scholar] [CrossRef]
- Plerhoples, W.; Zwemer, F.L., Jr.; Bazarian, J. Point of care pregnancy testing provides staff satisfaction but does not change ED length of stay. Am. J. Emerg. Med. 2004, 22, 460–464. [Google Scholar] [CrossRef]
- Cole, L.A.; Ladner, D.G. Background hCG in non-pregnant individuals: Need for more sensitive point-of-care and over-the-counter pregnancy tests. Clin. Biochem. 2009, 42, 168–175. [Google Scholar] [CrossRef] [PubMed]
- Kamer, S.M.; Foley, K.F.; Schmidt, R.L.; Greene, D.N. Analytical sensitivity of four commonly used hCG point of care devices. Clin. Biochem. 2015, 48, 448–452. [Google Scholar] [CrossRef] [PubMed]
- Greene, D.N.; Schmidt, R.L.; Kamer, S.M.; Grenache, D.G.; Hoke, C.; Lorey, T.S. Limitations in qualitative point of care hCG tests for detecting early pregnancy. Clin. Chim. Acta 2013, 415, 315. [Google Scholar] [CrossRef]
- Brun, M.M.; Holloway, L.; Oleksy, A.; Dayton, J.; Estey, M.P.; Goudreau, B.L.; Füzéry, A.K. Analytical evaluation of the Radiometer AQT90 FLEX βhCG assay. Pract. Lab. Med. 2019, 14, e00116. [Google Scholar] [CrossRef]
- Azzazy, H.M.; Romero, L.F.; Hall, L.; Ruttle, D.; Christenson, R.H. Two-center clinical evaluation of a new automated fluorometric immunoassay for the quantitative analysis of total beta-human chorionic gonadotropin. Clin. Biochem. 2003, 36, 523–528. [Google Scholar] [CrossRef]
- Sowder, A.M.; Yarbrough, M.L.; Nerenz, R.D.; Mitsios, J.V.; Mortensen, R.; Gronowski, A.M.; Grenache, D.G. Analytical performance evaluation of the i-STAT Total β-human chorionic gonadotropin immunoassay. Clin. Chim. Acta 2015, 446, 165–170. [Google Scholar] [CrossRef] [PubMed]
- AQT90 FLEX βhCG Test Kit [Package Insert]; Radiometer Medical ApS: Brønshøj, Denmark, 2018.
- User Verification of Precision and Estimation of Bias, Approved Guideline, 3rd ed.; CLSI document EP15-A3; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2014.
- Elecsys HCG+β [Package Insert]; Roche: Mannheim, Germany, 2022.
- Bland, J.M.; Altman, D.G. Measuring agreement in method comparison studies. Stat. Methods Med. Res. 1999, 8, 135–160. [Google Scholar] [CrossRef]
- Cole, L.A.; DuToit, S.; Higgins, T.N. Total hCG tests. Clin. Chim. Acta 2011, 412, 2216–2222. [Google Scholar] [CrossRef]
- Whittington, J.; Fantz, C.R.; Gronowski, A.M.; McCudden, C.; Mullins, R.; Sokoll, L.; Wiley, C.; Wilson, A.; Grenache, D.G. The analytical specificity of human chorionic gonadotropin assays determined using WHO International Reference Reagents. Clin. Chim. Acta 2010, 411, 81–85. [Google Scholar] [CrossRef]
- Storring, P.L.; Gaines-Das, R.E.; Bangham, D.R. International Reference Preparation of Human Chorionic Gonadotrophin for Immunoassay: Potency estimates in various bioassay and protein binding assay systems; and International Reference Preparations of the alpha and beta subunits of human chorionic gonadotrophin for immunoassay. J. Endocrinol. 1980, 84, 295–310. [Google Scholar]
- Sturgeon, C.M.; Berger, P.; Bidart, J.M.; Birken, S.; Burns, C.; Norman, R.J.; Stenman, U.H. IFCC Working Group on hCG. Differences in recognition of the 1st WHO international reference reagents for hCG-related isoforms by diagnostic immunoassays for human chorionic gonadotropin. Clin. Chem. 2009, 55, 1484–1491. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Snyder, J.A.; Haymond, S.; Parvin, C.A.; Gronowski, A.M.; Grenache, D.G. Diagnostic considerations in the measurement of human chorionic gonadotropin in aging women. Clin. Chem. 2005, 51, 1830–1835. [Google Scholar] [CrossRef] [PubMed]
- Grenache, D.G. Current Practices When Reporting Quantitative Human Chorionic Gonadotropin Test Results. J. Appl. Lab. Med. 2020, 5, 850–857. [Google Scholar] [CrossRef] [PubMed]
- Kirk, E.; Bottomley, C.; Bourne, T. Diagnosing ectopic pregnancy and current concepts in the management of pregnancy of unknown location. Hum. Reprod. Update 2014, 20, 250–261. [Google Scholar] [CrossRef]
- Hausfater, P.; Hajage, D.; Bulsei, J.; Canavaggio, P.; Lafourcade, A.; Paquet, A.L.; Arock, M.; Durand-Zaleski, I.; Riou, B.; Oueidat, N. Impact of Point-of-care Testing on Length of Stay of Patients in the Emergency Department: A Cluster-randomized Controlled Study. Acad. Emerg. Med. 2020, 27, 974–983. [Google Scholar] [CrossRef]
- Lee-Lewandrowski, E.; Nichols, J.; Van Cott, E.; Grisson, R.; Louissaint, A.; Benzer, T.; Lewandrowski, K. Implementation of a rapid whole blood D-dimer test in the emergency department of an urban academic medical center: Impact on ED length of stay and ancillary test utilization. Am. J. Clin. Pathol. 2009, 132, 326–331. [Google Scholar] [CrossRef] [Green Version]
- Singer, A.J.; Taylor, M.; LeBlanc, D.; Meyers, K.; Perez, K.; Thode, H.C., Jr.; Pines, J.M. Early Point-of-Care Testing at Triage Reduces Care Time in Stable Adult Emergency Department Patients. J. Emerg. Med. 2018, 55, 172–178. [Google Scholar] [CrossRef]
- Bargnoux, A.S.; Beaufils, O.; Oguike, M.; Lopasso, A.; Dupuy, A.M.; Sebbane, M.; Badiou, S.; Fesler, P.; Cristol, J.P. Point-of-care creatinine testing in patients receiving contrast-enhanced computed tomography scan. Clin. Chim. Acta 2018, 478, 111–113. [Google Scholar] [CrossRef]
- Kankaanpää, M.; Raitakari, M.; Muukkonen, L.; Gustafsson, S.; Heitto, M.; Palomäki, A.; Suojanen, K.; Harjola, V.P. Use of point-of-care testing and early assessment model reduces length of stay for ambulatory patients in an emergency department. Scand. J. Trauma. Resusc. Emerg. Med. 2016, 24, 125. [Google Scholar] [CrossRef] [Green Version]
- Asha, S.E.; Chan, A.C.; Walter, E.; Kelly, P.J.; Morton, R.L.; Ajami, A.; Wilson, R.D.; Honneyman, D. Impact from point-of-care devices on emergency department patient processing times compared with central laboratory testing of blood samples: A randomised controlled trial and cost-effectiveness analysis. Emerg. Med. J. 2014, 31, 714–719. [Google Scholar] [CrossRef] [Green Version]
- Wilgen, U.; Pretorius, C.J.; Gous, R.S.; Martin, C.; Hale, V.J.; Ungerer, J.P. Hook effect in Abbott i-STAT β-human chorionic gonadotropin (β-hCG) point of care assay. Clin. Biochem. 2014, 47, 1320–1322. [Google Scholar] [CrossRef] [PubMed]
- Al-Mahdili, H.A.; Jones, G.R. High-dose hook effect in six automated human chorionic gonadotrophin assays. Ann. Clin. Biochem. 2010, 47, 383–385. [Google Scholar] [CrossRef] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Brousse, M.; Bargnoux, A.-S.; Courtais-Coulon, C.; Badiou, S.; Kuster, N.; Compan, C.; Fuchs, F.; Cristol, J.-P. Optimization of Patient Management in the Gynecology Emergency Department Using Point-of-Care Beta hCG. Diagnostics 2022, 12, 1670. https://doi.org/10.3390/diagnostics12071670
Brousse M, Bargnoux A-S, Courtais-Coulon C, Badiou S, Kuster N, Compan C, Fuchs F, Cristol J-P. Optimization of Patient Management in the Gynecology Emergency Department Using Point-of-Care Beta hCG. Diagnostics. 2022; 12(7):1670. https://doi.org/10.3390/diagnostics12071670
Chicago/Turabian StyleBrousse, Mehdi, Anne-Sophie Bargnoux, Caroline Courtais-Coulon, Stéphanie Badiou, Nils Kuster, Clara Compan, Florent Fuchs, and Jean-Paul Cristol. 2022. "Optimization of Patient Management in the Gynecology Emergency Department Using Point-of-Care Beta hCG" Diagnostics 12, no. 7: 1670. https://doi.org/10.3390/diagnostics12071670
APA StyleBrousse, M., Bargnoux, A.-S., Courtais-Coulon, C., Badiou, S., Kuster, N., Compan, C., Fuchs, F., & Cristol, J.-P. (2022). Optimization of Patient Management in the Gynecology Emergency Department Using Point-of-Care Beta hCG. Diagnostics, 12(7), 1670. https://doi.org/10.3390/diagnostics12071670





