Pacinian Corpuscles as a Diagnostic Clue of Ledderhose Disease—A Case Report and Mapping of Pacinian Corpuscles of the Sole
Abstract
:1. Introduction
2. Materials and Methods
3. Results
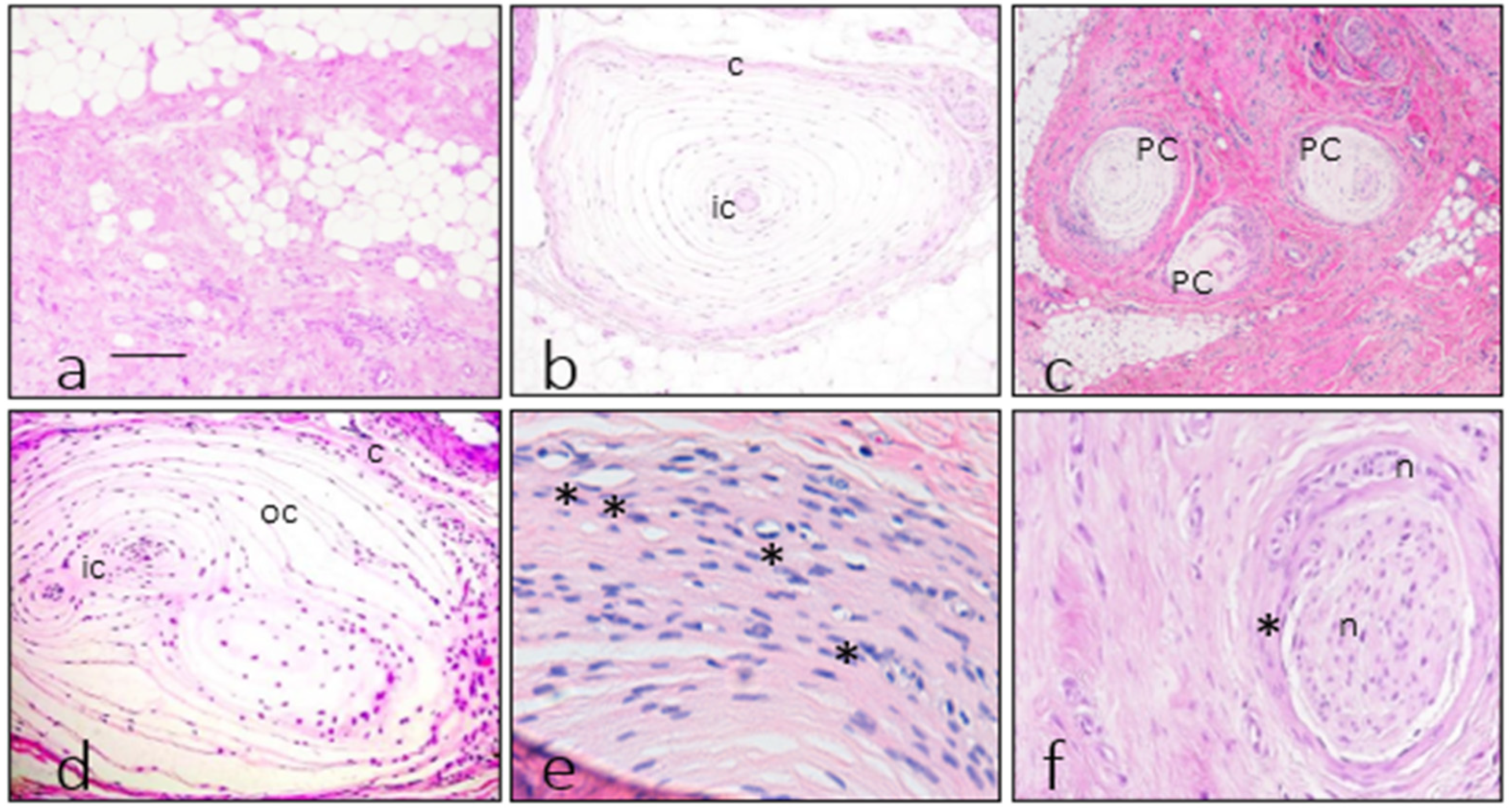
3.1. Morphology of Fibromatosis and Control Corpuscles
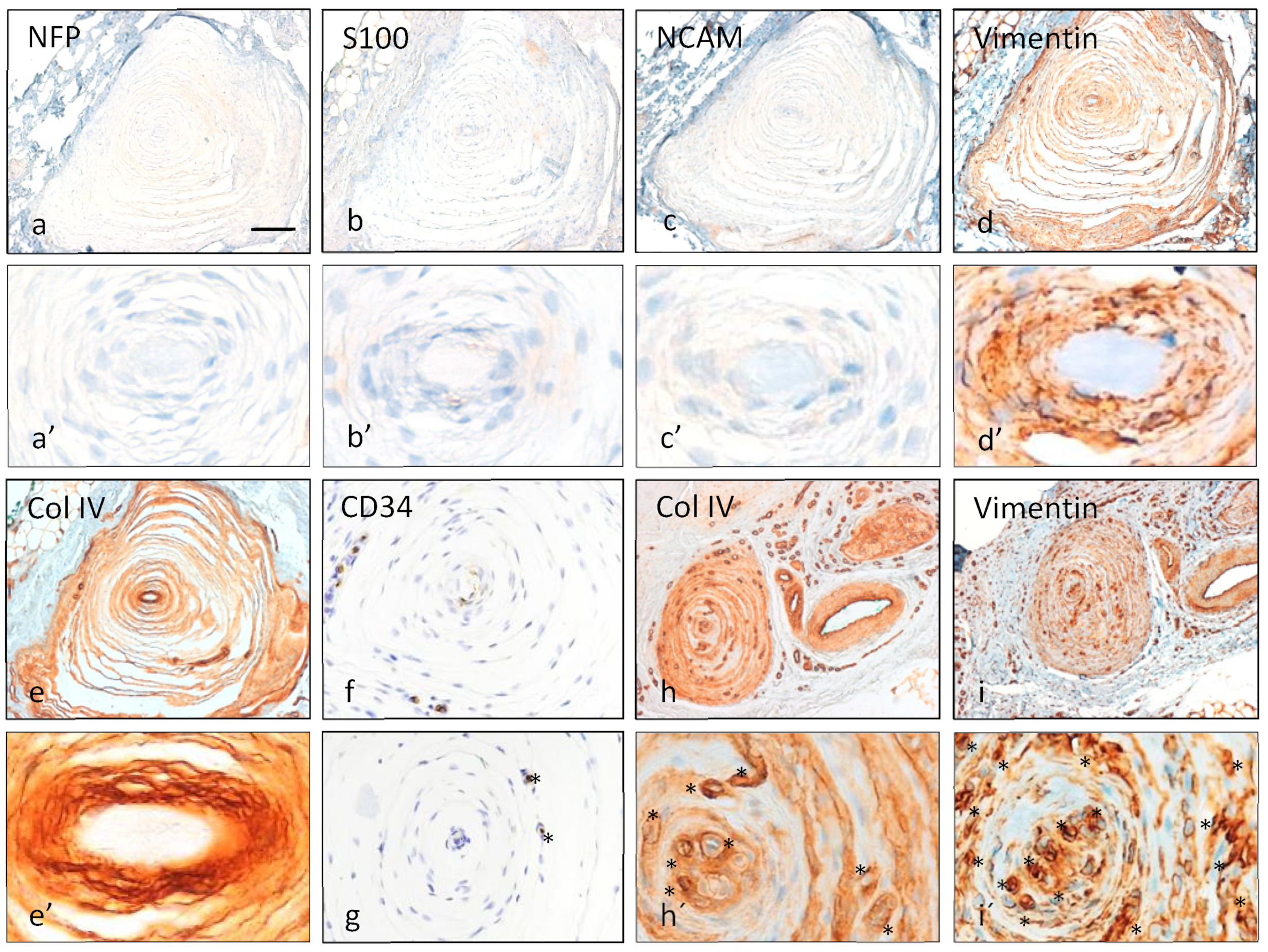
3.2. Immunohistochemistry of Pacinian Corpuscles
3.3. Measurements of Pacinian Corpuscles
3.4. Statistical Analysis
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Glodblum, J.R.; Folpe, A.L.; Weiss, S.W. Benign fibroblastic/myofibroblastic proliferations, including superficial fibromatoses. In Enzinger and Weiss’s Soft Tissue Tumors; Elsevier: Philadelphia, PA, USA, 2020. [Google Scholar]
- Goldblum, J.R.; Fletcher, J.A. Palmar/plantar fibromatosis. In WHO Classification of Tumours of Soft Tissue and Bone; IARC Press: Lyon, France, 2013. [Google Scholar]
- Thway, K.; Nascimento, A.F. Palmar/plantar fibromatosis. In WHO Classification of Tumours. Soft Tissue and Bone Tumours; IARC Press: Lyon, France, 2020. [Google Scholar]
- Liu, Q.; Fang, L.; Li, B. Desmoid fibromatosis in the foot. Medicine 2018, 97, e13109. [Google Scholar] [CrossRef]
- Bourne, M.; Talkad, A.; Varacallo, M. Anatomy, Bony Pelvis and Lower Limb, Foot Fascia; StatPearls Publishing: Treasure Island, FL, USA, 2022. [Google Scholar]
- Wang, L.; Zhu, H. Clonal analysis of palmar fibromatosis: A study whether palmar fibromatosis is a real tumor. J. Transl. Med. 2006, 4, 21. [Google Scholar] [CrossRef] [Green Version]
- Akyürek, N.; Ataoğlu, O.; Cenetoğlu, S.; Ozmen, S.; Cavuşoğlu, T.; Yavuzer, R. Pacinian corpuscle hyperplasia coexisting with Dupuytren’s contracture. Ann. Plast. Surg. 2000, 45, 220–222. [Google Scholar] [PubMed]
- Ehrmantant, W.R.; Graham, W.P., 3rd; Towfighi, J.; Mackay, D.R.; Ehrlich, H.P. A histological and anatomical profile of pacinian corpuscles from Dupuytren’s contracture and the expression of nerve growth factor receptor. Plat. Reconstr. Surg. 2004, 114, 721–727. [Google Scholar] [CrossRef] [PubMed]
- Yenidunya, M.O.; Yenidunya, S.; Seven, E. Pacinian hypertrophy in a type 2A hand burn contracture and Pacinian hypertrophy and hyperplasia in a Dupuytren’s contracture. Burns 2009, 35, 446–450. [Google Scholar] [CrossRef] [PubMed]
- Von Campe, A.; Mende, K.; Omaren, H.; Meuli-Simmen, C. Painful nodules and cords in Dupuytren disease. J. Hand Surg. Am. 2012, 37, 1313–1318. [Google Scholar] [CrossRef]
- Stecco, C.; Macchi, V.; Barbieri, A.; Tiengo, C.; Porzionato, A.; De Caro, R. Hand fasciae innervation: The palmar aponeurosis. Clin. Anat. 2018, 31, 677–683. [Google Scholar] [CrossRef]
- García-Martínez, I.; García-Mesa, Y.; García-Piqueras, J.; Martínez-Pubil, A.; Cobo, J.L.; Feito, J.; García-Suárez, O.; Vega, J.A. Sensory innervation of the human palmar aponeurosis in healthy individuals and patients with palmar fibromatosis. J. Anat. 2022, 240, 972–984. [Google Scholar] [CrossRef]
- Young, J.R.; Sternbach, S.; Willinger, M.; Hutchinson, I.D.; Rosenbaum, A. The etiology, evaluation and management of plantar fibromatosis. Orthop. Res. Rev. 2018, 11, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Stewart, D.; Nascimento, A.F. Palmar and plantar fibromatosis: A review. J. Pathol. Transl. Med. 2021, 55, 265–270. [Google Scholar] [CrossRef]
- Bojsen-Moller, F.; Flagstad, K.E. Plantar aponeurosis and internal architecture of the ball of the foot. J. Anat. 1976, 121, 599–611. [Google Scholar]
- Stecco, C.; Corradin, M.; Macchi, V.; Morra, A.; Porzionato, A.; Biz, C.; De Caro, R. Plantar fascia anatomy and its relationship with Achilles tendon and paratenon. J. Anat. 2013, 223, 665–676. [Google Scholar] [CrossRef]
- Strzalkowski, N.D.J.; Peters, R.M.; Inglis, J.T.; Bent, L.R. Cutaneous afferent innervation of the human foot sole: What can we learn from single-unit recordings? J. Neurophysiol. 2018, 120, 1233–1246. [Google Scholar] [CrossRef]
- Corniani, G.; Saal, H.P. Tactile innervation densities across the whole body. J. Neurophysiol. 2020, 124, 1229–1240. [Google Scholar] [CrossRef] [PubMed]
- Kowalzik, R.; Hermann, B.; Biedermann, H.; Peiper, U. Two-point discrimination of vibratory perception on the sole of the human foot. Foot Ankle Int. 1996, 17, 629–634. [Google Scholar] [CrossRef]
- Gu, C.; Griffin, M.J. Spatial summation of vibrotactile sensations at the foot. Med. Eng. Phys. 2013, 35, 1221–1227. [Google Scholar] [CrossRef] [Green Version]
- Jin, Z.W.; Cho, K.H.; Xu, D.Y.; You, Y.Q.; Kim, J.H.; Murakami, G.; Abe, H. Pacinian corpuscles in the human fetal foot: A study using 3D reconstruction and immunohistochemistry. Ann. Anat. 2020, 227, 151421. [Google Scholar] [CrossRef]
- Sugai, N.; Cho, K.H.; Murakami, G.; Abe, H.; Uchiyama, E.; Kura, H. Distribution of sole Pacinian corpuscles: A histological study using near-term human feet. Surg. Radiol. Anat. 2021, 43, 10311039. [Google Scholar] [CrossRef]
- Germann, C.; Sutter, R.; Nanz, D. Novel observations of Pacinian corpuscle distribution in the hands and feet based on high—Resolution 7-T MRI in healthy volunteers. Skeletal. Radiol. 2021, 50, 1249–1255. [Google Scholar] [CrossRef]
- Toepfer, A.; Harrasser, N.; Dreyer, F.; Mogler, C.; Walther, M.; von Eisenhart-Rothe, R. Epithelioid sarcoma of the plantar fascia mimicking morbus Ledderhose—A severe pitfall for clinical and histopathological misinterpretation. Foot Ankle Surg. 2017, 23, e25–e30. [Google Scholar] [CrossRef]
- Feito, J.; Ramos-García, J.L.; Gago, Á.; Cobo, J.L.; García-Suárez, O.; Junquera, L.M.; Vega, J.A. Pacinian Corpuscles in a Cervical Chondrocutaneous Remnant: A Case Report and Update of Pacinian Corpuscles. Am. J. Dermatopathol. 2016, 38, 231–235. [Google Scholar] [CrossRef]
- Cobo, R.; García-Piqueras, J.; Cobo, J.; Vega, J.A. The Human Cutaneous Sensory Corpuscles: An Update. J. Clin. Med. 2021, 10, 227. [Google Scholar] [CrossRef] [PubMed]
- Mikusev, I.E. New facts about the pathogenesis of Dupuytren’s contracture. Acta Chir. Plast. 1989, 31, 1–14. [Google Scholar] [PubMed]
- Carroll, P.; Henshaw, R.; Garwood, C.; Raspovic, K.; Kumar, D. Plantar fibromatosis: Pathophysiology, surgical and nonsurgical therapies: An evidence-based review. Foot Ankle Spec. 2018, 11, 168–176. [Google Scholar] [CrossRef]
- Fausto de Souza, D.; Micaelo, L.; Cuzzi, T.; Silva, M.R.E. Ledderhose disease: An unusual presentation. J. Clin. Aesthet. Dermatol. 2010, 3, 45–47. [Google Scholar]
- Miceli, A.J.; Junkins-Hopkins, J.M.; Polley, D.C.; Elston, D.M. Multiple nodules on the sole of the foot. Indian Dermatol. Online J. 2015, 6, 422–424. [Google Scholar] [CrossRef]
- Ocampo-Garza, J.; Cunha, R.A.; Pereira, C.S.; Sanderson, A.; Pinto-Desmond, K.; Machado-Filho, C.D.A.; Haneke, E.; de Carvalho, M. Plantar fibromatosis: Surgical approach of a giant bilateral case. Int. J. Dermatol. 2018, 57, 365–367. [Google Scholar] [CrossRef]
- Neagu, T.P.; Tiglis, M.; Popescu, A.; Enache, V.; Popescu, S.A.; Lascar, I. Clinical, histological and therapeutic modern approach of Ledderhose disease. Rom. J. Morphol. Embryol. 2018, 59, 691–697. [Google Scholar]
- Gerosa, T.; Pierrart, J.; Serane-Fresnel, J.; Amsallem, L.; Masmejean, E.H. Distal sensory disorders in Dupuytren’s disease. Orthop. Traumatol. Surg. Res. 2018, 104, 897–900. [Google Scholar] [CrossRef]
- Lubahn, J.D.; Pollard, M.; Cooney, T. Immunohistochemical evidence of nerve growth factor in Dupuytren’s disease palmar fascia. J. Hand Surg. Am. 2007, 32, 337–342. [Google Scholar] [CrossRef]
- Schubert, T.E.O.; Weidler, C.; Borisch, N.; Schubert, C.; Hofstädter, F.; Straub, R.H. Dupuytren’s contracture is associated with sprouting of substance P positive nerve fibres and infiltration by mast cells. Ann. Rheum. Dis. 2006, 65, 414–415. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chambers, A.R.; Dreyer, M.A.; Song, K.Y.; Lam, K.K. Bilateral symptomatic Pacinian corpuscle hyperplasia of the adult foot. Foot 2020, 49, 101709. [Google Scholar] [CrossRef] [PubMed]
- Hughes, P.; Miranda, R.; Doyle, A.J. MRI imaging of soft tissue tumours of the foot and ankle. Insights Imaging 2019, 10, 60. [Google Scholar] [CrossRef] [PubMed]
- Ahlawat, S.; Fayad, L.M. Revisiting the WHO classification system of soft tissue tumours: Emphasis on advanced magnetic resonance imaging sequences. Part 1. Pol. J. Radiol. 2020, 85, e396–e408. [Google Scholar] [CrossRef]



| Antigen | Origin | Dilution | Supplier |
|---|---|---|---|
| Glut1 (polyclonal) | Rabbit | prediluted | Cell Marque 1 |
| EMA (E29) | Mouse | prediluted | Cell Marque 1 |
| N-CAM (123C3) | Mouse | prediluted | Ventana Medical Systems 2 |
| NFP (2F11) | Mouse | prediluted | Cell Marque 1 |
| S-100 protein (polyclonal) | Rabbit | prediluted | Ventana Medical Systems 2 |
| Vimentin (V9) | Mouse | prediluted | Cell Marque 1 |
| Actin, muscle specific (HHF35) | Mouse | prediluted | Ventana Medical Systems 2 |
| Actin, smooth muscle (1A4) | Mouse | prediluted | Ventana Medical Systems 2 |
| Desmin (D33) | Mouse | prediluted | Cell Marque 1 |
| Collagen type IV (CIV22) | Mouse | prediluted | Ventana Medical Systems 2 |
| CD34 (QBEnd/10) | Mouse | prediluted | Master Diagnóstica 3 |
| NTrk (EPR17341) | Mouse | prediluted | Ventana Medical Systems 2 |
| Antigen | Axon | IC | IL | OC | C | |
|---|---|---|---|---|---|---|
| NFP | Control | +++ | − | − | − | − |
| PF | − (1) | − | − | − | − | |
| S100P | Control | − | +++ | − | − | − |
| PF | − | − (1) | − | − | − | |
| EMA | Control | − | − | − | ++ | ++ |
| PF | − | − | − | +/++ | +/++ | |
| CD34 | Control | − | − | +++ | − | − |
| PF | − | − | −/+ | − (2) | − (2) | |
| VIM | Control | − | ++ | +++ | +++ | +++ |
| PF | − | −/+++ (3) | +++ | +++ | +++ | |
| Actin | Control | − | − | − | − | − |
| PF | − | − | − | − | − | |
| Desmin | Control | − | − | − | − | − |
| PF | − | − | − | − | − | |
| IV COL | Control | − | ++ (4) | − | − | − |
| PF | − | −/+ (4) | − | − | − | |
| Glut1 | Control | − | − | − | + | +++ |
| PF | − | +++ (3) | − | + | ++ | |
| N-CAM | Control | − | ++ | − | − | − |
| PF | − | − | − | − | − | |
| NTrk | Control | + | + | + | + | + |
| PF | − | − | − | − | − | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Feito, J.; Esteban, R.; García-Martínez, M.L.; García-Alonso, F.J.; Rodríguez-Martín, R.; Rivas-Marcos, M.B.; Cobo, J.L.; Martín-Biedma, B.; Lahoz, M.; Vega, J.A. Pacinian Corpuscles as a Diagnostic Clue of Ledderhose Disease—A Case Report and Mapping of Pacinian Corpuscles of the Sole. Diagnostics 2022, 12, 1705. https://doi.org/10.3390/diagnostics12071705
Feito J, Esteban R, García-Martínez ML, García-Alonso FJ, Rodríguez-Martín R, Rivas-Marcos MB, Cobo JL, Martín-Biedma B, Lahoz M, Vega JA. Pacinian Corpuscles as a Diagnostic Clue of Ledderhose Disease—A Case Report and Mapping of Pacinian Corpuscles of the Sole. Diagnostics. 2022; 12(7):1705. https://doi.org/10.3390/diagnostics12071705
Chicago/Turabian StyleFeito, Jorge, Ruth Esteban, María Lourdes García-Martínez, Francisco J. García-Alonso, Raquel Rodríguez-Martín, María Belén Rivas-Marcos, Juan L. Cobo, Benjamín Martín-Biedma, Manuel Lahoz, and José A. Vega. 2022. "Pacinian Corpuscles as a Diagnostic Clue of Ledderhose Disease—A Case Report and Mapping of Pacinian Corpuscles of the Sole" Diagnostics 12, no. 7: 1705. https://doi.org/10.3390/diagnostics12071705






