Association of the rs17574 DPP4 Polymorphism with Premature Coronary Artery Disease in Diabetic Patients: Results from the Cohort of the GEA Mexican Study
Abstract
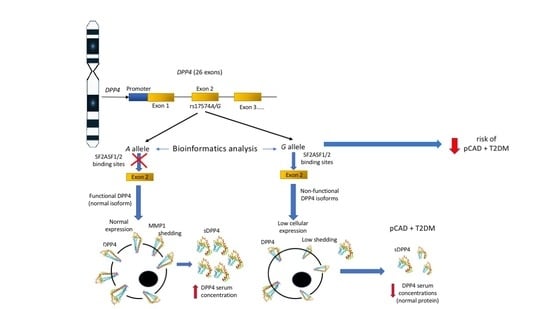
:1. Introduction
2. Materials and Methods
2.1. Description of the Studied Population and Determination of DPP4 Polymorphisms and Concentrations
2.2. Statistical Analysis
3. Results
3.1. General Characteristics of the Population Stratified by Group
3.2. DPP4 Serum Concentration Stratified by rs17574 DPP4 Genotypes
3.3. Association of the rs17574 DPP4 Polymorphism with pCAD + T2DM
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Teo, K.K.; Rafiq, T. Cardiovascular Risk Factors and Prevention: A Perspective From Developing Countries. Can. J. Cardiol. 2021, 37, 733–743. [Google Scholar] [CrossRef] [PubMed]
- Preis, S.R.; Hwang, S.J.; Coady, S.; Pencina, M.J.; D’Agostino, R.B.; Savage, P.J.; Levy, D.; Fox, C.S. Trends in all-cause and cardiovascular disease mortality among women and men with and without diabetes mellitus in the Framingham Heart Study, 1950 to 2005. Circulation 2009, 119, 1728–1735. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Malmberg, K.; Yusuf, S.; Gerstein, H.C.; Brown, J.; Zhao, F.; Hunt, D.; Piegas, L.; Calvin, J.; Keltai, M.; Budaj, A. Impact of diabetes on long-term prognosis in patients with unstable angina and non-Q-wave myocardial infarction: Results of the OASIS (Organization to Assess Strategies for Ischemic Syndromes) Registry. Circulation 2000, 102, 1014–1019. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rivas Rios, J.R.; Franchi, F.; Rollini, F.; Angiolillo, D.J. Diabetes and antiplatelet therapy: From bench to bedside. Cardiovasc. Diagn. Ther. 2018, 8, 594–609. [Google Scholar] [CrossRef] [PubMed]
- Chehade, J.M.; Gladysz, M.; Mooradian, A.D. Dyslipidemia in type 2 diabetes: Prevalence, pathophysiology, and management. Drugs 2013, 73, 327–339. [Google Scholar] [CrossRef]
- Genest, J.J.; Martin-Munley, S.S.; McNamara, J.R.; Ordovas, J.M.; Jenner, J.; Myers, R.H.; Silberman, S.R.; Wilson, P.W.F.; Salem, D.N.; Schaefer, E.J. Familial lipoprotein disorders in patients with premature coronary artery disease. Circulation 1992, 85, 2025–2033. [Google Scholar] [CrossRef] [Green Version]
- Augustyns, K.; Bal, G.; Thonus, G.; Belyaev, A.; Zhang, X.; Bollaert, W.; Lambeir, A.; Durinx, C.; Goossens, F.; Haemers, A. The unique properties of dipeptidyl-peptidase IV (DPP IV/CD26) and the therapeutic potential of DPP IV inhibitors. Curr. Med. Chem 1999, 6, 311–327. [Google Scholar] [CrossRef]
- Mulvihill, E.E.; Drucker, D.J. Pharmacology, Physiology, and Mechanisms of Action of Dipeptidyl Peptidase-4 Inhibitors. Endocr. Rev. 2014, 35, 992–1019. [Google Scholar] [CrossRef] [Green Version]
- Röhrborn, D.; Eckel, J.; Sell, H. Shedding of Dipeptidyl Peptidase 4 Is Mediated by Metalloproteases and Up-Regulated by Hypoxia in Human Adipocytes and Smooth Muscle Cells. FEBS Lett. 2014, 588, 3870–3877. [Google Scholar] [CrossRef] [Green Version]
- Nargis, T.; Kumar, K.; Ghosh, A.R.; Sharma, A.; Rudra, D.; Sen, D.; Chakrabarti, S.; Mukhopadhyay, S.; Ganguly, D.; Chakrabarti, P. KLK5 induces shedding of DPP4 from circulatory Th17 cells in type 2 diabetes. Mol. Metab. 2017, 6, 1529–1539. [Google Scholar] [CrossRef]
- Miyazaki, M.; Kato, M.; Tanaka, K.; Tanaka, M.; Kohjima, M.; Nakamura, K.; Enjoji, M.; Nakamuta, M.; Kotoh, K.; Takayanagi, R. Increased hepatic expression of dipeptidyl peptidase-4 in non-alcoholic fatty liver disease and its association with insulin resistance and glucose metabolism. Mol. Med. Rep. 2012, 5, 729–733. [Google Scholar] [CrossRef] [PubMed]
- Baumeier, C.; Saussenthaler, S.; Kammel, A.; Jähnert, M.; Schlüter, L.; Hesse, D.; Canouil, M.; Lobbens, S.; Caiazzo, R.; Raverdy, V.; et al. Hepatic DPP4 DNA Methylation Associates With Fatty Liver. Diabetes 2017, 66, 25–35. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zheng, T.; Chen, T.; Liu, Y.; Gao, Y.; Tian, H. Increased plasma DPP4 activity predicts new-onset hypertension in Chinese over a 4-year period: Possible associations with inflammation and oxidative stress. J. Hum. Hypertens. 2015, 29, 424–429. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Li, P.; Xin, M.; Jin, X.; Zhao, L.; Nan, Y.; Cheng, X.W. Dipeptidyl peptidase-4 inhibition prevents lung injury in mice under chronic stress via the modulation of oxidative stress and inflammation. Exp. Anim. 2021, 70, 541–552. [Google Scholar] [CrossRef]
- Reinhold, D.; Goihl, A.; Wrenger, S.; Reinhold, A.; Kühlmann, U.C.; Faust, J.; Neubert, K.; Thielitz, A.; Brocke, S.; Täger, M.; et al. Role of dipeptidyl peptidase IV (DP IV)-like enzymes in T lymphocyte activation: Investigations in DP IV/CD26-knockout mice. Clin. Chem. Lab. Med. 2009, 47, 268–274. [Google Scholar] [CrossRef]
- Duan, L.; Rao, X.; Xia, C.; Rajagopalan, S.; Zhong, J. The regulatory role of DPP4 in atherosclerotic disease. Cardiovasc. Diabetol. 2017, 16, 1–8. [Google Scholar] [CrossRef]
- Matheeussen, V.; Waumans, Y.; Martinet, W.; Van Goethem, S.; Van Der Veken, P.; Scharpé, S.; Augustyns, K.; De Meyer, G.R.Y.; De Meester, I. Dipeptidyl peptidases in atherosclerosis: Expression and role in macrophage differentiation, activation and apoptosis. Basic Res. Cardiol. 2013, 108, 350. [Google Scholar] [CrossRef]
- Nargis, T.; Chakrabarti, P. Significance of circulatory DPP4 activity in metabolic diseases. IUBMB Life 2018, 70, 112–119. [Google Scholar] [CrossRef] [Green Version]
- Kadoglou, N.P.E.; Korakas, E.; Lampropoulos, S.; Maratou, E.; Kassimis, G.; Patsourakos, N.; Plotas, P.; Moutsatsou, P.; Lambadiari, V. Plasma nesfatin-1 and DDP-4 levels in patients with coronary artery disease: Kozani study. Cardiovasc. Diabetol. 2021, 20, 166. [Google Scholar] [CrossRef]
- Barchetta, I.; Ceccarelli, V.; Cimini, F.A.; Barone, E.; Sentinelli, F.; Coluzzi, M.; Chiappetta, C.; Bertoccini, L.; Tramutola, A.; Labbadia, G.; et al. Circulating dipeptidyl peptidase-4 is independently associated with the presence and severity of NAFLD/NASH in individuals with and without obesity and metabolic disease. J. Endocrinol. Investig. 2021, 44, 979–988. [Google Scholar] [CrossRef]
- Yang, G.; Li, Y.; Cui, L.; Jiang, H.; Li, X.; Jin, C.; Jin, D.; Zhao, G.; Jin, J.; Sun, R.; et al. Increased Plasma Dipeptidyl Peptidase-4 Activities in Patients with Coronary Artery Disease. PLoS ONE 2016, 11, e0163027. [Google Scholar] [CrossRef] [PubMed]
- Bailey, S.D.; Xie, C.; Paré, G.; Montpetit, A.; Mohan, V.; Yusuf, S.; Gerstein, H.; Engert, J.C.; Anand, S.S. EpiDREAM and INTERHEART Investigators Variation at the DPP4 locus influences apolipoprotein B levels in South Asians and exhibits heterogeneity in Europeans related to BMI. Diabetologia 2014, 57, 738–745. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, R.H.; Huri, H.Z.; Al-Hamodi, Z.; Salem, S.D.; Al-Absi, B.; Muniandy, S. Association of DPP4 gene polymorphisms with type 2 diabetes mellitus in Malaysian subjects. PLoS ONE 2016, 11, 1–12. [Google Scholar] [CrossRef]
- Aghili, N.; Devaney, J.M.; Alderman, L.O.; Zukowska, Z.; Epstein, S.E.; Burnett, M.S.; Li, M.; Reilly, M.; Rader, D.; Zukowska, Z.; et al. Polymorphisms in dipeptidyl peptidase IV gene are associated with the risk of myocardial infarction in patients with atherosclerosis. Neuropeptides 2012, 46, 367–371. [Google Scholar] [CrossRef] [PubMed]
- Vargas-Alarcón, G.; González-Salazar, M.d.C.; Vázquez-Vázquez, C.; Hernández-Díaz Couder, A.; Sánchez-Muñoz, F.; Reyes-Barrera, J.; Criales-Vera, S.A.; Sánchez-Guerra, M.; Osorio-Yáñez, C.; Posadas-Sánchez, R. The rs12617336 and rs17574 Dipeptidyl Peptidase-4 Polymorphisms Are Associated With Hypoalphalipoproteinemia and Dipeptidyl Peptidase-4 Serum Levels: A Case-Control Study of the Genetics of Atherosclerotic Disease (GEA) Cohort. Front. Genet. 2021, 12, 686. [Google Scholar] [CrossRef] [PubMed]
- Medina-Urrutia, A.; Posadas-Romero, C.; Posadas-Sánchez, R.; Jorge-Galarza, E.; Villarreal-Molina, T.; González-Salazar, M.C.; Cardoso-Saldaña, G.; Vargas-Alarcón, G.; Torres-Tamayo, M.; Juárez-Rojas, J.G. Role of adiponectin and free fatty acids on the association between abdominal visceral fat and insulin resistance. Cardiovasc. Diabetol. 2015, 14, 20. [Google Scholar] [CrossRef] [Green Version]
- Posadas-Sánchez, R.; López-Uribe, Á.R.; Posadas-Romero, C.; Pérez-Hernández, N.; Rodríguez-Pérez, J.M.; Ocampo-Arcos, W.A.; Fragoso, J.M.; Cardoso-Saldaña, G.; Vargas-Alarcón, G. Association of the I148M/PNPLA3 (rs738409) polymorphism with premature coronary artery disease, fatty liver, and insulin resistance in type 2 diabetic patients and healthy controls. The GEA study. Immunobiology 2017, 222, 960–966. [Google Scholar] [CrossRef]
- Posadas-Sánchez, R.; Pérez-Hernández, N.; Angeles-Martínez, J.; López-Bautista, F.; Villarreal-Molina, T.; Rodríguez-Pérez, J.M.; Fragoso, J.M.; Posadas-Romero, C.; Vargas-Alarcón, G. Interleukin 35 Polymorphisms Are Associated with Decreased Risk of Premature Coronary Artery Disease, Metabolic Parameters, and IL-35 Levels: The Genetics of Atherosclerotic Disease (GEA) Study. Mediators Inflamm. 2017, 2017, 6012795. [Google Scholar] [CrossRef]
- Mautner, G.C.; Mautner, S.L.; Froehlich, J.; Feuerstein, I.M.; Proschan, M.A.; Roberts, W.C.; Doppman, J.L. Coronary artery calcification: Assessment with electron beam CT and histomorphometric correlation. Radiology 1994, 192, 619–623. [Google Scholar] [CrossRef]
- Baecke, J.A.; Burema, J.; Frijters, J.E. A short questionnaire for the measurement of habitual physical activity in epidemiological studies. Am. J. Clin. Nutr. 1982, 36, 936–942. [Google Scholar] [CrossRef]
- Athyros, V.G.; Doumas, M.; Imprialos, K.P.; Stavropoulos, K.; Georgianou, E.; Katsimardou, A.; Karagiannis, A. Diabetes and lipid metabolism. Hormones (Athens) 2018, 17, 61–67. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chiang, S.M.; Ueng, K.C.; Yang, Y.S. Gender differences in variables associated with dipeptidyl peptidase 4 genetic polymorphisms in coronary artery disease. Adv. Clin. Exp. Med. 2020, 29, 1181–1186. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Liu, Y.; Wang, W.; Qu, H.; Han, Y.; Hou, Y. Association of dipeptidyl peptidase IV polymorphism, serum lipid profile, and coronary artery stenosis in patients with coronary artery disease and type 2 diabetes. Medicine (Baltimore) 2021, 100, e25209. [Google Scholar] [CrossRef] [PubMed]
- Kazafeos, K. Incretin effect: GLP-1, GIP, DPP4. Diabetes Res. Clin. Pract. 2011, 93 (Suppl. 1), S32–S36. [Google Scholar] [CrossRef] [PubMed]
- Özyazgan, S.; Kutluata, N.; Afşar, S.; Özdaş, Ş.B.; Akkan, A.G. Effect of glucagon-like peptide-1(7-36) and exendin-4 on the vascular reactivity in streptozotocin/nicotinamide-induced diabetic rats. Pharmacology 2005, 74, 119–126. [Google Scholar] [CrossRef]
- Zhao, T.; Parikh, P.; Bhashyam, S.; Bolukoglu, H.; Poornima, I.; Shen, Y.-T.; Shannon, R.P. Direct effects of glucagon-like peptide-1 on myocardial contractility and glucose uptake in normal and postischemic isolated rat hearts. J. Pharmacol. Exp. Ther. 2006, 317, 1106–1113. [Google Scholar] [CrossRef]
- Nagashima, M.; Watanabe, T.; Terasaki, M.; Tomoyasu, M.; Nohtomi, K.; Kim-Kaneyama, J.; Miyazaki, A.; Hirano, T. Native incretins prevent the development of atherosclerotic lesions in apolipoprotein E knockout mice. Diabetologia 2011, 54, 2649–2659. [Google Scholar] [CrossRef] [Green Version]
- Nauck, M.A.; Quast, D.R.; Wefers, J.; Pfeiffer, A.F.H. The evolving story of incretins (GIP and GLP-1) in metabolic and cardiovascular disease: A pathophysiological update. Diabetes. Obes. Metab. 2021, 23 (Suppl. 3), 5–29. [Google Scholar] [CrossRef]
- Turcot, V.; Bouchard, L.; Faucher, G.; Tchernof, A.; Deshaies, Y.; Pérusse, L.; Bélisle, A.; Marceau, S.; Biron, S.; Lescelleur, O.; et al. DPP4 gene DNA methylation in the omentum is associated with its gene expression and plasma lipid profile in severe obesity. Obesity (Silver Spring) 2011, 19, 388–395. [Google Scholar] [CrossRef]
- Stengel, A.; Goebel-Stengel, M.; Teuffel, P.; Hofmann, T.; Buße, P.; Kobelt, P.; Rose, M.; Klapp, B.F. Obese patients have higher circulating protein levels of dipeptidyl peptidase IV. Peptides 2014, 61, 75–82. [Google Scholar] [CrossRef]
- da Silva Júnior, W.S.; de Souza, M.d.G.C.; Nogueira Neto, J.F.; Bouskela, E.; Kraemer-Aguiar, L.G. Constitutive DPP4 activity, inflammation, and microvascular reactivity in subjects with excess body weight and without diabetes. Microvasc. Res. 2018, 120, 94–99. [Google Scholar] [CrossRef] [PubMed]
- Lamers, D.; Famulla, S.; Wronkowitz, N.; Hartwig, S.; Lehr, S.; Ouwens, D.M.; Eckardt, K.; Kaufman, J.M.; Ryden, M.; Muller, S.; et al. Dipeptidyl Peptidase 4 Is a Novel Adipokine Potentially Linking Obesity to the Metabolic Syndrome. Diabetes 2011, 60, 1917–1925. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sell, H.; Blüher, M.; Klöting, N.; Schlich, R.; Willems, M.; Ruppe, F.; Knoefel, W.T.; Dietrich, A.; Fielding, B.A.; Arner, P.; et al. Adipose dipeptidyl peptidase-4 and obesity: Correlation with insulin resistance and depot-specific release from adipose tissue in vivo and in vitro. Diabetes Care 2013, 36, 4083–4090. [Google Scholar] [CrossRef] [Green Version]
- Wellen, K.E.; Hotamisligil, G.S. Inflammation, stress, and diabetes. J. Clin. Investig. 2005, 115, 1111–1119. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maggio, C.A.; Pi-Sunyer, F.X. Obesity and type 2 diabetes. Endocrinol. Metab. Clin. North Am. 2003, 32, 805–822. [Google Scholar] [CrossRef]
- Zhong, J.; Rao, X.; Deiuliis, J.; Braunstein, Z.; Narula, V.; Hazey, J.; Mikami, D.; Needleman, B.; Satoskar, A.; Rajagopalan, S. A Potential Role for Dendritic Cell/Macrophage-Expressing DPP4 in Obesity-Induced Visceral Inflammation. Diabetes 2013, 62, 149–157. [Google Scholar] [CrossRef] [Green Version]
- Love, K.M.; Liu, Z. DPP4 Activity, Hyperinsulinemia, and Atherosclerosis. J. Clin. Endocrinol. Metab. 2021, 106, 1553–1565. [Google Scholar] [CrossRef]
- Barchetta, I.; Ciccarelli, G.; Barone, E.; Cimini, F.A.; Ceccarelli, V.; Bertoccini, L.; Sentinelli, F.; Tramutola, A.; Del Ben, M.; Angelico, F.; et al. Greater circulating DPP4 activity is associated with impaired flow-mediated dilatation in adults with type 2 diabetes mellitus. Nutr. Metab. Cardiovasc. Dis. 2019, 29, 1087–1094. [Google Scholar] [CrossRef]
- Gorrell, M.; Gysbers, V.; McCaughan, G. CD26: A multifunctional integral membrane and secreted protein of activated lymphocytes. Scand. J. Immunol. 2001, 54, 249–264. [Google Scholar] [CrossRef]
- Posadas-Sánchez, R.; Sánchez-Muñoz, F.; Guzmán-Martín, C.A.; Hernández-Díaz Couder, A.; Rojas-Velasco, G.; Fragoso, J.M.; Vargas-Alarcón, G. Dipeptidylpeptidase-4 levels and DPP4 gene polymorphisms in patients with COVID-19. Association with disease and with severity. Life Sci. 2021, 276, 119410. [Google Scholar] [CrossRef]



| Premature Coronary Artery Disease | ||||
|---|---|---|---|---|
| No (n = 852) | Yes (n = 1141) | * p | ||
| Control Group | Non-T2DM (n = 736) | T2DM (n = 405) | ||
| Age (years) | 51 ± 9 | 53 ± 8 a,b | 56 ± 8 a | <0.001 |
| Sex (% male) | 40.5 | 85.4 a,b | 73.6 a | <0.001 |
| Body mass index (kg/m2) | 27.3 (24.9–30.29) | 28.1 (26.0–31.0) a | 28.8 (26.1–31.4) a | <0.001 |
| Waist circumference (cm) | 92.2 ± 11.1 | 97.4 ± 10.4 a,b | 98.9 ± 10.5 a | <0.001 |
| Abdominal visceral tissue (cm2) | 130 (98–172) | 162 (125–208) a,b | 180 (137–233) a | <0.001 |
| LDL cholesterol (mg/dL) | 116 (95–133) | 93 (71–117) a,b | 86 (64–115) a | <0.001 |
| HDL cholesterol (mg/dL) | 46 (37–56) | 37 (31–44) a | 37 (32–44) a | <0.001 |
| Triglycerides (mg/dL) | 138 (102–190) | 159 (116–212) a,b | 169 (124–231) a | <0.001 |
| Apolipoprotein A1 (mg/dL) | 134 (116–158) | 120 (101–136) a | 120 (102–142) a | <0.001 |
| Apolipoprotein B (mg/dL) | 92 (75 –111) | 80 (65–102) a,b | 79 (61–102) a | <0.001 |
| DPP4 (ng/mL) | 121 (93–155) | 107 (76–137) a | 93 (70–122) a | <0.001 |
| Type 2 diabetes mellitus (%) | 0 | 0 b | 100 a | <0.001 |
| Current smoking habit (%) | 23.4 | 12.2 a | 10.7 a | <0.001 |
| Physical activity | 7.9 (7.0–8.9) | 7.6 (6.8–8.5) a,b | 7.4 (6.5–8.4) a | <0.001 |
| rs17574 DPP4 genotypes | ||||
| AA (%) | 64.9 | 68.6 a | 72.6 a | |
| AG (%) | 31.7 | 28.1 a | 24.0 a | 0.018 |
| GG (%) | 3.4 | 3.5 | 3.5 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vargas-Alarcón, G.; González-Salazar, M.d.C.; Hernández-Díaz Couder, A.; Sánchez-Muñoz, F.; Ramírez-Bello, J.; Rodríguez-Pérez, J.M.; Posadas-Sánchez, R. Association of the rs17574 DPP4 Polymorphism with Premature Coronary Artery Disease in Diabetic Patients: Results from the Cohort of the GEA Mexican Study. Diagnostics 2022, 12, 1716. https://doi.org/10.3390/diagnostics12071716
Vargas-Alarcón G, González-Salazar MdC, Hernández-Díaz Couder A, Sánchez-Muñoz F, Ramírez-Bello J, Rodríguez-Pérez JM, Posadas-Sánchez R. Association of the rs17574 DPP4 Polymorphism with Premature Coronary Artery Disease in Diabetic Patients: Results from the Cohort of the GEA Mexican Study. Diagnostics. 2022; 12(7):1716. https://doi.org/10.3390/diagnostics12071716
Chicago/Turabian StyleVargas-Alarcón, Gilberto, Maria del Carmen González-Salazar, Adrian Hernández-Díaz Couder, Fausto Sánchez-Muñoz, Julian Ramírez-Bello, José Manuel Rodríguez-Pérez, and Rosalinda Posadas-Sánchez. 2022. "Association of the rs17574 DPP4 Polymorphism with Premature Coronary Artery Disease in Diabetic Patients: Results from the Cohort of the GEA Mexican Study" Diagnostics 12, no. 7: 1716. https://doi.org/10.3390/diagnostics12071716