Abstract
Obstructive sleep apnoea (OSA) is characterised by repetitive episodes of upper airway collapse and breathing cessation during sleep. Empty nose syndrome (ENS) is a surgically iatrogenic phenomenon of paradoxical nasal obstruction despite an objectively patent nasal airway. This study aimed to investigate sleep quality and the presence of OSA in ENS patients. Forty-eight ENS patients underwent full-night polysomnography. Total nasal resistance (TNR) was determined using anterior rhinomanometry. Symptoms and quality of life were evaluated by the empty nose syndrome 6-item questionnaire (ENS6Q), Sino-Nasal Outcome Test-22 (SNOT-22), and Epworth Sleepiness Scale questionnaires (ESS). Fourteen, twelve, and fourteen patients had mild, moderate, and severe OSA, respectively. The apnoea–hypopnoea index (AHI) and the lowest SpO2 were 23.8 ± 22.4/h and 85.9 ± 11.1%, respectively. N1, N2, N3 and rapid-eye-movement sleep comprised 30.2 ± 16.9%, 47.3 ± 15.5%, 2.1 ± 5.4%, and 20.0 ± 8.1% of the total sleep time. Body mass index, neck circumference, serum total immunoglobulin E, and ENS6Q score were significantly associated with AHI in the regression analysis. The ENS6Q scores correlated positively with AHI, arousal index, and ESS score, but negatively with TNR. ENS patients showed a high OSA prevalence and significant sleep impairment. The extent of OSA was associated with obesity levels and ENS symptom severity. The ENS6Q scores correlated negatively with nasal resistance, and positively with arousal frequency and daytime sleepiness. The recognition of individuals experiencing marked OSA and provision of appropriate intervention is critical to preventing long-term morbidity and mortality, and improving therapeutic outcomes in ENS patients.
1. Introduction
Obstructive sleep apnoea (OSA) is a complex disorder characterised by repetitive episodes of upper airway collapse and breathing cessation during sleep [1]. The drop in muscle tone during sleep leads to collapse of the upper airways and intermittent episodes of hypopnoea (partial obstruction) and/or apnoea (total obstruction). OSA not only causes symptoms such as mid-night arousal and excessive daytime sleepiness, but is also associated with a significant risk of cardiovascular, pulmonary, and neurocognitive morbidity and mortality [2].
Empty nose syndrome (ENS) is a surgically iatrogenic phenomenon of paradoxical nasal obstruction despite an objectively patent nasal airway [3]. The pathophysiology of abnormal sensations in ENS patients is complicated and not fully understood [4]. Hypothesised theories include changes in nasal airflow after surgical intervention and abnormalities in neurosensory systems owing to improper mucosal healing [4,5,6,7,8].
ENS patients may also experience dyspnoea, nasal and pharyngeal dryness, facial or nasal pain, rhinorrhoea or postnasal drip, crusting, hyposmia, headache, inability to concentrate, chronic fatigue, frustration, anxiety, and depression [1,9,10]. Affected patients often report significant psychological effects, loss of productivity, decreased quality of life (QOL), and lifestyle disruption [11,12,13]. Our previous questionnaire-based study revealed associations between significant sleep dysfunction and psychological effects in ENS patients [14,15]. However, there is no study that objectively measures sleep in the literature. As ENS affects the upper airway and exhibits significant sleep dysfunction, it is necessary to conduct an objective study to investigate sleep quality and the presence of OSA in these patients. As a result, we firstly used polysomnography (PSG), the gold standard for the evaluation of sleep architecture and diagnosis of OSA, in patients with ENS and identified the clinical risk factors for moderate-to-severe OSA in this study. This information could be helpful for sleep dysfunction management and optimisation of patient-centred care.
2. Materials and Methods
2.1. Patients
After approval from the institutional review board of Chang Gung Medical Foundation (IRB numbers: 201702048B0, 201802147A3 and 201902001A3), we recruited ENS patients who were managed in the Department of Otolaryngology between September 2018 and April 2021. All participants provided signed informed consent at enrolment. The diagnosis of ENS was based on subjective symptoms, including paradoxical nasal obstruction, and comparable findings in a cotton test (during which a moistened cotton ball was placed in the widest area of the common nasal cavity). Improvement in symptoms through 30 min of nasal inhalation was indicative of a positive cotton test [9,16]. Clinical characteristics and demographic data, including body mass index (BMI), neck circumference (NC), and serum total immunoglobulin E (sIgE), were documented. Nasal endoscopy, bilateral nasal resistance measurement, and computed tomography were used to evaluate the nasal airway. Patients with congenital craniofacial anomalies or other sinonasal diseases, such as rhinosinusitis or nasal polyps, were excluded.
2.2. Total Nasal Resistance (TNR)
Nasal resistance for each nostril was measured by active anterior rhinomanometry at 150 Pa, after a rest period in the seated position. A pressure sensor was placed in one nostril, while airflow during inspiration was measured in the other. Unilateral nasal resistance values of the left (RL) and right (RR) nostrils were calculated according to Ohm’s Law (flow = pressure/resistance). TNR was obtained using the following formula:
TNR = (RL × RR)/(RL + RR).
2.3. Polysomnography
Each patient underwent full-night polysomnography (Alice 5, Respironics using standard techniques). Sleep stages and arousals were scored according to the American Academy of Sleep Medicine criteria [17]. Apnoea was defined as more than a 90% reduction in airflow for more than 10 s. Hypopnoea was defined as more than a 30% decrease in airflow for at least 10 s with associated oxygen desaturation of more than 3% or associated arousal. The apnoea–hypopnoea index (AHI), which refers to episodes of obstructive apnoea and/or hypopnoea per hour of sleep, and the lowest observed oxy-haemoglobin saturation by pulse oximetry (lowest SpO2) are the main indicators for assessment of OSA severity. Based on the polysomnography results, mild, moderate, and severe OSA were defined as AHI ≥ 5, 15, and 30 per hour, respectively.
2.4. Subjective Evaluation
The empty nose syndrome 6-item questionnaire (ENS6Q) is a validated, ENS-specific instrument to identify patients suspected of having ENS [18,19]. The ENS6Q consists of six items, including dryness, lack of air sensation going through the nasal cavities, suffocation, nose feeling too open, nasal crusting, and nasal burning. Participants scored each item from 0 (no symptom) to 5 (severe symptom), with a maximum score of 30 points. An ENS6Q score ≥ 10.5 suggests the possible presence of ENS to the clinician.
The Sino-Nasal Outcome Test-22 (SNOT-22) questionnaire is a validated instrument developed to evaluate subjective measures of symptoms in sinonasal disorders [20]. The SNOT-22 questionnaire includes various symptoms, physical problems, functional limitations, and emotional consequences. Scores range from 0 to 5, with higher scores indicating more severe symptoms.
The Epworth Sleepiness Scale (ESS) is the instrument most commonly used for assessing daytime sleepiness [21]. This self-administered questionnaire calculates eight scores about the frequency of sleepiness on a 4-point scale (0–3) in eight different situations in daily life. ESS scores >10 indicate extensive daytime sleepiness [22].
2.5. Statistical Analyses
Data are presented as mean ± standard deviation (SD) and were statistically analysed using SPSS version 26.0 (IBM Corp, Armonk, NY, USA). Univariate and multivariate linear regression models determined the association between AHI and clinical variables. Correlations were found using Pearson’s correlation coefficient (r) or Spearman’s correlation coefficient (rs) when data did not pass the normality test. To identify and characterise the sensitivity and specificity of clinical metrics for detection of moderate-to-severe OSA in ENS participants, receiver operating characteristic (ROC) curves were analysed and the area under the ROC curve (AUC) was calculated. Statistical significance was set at p < 0.05.
3. Results
3.1. Clinical Characteristics of the Study Population
A total of 48 ENS patients with a mean (±SD) age of 44.5 ± 11.7 years, including 7 women and 41 men, were enrolled during the study period. Table 1 summarises the general characteristics of the participants. The mean (±SD) BMI and neck circumference were 24.0 ± 3.1 kg/m2 and 37.3 ± 3.1 cm, respectively, which indicated that the patients were slightly overweight. The mean (±SD) scores of questionnaires, including ESS, SNOT-22, and ENS6Q, were 10.8 ± 6, 63.9 ± 18.1, and 15.2 ± 5.0, respectively, indicating significant symptomatic burdens on patients’ QOL.

Table 1.
Clinical characteristics of the study participants.
3.2. PSG Results
Table 2 summarises the data collected from PSGs. The mean (±SD) AHI and the lowest SpO2 were 23.8 ± 22.4/h and 85.9 ± 11.1%, respectively, among 14 (29.2%), 12 (25.0%), and 14 (29.2%) patients with mild, moderate, and severe OSA. The mean (±SD) arousal index was 25.1 ± 15.9 per hour. In the sleep architecture analysis, stage N1, N2, N3, and rapid-eye-movement (REM) sleep comprised 30.2 ± 16.9%, 47.3 ± 15.5%, 2.1 ± 5.4%, and 20.0 ± 8.1% of the total sleep time. A relatively high proportion of stage N1 sleep and a low proportion of stage N3 sleep were observed in these patients when compared with the normal sleep architecture.

Table 2.
Polysomnographic results of the study participants.
3.3. Regression Analyses for AHI
In the univariate regression analysis, BMI (β = 0.52, p < 0.001), NC (β = 0.52, p < 0.001), sIgE (β = 0.32, p = 0.026), and ENS6Q score (β = 0.31, p = 0.032) were significantly associated with AHI (Table 3). Further analysis using a multivariate regression model (adjusted r2 = 0.378, p < 0.001) revealed that BMI (β = 0.48, p < 0.001) and sIgE (β = 0.32, p = 0.01) were significant predictors of AHI.

Table 3.
Linear regression analysis for an apnoea–hypopnoea index of the study participants.
3.4. Correlation Analyses of ENS6Q
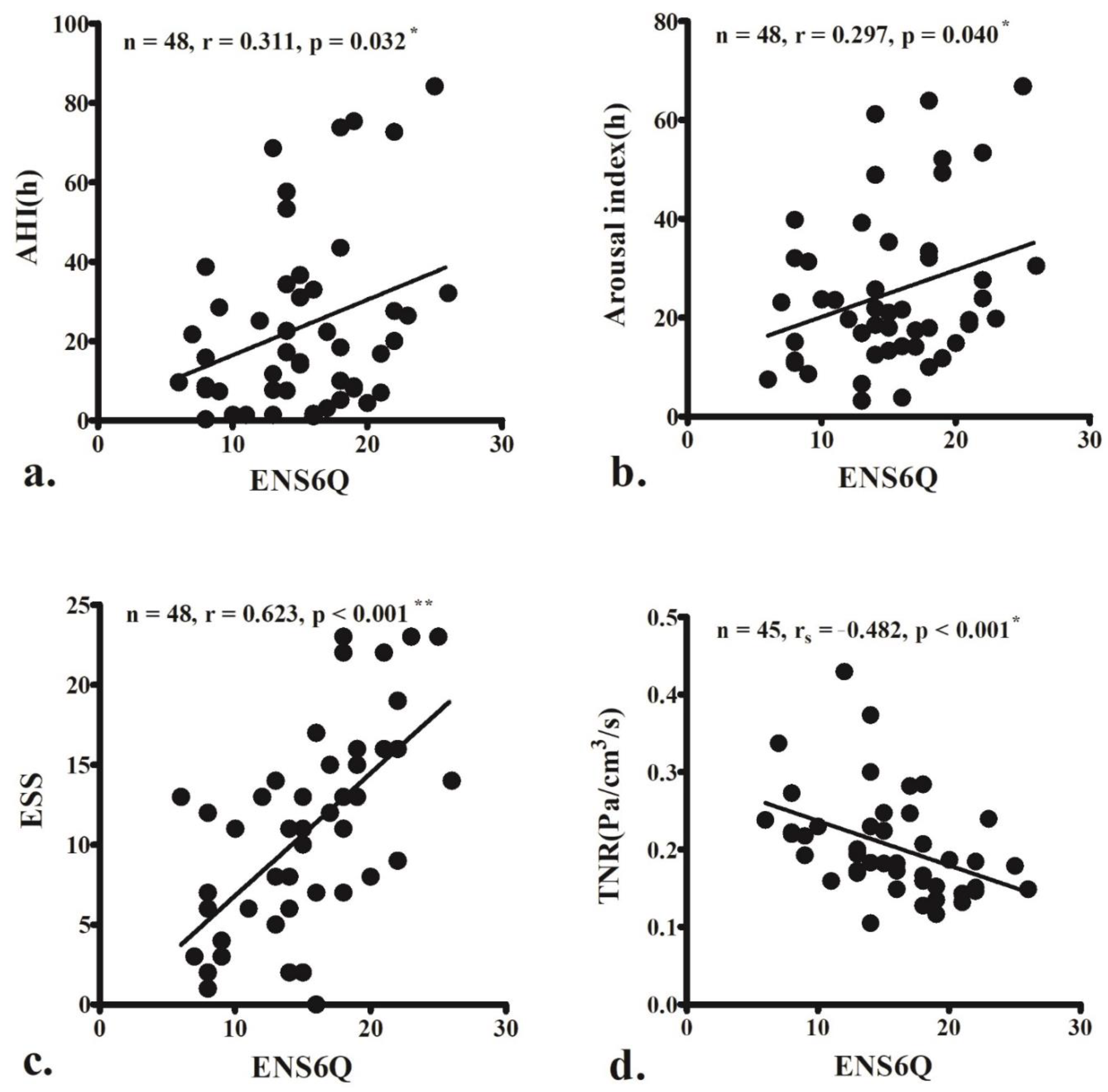
The ENS6Q scores significantly correlated with AHI (r = 0.311, p = 0.032; Figure 1a), arousal index (r = 0.297, p = 0.040; Figure 1b), and ESS score (r = 0.623, p < 0.001; Figure 1c). The ENS6Q scores also negatively correlated with TNR (rs = −0.482, p < 0.001; Figure 1d).
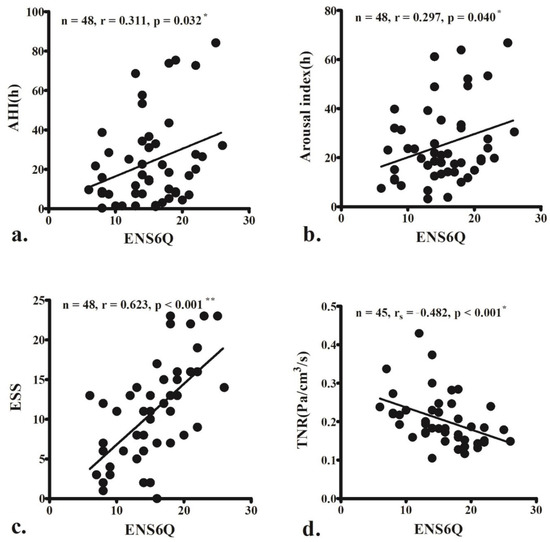
Figure 1.
The empty nose syndrome 6-item questionnaire (ENS6Q) scores are significantly associated with the apnoea–hypopnoea index (AHI) (a), arousal index (b), and Epworth Sleepiness Scale (ESS) score (c). ENS6Q is also negatively correlated with total nasal resistance (TNR) (d). The correlations were determined using Pearson’s correlation coefficient (r) or Spearman’s correlation coefficient (rs) if the data did not pass the normality test. (r, 0–0.2: very weak, 0.2–0.4: weak, 0.4–0.6: moderate, 0.6–0.8: strong, 0.8–1.0: very strong correlation) * p < 0.05, ** p < 0.001.
3.5. Using Clinical Metrics to Detect Moderate-to-Severe OSA
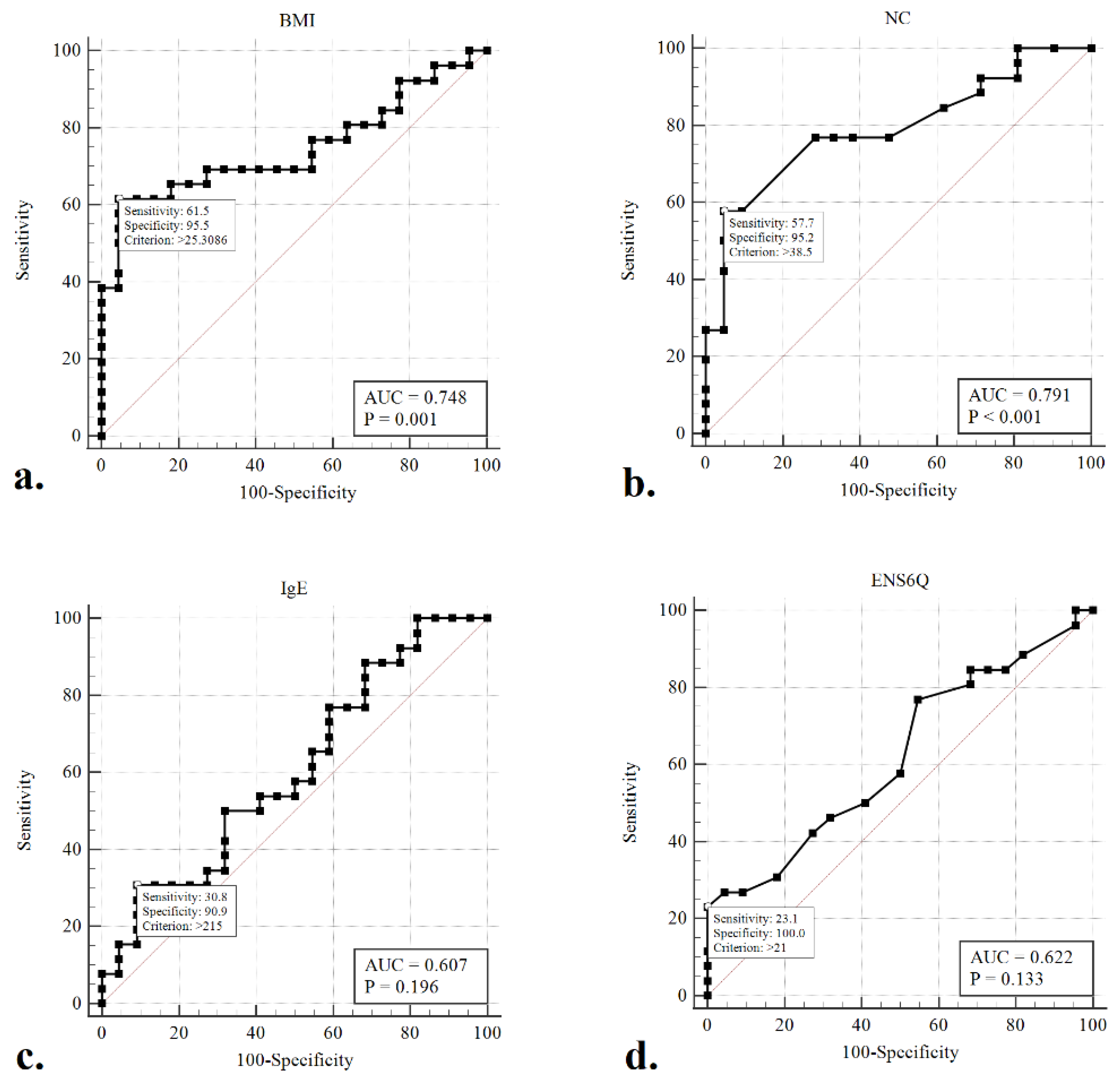
Given the concern that patients with moderate-to-severe OSA (AHI ≥ 15) warrant aggressive intervention and therapy, ROC curves were generated, and the AUC was calculated to evaluate whether any of the clinical variables would detect moderate-to-severe OSA in our participants better than a random test in a statistically significant manner (AUC > 0.5) (Figure 2). The ROC curves of BMI (AUC = 0.765, p < 0.001) and NC (AUC = 0.734, p < 0.001) were associated with AUCs significantly greater than 0.5. The optimal cut-offs for these variables (maximising the sum of sensitivity and specificity) were BMI > 25.3 kg/m2 (sensitivity: 61.5%, specificity: 95.5%) and NC > 38.5 cm (sensitivity: 57.7%, specificity: 95.2%).
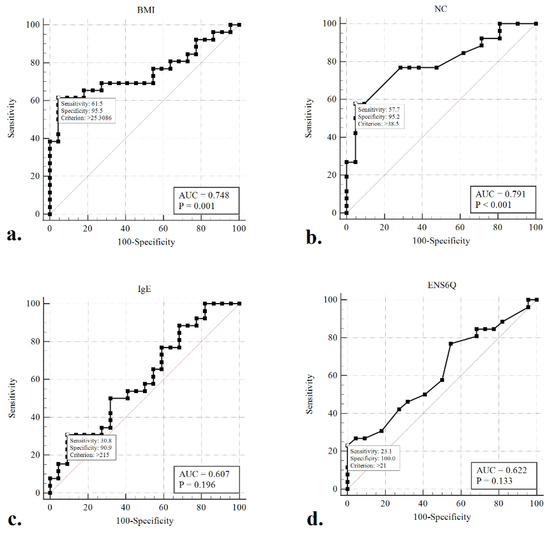
Figure 2.
Receiver operating characteristic (ROC) curves to detect moderate-to-severe obstructive sleep apneoa (AHI > 15) using the variables of BMI (a), NC (b), sIgE (c), and ENS6Q (d). The optimal cut-offs for these metrics (maximizing the sum of sensitivity and specificity) are indicated.
4. Discussion
In adults, approximately 5%, 50%, and 20% of the total sleep time is stage N1, N2, and N3 sleep, respectively. The remaining 25% is REM sleep [23,24]. In the present study, stages N1, N2, N3, and REM sleep made up 30.2%, 47.3%, 2.1%, and 20.0% of the total sleep time, respectively. A marked increase in stage N1 and a decrease in stage N3 sleep were observed. Stage N1 sleep is the transition from an awake state to sleep. A high proportion of stage N1 sleep is generally a result of frequent arousal and sleep fragmentation caused by sleep disorders, such as sleep apnoea. Stage N3, sometimes referred to as slow-wave sleep, is considered to be deep sleep [25]. Our results confirmed substantial sleep dysfunction in ENS patients, which, in earlier subjective studies, was found to be associated with psychological burdens such as anxiety and depression [14,15]. Differentiating the associations between disease-specific impairment of QOL is important and could allow for targeted symptom therapy to improve patient-centred care in ENS because of the heterogeneity of these patients.
This is the first study to evaluate sleep architecture and investigate the prevalence of OSA by PSG in ENS patients. Earlier studies have reported that significant sleep dysfunction is associated with psychological burden in ENS patients [13,14,15]. The results of the present study showed a high prevalence of OSA in ENS patients; 40 (83.3%) patients had AHI ≥ 5 and 26 (54.2%) patients experienced moderate-to-severe OSA (AHI ≥ 15). In addition, AHI was significantly associated with BMI, NC, sIgE, and ENS6Q scores in patients with ENS. In detecting moderate-to-severe OSA by clinical metrics, BMI and NC were identified as significant predictors, with AUCs significantly greater than 0.5. The optimal cut-off values for these variables were BMI > 25.3 kg/m2 and NC > 38.5 cm. Moreover, ENS6Q scores were positively associated with AHI, arousal index, and ESS scores, and negatively correlated with TNR. These results show that low TNR and severe ENS symptoms may lead to significant sleep dysfunction and arousal. The extent of OSA was associated with the degree of obesity and severity of ENS symptoms in these patients.
The association of ENS and OSA in our patient group may be attributed to aggressive manipulation of the inferior turbinates in OSA patients who undergo surgery for nasal obstruction, or to improve the use of nasal continuous positive airway pressure [26]. An earlier study reported that oropharyngeal surgery for OSA can lower TNR in patients with moderate-to-severe OSA [27]. This indicates that TNR may be caused by both nasal and oropharyngeal stenosis in patients with moderate-to-severe OSA. Thus, there is a higher chance of over or repeated resection of the turbinates in these patients if the surgeon does not evaluate their upper airway comprehensively.
Previous studies by computational fluid dynamics (CFD) demonstrated that total turbinectomy reduced nasal resistance, increased nasal airflow rate [28], and increased airflow distribution around the middle meatus [29]. Thus, another possible mechanism for the association between ENS and OSA is that the reduced TNR and increased rate of airflow after nasal surgery may decrease the supporting pressure and increase the collapsibility of the narrow oropharynges in OSA patients, according to Bernoulli’s law. However, further CFD studies on the relationship between nasal airflow and oropharyngeal flow, resistance, and collapsibility in OSA patients are required.
Our previous study also reported significant psychological burdens in ENS patients, including that 38.3% and 53% of ENS patients experienced moderate-to-severe depression and anxiety, respectively [14]. The association between OSA and depression has been previously reported [30,31,32]. For example, in subjects with OSA, the prevalence of depression may reach 63% [31], whereas the prevalence of OSA in individuals with major depression was 36.3% [32]. The prevalence of OSA in the general population is 1–14% (2–7% of women and 9–14% of men) [33]. Hence, OSA seems to be more common in individuals with depression than in the general population. The high prevalence of obesity and metabolic syndrome associated with a depressive disorder may be a possible aetiology of OSA [34]. However, increased alcohol consumption, smoking, and benzodiazepine drug use in patients with depression and anxiety also potentially promote the development of OSA because these factors may cause a decrease in muscle tone or an increase in mucus secretion in the upper airway [35]. This could be another explanation for the high prevalence of OSA in ENS patients.
In addition to excessive daytime sleepiness, moderate-to-severe OSA usually leads to significant impairments in QOL and cognitive performance, and to an increase in traffic or occupational accidents [36]. In addition, moderate-to-severe OSA is significantly associated with cardiovascular and metabolic diseases, such as systemic arterial hypertension, coronary artery disease, heart failure, and stroke. All these factors could lead to substantial morbidity and mortality [37,38]. As a result, early identification and treatment of patients with moderate-to-severe OSA are important. In a study of the general population, male sex, age ≥ 50 years, NC ≥ 14.5 inches (36.8 cm), and BMI ≥ 30 kg/m2 are risk factors for moderate-to-severe OSA [39]. In this study, the ROC curve analysis revealed that BMI > 25.3 kg/m2 and NC >38.5 cm were predictors of moderate-to-severe OSA. Recognition of individuals experiencing marked OSA and provision of appropriate intervention are also critical to prevent long-term morbidity and mortality in ENS patients.
Our results also showed an association between sIgE levels and AHI. The relationship between allergies and OSA remains controversial. Allergic rhinitis-related inflammation in the upper airway might worsen the extent of OSA, and treatment of allergic rhinitis may reduce the severity of OSA-impaired sleep quality and daytime sleepiness [40]. However, a previous study showed no significant difference in the frequency of allergic rhinitis and atopy between subjects with and without OSA [41]. Future studies on the relationship between allergic inflammation and OSA severity in patients with ENS are necessary.
A limitation of this study is the cross-sectional evaluation of the association between variables. The aetiology of OSA is usually multifactorial [1]. ENS6Q is one of the frequently used symptom scores in the evaluation of ENS. We found an association between AHI and ENS6Q with correlation and univariate regression analysis, but not with multivariate regression; this may be due to the strong influencing factor of obesity in OSA [22]. Future studies that longitudinally analyse the metrics before and after treatment, such as surgery, as well as comparison with a control group, may be beneficial in illustrating the interaction of these variables. This study reported a high prevalence of OSA and significant sleep dysfunction in patients with ENS.
5. Conclusions
We found a high prevalence of OSA and significant sleep impairment in patients with ENS. The extent of OSA was associated with the degree of obesity and severity of ENS symptoms in these patients. In addition, the ENS6Q scores were negatively correlated with nasal resistance and positively associated with arousal frequency and daytime sleepiness. Recognition of individuals experiencing marked OSA and provision of appropriate intervention are critical to preventing long-term morbidity and mortality and improving therapeutic outcomes in ENS patients.
Author Contributions
T.-J.L., C.-C.H. (Chi-Che Huang) and C.-C.H. (Chien-Chia Huang) participated in the study design. C.-C.H. (Chien-Chia Huang) and P.-W.W. performed the data collection and analysis and drafted the manuscript. P.-H.C., C.-H.F., C.-C.H. (Chi-Che Huang) and T.-J.L. helped in the enrolment of participants. C.-C.L., Y.-S.L., C.-C.C. and C.-C.H. (Chien-Chia Huang) contributed to data interpretation. All authors have read and agreed to the published version of the manuscript.
Funding
The authors received a research grant from the Ministry of Science and Technology (NMRPG3K0031) and Chang Gung Memorial Hospital (CMRPG3K2321), Taiwan. The funder had no role in the study design, data collection, analysis, decision to publish, or preparation of the manuscript.
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki and approved by the Institutional Review Board of Chang Gung Medical Foundation (IRB numbers: 201702048B0 (2/1/2018), 201802147A3 (13/2/2019) and 201902001A3 (13/2/2020)).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The dataset used and/or analysed during the current study is available from the corresponding author upon reasonable request.
Acknowledgments
The authors thank Meng-Chieh Tsai for assisting with data collection.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Veasey, S.C.; Rosen, I.M. Obstructive sleep apnea in adults. N. Engl. J. Med. 2019, 380, 1442–1449. [Google Scholar] [CrossRef]
- McEvoy, R.D.; Antic, N.A.; Heeley, E.; Luo, Y.; Ou, Q.; Zhang, X.; Mediano, O.; Chen, R.; Drager, L.F.; Liu, Z.; et al. CPAP for prevention of cardiovascular events in obstructive sleep apnea. N. Engl. J. Med. 2016, 375, 919–931. [Google Scholar] [CrossRef]
- Houser, S.M. Empty nose syndrome associated with middle turbinate resection. Otolaryngol.-Head Neck Surg. 2006, 135, 972–973. [Google Scholar] [CrossRef]
- Sozansky, J.; Houser, S.M. Pathophysiology of empty nose syndrome. Laryngoscope 2015, 125, 70–74. [Google Scholar] [CrossRef]
- Zhao, K.; Blacker, K.; Luo, Y.; Bryant, B.; Jiang, J. Perceiving nasal patency through mucosal cooling rather than air temperature or nasal resistance. PLoS ONE 2011, 6, e24618. [Google Scholar] [CrossRef]
- Chen, X.B.; Leong, S.C.; Lee, H.P.; Chong, V.F.; Wang, D.Y. Aerodynamic effects of inferior turbinate surgery on nasal airflow—A computational fluid dynamics model. Rhinol. J. 2010, 48, 394–400. [Google Scholar] [CrossRef]
- Konstantinidis, I.; Tsakiropoulou, E.; Chatziavramidis, A.; Ikonomidis, C.; Markou, K. Intranasal trigeminal function in patients with empty nose syndrome. Laryngoscope 2017, 127, 1263–1267. [Google Scholar] [CrossRef]
- Wu, C.L.; Fu, C.H.; Lee, T.J. Distinct histopathology characteristics in empty nose syndrome. Laryngoscope 2021, 131, E14–E18. [Google Scholar]
- Chhabra, N.; Houser, S.M. The diagnosis and management of empty nose syndrome. Otolaryngol. Clin. N. Am. 2009, 42, 311–330. [Google Scholar] [CrossRef]
- Jiang, C.; Wang, F.; Chen, K.; Shi, R. Assessment of surgical results in patients with empty nose syndrome using the 25-item sino-nasal outcome test evaluation. JAMA Otolaryngol.-Head Neck Surg. 2014, 140, 453–458. [Google Scholar] [CrossRef]
- Manji, J.; Nayak, J.V.; Thamboo, A. The functional and psychological burden of empty nose syndrome. Int. Forum Allergy Rhinol. 2018, 8, 707–712. [Google Scholar] [CrossRef]
- Mangin, D.; Bequignon, E.; Zerah-Lancner, F.; Isabey, D.; Louis, B.; Adnot, S.; Papon, J.-F.; Coste, A.; Boyer, L.; Du Mayne, M.D. Investigating hyperventilation syndrome in patients suffering from empty nose syndrome. Laryngoscope 2017, 127, 1983–1988. [Google Scholar] [CrossRef]
- Lee, T.-J.; Fu, C.-H.; Wu, C.-L.; Tam, Y.-Y.; Huang, C.-C.; Chang, P.-H.; Chen, Y.-W.; Wu, M.-H. Evaluation of depression and anxiety in empty nose syndrome after surgical treatment. Laryngoscope 2016, 126, 1284–1289. [Google Scholar] [CrossRef]
- Huang, C.-C.; Wu, P.-W.; Fu, C.-H.; Chang, P.-H.; Wu, C.-L.; Lee, T.-J. What drives depression in empty nose syndrome? A sinonasal outcome test-25 subdomain analysis. Rhinol. J. 2019, 57, 469–476. [Google Scholar] [CrossRef]
- Huang, C.C.; Wu, P.W.; Fu, C.H.; Huang, C.C.; Chang, P.H.; Lee, T.J. Impact of psychologic burden on surgical outcome in empty nose syndrome. Laryngoscope 2021, 131, E694–E701. [Google Scholar] [CrossRef]
- Amanian, A.; Hari, K.; Habib, A.; Dholakia, S.S.; Nayak, J.; Thamboo, A. The empty nose syndrome 6-item questionnaire (ENS6Q): A diagnostic tool to distinguish empty nose syndrome from primary nasal obstruction. Int. Forum Allergy Rhinol. 2021, 11, 1113–1115. [Google Scholar] [CrossRef]
- Berry, R.B.; Budhiraja, R.; Gottlieb, D.J.; Gozal, D.; Iber, C.; Kapur, V.K.; Marcus, C.L.; Mehra, R.; Parthasarathy, S.; Quan, S.F.; et al. Rules for scoring respiratory events in sleep: Update of the 2007 AASM manual for the scoring of sleep and associated events. Deliberations of the sleep apnea definitions task force of the american academy of sleep medicine. J. Clin. Sleep Med. 2012, 8, 597–619. [Google Scholar] [CrossRef]
- Velasquez, N.; Thamboo, A.; Habib, A.-R.R.; Huang, Z.; Nayak, J.V. The empty nose syndrome 6-item questionnaire (ENS6Q): A validated 6-item questionnaire as a diagnostic aid for empty nose syndrome patients. Int. Forum Allergy Rhinol. 2016, 7, 64–71. [Google Scholar] [CrossRef]
- Thamboo, A.; Velasquez, N.; Habib, A.R.R.; Zarabanda, D.; Paknezhad, H.; Nayak, J.V. Defining surgical criteria for empty nose syndrome: Validation of the office-based cotton test and clinical interpretability of the validated empty nose syndrome 6-item questionnaire. Laryngoscope 2017, 127, 1746–1752. [Google Scholar] [CrossRef]
- Toma, S.; Hopkins, C. Stratification of SNOT-22 scores into mild, moderate or severe and relationship with other subjective instruments. Rhinology 2016, 54, 129–133. [Google Scholar] [CrossRef]
- Johns, M.W. A new method for measuring daytime sleepiness: The epworth sleepiness scale. Sleep 1991, 14, 540–545. [Google Scholar] [CrossRef]
- Conradt, R.; Redhardt, F.; Beutel, B.; Hildebrandt, O.; Cassel, W.; Kesper, K.; Koehler, J.; Hildebrandt, W.; Koehler, U. Predisposing factors of daytime sleepiness in obese OSA patients. J. Sleep Res. 2021, 31, e13458. [Google Scholar] [CrossRef]
- Pressman, M.R. Primer of Polysomnogram Interpretation; Butterworth Heinemann: Boston, MA, USA, 2002. [Google Scholar]
- Iber, C.; Ancoli-Israel, S.; Chesson, A.; Quan, S.F. The AASM manual for the scoring of sleep and associated events: Rules. In Terminology and Technical Specifications; American Academy of Sleep Medicine: Westchester, NY, USA, 2007. [Google Scholar]
- Shrivastava, D.; Jung, S.; Saadat, M.; Sirohi, R.; Crewson, K. How to interpret the results of a sleep study. J. Community Hosp. Intern. Med. Perspect. 2014, 4, 24983. [Google Scholar] [CrossRef]
- Wu, J.; He, S.; Li, Y.; Wang, T.; Zhao, G.; Pan, Y.; Zang, H.; Han, D. Evaluation of the clinical efficacy of nasal surgery in the treatment of obstructive sleep apnoea. Am. J. Otolaryngol. 2021, 43, 103158. [Google Scholar] [CrossRef]
- Huang, C.-C.; Cheng, P.-W.; Liao, L.-J.; Huang, T.-W. Reduction of postural nasal resistance following oropharyngeal surgery in patients with moderate-severe obstructive sleep apnea. Rhinol. J. 2021, 59, 75–80. [Google Scholar] [CrossRef]
- Dayal, A.; Rhee, J.S.; Garcia, G.J.M. Impact of middle versus inferior total turbinectomy on nasal aerodynamics. Otolaryngol.-Head Neck Surg. 2016, 155, 18–525. [Google Scholar] [CrossRef]
- Malik, J.; Li, C.; Maza, G.; Farag, A.A.; Krebs, J.P.; McGhee, S.; Zappitelli, G.; Deshpande, B.; Otto, B.A.; Zhao, K. Computational fluid dynamic analysis of aggressive turbinate reductions: Is it a culprit of empty nose syndrome? Int. Forum Allergy Rhinol. 2019, 9, 891–899. [Google Scholar] [CrossRef]
- Yosunkaya, S.; Kutlu, R.; Cihan, F.G. Evaluation of depression and quality of life in patients with obstructive sleep apnea syndrome. Niger. J. Clin. Pract. 2016, 19, 573–579. [Google Scholar] [CrossRef]
- Saunamäki, T.; Jehkonen, M. Depression and anxiety in obstructive sleep apnea syndrome: A review. Acta Neurol. Scand. 2007, 116, 277–288. [Google Scholar] [CrossRef]
- Stubbs, B.; Vancampfort, D.; Veronese, N.; Solmi, M.; Gaughran, F.; Manu, P.; Rosenbaum, S.; De Hert, M.; Fornaro, M. The prevalence and predictors of obstructive sleep apnea in major depressive disorder, bipolar disorder and schizophrenia: A systematic review and meta-analysis. J. Affect. Disord. 2016, 197, 259–267. [Google Scholar] [CrossRef]
- Young, T.; Peppard, P.E.; Gottlieb, D.J. Epidemiology of obstructive sleep apnea: A population health perspective. Am. J. Respir. Crit. Care Med. 2002, 165, 1217–1239. [Google Scholar] [CrossRef]
- Hein, M.; Lanquart, J.-P.; Loas, G.; Hubain, P.; Linkowski, P. Prevalence and risk factors of moderate to severe obstructive sleep apnea syndrome in major depression: A observational and retrospective study on 703 subjects. BMC Pulm. Med. 2017, 17, 165. [Google Scholar] [CrossRef]
- Ong, J.C.; Gress, J.L.; Pedro-Salcedo, M.G.S.; Manber, R. Frequency and predictors of obstructive sleep apnea among individuals with major depressive disorder and insomnia. J. Psychosom. Res. 2009, 67, 135–141. [Google Scholar] [CrossRef]
- Mulgrew, A.T.; Nasvadi, G.; Butt, A.; Cheema, R.; Fox, N.; Fleetham, J.A.; Ryan, C.F.; Cooper, P.; Ayas, N.T. Risk and severity of motor vehicle crashes in patients with obstructive sleep apnoea/hypopnoea. Thorax 2008, 63, 536–541. [Google Scholar] [CrossRef]
- Kent, B.D.; Grote, L.; Ryan, S.; Pépin, J.L.; Bonsignore, M.R.; Tkacova, R.; Saaresranta, T.; Verbraecken, J.; Lévy, P.; Hedner, J.; et al. Diabetes mellitus prevalence and control in sleep-disordered breathing: The European sleep apnea cohort (ESADA) study. Chest 2014, 146, 982–990. [Google Scholar] [CrossRef]
- McNicholas, W.T.; Bonsignore, M.R.; Management Committee of EU Cost Action B26. Sleep apnoea as an independent risk factor for cardiovascular disease: Current evidence, basic mechanisms and research priorities. Eur. Respir. J. 2007, 29, 156–178. [Google Scholar] [CrossRef]
- Young, T.; Shahar, E.; Nieto, F.J.; Redline, S.; Newman, A.B.; Gottlieb, D.J.; Walsleben, J.A.; Finn, L.; Enright, P.; Samet, J.M. Predictors of sleep-disordered breathing in community-dwelling adults the sleep heart health study. Arch. Intern. Med. 2002, 162, 893–900. [Google Scholar] [CrossRef]
- Lavigne, F.; Petrof, B.J.; Johnson, J.R.; Lavigne, P.; Binothman, N.; Kassissia, G.O.; Al Samri, M.; Giordano, C.; Dubé, N.; Hercz, D.; et al. Effect of topical corticosteroids on allergic airway inflammation and disease severity in obstructive sleep apnoea. Clin. Exp. Allergy 2013, 43, 1124–1133. [Google Scholar]
- Gadi, G.; Wali, S.; Koshak, E.; Albar, M.; Fida, A.; Abdelaziz, M.; Alnoury, K.; Alama, N. The prevalence of allergic rhinitis and atopic markers in obstructive sleep apnea. J. Epidemiol. Glob. Health 2017, 7, 37–44. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).