Repeatability and Reproducibility of Retinal Fractal Dimension Measured with Swept-Source Optical Coherence Tomography Angiography in Healthy Eyes: A Proof-of-Concept Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Participants
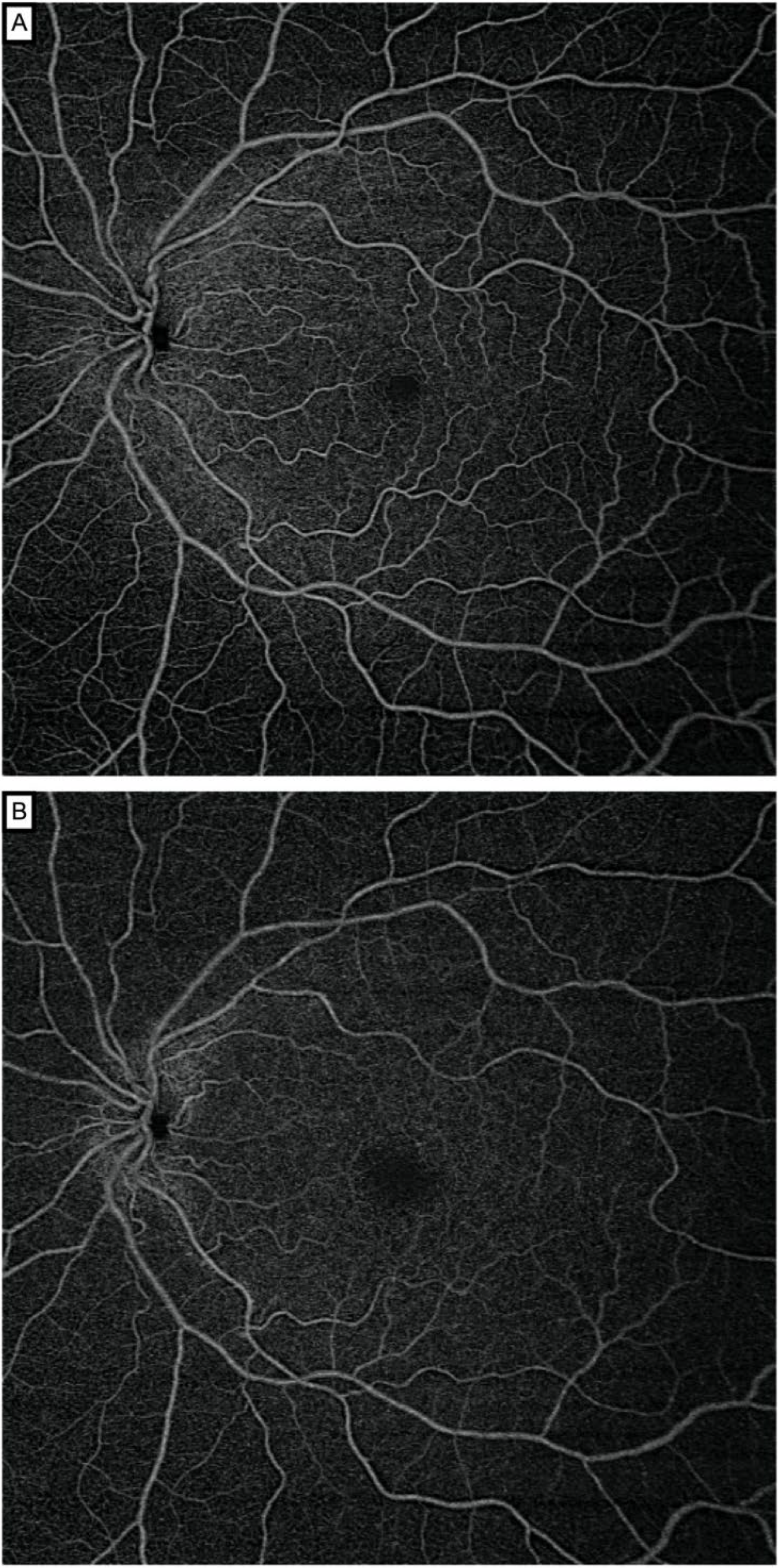
2.2. Image Acquisition and Processing
2.3. Retinal Vascular Network Fractal Dimension Analysis
2.4. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wong, T.Y.; Klein, R.; Klein, B.E.; Tielsch, J.M.; Hubbard, L.; Nieto, F.J. Retinal microvascular abnormalities and their relationship with hypertension, cardiovascular disease, and mortality. Surv. Ophthalmol. 2001, 46, 59–80. [Google Scholar] [CrossRef]
- Patton, N.; Aslam, T.; Macgillivray, T.; Pattie, A.; Deary, I.J.; Dhillon, B. Retinal vascular image analysis as a potential screening tool for cerebrovascular disease: A rationale based on homology between cerebral and retinal microvasculatures. J. Anat. 2005, 206, 319–348. [Google Scholar] [CrossRef] [PubMed]
- Witt, N.; Wong, T.Y.; Hughes, A.D.; Chaturvedi, N.; Klein, B.E.; Evans, R.; McNamara, M.; Thom, S.A.; Klein, R. Abnormalities of retinal microvascular structure and risk of mortality from ischemic heart disease and stroke. Hypertension 2006, 47, 975–981. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cheung, N.; Bluemke, D.A.; Klein, R.; Sharrett, A.R.; Islam, F.M.; Cotch, M.F.; Klein, B.E.; Criqui, M.H.; Wong, T.Y. Retinal arteriolar narrowing and left ventricular remodeling: The multi-ethnic study of atherosclerosis. J. Am. Coll. Cardiol. 2007, 50, 48–55. [Google Scholar] [CrossRef] [Green Version]
- Cheung, N.; Sharrett, A.R.; Klein, R.; Criqui, M.H.; Islam, F.M.; Macura, K.J.; Cotch, M.F.; Klein, B.E.; Wong, T.Y. Aortic distensibility and retinal arteriolar narrowing: The multi-ethnic study of atherosclerosis. Hypertension 2007, 50, 617–622. [Google Scholar] [CrossRef] [Green Version]
- Sasongko, M.B.; Wong, T.Y.; Nguyen, T.T.; Cheung, C.Y.; Shaw, J.E.; Wang, J.J. Retinal vascular tortuosity in persons with diabetes and diabetic retinopathy. Diabetologia 2011, 54, 2409–2416. [Google Scholar] [CrossRef]
- Seidelmann, S.B.; Claggett, B.; Bravo, P.E.; Gupta, A.; Farhad, H.; Klein, B.E.; Klein, R.; Di Carli, M.; Solomon, S.D. Retinal vessel calibers in predicting long-term cardiovascular outcomes: The atherosclerosis risk in communities study. Circulation 2016, 134, 1328–1338. [Google Scholar] [CrossRef] [Green Version]
- Lemmens, S.; Devulder, A.; Van Keer, K.; Bierkens, J.; De Boever, P.; Stalmans, I. Systematic review on fractal dimension of the retinal vasculature in neurodegeneration and stroke: Assessment of a potential biomarker. Front. Neurosci. 2020, 14, 16. [Google Scholar] [CrossRef] [Green Version]
- Mainster, M.A. The fractal properties of retinal vessels: Embryological and clinical implications. Eye 1990, 4, 235–241. [Google Scholar] [CrossRef] [Green Version]
- Masters, B.R. Fractal analysis of the vascular tree in the human retina. Annu. Rev. Biomed. Eng. 2004, 6, 427–452. [Google Scholar] [CrossRef] [Green Version]
- Liew, G.; Wang, J.J.; Cheung, N.; Zhang, Y.P.; Hsu, W.; Lee, M.L.; Mitchell, P.; Tikellis, G.; Taylor, B.; Wong, T.Y. The retinal vasculature as a fractal: Methodology, reliability, and relationship to blood pressure. Ophthalmology 2008, 115, 1951–1956. [Google Scholar] [CrossRef] [PubMed]
- Avakian, A.; Kalina, R.E.; Sage, E.H.; Rambhia, A.H.; Elliott, K.E.; Chuang, E.L.; Clark, J.I.; Hwang, J.N.; Parsons-Wingerter, P. Fractal analysis of region-based vascular change in the normal and non-proliferative diabetic retina. Curr. Eye Res. 2002, 24, 274–280. [Google Scholar] [CrossRef] [PubMed]
- Orlando, J.I.; Van Keer, K.; Barbosa-Breda, J.; Manterola, H.L.; Blaschko, M.B.; Clausse, A. Proliferative diabetic retinopathy characterization based on fractal features: Evaluation on a publicly available dataset. Med. Phys. 2017, 44, 6425–6434. [Google Scholar] [CrossRef] [PubMed]
- Rosenfeld, P.J.; Durbin, M.K.; Roisman, L.; Zheng, F.; Miller, A.; Robbins, G.; Schaal, K.B.; Gregori, G. ZEISS angioplex spectral domain optical coherence tomography angiography: Technical aspects. Dev. Ophthalmol. 2016, 56, 18–29. [Google Scholar] [CrossRef] [PubMed]
- Spaide, R.F.; Fujimoto, J.G.; Waheed, N.K.; Sadda, S.R.; Staurenghi, G. Optical coherence tomography angiography. Prog. Retin. Eye Res. 2018, 64, 1–55. [Google Scholar] [CrossRef]
- Spaide, R.F.; Klancnik, J.M., Jr.; Cooney, M.J. Retinal vascular layers imaged by fluorescein angiography and optical coherence tomography angiography. JAMA Ophthalmol. 2015, 133, 45–50. [Google Scholar] [CrossRef]
- Potsaid, B.; Baumann, B.; Huang, D.; Barry, S.; Cable, A.E.; Schuman, J.S.; Duker, J.S.; Fujimoto, J.G. Ultrahigh speed 1050 nm swept source/Fourier domain OCT retinal and anterior segment imaging at 100,000 to 400,000 axial scans per second. Opt. Express 2010, 18, 20029–20048. [Google Scholar] [CrossRef] [Green Version]
- Lei, J.; Durbin, M.K.; Shi, Y.; Uji, A.; Balasubramanian, S.; Baghdasaryan, E.; Al-Sheikh, M.; Sadda, S.R. Repeatability and reproducibility of superficial macular retinal vessel density measurements using optical coherence tomography angiography en face images. JAMA Ophthalmol. 2017, 135, 1092–1098. [Google Scholar] [CrossRef]
- Lee, M.W.; Kim, K.M.; Lim, H.B.; Jo, Y.J.; Kim, J.Y. Repeatability of vessel density measurements using optical coherence tomography angiography in retinal diseases. Br. J. Ophthalmol. 2019, 103, 704–710. [Google Scholar] [CrossRef] [Green Version]
- Xiao, H.; Liu, X.; Liao, L.; Tan, K.; Ling, Y.; Zhong, Y. Reproducibility of foveal avascular zone and superficial macular retinal vasculature measurements in healthy eyes determined by two different scanning protocols of optical coherence tomography angiography. Ophthalmic Res. 2020, 63, 244–251. [Google Scholar] [CrossRef]
- Corvi, F.; Pellegrini, M.; Erba, S.; Cozzi, M.; Staurenghi, G.; Giani, A. Reproducibility of vessel density, fractal dimension, and foveal avascular zone using 7 different optical coherence tomography angiography devices. Am. J. Ophthalmol. 2018, 186, 25–31. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Ma, Q.; Wu, C.; Tan, F.; Chen, F.; Wu, Q.; Zhou, R.; Zhuang, X.; Lu, F.; Qu, J.; et al. Macular vascular fractal dimension in the deep capillary layer as an early indicator of microvascular loss for retinopathy in type 2 diabetic patients. Investig. Ophthalmol. Vis. Sci. 2017, 58, 3785–3794. [Google Scholar] [CrossRef] [PubMed]
- Bhardwaj, S.; Tsui, E.; Zahid, S.; Young, E.; Mehta, N.; Agemy, S.; Garcia, P.; Rosen, R.B.; Young, J.A. Value of fractal analysis of optical coherence tomography angiography in various stages of diabetic retinopathy. Retina 2018, 38, 1816–1823. [Google Scholar] [CrossRef] [PubMed]
- Sng, C.C.A.; Wong, W.L.; Cheung, C.Y.; Lee, J.; Tai, E.S.; Wong, T.Y. Retinal vascular fractal and blood pressure in a multiethnic population. J. Hypertens. 2013, 31, 2036–2042. [Google Scholar] [CrossRef]
- Arnould, L.; Binquet, C.; Guenancia, C.; Alassane, S.; Kawasaki, R.; Daien, V.; Tzourio, C.; Kawasaki, Y.; Bourredjem, A.; Bron, A.; et al. Association between the retinal vascular network with Singapore “I” Vessel Assessment (SIVA) software, cardiovascular history and risk factors in the elderly: The Montrachet study, population-based study. PLoS ONE 2018, 13, e0194694. [Google Scholar] [CrossRef] [Green Version]
- Shi, C.; Chen, Y.; Kwapong, W.R.; Tong, Q.; Wu, S.; Zhou, Y.; Miao, H.; Shen, M.; Ye, H. Characterization by fractal dimension analysis of the retinal capillary network in parkinson disease. Retina 2020, 40, 1483–1491. [Google Scholar] [CrossRef]
- Talu, S.; Calugaru, D.M.; Lupascu, C.A. Characterisation of human non-proliferative diabetic retinopathy using the fractal analysis. Int. J. Ophthalmol. 2015, 8, 770–776. [Google Scholar] [CrossRef]
- Plesser, H.E. Reproducibility vs. Replicability: A Brief History of a Confused Terminology. Front. Neuroinform. 2018, 11, 76. [Google Scholar] [CrossRef] [Green Version]
- Kottner, J.; Kottner, L.; Brorson, S.; Donner, A.; Gajewski, B.J.; Hróbjartsson, A.; Roberts, C.; Shoukri, M.; Streiner, D.L. Guidelines for reporting reliability and agreement studies (GRRAS) were proposed. J. Clin. Epidemiol. 2011, 64, 96–106. [Google Scholar] [CrossRef]
- Landini, G.; Misson, G.P.; Murray, P.I. Fractal analysis of the normal human retinal fluorescein angiogram. Curr. Eye Res. 1993, 12, 23–27. [Google Scholar] [CrossRef]
- Cicchetti, D.V. Guidelines, criteria, and rules of thumb for evaluating normed and standardized assessment instruments in psychology. Psychol. Assess. 1994, 6, 284–290. [Google Scholar] [CrossRef]
- Bland, J.M.; Altman, D.G. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet 1986, 1, 307–310. [Google Scholar] [CrossRef]
- Hirano, T.; Kitahara, J.; Toriyama, Y.; Kasamatsu, H.; Murata, T.; Sadda, S. Quantifying vascular density and morphology using different swept-source optical coherence tomography angiographic scan patterns in diabetic retinopathy. Br. J. Ophthalmol. 2019, 103, 216–221. [Google Scholar] [CrossRef] [PubMed]
- Bonnin, S.; Mané, V.; Couturier, A.; Julien, M.; Paques, M.; Tadayoni, R.; Gaudric, A. New insight into the macular deep vascular plexus imaged by optical coherence tomography angiography. Retina 2015, 35, 2347–2352. [Google Scholar] [CrossRef]
- Lu, Y.; Wang, J.C.; Cui, Y.; Zhu, Y.; Zeng, R.; Lu, E.S.; Katz, R.; Husain, D.; Vavvas, D.G.; Kim, L.A.; et al. A quantitative comparison of four optical coherence tomography angiography devices in healthy eyes. Graefes Arch. Clin. Exp. Ophthalmol. 2020, 259, 1493–1501. [Google Scholar] [CrossRef]
- Hong, J.; Ke, M.; Tan, B.; Lau, A.; Wong, D.; Yao, X.; Liu, X.; Schmetterer, L.; Chua, J. Effect of vessel enhancement filters on the repeatability of measurements obtained from widefield swept-source optical coherence tomography angiography. Sci. Rep. 2020, 10, 22179. [Google Scholar] [CrossRef]
- Hong, J.; Tan, B.; Quang, N.D.; Gupta, P.; Lin, E.; Wong, D.; Ang, M.; Lamoureux, E.; Schmetterer, L.; Chua, J. Intra-session repeatability of quantitative metrics using widefield optical coherence tomography angiography (OCTA) in elderly subjects. Acta Ophthalmol. 2019, 98, e570–e578. [Google Scholar] [CrossRef] [Green Version]
- Karst, S.G.; Heisler, M.; Lo, J.; Schuck, N.; Safari, A.; Sarunic, M.V.; Maberley, D.A.L.; Navajas, E.V. Evaluating signs of microangiopathy secondary to diabetes in different areas of the retina with swept source OCTA. Investig. Ophthalmol. Vis. Sci. 2020, 61, 8. [Google Scholar] [CrossRef]
- Coscas, F.; Cabral, D.; Pereira, T.; Geraldes, C.; Narotamo, H.; Miere, A.; Lupidi, M.; Sellam, A.; Papoila, A.; Coscas, G.; et al. Quantitative optical coherence tomography angiography biomarkers for neovascular age-related macular degeneration in remission. PLoS ONE 2018, 13, e0205513. [Google Scholar] [CrossRef] [Green Version]
- Spaide, R.F.; Fujimoto, J.G.; Waheed, N.K. Image artifacts in optical coherence tomography angiography. Retina 2015, 35, 2163–2180. [Google Scholar] [CrossRef]






| Baseline Characteristics | |
|---|---|
| Age, years | 30 ± 6.2 |
| Sex, female | 24 (55.82) |
| Study eye, right | 29 (67.44) |
| Axial length, mm | 23.7 ± 1.4 |
| Body mass index, kg/m2 | 21.8 ± 2.1 |
| Systolic blood pressure, mmHg | 128.9 ± 7.9 |
| Diastolic blood pressure, mmHg | 79.3 ± 10.2 |
| Observer 1 | Observer 2 | p-Value | |
|---|---|---|---|
| Average fractal dimension | |||
| SCP | 1.694 ± 0.010 | 1.693 ± 0.014 | 0.59 |
| DCP | 1.695 ± 0.007 | 1.693 ± 0.011 | 0.41 |
| Coefficient of variation | |||
| SCP | 0.60 | 0.43 | |
| DCP | 0.83 | 0.64 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Arnould, L.; Haddad, D.; Baudin, F.; Gabrielle, P.-H.; Sarossy, M.; Bron, A.M.; Aliahmad, B.; Creuzot-Garcher, C. Repeatability and Reproducibility of Retinal Fractal Dimension Measured with Swept-Source Optical Coherence Tomography Angiography in Healthy Eyes: A Proof-of-Concept Study. Diagnostics 2022, 12, 1769. https://doi.org/10.3390/diagnostics12071769
Arnould L, Haddad D, Baudin F, Gabrielle P-H, Sarossy M, Bron AM, Aliahmad B, Creuzot-Garcher C. Repeatability and Reproducibility of Retinal Fractal Dimension Measured with Swept-Source Optical Coherence Tomography Angiography in Healthy Eyes: A Proof-of-Concept Study. Diagnostics. 2022; 12(7):1769. https://doi.org/10.3390/diagnostics12071769
Chicago/Turabian StyleArnould, Louis, Déa Haddad, Florian Baudin, Pierre-Henry Gabrielle, Marc Sarossy, Alain M. Bron, Behzad Aliahmad, and Catherine Creuzot-Garcher. 2022. "Repeatability and Reproducibility of Retinal Fractal Dimension Measured with Swept-Source Optical Coherence Tomography Angiography in Healthy Eyes: A Proof-of-Concept Study" Diagnostics 12, no. 7: 1769. https://doi.org/10.3390/diagnostics12071769
APA StyleArnould, L., Haddad, D., Baudin, F., Gabrielle, P.-H., Sarossy, M., Bron, A. M., Aliahmad, B., & Creuzot-Garcher, C. (2022). Repeatability and Reproducibility of Retinal Fractal Dimension Measured with Swept-Source Optical Coherence Tomography Angiography in Healthy Eyes: A Proof-of-Concept Study. Diagnostics, 12(7), 1769. https://doi.org/10.3390/diagnostics12071769







