Anatomical Features of the Parotid Duct in Sialography as an Aid to Endoscopy—A Retrospective Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Group
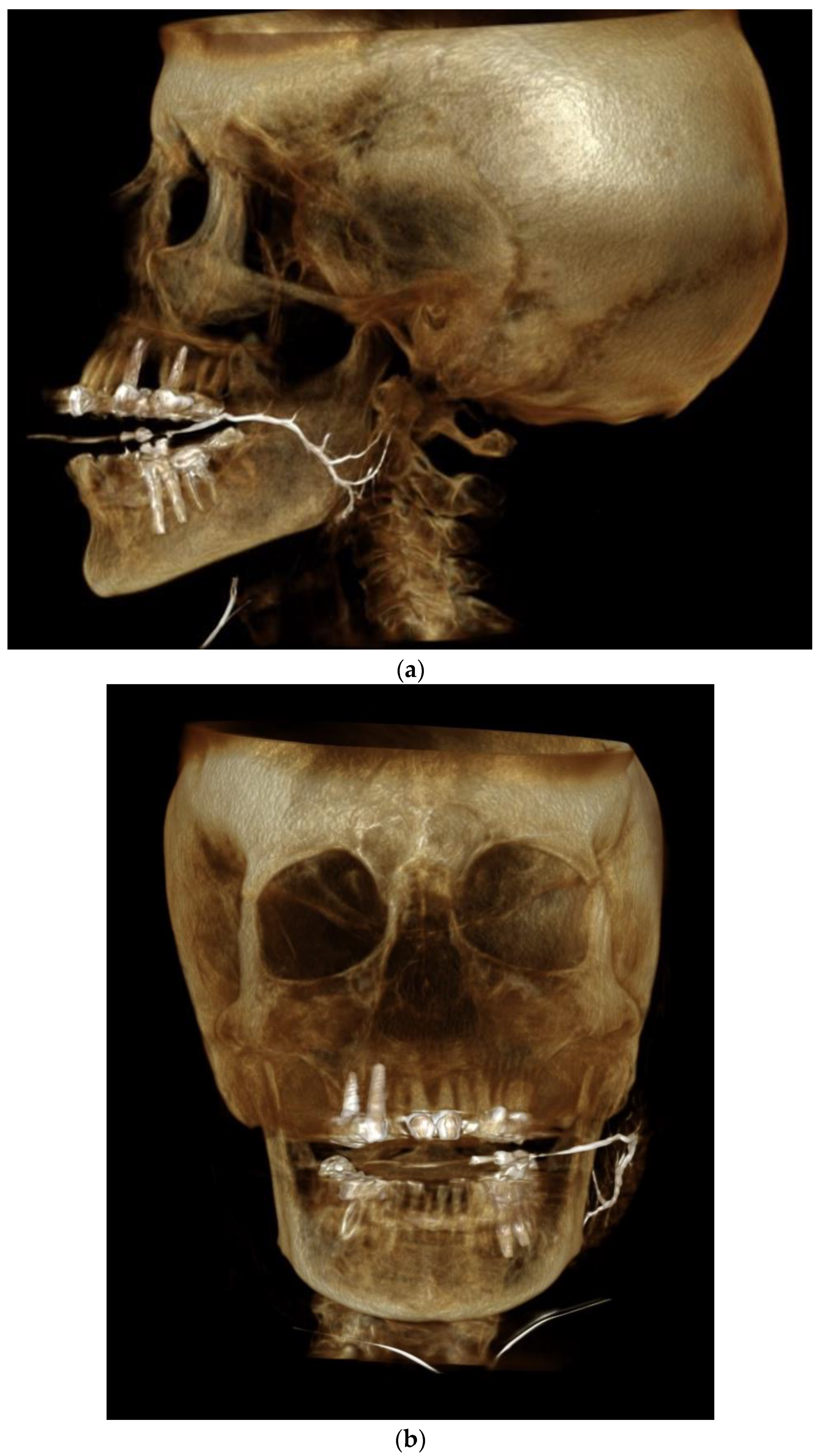
2.2. Sialography Technique
2.3. Inclusion Criteria
- Patients over 18 years old.
- Available sialography demonstrating the entire length of the parotid gland duct.
2.4. Exclusion Criteria
- Insufficient demonstration of the parotid gland duct.
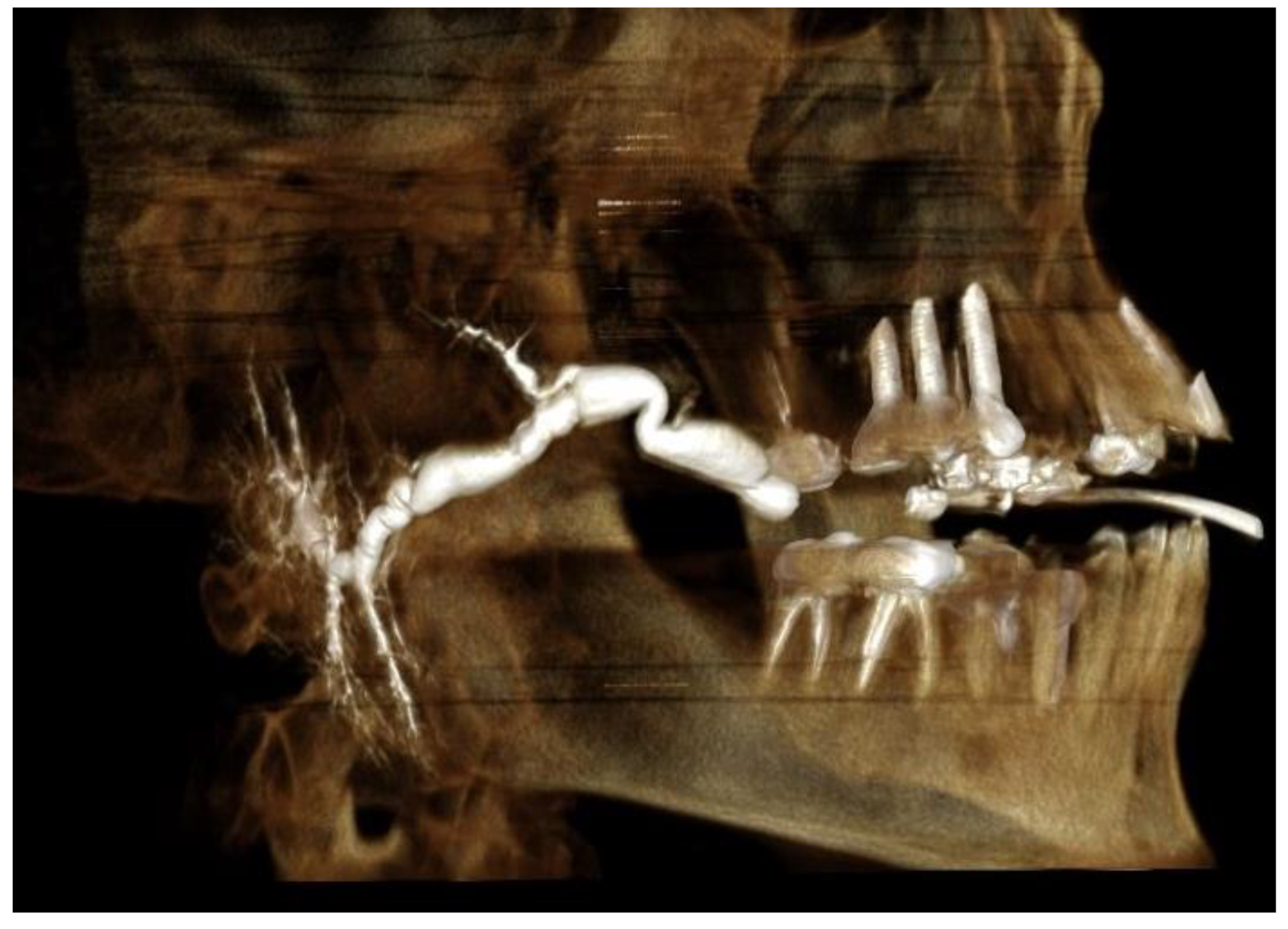
- Mega duct—a pathological status of the duct, which renders length measurement impossible (Figure 1).
- Distinct sialectases (widening of the proximal ducts).
- Known diagnosis or sialographic features of Sjögren’s syndrome.
- Rupture of the duct during infusion of the contrast medium.
2.5. Data Collection
2.6. Data Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Avishai, G.; Ben-Zvi, Y.; Chaushu, G.; Rosenfeld, E.; Gillman, L.; Reiser, V.; Gilat, H. The unique characteristics of sialolithiasis following drug-induced hyposalivation. Clin. Oral. Investig. 2021, 25, 4369–4376. [Google Scholar] [CrossRef] [PubMed]
- Marchal, F.; Dulguerov, P. Sialolithiasis management: The state of the art. Arch. Otolaryngol. Head Neck. Surg. 2003, 129, 951–956. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Burke, C.J.; Thomas, R.H.; Howlett, D. Imaging the major salivary glands. Br. J. Oral Maxillofac. Surg. 2011, 49, 261–269. [Google Scholar] [CrossRef] [PubMed]
- Afzelius, P.; Nielsen, M.Y.; Ewertsen, C.; Bloch, K.P. Imaging of the major salivary glands. Clin. Physiol. Funct. Imaging 2016, 36, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Thomas, N.; Kaur, A.; Reddy, S.S.; Nagaraju, R.; Nagi, R.; Shankar, V.G. Three-dimensional cone-beam computed tomographic sialography in the diagnosis and management of primary Sjogren syndrome: Report of 3 cases. Imaging Sci. Dent. 2021, 51, 209–216. [Google Scholar] [CrossRef] [PubMed]
- Miracle, A.C.; Mukherji, S.K. Conebeam CT of the head and neck, part 1: Physical principles. AJNR Am. J. Neuroradiol. 2009, 30, 1088–1095. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abdalla-Aslan, R.; Keshet, N.; Zadik, Y.; Aframian, D.J.; Nadler, C. Standardization of terminology, imaging features, and interpretation of CBCT sialography of major salivary glands: A clinical review. Quintessence Int. 2021, 52, 728–740. [Google Scholar] [CrossRef] [PubMed]
- Hasson, O. Modern sialography for screening of salivary gland obstruction. J. Oral Maxillofac. Surg. 2010, 68, 276–280. [Google Scholar] [CrossRef] [PubMed]
- Nahlieli, O.; Baruchin, A.M. Endoscopic technique for the diagnosis and treatment of obstructive salivary gland diseases. J. Oral Maxillofac. Surg. 1999, 57, 1394–1401, discussion 1401–1392. [Google Scholar] [CrossRef]
- Singh, P.P.; Gupta, V. Sialendoscopy: Introduction, indications and technique. Indian J. Otolaryngol. Head Neck Surg. 2014, 66, 74–78. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pogrel, M.A.; Schmidt, B.; Ammar, A. The relationship of the buccal branch of the facial nerve to the parotid duct. J. Oral Maxillofac. Surg. 1996, 54, 71–73. [Google Scholar] [CrossRef]
- Richards, A.T.; Digges, N.; Norton, N.S.; Quinn, T.H.; Say, P.; Galer, C.; Lydiatt, K. Surgical anatomy of the parotid duct with emphasis on the major tributaries forming the duct and the relationship of the facial nerve to the duct. Clin. Anat. 2004, 17, 463–467. [Google Scholar] [CrossRef] [PubMed]
- Horsburgh, A.; Massoud, T.F. The salivary ducts of Wharton and Stenson: Analysis of normal variant sialographic morphometry and a historical review. Ann. Anat. 2013, 195, 238–242. [Google Scholar] [CrossRef]
- Conn, I.G.; Wiesenfeld, D.; Ferguson, M.M. The anatomy of the facial nerve in relation to CT/sialography of the parotid gland. Br. J. Radiol. 1983, 56, 901–905. [Google Scholar] [CrossRef] [PubMed]


| Average | SD | Minimum | Maximum | Normal Distribution | Q1 (25%) | Q2 Median (50%) | Q3 (75%) | |
|---|---|---|---|---|---|---|---|---|
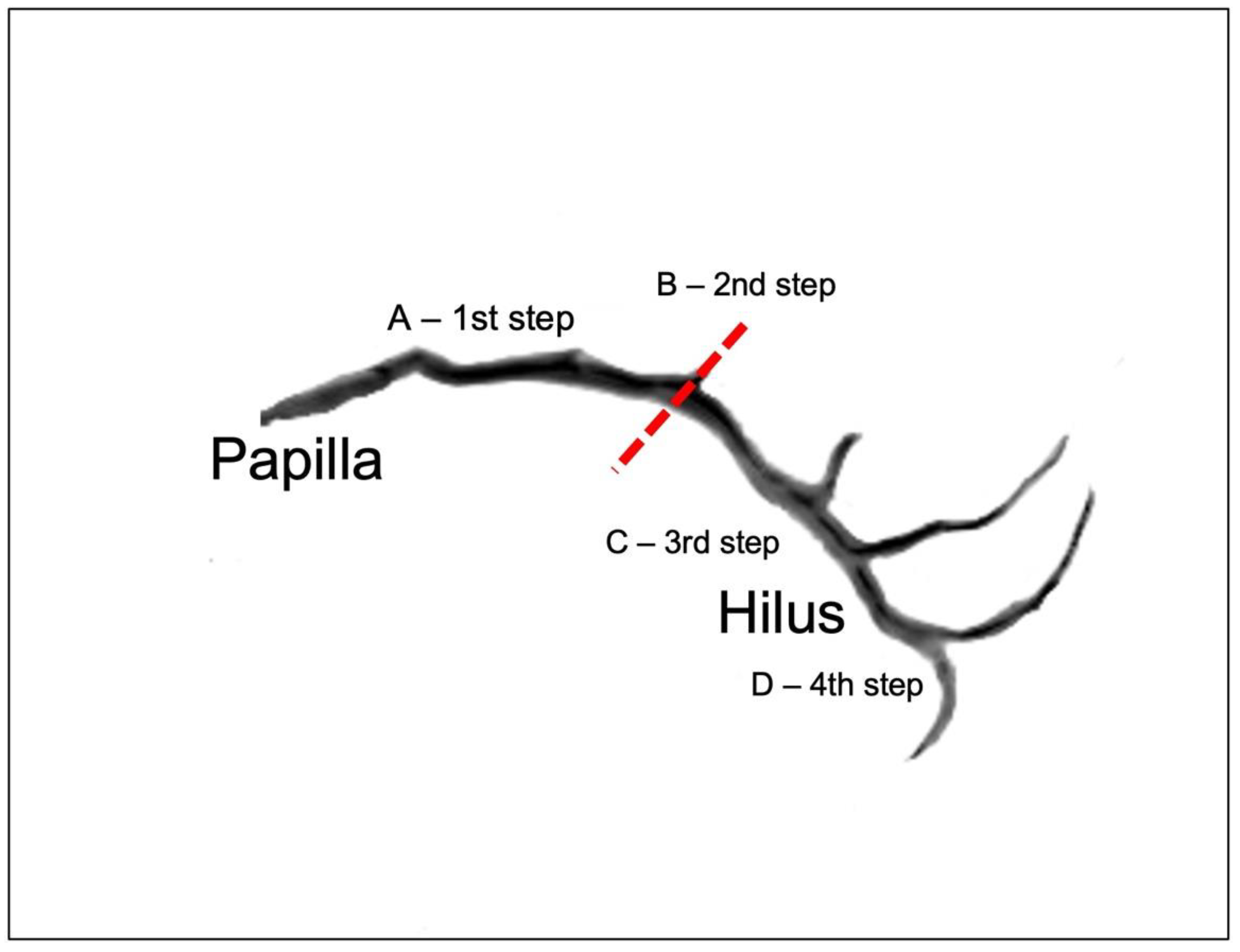
| Distance Between Papilla and Curvature | 35.4 | 52.9 | NO | 36.97 | 41.5 | 45.32 | ||
| Angle of Curvature ( α ) | 69.3 | 155 | NO | 107.58 | 126.9 | 135.6 | ||
| Distance Between Curvature and Hilus | 35.25 | 7.81 | 19.4 | 53.8 | YES | |||
| Distance Between Papilla and Hilus | 63.6 | 100.6 | NO | 71.2 | 75.4 | 80.77 | ||
| Number of Tributaries Pre-Hilus | 0 | 4 | NO | 0.75 | 2 | 2 | ||
| Number of Tributaries Post-Hilus | 2 | 3 | NO | 2 | 2 | 2 | ||
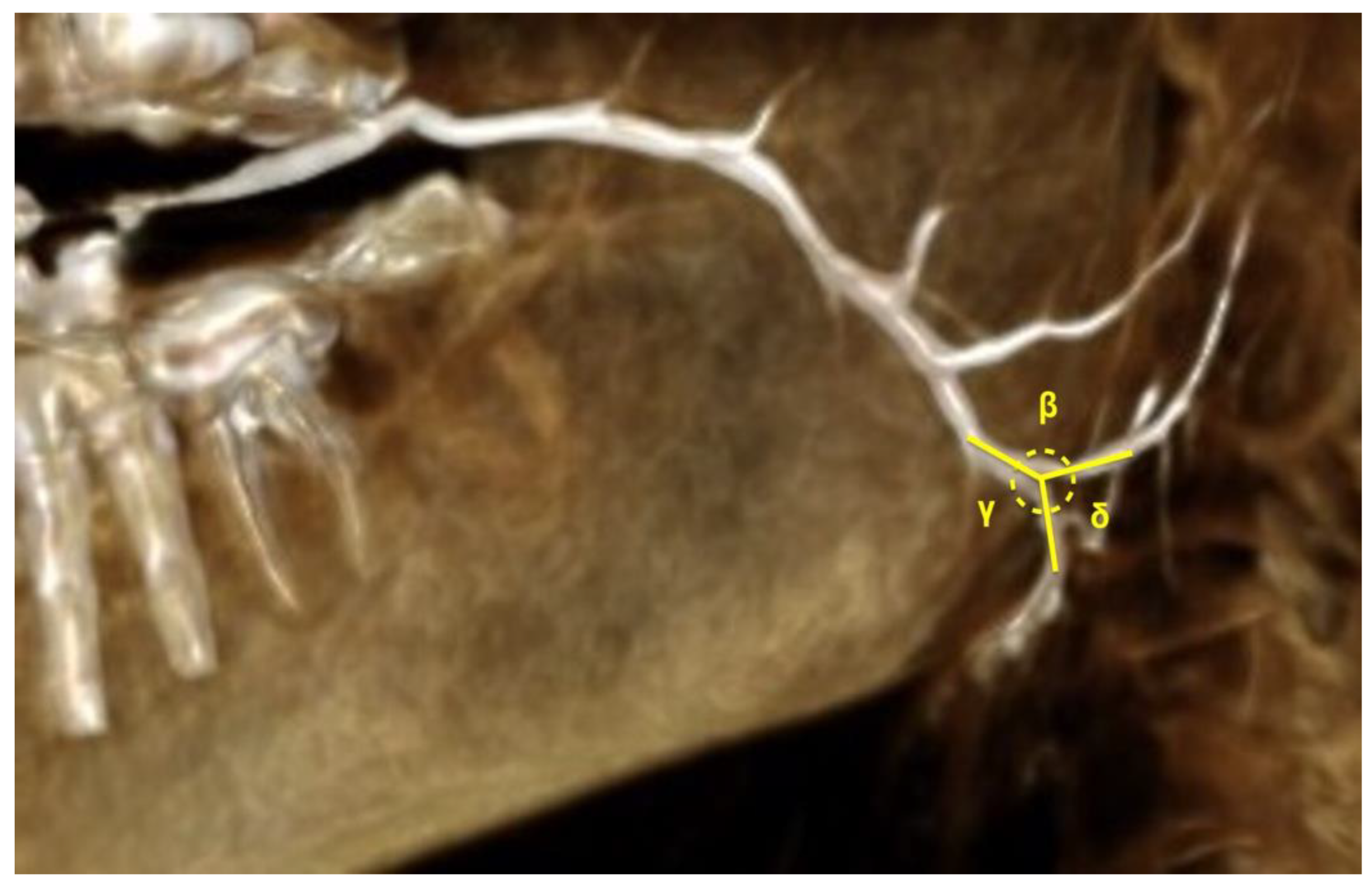
| Angle between PD and SPD (β) | 123.68 | 22.68 | 83.11 | 167.9 | YES | |||
| Angle between PD and IPD (γ) | 136.94 | 25.22 | 64.54 | 177.48 | YES | 124.53 | 140.88 | 154.62 |
| Angle between SPD and IPD (δ) | 41.5 | 202 | NO | 60.59 | 90.6 | 130.99 | ||
| Duct Width—Maximal | 0.9 | 4 | NO | 1.4 | 2 | 2.2 | ||
| Duct Width—Minimal | 0.5 | 2.5 | NO | 0.7 | 0.8 | 1.15 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Avishai, G.; Younes, M.; Gilat, H.; Gillman, L.; Reiser, V.; Rosenfeld, E.; Chaushu, G.; Masri, D. Anatomical Features of the Parotid Duct in Sialography as an Aid to Endoscopy—A Retrospective Study. Diagnostics 2022, 12, 1868. https://doi.org/10.3390/diagnostics12081868
Avishai G, Younes M, Gilat H, Gillman L, Reiser V, Rosenfeld E, Chaushu G, Masri D. Anatomical Features of the Parotid Duct in Sialography as an Aid to Endoscopy—A Retrospective Study. Diagnostics. 2022; 12(8):1868. https://doi.org/10.3390/diagnostics12081868
Chicago/Turabian StyleAvishai, Gal, Muhammad Younes, Hanna Gilat, Leon Gillman, Vadim Reiser, Eli Rosenfeld, Gavriel Chaushu, and Daya Masri. 2022. "Anatomical Features of the Parotid Duct in Sialography as an Aid to Endoscopy—A Retrospective Study" Diagnostics 12, no. 8: 1868. https://doi.org/10.3390/diagnostics12081868
APA StyleAvishai, G., Younes, M., Gilat, H., Gillman, L., Reiser, V., Rosenfeld, E., Chaushu, G., & Masri, D. (2022). Anatomical Features of the Parotid Duct in Sialography as an Aid to Endoscopy—A Retrospective Study. Diagnostics, 12(8), 1868. https://doi.org/10.3390/diagnostics12081868








