Salivary Gland Toxicity of PSMA-Targeted Radioligand Therapy with 177Lu-PSMA and Combined 225Ac- and 177Lu-Labeled PSMA Ligands (TANDEM-PRLT) in Advanced Prostate Cancer: A Single-Center Systematic Investigation
Abstract
:1. Introduction
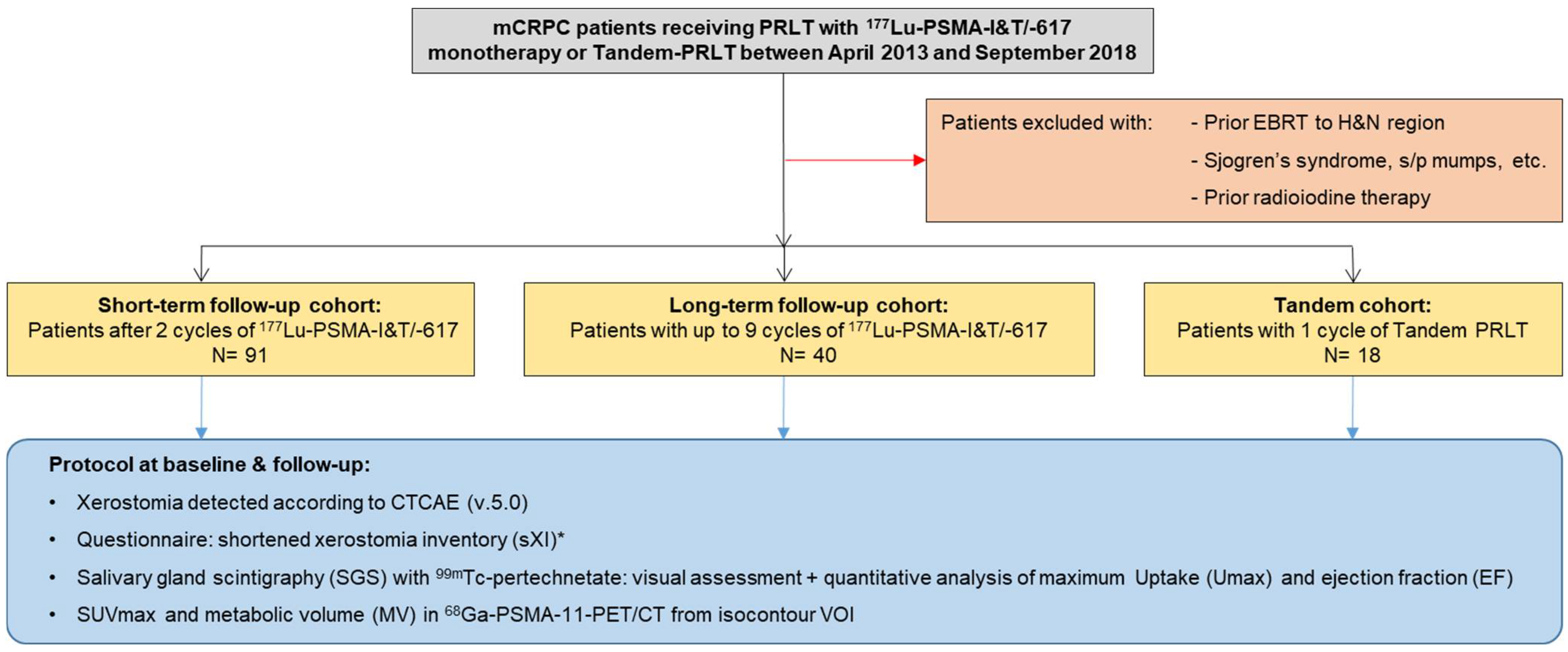
2. Material and Methods
2.1. Study Design
2.2. Patient Population
2.3. Assessment of Salivary Gland Function
2.4. Statistical Analysis
3. Results
3.1. Short-Term Follow-Up Cohort
3.2. Long-Term Follow-Up Cohort
3.3. Tandem-Cohort PRLT
3.4. Co-Factors for Salivary Gland Toxicity
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Loeb, S.; Schaeffer, E.M.; Trock, B.J.; Epstein, J.I.; Humphreys, E.B.; Walsh, P.C. What Are the Outcomes of Radical Prostatectomy for High-risk Prostate Cancer? Urology 2010, 76, 710–714. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weiner, A.B.; Matulewicz, R.S.; Eggener, S.E.; Schaeffer, E.M. Increasing incidence of metastatic prostate cancer in the United States (2004–2013). Prostate Cancer Prostatic Dis. 2016, 19, 395–397. [Google Scholar] [CrossRef] [Green Version]
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2019. CA Cancer J. Clin. 2019, 69, 7–34. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Halabi, S.; Vogelzang, N.J.; Kornblith, A.B.; Ou, S.S.; Kantoff, P.W.; Dawson, N.A.; Small, E.J. Pain Predicts Overall Survival in Men With Metastatic Castration-Refractory Prostate Cancer. J. Clin. Oncol. 2008, 26, 2544–2549. [Google Scholar] [CrossRef] [PubMed]
- Silver, D.A.; Pellicer, I.; Fair, W.R.; Heston, W.D.; Cordon-Cardo, C. Prostate-specific membrane antigen expression in normal and malignant human tissues. Clin. Cancer Res. 1997, 3, 81–85. [Google Scholar] [PubMed]
- Bostwick, D.G.; Pacelli, A.; Blute, M.; Roche, P.; Murphy, G.P. Prostate specific membrane antigen expression in prostatic intraepithelial neoplasia and adenocarcinoma: A study of 184 cases. Cancer 1998, 82, 2256–2261. [Google Scholar] [CrossRef]
- Bander, N.H.; Trabulsi, E.J.; Kostakoglu, L.; Yao, D.; Vallabhajosula, S.; Smith-Jones, P.; Joyce, M.A.; Milowsky, M.; Nanus, D.M.; Goldsmith, S.J. Targeting Metastatic Prostate Cancer With Radiolabeled Monoclonal Antibody J591 to the Extracellular Domain of Prostate Specific Membrane Antigen. J. Urol. 2003, 170, 1717–1721. [Google Scholar] [CrossRef]
- Mannweiler, S.; Amersdorfer, P.; Trajanoski, S.; Terrett, J.A.; King, D.; Mehes, G. Heterogeneity of Prostate-Specific Membrane Antigen (PSMA) Expression in Prostate Carcinoma with Distant Metastasis. Pathol. Oncol. Res. 2009, 15, 167–172. [Google Scholar] [CrossRef] [PubMed]
- Pomper, M.G.; Musachio, J.L.; Zhang, J.; Scheffel, U.; Zhou, Y.; Hilton, J.; Maini, A.; Dannals, R.F.; Wong, D.F.; Kozikowski, A.P. 11C-MCG: Synthesis, Uptake Selectivity, and Primate PET of a Probe for Glutamate Carboxypeptidase II (NAALADase). Mol. Imaging 2002, 1, 96–101. [Google Scholar] [CrossRef] [PubMed]
- Eder, M.; Schafer, M.; Bauder-Wust, U.; Hull, W.E.; Wangler, C.; Mier, W.; Haberkorn, U.; Eisenhut, M. 68Ga-Complex Lipophilicity and the Targeting Property of a Urea-Based PSMA Inhibitor for PET Imaging. Bioconjug. Chem. 2012, 23, 688–697. [Google Scholar] [CrossRef] [PubMed]
- Afshar-Oromieh, A.; Malcher, A.; Eder, M.; Eisenhut, M.; Linhart, H.G.; Hadaschik, B.A.; Holland-Letz, T.; Giesel, F.L.; Kratochwil, C.; Haufe, S.; et al. PET imaging with a [68Ga]gallium-labelled PSMA ligand for the diagnosis of prostate cancer: Biodistribution in humans and first evaluation of tumour lesions. Eur. J. Nucl. Med. Mol. Imaging 2013, 40, 486–495. [Google Scholar] [CrossRef] [PubMed]
- Zechmann, C.M.; Afshar-Oromieh, A.; Armor, T.; Stubbs, J.B.; Mier, W.; Hadaschik, B.; Joyal, J.; Kopka, K.; Debus, J.; Babich, J.W.; et al. Radiation dosimetry and first therapy results with a 124I/131I-labeled small molecule (MIP-1095) targeting PSMA for prostate cancer therapy. Eur. J. Nucl. Med. Mol. Imaging 2014, 41, 1280–1292. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baum, R.P.; Kulkarni, H.R.; Schuchardt, C.; Singh, A.; Wirtz, M.; Wiessalla, S.; Schottelius, M.; Mueller, D.; Klette, I.; Wester, H.J. 177Lu-Labeled Prostate-Specific Membrane Antigen Radioligand Therapy of Metastatic Castration-Resistant Prostate Cancer: Safety and Efficacy. J. Nucl. Med. 2016, 57, 1006–1013. [Google Scholar] [CrossRef] [Green Version]
- Kratochwil, C.; Giesel, F.L.; Stefanova, M.; Benesova, M.; Bronzel, M.; Afshar-Oromieh, A.; Mier, W.; Eder, M.; Kopka, K.; Haberkorn, U. PSMA-Targeted Radionuclide Therapy of Metastatic Castration-Resistant Prostate Cancer with 177Lu-Labeled PSMA-617. J. Nucl. Med. 2016, 57, 1170–1176. [Google Scholar] [CrossRef] [Green Version]
- Rahbar, K.; Ahmadzadehfar, H.; Kratochwil, C.; Haberkorn, U.; Schafers, M.; Essler, M.; Baum, R.P.; Kulkarni, H.R.; Schmidt, M.; Drzezga, A.; et al. German Multicenter Study Investigating 177Lu-PSMA-617 Radioligand Therapy in Advanced Prostate Cancer Patients. J. Nucl. Med. 2017, 58, 85–90. [Google Scholar] [CrossRef] [Green Version]
- Heck, M.M.; Tauber, R.; Schwaiger, S.; Retz, M.; D’Alessandria, C.; Maurer, T.; Gafita, A.; Wester, H.J.; Gschwend, J.E.; Weber, W.A.; et al. Treatment Outcome, Toxicity, and Predictive Factors for Radioligand Therapy with 177Lu-PSMA-I&T in Metastatic Castration-resistant Prostate Cancer. Eur. Urol. 2019, 75, 920–926. [Google Scholar] [CrossRef]
- Hofman, M.S.; Violet, J.; Hicks, R.J.; Ferdinandus, J.; Thang, S.P.; Akhurst, T.; Iravani, A.; Kong, G.; Kumar, A.R.; Murphy, D.G.; et al. [177Lu]-PSMA-617 radionuclide treatment in patients with metastatic castration-resistant prostate cancer (LuPSMA trial): A single-centre, single-arm, phase 2 study. Lancet Oncol. 2018, 19, 825–833. [Google Scholar] [CrossRef]
- Sartor, O.; de Bono, J.; Chi, K.N.; Fizazi, K.; Herrmann, K.; Rahbar, K.; Tagawa, S.T.; Nordquist, L.T.; Vaishampayan, N.; El-Haddad, G.; et al. Lutetium-177–PSMA-617 for Metastatic Castration-Resistant Prostate Cancer. N. Engl. J. Med. 2021, 385, 1091–1103. [Google Scholar] [CrossRef]
- Kratochwil, C.; Bruchertseifer, F.; Giesel, F.L.; Weis, M.; Verburg, F.A.; Mottaghy, F.; Kopka, K.; Apostolidis, C.; Haberkorn, U.; Morgenstern, A. 225Ac-PSMA-617 for PSMA-Targeted α-Radiation Therapy of Metastatic Castration-Resistant Prostate Cancer. J. Nucl. Med. 2016, 57, 1941–1944. [Google Scholar] [CrossRef] [Green Version]
- Kratochwil, C.; Bruchertseifer, F.; Rathke, H.; Bronzel, M.; Apostolidis, C.; Weichert, W.; Haberkorn, U.; Giesel, F.L.; Morgenstern, A. Targeted α-Therapy of Metastatic Castration-Resistant Prostate Cancer with 225Ac-PSMA-617: Dosimetry Estimate and Empiric Dose Finding. J. Nucl. Med. 2017, 58, 1624–1631. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kratochwil, C.; Bruchertseifer, F.; Rathke, H.; Hohenfellner, M.; Giesel, F.L.; Haberkorn, U.; Morgenstern, A. Targeted α-Therapy of Metastatic Castration-Resistant Prostate Cancer with 225Ac-PSMA-617: Swimmer-Plot Analysis Suggests Efficacy Regarding Duration of Tumor Control. J. Nucl. Med. 2018, 59, 795–802. [Google Scholar] [CrossRef] [Green Version]
- Feuerecker, B.; Tauber, R.; Knorr, K.; Heck, M.; Beheshti, A.; Seidl, C.; Bruchertseifer, F.; Pickhard, A.; Gafita, A.; Kratochwil, C.; et al. Activity and Adverse Events of Actinium-225-PSMA-617 in Advanced Metastatic Castration-resistant Prostate Cancer After Failure of Lutetium-177-PSMA. Eur. Urol. 2021, 79, 343–350. [Google Scholar] [CrossRef] [PubMed]
- Kulkarni, H.; Zhang, J.; Langbein, T.; Schuchardt, C.; Singh, A.; Mueller, D.; Baum, R. Radioligand therapy using combination of Ac-225 and Lu-177 labelled PSMA ligands for progressive end-stage metastatic prostate cancer: Effective trade-off between response and toxicity. J. Nucl. Med. 2019, 60, 464. [Google Scholar]
- Tonnesmann, R.; Meyer, P.T.; Eder, M.; Baranski, A.C. [177Lu]Lu-PSMA-617 Salivary Gland Uptake Characterized by Quantitative In Vitro Autoradiography. Pharmaceuticals 2019, 12, 18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rupp, N.J.; Umbricht, C.A.; Pizzuto, D.A.; Lenggenhager, D.; Topfer, A.; Muller, J.; Muhlematter, U.J.; Ferraro, D.A.; Messerli, M.; Morand, G.B.; et al. First Clinicopathological Evidence of a Non–PSMA-Related Uptake Mechanism for 68Ga-PSMA-11 in Salivary Glands. J. Nucl. Med. 2019, 60, 1270–1276. [Google Scholar] [CrossRef] [Green Version]
- Dirix, P.; Nuyts, S.; Van den Bogaert, W. Radiation-induced xerostomia in patients with head and neck cancer: A literature review. Cancer 2006, 107, 2525–2534. [Google Scholar] [CrossRef]
- Mandel, S.J.; Mandel, L. Radioactive Iodine and the Salivary Glands. Thyroid 2003, 13, 265–271. [Google Scholar] [CrossRef] [Green Version]
- Van Nostrand, D. Sialoadenitis secondary to 131I therapy for well-differentiated thyroid cancer. Oral Dis. 2011, 17, 154–161. [Google Scholar] [CrossRef]
- Solans, R.; Bosch, J.-A.; Galofre, P.; Porta, F.; Jose, M.V. Salivary and Lacrimal Gland Dysfunction (Sicca Syndrome) After Radioiodine Therapy. J. Nucl. Med. 2001, 42, 738–743. [Google Scholar]
- Kulkarni, H.R.; Singh, A.; Schuchardt, C.; Niepsch, K.; Sayeg, M.; Leshch, Y.; Wester, H.J.; Baum, R.P. PSMA-Based Radioligand Therapy for Metastatic Castration-Resistant Prostate Cancer: The Bad Berka Experience Since 2013. J. Nucl. Med. 2016, 57, 97S–104S. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fendler, W.P.; Kratochwil, C.; Ahmadzadehfar, H.; Rahbar, K.; Baum, R.P.; Schmidt, M.; Pfestroff, A.; Lutzen, U.; Prasad, V.; Heinzel, A.; et al. 177Lu-PSMA-617 therapy, dosimetry and follow-up in patients with metastatic castration-resistant prostate cancer. Nukl. Nucl. 2016, 55, 123–128. [Google Scholar]
- Kratochwil, C.; Fendler, W.P.; Eiber, M.; Baum, R.; Bozkurt, M.F.; Czernin, J.; Bolton, R.C.D.; Ezziddin, S.; Forrer, F.; Hicks, R.J.; et al. EANM procedure guidelines for radionuclide therapy with 177Lu-labelled PSMA-ligands (177Lu-PSMA-RLT). Eur. J. Nucl. Med. Mol. Imaging 2019, 46, 2536–2544. [Google Scholar] [CrossRef]
- Weineisen, M.; Simecek, J.; Schottelius, M.; Schwaiger, M.; Wester, H.J. Synthesis and preclinical evaluation of DOTAGA-conjugated PSMA ligands for functional imaging and endoradiotherapy of prostate cancer. EJNMMI Res. 2014, 4, 63. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gaertner, F.C.; Halabi, K.; Ahmadzadehfar, H.; Kurpig, S.; Eppard, E.; Kotsikopoulos, C.; Liakos, N.; Bundschuh, R.A.; Strunk, H.; Essler, M. Uptake of PSMA-ligands in normal tissues is dependent on tumor load in patients with prostate cancer. Oncotarget 2017, 8, 55094–55103. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thomson, W.M.; van der Putten, G.J.; de Baat, C.; Ikebe, K.; Matsuda, K.I.; Enoki, K.; Hopcraft, M.S.; Ling, G.Y. Shortening the Xerostomia Inventory. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endodontology 2011, 112, 322–327. [Google Scholar] [CrossRef] [Green Version]
- Rathke, H.; Kratochwil, C.; Hohenberger, R.; Giesel, F.L.; Bruchertseifer, F.; Flechsig, P.; Morgenstern, A.; Hein, M.; Plinkert, P.; Haberkorn, U.; et al. Initial clinical experience performing sialendoscopy for salivary gland protection in patients undergoing 225Ac-PSMA-617 RLT. Eur. J. Nucl. Med. Mol. Imaging 2019, 46, 139–147. [Google Scholar] [CrossRef]
- Bohuslavizki, K.H.; Brenner, W.; Lassmann, S.; Tinnemeyer, S.; Kalina, S.; Clausen, M.; Henze, E. Quantitative salivary gland scintigraphy--a recommended examination prior to and after radioiodine therapy. Nukl. Nucl. 1997, 36, 103–109. [Google Scholar]
- Klutmann, S.; Bohuslavizki, K.H.; Kroger, S.; Bleckmann, C.; Brenner, W.; Mester, J.; Clausen, M. Quantitative salivary gland scintigraphy. J. Nucl. Med. Technol. 1999, 27, 20–26. [Google Scholar]
- Scarpa, L.; Buxbaum, S.; Kendler, D.; Fink, K.; Bektic, J.; Gruber, L.; Decristoforo, C.; Uprimny, C.; Lukas, P.; Horninger, W.; et al. The 68Ga/177Lu theragnostic concept in PSMA targeting of castration-resistant prostate cancer: Correlation of SUVmax values and absorbed dose estimates. Eur. J. Nucl. Med. Mol. Imaging 2017, 44, 788–800. [Google Scholar] [CrossRef]
- Zhao, Y.; Xia, Y.; Liu, H.; Wang, Z.; Chen, Y.; Zhang, W. Potential Applications of 68Ga-PSMA-11 PET/CT in the Evaluation of Salivary Gland Uptake Function: Preliminary Observations and Comparison with 99mTcO4−Salivary Gland Scintigraphy. Contrast Media Mol. Imaging 2020, 2020, 1097516. [Google Scholar] [CrossRef] [PubMed]
- Roesink, J.M.; Moerland, M.A.; Hoekstra, A.; Van Rijk, P.P.; Terhaard, C.H. Scintigraphic assessment of early and late parotid gland function after radiotherapy for head-and-neck cancer: A prospective study of dose–volume response relationships. Int. J. Radiat. Oncol. Biol. Phys. 2004, 58, 1451–1460. [Google Scholar] [CrossRef]
- Münter, M.W.; Karger, C.P.; Hoffner, S.G.; Hof, H.; Thilmann, C.; Rudat, V.; Nill, S.; Wannenmacher, M.; Debus, J. Evaluation of salivary gland function after treatment of head-and-neck tumors with intensity-modulated radiotherapy by quantitative pertechnetate scintigraphy. Int. J. Radiat. Oncol. Biol. Phys. 2004, 58, 175–184. [Google Scholar] [CrossRef]
- Bussels, B.; Maes, A.; Flamen, P.; Lambin, P.; Erven, K.; Hermans, R.; Nuyts, S.; Weltens, C.; Cecere, S.; Lesaffre, E.; et al. Dose–response relationships within the parotid gland after radiotherapy for head and neck cancer. Radiother. Oncol. J. Eur. Soc. Ther. Radiol. Oncol. 2004, 73, 297–306. [Google Scholar] [CrossRef] [PubMed]
- Badam, R.K.; Suram, J.; Babu, D.B.; Waghray, S.; Marshal, R.; Bontha, S.C.; Lavanya, R.; Kanth, S. Assessment of Salivary Gland Function Using Salivary Scintigraphy in Pre and Post Radioactive Iodine Therapy in Diagnosed Thyroid Carcinoma Patients. J. Clin. Diagn. Res. 2016, 10, ZC60–ZC62. [Google Scholar] [CrossRef] [PubMed]
- Kohn, W.G.; Ship, J.A.; Atkinson, J.C.; Patton, L.L.; Fox, P.C. Salivary gland 99mTc-scintigraphy: A grading scale and correlation with major salivary gland flow rates. J. Oral Pathol. Med. 1992, 21, 70–74. [Google Scholar] [CrossRef]
- Jeong, S.Y.; Kim, H.W.; Lee, S.W.; Ahn, B.C.; Lee, J. Salivary Gland Function 5 Years After Radioactive Iodine Ablation in Patients with Differentiated Thyroid Cancer: Direct Comparison of Pre- and Postablation Scintigraphies and Their Relation to Xerostomia Symptoms. Thyroid 2013, 23, 609–616. [Google Scholar] [CrossRef]
- Nulent, T.J.W.K.; Valstar, M.H.; de Keizer, B.; Willems, S.M.; Smit, L.A.; Al-Mamgani, A.; Smeele, L.E.; van Es, R.J.J.; de Bree, R.; Vogel, W.V. Physiologic distribution of PSMA-ligand in salivary glands and seromucous glands of the head and neck on PET/CT. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2018, 125, 478–486. [Google Scholar] [CrossRef]
- Filss, C.; Heinzel, A.; Miiller, B.; Vogg, A.T.J.; Langen, K.J.; Mottaghy, F.M. Relevant tumor sink effect in prostate cancer patients receiving 177Lu-PSMA-617 radioligand therapy. Nukl. Nucl. 2018, 57, 19–25. [Google Scholar] [CrossRef]
- Begum, N.J.; Thieme, A.; Eberhardt, N.; Tauber, R.; D’Alessandria, C.; Beer, A.J.; Glatting, G.; Eiber, M.; Kletting, P. The Effect of Total Tumor Volume on the Biologically Effective Dose to Tumor and Kidneys for 177Lu-Labeled PSMA Peptides. J. Nucl. Med. 2018, 59, 929–933. [Google Scholar] [CrossRef] [Green Version]
- Barber, T.W.; Singh, A.; Kulkarni, H.R.; Niepsch, K.; Billah, B.; Baum, R.P. Clinical outcomes of (177)Lu-PSMA radioligand therapy in taxane chemotherapy pretreated and taxane chemotherapy naive patients with metastatic castration resistant prostate cancer. J. Nucl. Med. 2019, 60, 955–962. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Okamoto, S.; Thieme, A.; Allmann, J.; D’Alessandria, C.; Maurer, T.; Retz, M.; Tauber, R.; Heck, M.M.; Wester, H.J.; Tamaki, N.; et al. Radiation Dosimetry for177Lu-PSMA I&T in Metastatic Castration-Resistant Prostate Cancer: Absorbed Dose in Normal Organs and Tumor Lesions. J. Nucl. Med. 2017, 58, 445–450. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Violet, J.; Jackson, P.; Ferdinandus, J.; Sandhu, S.; Akhurst, T.; Iravani, A.; Kong, G.; Kumar, A.R.; Thang, S.P.; Eu, P.; et al. Dosimetry of 177Lu-PSMA-617 in Metastatic Castration-Resistant Prostate Cancer: Correlations Between Pretherapeutic Imaging and Whole-Body Tumor Dosimetry with Treatment Outcomes. J. Nucl. Med. 2019, 60, 517–523. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schuchardt, C.; Zhang, J.; Kulkarni, H.R.; Chen, X.; Müller, D.; Baum, R.P. Prostate-specific membrane antigen radioligand therapy using 177Lu-PSMA I&T and 177Lu-PSMA-617 in patients with metastatic castration-resistant prostate cancer: Comparison of safety, biodistribution and dosimetry. J. Nucl. Med. 2022, 63, 1199–1207. [Google Scholar] [CrossRef]
- Eisbruch, A.; Haken, R.K.T.; Kim, H.M.; Marsh, L.H.; Ship, J.A. Dose, volume, and function relationships in parotid salivary glands following conformal and intensity-modulated irradiation of head and neck cancer. Int. J. Radiat. Oncol. Biol. Phys. 1999, 45, 577–587. [Google Scholar] [CrossRef]
- Li, Y.; Taylor, J.M.; Haken, R.K.T.; Eisbruch, A. The impact of dose on parotid salivary recovery in head and neck cancer patients treated with radiation therapy. Int. J. Radiat. Oncol. Biol. Phys. 2007, 67, 660–669. [Google Scholar] [CrossRef] [Green Version]
- Sathekge, M.; Bruchertseifer, F.; Vorster, M.; Lawal, I.O.; Knoesen, O.; Mahapane, J.; Davis, C.; Reyneke, F.; Maes, A.; Kratochwil, C.; et al. Predictors of Overall and Disease-Free Survival in Metastatic Castration-Resistant Prostate Cancer Patients Receiving 225Ac-PSMA-617 Radioligand Therapy. J. Nucl. Med. 2019, 61, 62–69. [Google Scholar] [CrossRef]
- Khreish, F.; Ebert, N.; Ries, M.; Maus, S.; Rosar, F.; Bohnenberger, H.; Stemler, T.; Saar, M.; Bartholoma, M.; Ezziddin, S. 225Ac-PSMA-617/177Lu-PSMA-617 tandem therapy of metastatic castration-resistant prostate cancer: Pilot experience. Eur. J. Nucl. Med. Mol. Imaging 2020, 47, 721–728. [Google Scholar] [CrossRef]
- Cung, T.D.; Lai, W.; Svider, P.F.; Hanba, C.; Samantray, J.; Folbe, A.J.; Shkoukani, M.; Raza, S.N. Sialendoscopy in the Management of Radioiodine Induced Sialadenitis: A Systematic Review. Ann. Otol. Rhinol. Laryngol. 2017, 126, 768–773. [Google Scholar] [CrossRef]
- Taieb, D.; Foletti, J.M.; Bardies, M.; Rocchi, P.; Hicks, R.J.; Haberkorn, U. PSMA-Targeted Radionuclide Therapy and Salivary Gland Toxicity: Why Does It Matter? J. Nucl. Med. 2018, 59, 747–748. [Google Scholar] [CrossRef] [Green Version]
- Langbein, T.; Chausse, G.; Baum, R.P. Salivary Gland Toxicity of PSMA Radioligand Therapy: Relevance and Preventive Strategies. J. Nucl. Med. 2018, 59, 1172–1173. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yilmaz, B.; Nisli, S.; Ergul, N.; Gursu, R.U.; Acikgoz, O.; Cermik, T.F. Effect of external cooling on Lu-177 PSMA uptake for parotid glands. J. Nucl. Med. 2019, 60, 1388–1393. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Kalmthout, L.W.M.; Lam, M.G.E.H.; de Keizer, B.; Krijger, G.C.; Ververs, T.F.T.; De Roos, R.; Braat, A.J.A.T. Impact of external cooling with icepacks on 68Ga-PSMA uptake in salivary glands. EJNMMI Res. 2018, 8, 56. [Google Scholar] [CrossRef] [PubMed]
- Rousseau, E.; Lau, J.; Kuo, H.T.; Zhang, Z.; Merkens, H.; Hundal-Jabal, N.; Colpo, N.; Lin, K.S.; Benard, F. Monosodium Glutamate Reduces 68Ga-PSMA-11 Uptake in Salivary Glands and Kidneys in a Preclinical Prostate Cancer Model. J. Nucl. Med. 2018, 59, 1865–1868. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Harsini, S.; Saprunoff, H.; Alden, T.M.; Mohammadi, B.; Wilson, D.; Benard, F. The Effects of Monosodium Glutamate on PSMA Radiotracer Uptake in Men with Recurrent Prostate Cancer: A Prospective, Randomized, Double-Blind, Placebo-Controlled Intraindividual Imaging Study. J. Nucl. Med. 2020, 62, 81–87. [Google Scholar] [CrossRef] [PubMed]
- Baum, R.P.; Langbein, T.; Singh, A.; Shahinfar, M.; Schuchardt, C.; Volk, G.F.; Kulkarni, H. Injection of Botulinum Toxin for Preventing Salivary Gland Toxicity after PSMA Radioligand Therapy: An Empirical Proof of a Promising Concept. Nucl. Med. Mol. Imaging 2018, 52, 80–81. [Google Scholar] [CrossRef]
- Mueller, J.; Langbein, T.; Mishra, A.; Baum, R.P. Safety of High-Dose Botulinum Toxin Injections for Parotid and Submandibular Gland Radioprotection. Toxins 2022, 14, 64. [Google Scholar] [CrossRef]




| Characteristic | Short-Term Cohort; n = 91 | Long-Term Cohort; n = 40 | Tandem Cohort; n = 18 |
|---|---|---|---|
| Age at first cycle of PRLT (median; range); years | 68 (46–90) | 68 (50–90) | 65 (52–82) |
| Initial Gleason score (median; range) | 8 (6–10) | 8 (5–10) | 8 (6–10) |
| Metastases at baseline PET/CT | |||
| Bone metastases | 77 (84.6%) | 35 (87.5%) | 17 (94.4%) |
| Lymph node metastases | 73 (80.2%) | 33 (82.5%) | 15 (83.3%) |
| Visceral metastases | 23 (25.3%) | 11 (27.5%) | 5 (27.8%) |
| Tumor burden based on base PSMA PET/CT | |||
| Low | 34 (37.4%) | 15 (37.5%) | 0 (0%) |
| Moderate | 30 (33.0%) | 13 (32.5%) | 5 (27.8%) |
| High | 27 (29.7%) | 12 (30.0%) | 13 (72.2%) |
| mCRPC pretreatments | |||
| Chemotherapy | 44 (48.4%) | 19 (47.5%) | 7 (38.9%) |
| Docetaxel | 40 (44.0%) | 19 (47.5%) | 6 (33.3%) |
| Cabazitaxel | 16 (17.6%) | 4 (10.0%) | 3 (16.7%) |
| androgen receptor axis-targeted agents | 56 (61.5%) | 32 (80.0%) | 14 (77.8%) |
| Enzalutamide | 45 (49.5%) | 22 (55.0%) | 11 (61.1%) |
| Abiraterone | 37 (40.7%) | 23 (57.5%) | 10 (55.6%) |
| Prior 177Lu-PSMA-I&T/-617 monotherapy | n/a | n/a | 14 (77.8%) |
| Supportive treatments during PRLT | |||
| Bisphosphonates | 27 (29.7%) | 14 (35.0%) | 7 (38.9%) |
| Denosumab | 24 (26.4%) | 9 (22.5%) | 3 (16.7%) |
| Cumulative administered 177Lu-PSMA-I&T/-617 (median; range); GBq | 14.3 (9.5–20.2) | 35.3 (9.9–61.8) | |
| Administered 225Ac-PSMA-617 (median, range); MBq | n/a | n/a | 4.0 (2.0–7.0) |
| Follow-up (median; IQR); months | 2.3 (1.9–2.7) | 22.7 (16.4–30.2) | 2.5 (2.0–3.2) |
| Total cycles of 177Lu-PSMA-I&T/-617 applied (median, range) | 2 | 5.5 (2–9) | n/a |
| Umax | p * | EF | p * | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Baseline | Follow-Up | Baseline | Follow-Up | |||||||||||
| Mean | Min | Max | Mean | Min | Max | Mean | Min | Max | Mean | Min | Max | |||
| right PG | 0.32 | 0.11 | 0.73 | 0.31 | 0.12 | 0.63 | n.s. | 57.9 | 2.4 | 88.8 | 54.9 | 13.9 | 83.5 | n.s. |
| left PG | 0.35 | 0.10 | 0.82 | 0.34 | 0.12 | 0.73 | n.s. | 57.1 | 23.2 | 82.7 | 52.5 | 5.7 | 73.9 | n.s. |
| right SMG | 0.31 | 0.15 | 0.84 | 0.32 | 0.10 | 0.85 | n.s. | 49.9 | 24.9 | 67.9 | 49.1 | 19.7 | 67.7 | n.s. |
| left SMG | 0.33 | 0.13 | 0.67 | 0.33 | 0.10 | 0.91 | n.s. | 48.8 | 20.7 | 70.1 | 47.5 | 6.1 | 68.4 | n.s. |
| SUVmax | p * | MV (cm3) | p * | |||||||||||
| Baseline | Follow-Up | Baseline | Follow-Up | |||||||||||
| Mean | Min | Max | Mean | Min | Max | Mean | Min | Max | Mean | Min | Max | |||
| right PG | 21.3 | 5.4 | 41.9 | 20.5 | 9.3 | 37.0 | n.s. | 36.7 | 11.2 | 61.0 | 33.2 | 3.1 | 57.1 | <0.001 |
| left PG | 21.1 | 7.7 | 38.6 | 20.2 | 8.3 | 37.3 | n.s. | 37.1 | 22.7 | 60.6 | 33.8 | 17.4 | 59.5 | <0.001 |
| right SMG | 23.2 | 10.1 | 44.6 | 23.4 | 9.8 | 52.1 | n.s. | 13.0 | 2.7 | 24.2 | 11.9 | 3.6 | 20.1 | <0.001 |
| left SMG | 23.8 | 10.1 | 49.8 | 23.8 | 9.3 | 45.3 | n.s. | 13.0 | 7.6 | 26.6 | 11.9 | 5.1 | 19.8 | <0.001 |
| Umax | p * | Ef | p * | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Baseline | Follow-Up | Baseline | Follow-Up | |||||||||||
| Mean | Min | Max | Mean | Min | Max | Mean | Min | Max | Mean | Min | Max | |||
| right PG | 0.29 | 0.12 | 0.50 | 0.35 | 0.13 | 0.70 | n.s. | 53.1 | 27.2 | 75.1 | 48.5 | 1.9 | 72.2 | n.s. |
| left PG | 0.35 | 0.15 | 0.82 | 0.36 | 0.12 | 0.66 | n.s. | 52.6 | 36.3 | 72.1 | 48.8 | 8.0 | 71.1 | n.s. |
| right SMG | 0.32 | 0.20 | 0.48 | 0.34 | 0.17 | 0.51 | n.s. | 45.5 | 15.7 | 67.0 | 44.6 | 19.7 | 63.7 | n.s. |
| left SMG | 0.34 | 0.22 | 0.58 | 0.35 | 0.19 | 0.56 | n.s. | 45.3 | 34.6 | 62.6 | 46.7 | 16.1 | 65.1 | n.s. |
| SUVmax | p * | MV (cm3) | p * | |||||||||||
| Baseline | Follow-Up | Baseline | Follow-Up | |||||||||||
| Mean | Min | Max | Mean | Min | Max | Mean | Min | Max | Mean | Min | Max | |||
| right PG | 20.0 | 5.4 | 37.3 | 18.6 | 7.1 | 38.2 | n.s. | 40.5 | 23.6 | 60.7 | 34.5 | 16.5 | 56.4 | <0.001 |
| left PG | 20.1 | 7.6 | 38.6 | 18.0 | 4.9 | 35.6 | n.s. | 38.9 | 5.7 | 60.7 | 33.9 | 5.9 | 55.9 | <0.001 |
| right SMG | 21.3 | 9.8 | 39.4 | 20.6 | 10.8 | 46.9 | n.s. | 14.1 | 9.3 | 27.4 | 11.9 | 7.2 | 20.2 | <0.001 |
| left SMG | 21.6 | 10.5 | 38.9 | 21.0 | 9.2 | 46.5 | n.s. | 14.1 | 7.6 | 28.2 | 11.9 | 6.6 | 22.5 | <0.001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Langbein, T.; Kulkarni, H.R.; Schuchardt, C.; Mueller, D.; Volk, G.F.; Baum, R.P. Salivary Gland Toxicity of PSMA-Targeted Radioligand Therapy with 177Lu-PSMA and Combined 225Ac- and 177Lu-Labeled PSMA Ligands (TANDEM-PRLT) in Advanced Prostate Cancer: A Single-Center Systematic Investigation. Diagnostics 2022, 12, 1926. https://doi.org/10.3390/diagnostics12081926
Langbein T, Kulkarni HR, Schuchardt C, Mueller D, Volk GF, Baum RP. Salivary Gland Toxicity of PSMA-Targeted Radioligand Therapy with 177Lu-PSMA and Combined 225Ac- and 177Lu-Labeled PSMA Ligands (TANDEM-PRLT) in Advanced Prostate Cancer: A Single-Center Systematic Investigation. Diagnostics. 2022; 12(8):1926. https://doi.org/10.3390/diagnostics12081926
Chicago/Turabian StyleLangbein, Thomas, Harshad R. Kulkarni, Christiane Schuchardt, Dirk Mueller, Gerd Fabian Volk, and Richard P. Baum. 2022. "Salivary Gland Toxicity of PSMA-Targeted Radioligand Therapy with 177Lu-PSMA and Combined 225Ac- and 177Lu-Labeled PSMA Ligands (TANDEM-PRLT) in Advanced Prostate Cancer: A Single-Center Systematic Investigation" Diagnostics 12, no. 8: 1926. https://doi.org/10.3390/diagnostics12081926







