Screening for Myocardial Injury after Mild SARS-CoV-2 Infection with Advanced Transthoracic Echocardiography Modalities
Abstract
:1. Introduction
2. Patients and Methods
2.1. Patient Population
2.2. Echocardiographic Protocol
2.3. Statistical Analysis
3. Results
3.1. Dimensional Parameters of the Left-Side of the Heart
3.2. Functional Parameters of the Left-Side of the Heart
3.3. Myocardial Work Parameters
3.4. Dimensional and Functional Parameters of the Right-Side of the Heart
3.5. Valvular Alterations
4. Discussion
5. Study Limitations
6. Conclusions
- -
- During the post-acute phase of even mild COVID-19 subtle functional alterations can be detected by extended echocardiographic protocols including advanced deformation imaging;
- -
- Although altered echocardiographic parameters may include traditional echocardiographic parameters (e.g., LV ejection fraction, LV end diastolic diameter, etc.), their relative change is generally modest;
- -
- Along with non-invasive measurement of stroke volume, deformation imaging appears to be able to detect the most pronounced relative difference for both left and right ventricular function, with left ventricular global myocardial work index and right ventricular free wall strain being the most robust alteration;
- -
- These minor changes are difficult to utilize on a single patient basis, however, LV myocardial work and RV free wall strain seem to be the most sensitive and reproducible 2D echo-based functional parameters for screening for cardiac injury and follow-up;
- -
- Echocardiography, even advanced investigations requiring expert echocardiog-raphers, is less time consuming and more available than cardiac magnetic resonance imaging, and thus more suited for larger scale screening and follow-up for myocardial injury after COVID-19 infection;
- -
- Despite widespread vaccination has reduced the severity of subsequent waves of the pandemic, it is expected to remain a global health issue, and thus further studies and ongoing research into the cardiac involvement of the disease is warranted.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Guan, W.J.; Ni, Z.Y.; Hu, Y.; Liang, W.H.; Ou, C.Q.; He, J.X.; Liu, L.; Shan, H.; Lei, C.L.; Hui, D.S.C.; et al. Clinical Characteristics of Coronavirus Disease 2019 in China. N. Engl. J. Med. 2020, 382, 1708–1720. [Google Scholar] [CrossRef] [PubMed]
- Guo, T.; Fan, Y.; Chen, M.; Wu, X.; Zhang, L.; He, T.; Wang, H.; Wan, J.; Wang, X.; Lu, Z. Cardiovascular Implications of Fatal Outcomes of Patients with Coronavirus Disease 2019 (COVID-19). JAMA Cardiol. 2020, 5, 811–818. [Google Scholar] [CrossRef] [PubMed]
- Nishiga, M.; Wang, D.W.; Han, Y.; Lewis, D.B.; Wu, J.C. COVID-19 and cardiovascular disease: From basic mechanisms to clinical perspectives. Nat. Rev. Cardiol. 2020, 17, 543–558. [Google Scholar] [CrossRef] [PubMed]
- Kuck, K.H. Arrhythmias and sudden cardiac death in the COVID-19 pandemic. Herz 2020, 45, 325–326. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Zhao, P.; Tang, D.; Zhu, T.; Han, R.; Zhan, C.; Liu, W.; Zeng, H.; Tao, Q.; Xia, L. Cardiac Involvement in Patients Recovered From COVID-2019 Identified Using Magnetic Resonance Imaging. JACC Cardiovasc. Imaging 2020, 13, 2330–2339. [Google Scholar] [CrossRef] [PubMed]
- Puntmann, V.O.; Carerj, M.L.; Wieters, I.; Fahim, M.; Arendt, C.; Hoffmann, J.; Shchendrygina, A.; Escher, F.; Vasa-Nicotera, M.; Zeiher, A.M.; et al. Outcomes of Cardiovascular Magnetic Resonance Imaging in Patients Recently Recovered from Coronavirus Disease 2019 (COVID-19). JAMA Cardiol. 2020, 5, 1265–1273. [Google Scholar] [CrossRef]
- Petersen, S.E.; Friedrich, M.G.; Leiner, T.; Elias, M.D.; Ferreira, V.M.; Fenski, M.; Flamm, S.D.; Fogel, M.; Garg, R.; Halushka, M.K.; et al. Cardiovascular Magnetic Resonance for Patients With COVID-19. JACC Cardiovasc. Imaging 2022, 15, 685–699. [Google Scholar] [CrossRef]
- Shafiabadi Hassani, N.; Talakoob, H.; Karim, H.; Mozafari Bazargany, M.H.; Rastad, H. Cardiac Magnetic Resonance Imaging Findings in 2954 COVID-19 Adult Survivors: A Comprehensive Systematic Review. J. Magn. Reson. Imaging 2022, 55, 866–880. [Google Scholar] [CrossRef]
- Papadopoulos, K.; Ozden Tok, O.; Mitrousi, K.; Ikonomidis, I. Myocardial Work: Methodology and Clinical Applications. Diagnostics 2021, 11, 573. [Google Scholar] [CrossRef]
- Russo, C.; Jin, Z.; Elkind, M.S.; Rundek, T.; Homma, S.; Sacco, R.L.; Di Tullio, M.R. Prevalence and prognostic value of subclinical left ventricular systolic dysfunction by global longitudinal strain in a community-based cohort. Eur. J. Heart Fail. 2014, 16, 1301–1309. [Google Scholar] [CrossRef]
- Carrizales-Sepulveda, E.F.; Vera-Pineda, R.; Flores-Ramirez, R.; Hernandez-Guajardo, D.A.; Perez-Contreras, E.; Lozano-Ibarra, M.M.; Ordaz-Farias, A. Echocardiographic Manifestations in COVID-19: A Review. Heart Lung Circ. 2021, 30, 1117–1129. [Google Scholar] [CrossRef]
- Dweck, M.R.; Bularga, A.; Hahn, R.T.; Bing, R.; Lee, K.K.; Chapman, A.R.; White, A.; Salvo, G.D.; Sade, L.E.; Pearce, K.; et al. Global evaluation of echocardiography in patients with COVID-19. Eur. Heart J. Cardiovasc. Imaging 2020, 21, 949–958. [Google Scholar] [CrossRef] [PubMed]
- Giustino, G.; Croft, L.B.; Stefanini, G.G.; Bragato, R.; Silbiger, J.J.; Vicenzi, M.; Danilov, T.; Kukar, N.; Shaban, N.; Kini, A.; et al. Characterization of Myocardial Injury in Patients With COVID-19. J. Am. Coll. Cardiol. 2020, 76, 2043–2055. [Google Scholar] [CrossRef] [PubMed]
- Maiese, A.; Frati, P.; Del Duca, F.; Santoro, P.; Manetti, A.C.; La Russa, R.; Di Paolo, M.; Turillazzi, E.; Fineschi, V. Myocardial Pathology in COVID-19-Associated Cardiac Injury: A Systematic Review. Diagnostics 2021, 11, 1647. [Google Scholar] [CrossRef] [PubMed]
- Pellegrini, D.; Kawakami, R.; Guagliumi, G.; Sakamoto, A.; Kawai, K.; Gianatti, A.; Nasr, A.; Kutys, R.; Guo, L.; Cornelissen, A.; et al. Microthrombi as a Major Cause of Cardiac Injury in COVID-19: A Pathologic Study. Circulation 2021, 143, 1031–1042. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Wang, J.; Yan, Y.; Zhang, Z.; Gong, W.; Nie, S. Clinical Characterization and Possible Pathological Mechanism of Acute Myocardial Injury in COVID-19. Front. Cardiovasc. Med. 2022, 9, 862571. [Google Scholar] [CrossRef]
- Patel, P.; DeCuir, J.; Abrams, J.; Campbell, A.P.; Godfred-Cato, S.; Belay, E.D. Clinical Characteristics of Multisystem Inflammatory Syndrome in Adults: A Systematic Review. JAMA Netw. Open 2021, 4, e2126456. [Google Scholar] [CrossRef]
- Caro-Codon, J.; Rey, J.R.; Buno, A.; Iniesta, A.M.; Rosillo, S.O.; Castrejon-Castrejon, S.; Rodriguez-Sotelo, L.; Martinez, L.A.; Marco, I.; Merino, C.; et al. Characterization of NT-proBNP in a large cohort of COVID-19 patients. Eur. J. Heart Fail. 2021, 23, 456–464. [Google Scholar] [CrossRef]
- Bienstock, S.W.; Tandon, P.; Govindarajulu, U.; Leibner, E.; Glicksberg, B.S.; Samtani, R.; Giustino, G.; Gidwani, U.; Kohli-Seth, R.; Goldman, M.E. Impact of Myocardial Injury in Hospitalized Patients With COVID-19 in 2 Peak Time Periods. J. Am. Coll. Cardiol. 2021, 78, 1482–1483. [Google Scholar] [CrossRef]
- Vrettou, A.R.; Parissis, J.; Ikonomidis, I. The Dual Role of Echocardiography in the Diagnosis of Acute Cardiac Complications and Treatment Monitoring for Coronavirus Disease 2019 (COVID-19). Front. Cardiovasc. Med. 2020, 7, 129. [Google Scholar] [CrossRef]
- Rath, D.; Petersen-Uribe, A.; Avdiu, A.; Witzel, K.; Jaeger, P.; Zdanyte, M.; Heinzmann, D.; Tavlaki, E.; Muller, K.; Gawaz, M.P. Impaired cardiac function is associated with mortality in patients with acute COVID-19 infection. Clin. Res. Cardiol. 2020, 109, 1491–1499. [Google Scholar] [CrossRef] [PubMed]
- Szekely, Y.; Lichter, Y.; Taieb, P.; Banai, A.; Hochstadt, A.; Merdler, I.; Gal Oz, A.; Rothschild, E.; Baruch, G.; Peri, Y.; et al. Spectrum of Cardiac Manifestations in COVID-19: A Systematic Echocardiographic Study. Circulation 2020, 142, 342–353. [Google Scholar] [CrossRef] [PubMed]
- Baycan, O.F.; Barman, H.A.; Atici, A.; Tatlisu, A.; Bolen, F.; Ergen, P.; Icten, S.; Gungor, B.; Caliskan, M. Evaluation of biventricular function in patients with COVID-19 using speckle tracking echocardiography. Int. J. Cardiovasc. Imaging 2021, 37, 135–144. [Google Scholar] [CrossRef]
- Janus, S.E.; Hajjari, J.; Karnib, M.; Tashtish, N.; Al-Kindi, S.G.; Hoit, B.D. Prognostic Value of Left Ventricular Global Longitudinal Strain in COVID-19. Am. J. Cardiol. 2020, 131, 134–136. [Google Scholar] [CrossRef]
- Gul, M.; Inci, S.; Aktas, H.; Yildirim, O.; Alsancak, Y. Hidden danger of COVID-19 outbreak: Evaluation of subclinical myocardial dysfunction in patients with mild symptoms. Int. J. Cardiovasc. Imaging 2021, 37, 2957–2964. [Google Scholar] [CrossRef]
- Uzieblo-Zyczkowska, B.; Krzesinski, P.; Domino, B.; Chcialowski, A.; Maciorowska, M.; Gielerak, G. Echocardiographic assessment of cardiac function after mild coronavirus disease 2019: A preliminary report. J. Clin. Ultrasound 2022, 50, 17–24. [Google Scholar] [CrossRef] [PubMed]
- Mahajan, S.; Kunal, S.; Shah, B.; Garg, S.; Palleda, G.M.; Bansal, A.; Batra, V.; Yusuf, J.; Mukhopadhyay, S.; Kumar, S.; et al. Left ventricular global longitudinal strain in COVID-19 recovered patients. Echocardiography 2021, 38, 1722–1730. [Google Scholar] [CrossRef]
- Ikonomidis, I.; Lambadiari, V.; Mitrakou, A.; Kountouri, A.; Katogiannis, K.; Thymis, J.; Korakas, E.; Pavlidis, G.; Kazakou, P.; Panagopoulos, G.; et al. Myocardial work and vascular dysfunction are partially improved at 12 months after COVID-19 infection. Eur. J. Heart Fail. 2022, 24, 727–729. [Google Scholar] [CrossRef]
- Tudoran, C.; Tudoran, M.; Cut, T.G.; Lazureanu, V.E.; Oancea, C.; Marinescu, A.R.; Pescariu, S.A.; Pop, G.N.; Bende, F. Evolution of Echocardiographic Abnormalities Identified in Previously Healthy Individuals Recovering from COVID-19. J. Pers Med. 2022, 12, 46. [Google Scholar] [CrossRef]
- Jaglan, A.; Roemer, S.; Khandheria, B. Myocardial work index: It works. Eur. Heart J. Cardiovasc. Imaging 2020, 21, 1049. [Google Scholar] [CrossRef]
- Minhas, A.S.; Gilotra, N.A.; Goerlich, E.; Metkus, T.; Garibaldi, B.T.; Sharma, G.; Bavaro, N.; Phillip, S.; Michos, E.D.; Hays, A.G. Myocardial Work Efficiency, A Novel Measure of Myocardial Dysfunction, Is Reduced in COVID-19 Patients and Associated with In-Hospital Mortality. Front. Cardiovasc Med. 2021, 8, 667721. [Google Scholar] [CrossRef] [PubMed]
- Akkaya, F.; Yenercag, F.N.T.; Kaya, A.; Sener, Y.Z.; Bagci, A. Long term effects of mild severity COVID19 on right ventricular functions. Int. J. Cardiovasc. Imaging 2021, 37, 3451–3457. [Google Scholar] [CrossRef] [PubMed]
| Control Group (n = 60) | Post-COVID Group (n = 86) | Relative Difference (%) † | |
|---|---|---|---|
| age, year | 40.3 ± 11.0 | 39.5 ± 13.0 | NA |
| male sex | 24 (40.0) | 30 (34.9) | NA |
| BSA, m2 | 1.9 ± 0.3 | 1.9 ± 0.3 | NA |
| systolic blood pressure, Hgmm | 130.3 ± 12.8 | 132.2 ± 15.8 | NA |
| diastolic blood pressure, Hgmm | 75.0 (67.0–82.0) | 78.0 (67.8–86.0) | NA |
| previously treated/diagnosed hypertension | 7 (11.7) | 10 (11.8) | NA |
| heart rate, bpm | 70.9 ± 10.8 | 75.6 ± 13.4 * | 9.5 |
| Control Group (n = 60) | Post-COVID Group (n = 86) | Relative Difference (%) † | |
|---|---|---|---|
| left atrial volume, mL | 50.0 (42.3–60.8) | 50.0 (40.0–60.0) | NA |
| left atrial volume index, mL/m2 | 26.0 (23.0–31.0) | 27.0 (22.0–32.0) | NA |
| left ventricular end diastolic diameter, mm | 46.2 ± 4.2 | 47.9 ± 4.2 * | 3.2 |
| left ventricular end systolic diameter, mm | 30.0 (27.5–33.0) | 30.0 (27.0–33.0) | NA |
| left ventricular end diastolic volume, mL | 91.5 (72.0–118.5) | 97.0 (81.0–114.0) | NA |
| left ventricular end diastolic volume index, mL/m2 | 49.5 ± 11.2 | 51.9 ± 12.8 | NA |
| left ventricular end systolic volume, mL | 32.0 (24.0–36.5) | 32.5 (27.0–41.0) | NA |
| left ventricular end systolic volume index, mL/m2 | 15.5 (13.3–18.6) | 17.1 (15.2–21.0) * | 9.9 |
| interventricular septum, mm | 9.0 (8.0–9.5) | 9.0 (8.0–10.0) | NA |
| posterior wall, mm | 8.5 (8.0–9.0) | 9.0 (8.0–10.0) * | 5.9 |
| Control Group (n = 60) | Post-COVID Group (n = 86) | Relative Difference (%) † | |
|---|---|---|---|
| LV ejection fraction, % | 68.0 (65.0–70.0) | 66.0 (60.0–70.0) * | 2.9 |
| LVOT velocity time integral, cm | 23.0 (21.2–24.5) | 22.2 (20.2–24.9) | NA |
| LV stroke volume, mL | 75.5 (70.0–87.0) | 70.5 (61.0–78.0) ** | 6.6 |
| LV stroke volume index, mL/m2 | 41.6 (38.9–43.7) | 37.4 (33.5–41.8) *** | 10.0 |
| LV cardiac output, L/min | 5.5 ± 1.1 | 5.4 ± 1.2 | NA |
| LV cardiac index, L/min/m2 | 2.9 ± 0.5 | 2.9 ± 0.6 | NA |
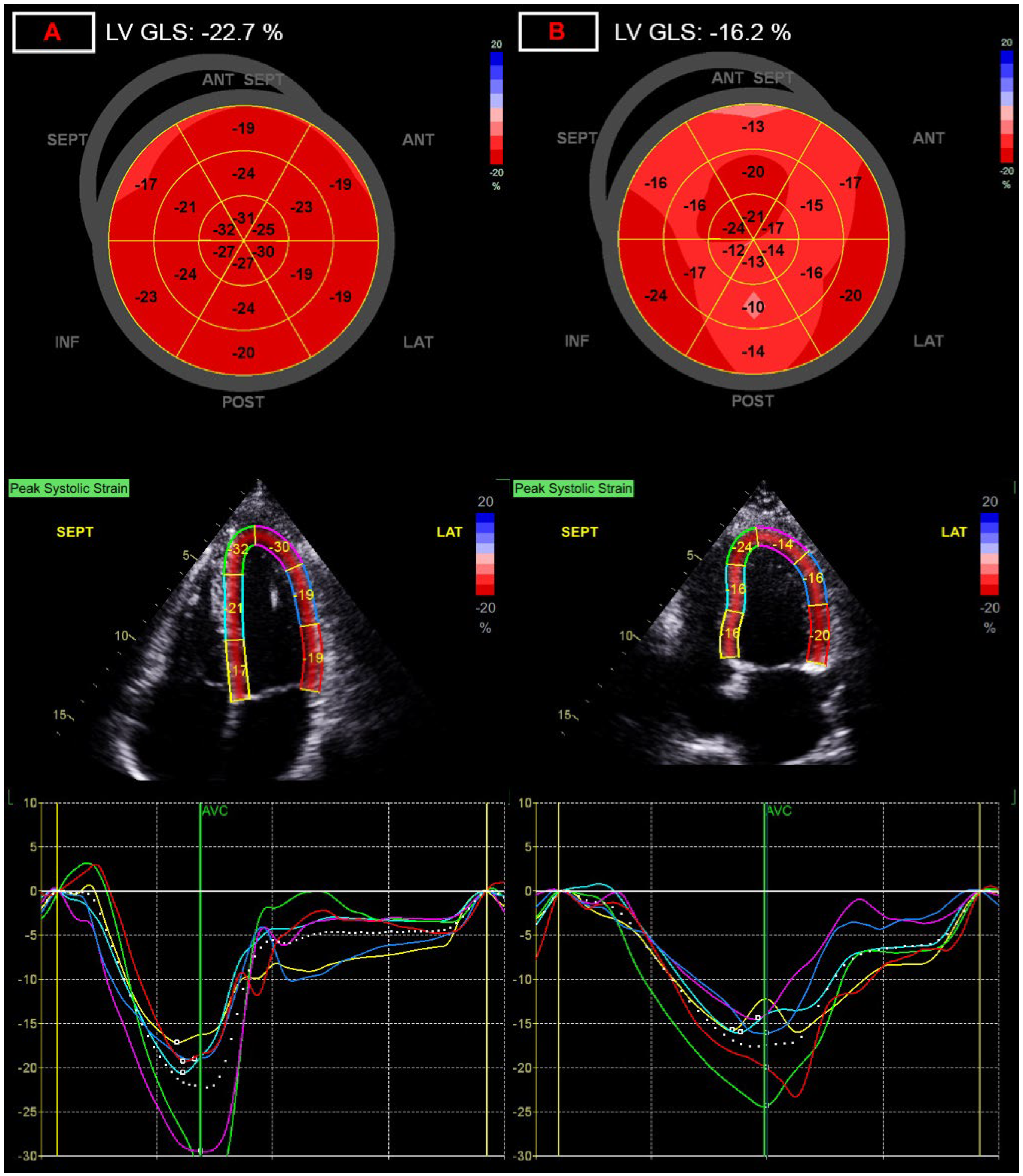
| LV global longitudinal strain, % | −20.3 (−21.1–−19.0) | −19.1 (−20.4–−17.6) *** | 5.9 |
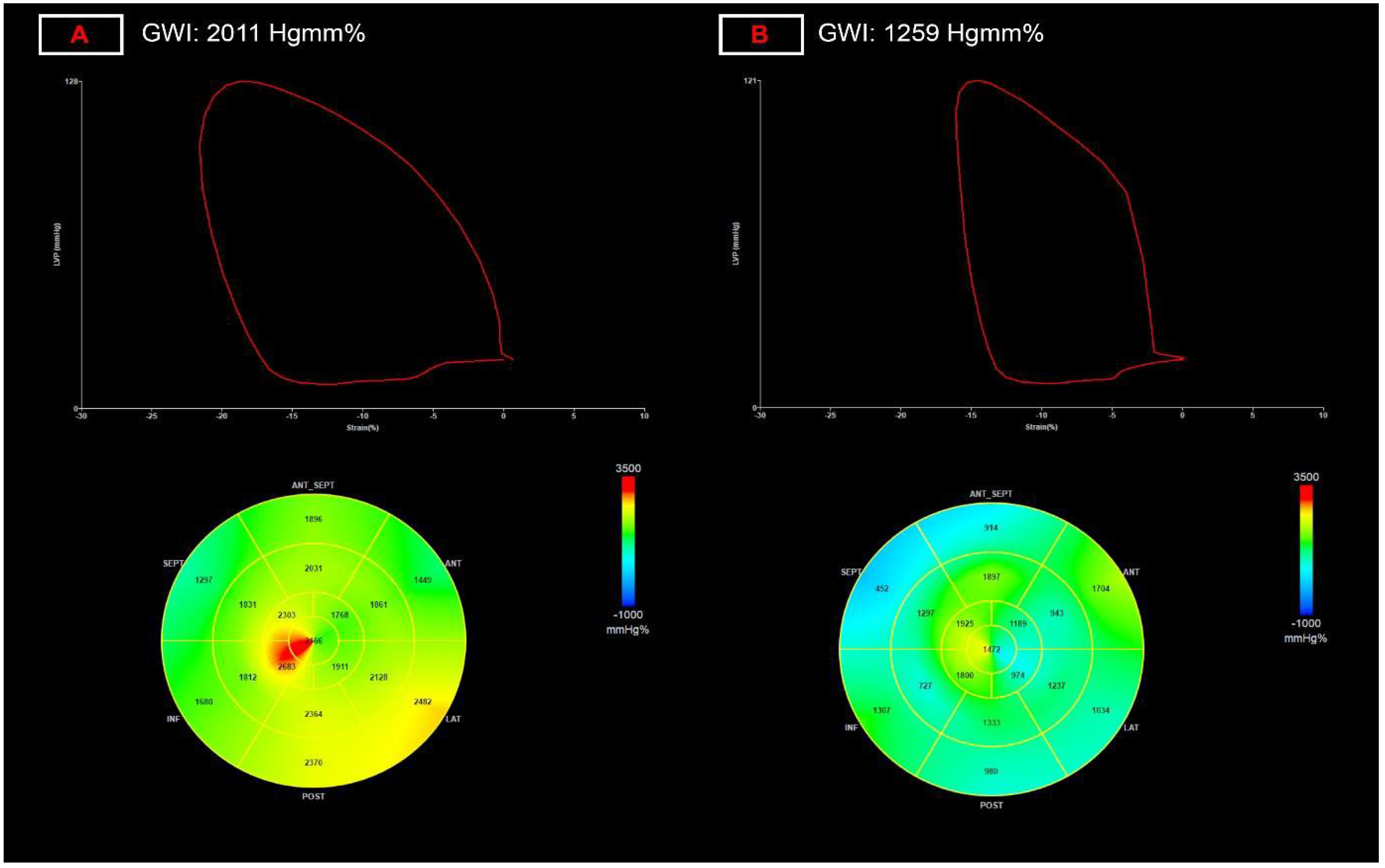
| LV global work index, Hgmm% | 1975 (1789–2105) | 1829 (1656–2057) ** | 7.4 |
| LV global constructive work, Hgmm% | 2383 (2226–2577) | 2341 (2094–2559) | NA |
| LV global wasted work, Hgmm% | 99 (63–129) | 107 (77–151) | NA |
| LV global work efficiency, % | 96 (94–97) | 95 (93–96) * | 1.0 |
| transmitral E velocity, cm/s | 82.0 ± 13.5 | 82.21 ± 15.7 | NA |
| transmitral A velocity, cm/s | 59.0 (51.3–70.5) | 61.0 (54.0–76.0) | NA |
| E/A | 1.35 (1.15–1.63) | 1.31 (1.07–1.63) | NA |
| mitral annulus e’ velocity, cm/s | 14.5 (12.0–16.0) | 13.0 (11.0–17.0) | NA |
| mitral annulus a’ velocity, cm/s | 9.0 (8.0–12−0) | 10.0 (8.0–12.0) | NA |
| mitral annulus s’ velocity, cm/s | 11.0 (10.0–13.0) | 10.0 (9.0–12.0) | NA |
| E/e’ | 5.6 (4.9–6.8) | 6.0 (5.2–7.3) | NA |
| Control Group (n = 60) | Post-COVID Group (n = 86) | Relative Difference (%) † | |
|---|---|---|---|
| right atrial area, cm2 | 14.0 (11.0–16.4) | 14.0 (12.0–16.7) | NA |
| right ventricular basal diameter, mm | 35.0 ± 4.5 | 35.6 ± 5.6 | NA |
| right ventricular diameter at the level of the papillary muscles, mm | 29.0 ± 5.1 | 29.7 ± 4.7 | NA |
| tricuspid annular plane systolic excursion, mm | 23.75 ± 2.8 | 22.5 ± 3.4 * | 5.3 |
| tricuspid annular s’ velocity, mm | 14.0 (13.0–15.0) | 13.0 (12.0–15.0) | NA |
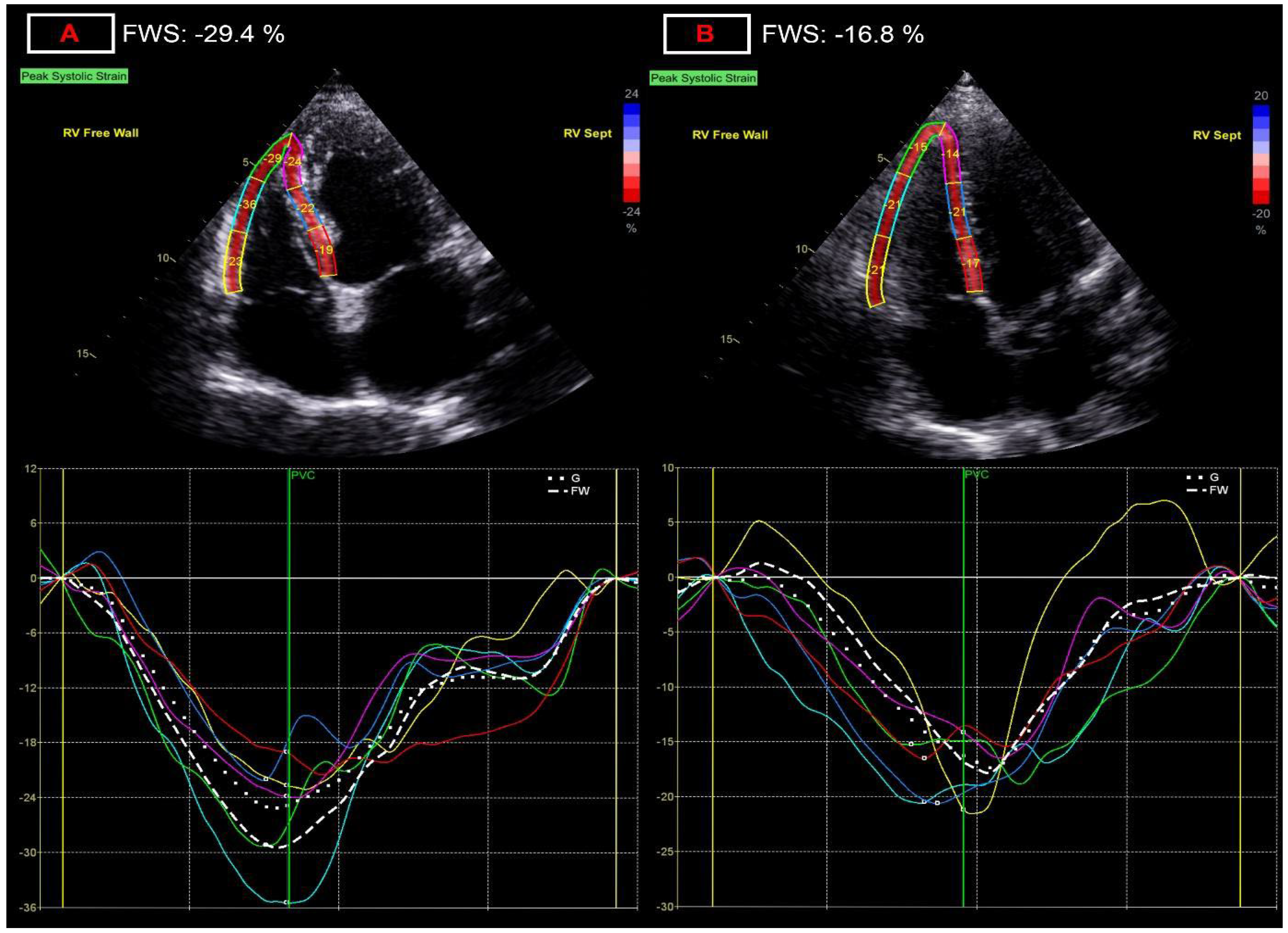
| right ventricular free wall strain, % | −26.6 ± 3.80 | −23.8 ± 4.0 *** | 11.7 |
| GWI | GWE | GLS | ||||
|---|---|---|---|---|---|---|
| Univariate Correlation | Multivariate Correlation | Univariate Correlation | Multivariate Correlation | Univariate Correlation | Multivariate Correlation | |
| LV ejection fraction, % | 0.220 * | NS | 0.214 * | NS | −0.252 * | NS |
| LVOT velocity time integral, cm | 0.336 ** | NS | NS | NS | NS | NS |
| LV stroke volume index, mL/m2 | 0.336 ** | NS | NS | NS | −0.387 *** | −0.284 * |
| LV cardiac index, L/min/m2 | NS | NS | NS | NS | −0.262 * | NS |
| LV global longitudinal strain, % | −0.551 **** | NA | −0.561 **** | NA | NA | NA |
| LV global work index, Hgmm% | NA | NA | NA | NA | −0.551 **** | NA |
| transmitral E velocity, cm/s | NS | NS | 0.326 ** | NS | −0.267 * | NS |
| E/A | NS | NS | NS | NS | −0.252 * | NS |
| mitral annulus e’ velocity, cm/s | NS | NS | 0.381 *** | NS | −0.328 ** | NS |
| mitral annulus s’ velocity, cm/s | NS | NS | 0.240 * | NS | −0.219 * | NS |
| E/e’ | 0.247 * | NS | NS | NS | NS | NS |
| left atrial diameter, medio-lateral, mm | 0.334 ** | NS | NS | NS | NS | NS |
| left atrial height, mm | 0.248 * | NS | NS | NS | NS | NS |
| left atrial volume, mL | 0.249 * | NS | 0.233 * | NS | NS | NS |
| left atrial volume index, mL/m2 | 0.321 ** | NS | 0.286 ** | NS | −0.263 * | −0.343 ** |
| left ventricular end systolic volume, mL | NS | NS | NS | NS | 0.242 * | NS |
| systolic blood pressure, Hgmm | 0.614 **** | NA | NS | NS | NS | NS |
| diastolic blood pressure, Hgmm | 0.479 **** | NA | NS | NS | NS | NS |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rácz, G.; Takács, H.; Kormányos, Á.; Polestyuk, B.; Borbás, J.; Gyenes, N.; Schvartz, N.; Németh, G.; Kincses, Z.T.; Sepp, R.; et al. Screening for Myocardial Injury after Mild SARS-CoV-2 Infection with Advanced Transthoracic Echocardiography Modalities. Diagnostics 2022, 12, 1941. https://doi.org/10.3390/diagnostics12081941
Rácz G, Takács H, Kormányos Á, Polestyuk B, Borbás J, Gyenes N, Schvartz N, Németh G, Kincses ZT, Sepp R, et al. Screening for Myocardial Injury after Mild SARS-CoV-2 Infection with Advanced Transthoracic Echocardiography Modalities. Diagnostics. 2022; 12(8):1941. https://doi.org/10.3390/diagnostics12081941
Chicago/Turabian StyleRácz, Gergely, Hedvig Takács, Árpád Kormányos, Bianka Polestyuk, János Borbás, Nándor Gyenes, Noémi Schvartz, Gergely Németh, Zsigmond Tamás Kincses, Róbert Sepp, and et al. 2022. "Screening for Myocardial Injury after Mild SARS-CoV-2 Infection with Advanced Transthoracic Echocardiography Modalities" Diagnostics 12, no. 8: 1941. https://doi.org/10.3390/diagnostics12081941






