Deep Learning Artificial Intelligence to Predict the Need for Tracheostomy in Patients of Deep Neck Infection Based on Clinical and Computed Tomography Findings—Preliminary Data and a Pilot Study
Abstract
:1. Introduction
2. Materials and Methods
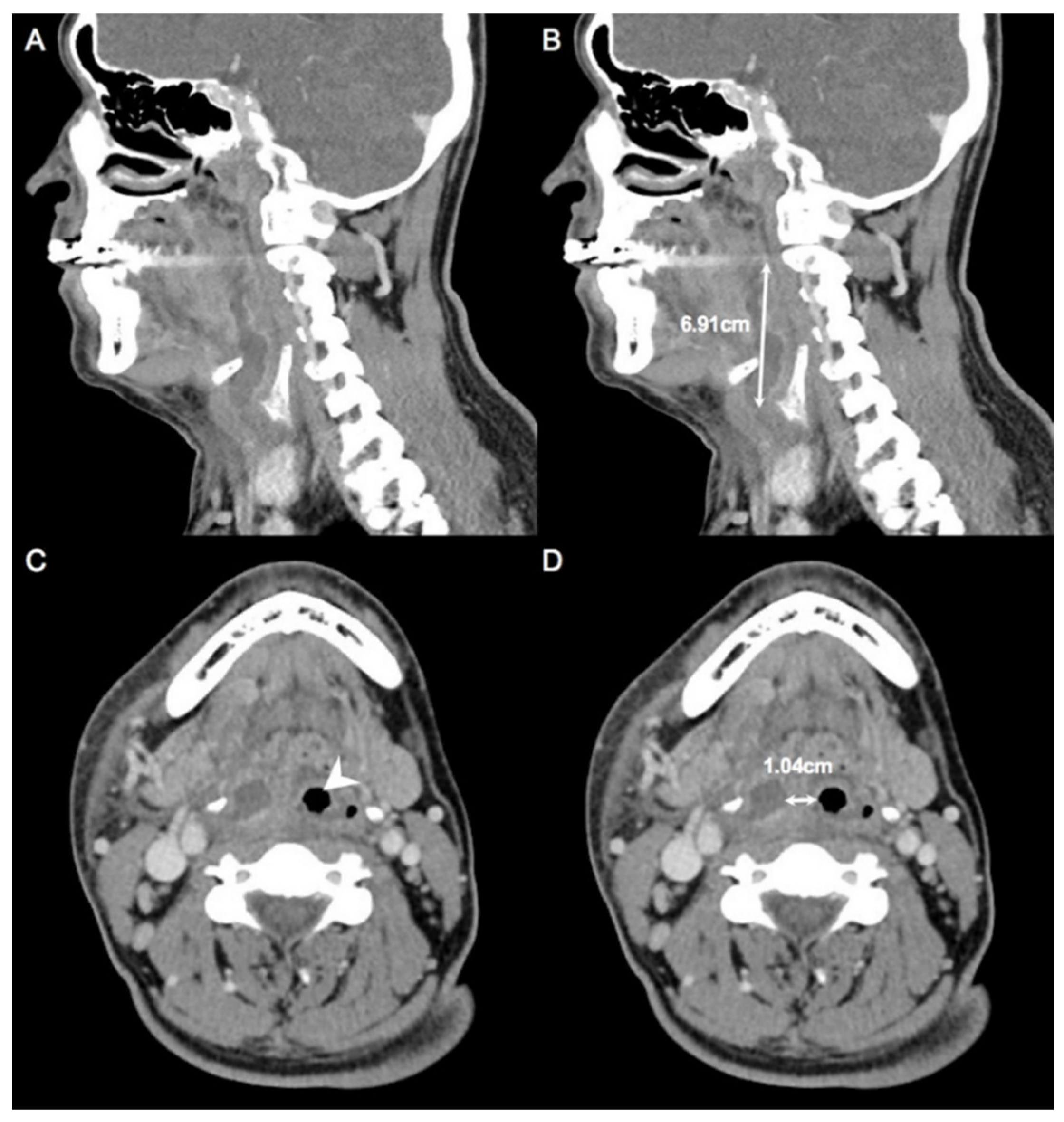
2.1. Measurement of CT
2.2. Data Collection
2.3. k-Nearest Neighbor Method
2.4. Exclusion Criteria
2.5. Statistical Analysis
3. Results
4. Discussion
Study Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Velhonoja, J.; Laaveri, M.; Soukka, T.; Irjala, H.; Kinnunen, I. Deep neck space infections: An upward trend and changing characteristics. Eur. Arch. Otorhinolaryngol. 2020, 277, 863–872. [Google Scholar] [CrossRef]
- Tapiovaara, L.; Back, L.; Aro, K. Comparison of intubation and tracheotomy in patients with deep neck infection. Eur. Arch. Otorhinolaryngol. 2017, 274, 3767–3772. [Google Scholar] [CrossRef]
- Bur, A.M.; Shew, M.; New, J. Artificial Intelligence for the Otolaryngologist: A State of the Art Review. Otolaryngol. Head Neck Surg. 2019, 160, 603–611. [Google Scholar] [CrossRef]
- Wilson, M.B.; Ali, S.A.; Kovatch, K.J.; Smith, J.D.; Hoff, P.T. Machine Learning Diagnosis of Peritonsillar Abscess. Otolaryngol. Head Neck Surg. 2019, 161, 796–799. [Google Scholar] [CrossRef]
- Laios, A.; Gryparis, A.; DeJong, D.; Hutson, R.; Theophilou, G.; Leach, C. Predicting complete cytoreduction for advanced ovarian cancer patients using nearest-neighbor models. J. Ovarian Res. 2020, 13, 117. [Google Scholar] [CrossRef]
- Crowson, M.G.; Ranisau, J.; Eskander, A.; Babier, A.; Xu, B.; Kahmke, R.R.; Chen, J.M.; Chan, T.C.Y. A contemporary review of machine learning in otolaryngology-head and neck surgery. Laryngoscope 2020, 130, 45–51. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.M.; Li, Y.; Cheng, Y.S.; He, Z.Y.; Yang, J.M.; Xu, J.H.; Chi, Z.C.; Chi, F.L.; Ren, D.D. Deep Learning in Automated Region Proposal and Diagnosis of Chronic Otitis Media Based on Computed Tomography. Ear Hear. 2020, 41, 669–677. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.L.; Young, C.K.; Tsai, T.Y.; Chien, H.T.; Kang, C.J.; Liao, C.T.; Huang, S.F. Factors Affecting the Necessity of Tracheostomy in Patients with Deep Neck Infection. Diagnostics 2021, 11, 1536. [Google Scholar] [CrossRef]
- Yang, S.W.; Lee, M.H.; See, L.C.; Huang, S.H.; Chen, T.M.; Chen, T.A. Deep neck abscess: An analysis of microbial etiology and the effectiveness of antibiotics. Infect. Drug Resist. 2008, 1, 1–8. [Google Scholar] [CrossRef]
- Chen, S.L.; Young, C.K.; Liao, C.T.; Tsai, T.Y.; Kang, C.J.; Huang, S.F. Parotid Space, a Different Space from Other Deep Neck Infection Spaces. Microorganisms 2021, 9, 2361. [Google Scholar] [CrossRef]
- Garcia-Carretero, R.; Vigil-Medina, L.; Mora-Jimenez, I.; Soguero-Ruiz, C.; Barquero-Perez, O.; Ramos-Lopez, J. Use of a K-nearest neighbors model to predict the development of type 2 diabetes within 2 years in an obese, hypertensive population. Med. Biol. Eng. Comput. 2020, 58, 991–1002. [Google Scholar] [CrossRef] [PubMed]
- Cover, T.; Hart, P. Nearest neighbor pattern classification. IEEE Trans. Inf. Theory 1967, 13, 21–27. [Google Scholar] [CrossRef]
- Luz, C.F.; Vollmer, M.; Decruyenaere, J.; Nijsten, M.W.; Glasner, C.; Sinha, B. Machine learning in infection management using routine electronic health records: Tools, techniques, and reporting of future technologies. Clin. Microbiol. Infect. 2020, 26, 1291–1299. [Google Scholar] [CrossRef] [PubMed]
- Hu, L.Y.; Huang, M.W.; Ke, S.W.; Tsai, C.F. The distance function effect on k-nearest neighbor classification for medical datasets. Springerplus 2016, 5, 1304. [Google Scholar] [CrossRef] [PubMed]
- Rajaguru, H.; Sr, R.S. Analysis of Decision Tree and K-Nearest Neighbor Algorithm in the Classification of Breast Cancer. Asian Pac. J. Cancer Prev. 2019, 20, 3777–3781. [Google Scholar] [CrossRef]
- Zhang, Z. Introduction to machine learning: K-nearest neighbors. Ann. Transl. Med. 2016, 4, 218. [Google Scholar] [CrossRef] [PubMed]
- Abu Alfeilat, H.A.; Hassanat, A.B.A.; Lasassmeh, O.; Tarawneh, A.S.; Alhasanat, M.B.; Eyal Salman, H.S.; Prasath, V.B.S. Effects of Distance Measure Choice on K-Nearest Neighbor Classifier Performance: A Review. Big Data 2019, 7, 221–248. [Google Scholar] [CrossRef] [PubMed]
- Campillo-Gimenez, B.; Bayat, S.; Cuggia, M. Coupling K-nearest neighbors with logistic regression in case-based reasoning. Stud. Health Technol. Inform. 2012, 180, 275–279. [Google Scholar]
- Singh, H.; Sharma, V.; Singh, D. Comparative analysis of proficiencies of various textures and geometric features in breast mass classification using k-nearest neighbor. Vis. Comput. Ind. Biomed. Art 2022, 5, 3. [Google Scholar] [CrossRef] [PubMed]
- Short, R.; Fukunaga, K. The optimal distance measure for nearest neighbor classification. IEEE Trans. Inf. Theory 1981, 27, 622–627. [Google Scholar] [CrossRef]
- Chen, L.; Wang, C.; Chen, J.; Xiang, Z.; Hu, X. Voice Disorder Identification by using Hilbert-Huang Transform (HHT) and K Nearest Neighbor (KNN). J. Voice 2021, 35, 932.e1–932.e11. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.H.; Huang, W.T.; Tan, T.H.; Chang, C.C.; Chang, Y.J. Using K-Nearest Neighbor Classification to Diagnose Abnormal Lung Sounds. Sensors 2015, 15, 13132–13158. [Google Scholar] [CrossRef] [PubMed]
- Hatem, M.Q. Skin lesion classification system using a K-nearest neighbor algorithm. Vis. Comput. Ind. Biomed. Art 2022, 5, 7. [Google Scholar] [CrossRef] [PubMed]
- Enriko, I.K.A.; Suryanegara, M.; Gunawan, D. Heart disease prediction system using k-Nearest neighbor algorithm with simplified patient’s health parameters. J. Telecommun. Electron. Comput. Electron. Comput. Eng. 2016, 8, 59–65. Available online: https://scholar.ui.ac.id/en/publications/heart-disease-prediction-system-using-k-nearest-neighbor-algorith (accessed on 31 May 2022).
- Brito, T.P.; Guimaraes, A.C.; Oshima, M.M.; Chone, C.T. Mediastinitis: Parotid abscess complication. Braz. J. Otorhinolaryngol. 2014, 80, 268–269. [Google Scholar] [CrossRef]
- Ho, C.Y.; Wang, Y.C.; Chin, S.C.; Chen, S.L. Factors Creating a Need for Repeated Drainage of Deep Neck Infections. Diagnostics 2022, 12, 940. [Google Scholar] [CrossRef]
- Chen, S.L.; Ho, C.Y.; Chin, S.C.; Wang, Y.C. Factors affecting perforation of the esophagus in patients with deep neck infection. BMC Infect. Dis. 2022, 22, 501. [Google Scholar] [CrossRef]
- Wang, L.F.; Kuo, W.R.; Tsai, S.M.; Huang, K.J. Characterizations of life-threatening deep cervical space infections: A review of one hundred ninety-six cases. Am. J. Otolaryngol. 2003, 24, 111–117. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, I.G.; Weber, M.B.; Bonamigo, R.R. History of dermatology: The study of skin diseases over the centuries. An. Bras. Dermatol. 2021, 96, 332–345. [Google Scholar] [CrossRef] [PubMed]
- Lotsch, J.; Sipila, R.; Tasmuth, T.; Kringel, D.; Estlander, A.M.; Meretoja, T.; Kalso, E.; Ultsch, A. Machine-learning-derived classifier predicts absence of persistent pain after breast cancer surgery with high accuracy. Breast Cancer Res. Treat. 2018, 171, 399–411. [Google Scholar] [CrossRef]
- Kleiman, R.S.; LaRose, E.R.; Badger, J.C.; Page, D.; Caldwell, M.D.; Clay, J.A.; Peissig, P.L. Using Machine Learning Algorithms to Predict Risk for Development of Calciphylaxis in Patients with Chronic Kidney Disease. AMIA Jt. Summits Transl. Sci. Proc. 2018, 2017, 139–146. [Google Scholar] [PubMed]
- Hsieh, C.H.; Lu, R.H.; Lee, N.H.; Chiu, W.T.; Hsu, M.H.; Li, Y.C. Novel solutions for an old disease: Diagnosis of acute appendicitis with random forest, support vector machines, and artificial neural networks. Surgery 2011, 149, 87–93. [Google Scholar] [CrossRef]
- Chan, S.; Reddy, V.; Myers, B.; Thibodeaux, Q.; Brownstone, N.; Liao, W. Machine Learning in Dermatology: Current Applications, Opportunities, and Limitations. Dermatol. Ther. 2020, 10, 365–386. [Google Scholar] [CrossRef]
- Howard, F.M.; Kochanny, S.; Koshy, M.; Spiotto, M.; Pearson, A.T. Machine Learning-Guided Adjuvant Treatment of Head and Neck Cancer. JAMA Netw. Open 2020, 3, e2025881. [Google Scholar] [CrossRef]
- Angus, D.C. Fusing Randomized Trials with Big Data: The Key to Self-learning Health Care Systems? JAMA 2015, 314, 767–768. [Google Scholar] [CrossRef]
- Cruz, J.A.; Wishart, D.S. Applications of machine learning in cancer prediction and prognosis. Cancer Inform. 2007, 2, 59–77. [Google Scholar] [CrossRef] [PubMed]
- Tan, A.C.; Gilbert, D. Ensemble machine learning on gene expression data for cancer classification. Appl. Bioinform. 2003, 2, S75–S83. [Google Scholar]
- Rajkomar, A.; Oren, E.; Chen, K.; Dai, A.M.; Hajaj, N.; Hardt, M.; Liu, P.J.; Liu, X.; Marcus, J.; Sun, M.; et al. Scalable and accurate deep learning with electronic health records. NPJ Digit. Med. 2018, 1, 18. [Google Scholar] [CrossRef]
- Elfiky, A.A.; Pany, M.J.; Parikh, R.B.; Obermeyer, Z. Development and Application of a Machine Learning Approach to Assess Short-term Mortality Risk Among Patients With Cancer Starting Chemotherapy. JAMA Netw. Open 2018, 1, e180926. [Google Scholar] [CrossRef]
- Peiffer-Smadja, N.; Rawson, T.M.; Ahmad, R.; Buchard, A.; Georgiou, P.; Lescure, F.X.; Birgand, G.; Holmes, A.H. Machine learning for clinical decision support in infectious diseases: A narrative review of current applications. Clin. Microbiol. Infect. 2020, 26, 584–595. [Google Scholar] [CrossRef]
- Yu, Z.; Chen, H.; Liuxs, J.; You, J.; Leung, H.; Han, G. Hybrid k-Nearest Neighbor Classifier. IEEE Trans. Cybern. 2016, 46, 1263–1275. [Google Scholar] [CrossRef]
- Bhatia, N.; Vandana. Survey of Nearest Neighbor Techniques. Int. J. Comput. Sci. Inf. Secur. 2010, 8, 302–305. [Google Scholar] [CrossRef]
- Wu, X.; Kumar, V.; Quinlan, J.R.; Ghosh, J.; Yang, Q.; Motoda, H.; McLachlan, G.J.; Ng, A.; Liu, B.; Yu, P.S.; et al. Top 10 algorithms in data mining. Knowl. Inf. Syst. Vol. 2007, 14, 1–37. [Google Scholar] [CrossRef]
- Przybyla-Kasperek, M.; Marfo, K.F. Neural Network Used for the Fusion of Predictions Obtained by the K-Nearest Neighbors Algorithm Based on Independent Data Sources. Entropy 2021, 23, 1568. [Google Scholar] [CrossRef] [PubMed]
- Przybyła-Kasperek, M. Three Conflict Methods in Multiple Classifiers that Use Dispersed Knowledge. Int. J. Inf. Technol. Decis. Mak. 2019, 18, 555–599. [Google Scholar] [CrossRef]
- Ho, C.Y.; Chin, S.C.; Wang, Y.C.; Chen, S.L. Factors affecting patients with concurrent deep neck infection and aspiration pneumonia. Am. J. Otolaryngol. 2022, 43, 103463. [Google Scholar] [CrossRef]
- Chen, M.K.; Wen, Y.S.; Chang, C.C.; Lee, H.S.; Huang, M.T.; Hsiao, H.C. Deep neck infections in diabetic patients. Am. J. Otolaryngol. 2000, 21, 169–173. [Google Scholar] [CrossRef]
- Chen, S.L.; Chin, S.C.; Wang, Y.C.; Ho, C.Y. Factors Affecting Patients with Concurrent Deep Neck Infection and Lemierre’s Syndrome. Diagnostics 2022, 12, 928. [Google Scholar] [CrossRef]
- Chowdhury, N.I.; Smith, T.L.; Chandra, R.K.; Turner, J.H. Automated classification of osteomeatal complex inflammation on computed tomography using convolutional neural networks. Int. Forum Allergy Rhinol. 2019, 9, 46–52. [Google Scholar] [CrossRef]
- Benitez, J.M.; Castro, J.L.; Requena, I. Are artificial neural networks black boxes? IEEE Trans. Neural Netw. 1997, 8, 1156–1164. [Google Scholar] [CrossRef] [PubMed]
- Tickle, A.B.; Andrews, R.; Golea, M.; Diederich, J. The truth will come to light: Directions and challenges in extracting the knowledge embedded within trained artificial neural networks. IEEE Trans. Neural Netw. 1998, 9, 1057–1068. [Google Scholar] [CrossRef]


| Characteristics | N (%) |
|---|---|
| Gender | 392 (100.0) |
| Male | 261 (66.58) |
| Female | 131 (33.42) |
| Age, years ± SD | 51.36 ± 18.74 |
| Chief complaint period, days ± SD | 5.04 ± 4.49 |
| WBC, uL ± SD | 15,007.39 ± 5801.19 |
| CRP, mg/L ± SD | 156.94 ± 99.61 |
| Blood sugar, mg/dL ± SD | 142.66 ± 72.46 |
| Diabetes mellitus | 147 (37.50) |
| Deep neck infection space involved | |
| Single space | 108 (27.55) |
| Double spaces | 151 (38.52) |
| Multiple spaces, ≥3 | 133 (33.93) |
| Mediastinitis | 20 (5.10) |
| Maximum diameter of abscess, cm ± SD | 6.36 ± 3.08 |
| Nearest distance from abscess to inlet of trachea, cm ± SD | 1.41 ± 1.35 |
| Tracheostomy performance | 50 (12.75) |
| Characteristics | Training Group; N (%) | Test Group; N (%) | p Value |
|---|---|---|---|
| Gender | 317 (100.0) | 75 (100.0) | |
| Male | 215 (67.82) | 46 (61.33) | 0.340 |
| Female | 102 (32.18) | 29 (38.67) | |
| Age, years ± SD | 50.88 ± 18.89 | 53.40 ± 18.06 | 0.364 |
| Chief complaint period, days ± SD | 5.20 ± 4.79 | 4.34 ± 2.79 | 0.455 |
| WBC, μL ± SD | 14,824.91 ± 5732.75 | 15,778.66 ± 6060.84 | 0.240 |
| CRP, mg/L ± SD | 155.08 ± 98.23 | 164.81 ± 105.52 | 0.511 |
| Blood sugar, mg/dL ± SD | 140.51 ± 70.13 | 151.77 ± 81.46 | 0.080 |
| Diabetes mellitus | 0.598 | ||
| Yes | 121 (38.17) | 26 (34.66) | |
| No | 196 (61.83) | 49 (65.34) | |
| Deep neck infection space involved | |||
| Single space | 92 (29.02) | 16 (21.33) | 0.198 |
| Double spaces | 120 (37.85) | 31 (41.33) | 0.599 |
| Multiple spaces, ≥3 | 105 (33.13) | 28 (37.34) | 0.499 |
| Mediastinitis | 0.557 | ||
| Yes | 15 (4.73) | 5 (6.66) | |
| No | 302 (95.27) | 70 (93.34) | |
| Maximum diameter of abscess, cm ± SD | 6.23 ± 2.91 | 6.92 ± 3.71 | 0.293 |
| Nearest distance from abscess to inlet of trachea, cm ± SD | 1.49 ± 1.44 | 1.03 ± 0.79 | 0.169 |
| Tracheostomy performance | 0.700 | ||
| Yes | 42 (13.24) | 8 (10.66) | |
| No | 275 (86.76) | 67 (89.34) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, S.-L.; Chin, S.-C.; Ho, C.-Y. Deep Learning Artificial Intelligence to Predict the Need for Tracheostomy in Patients of Deep Neck Infection Based on Clinical and Computed Tomography Findings—Preliminary Data and a Pilot Study. Diagnostics 2022, 12, 1943. https://doi.org/10.3390/diagnostics12081943
Chen S-L, Chin S-C, Ho C-Y. Deep Learning Artificial Intelligence to Predict the Need for Tracheostomy in Patients of Deep Neck Infection Based on Clinical and Computed Tomography Findings—Preliminary Data and a Pilot Study. Diagnostics. 2022; 12(8):1943. https://doi.org/10.3390/diagnostics12081943
Chicago/Turabian StyleChen, Shih-Lung, Shy-Chyi Chin, and Chia-Ying Ho. 2022. "Deep Learning Artificial Intelligence to Predict the Need for Tracheostomy in Patients of Deep Neck Infection Based on Clinical and Computed Tomography Findings—Preliminary Data and a Pilot Study" Diagnostics 12, no. 8: 1943. https://doi.org/10.3390/diagnostics12081943
APA StyleChen, S.-L., Chin, S.-C., & Ho, C.-Y. (2022). Deep Learning Artificial Intelligence to Predict the Need for Tracheostomy in Patients of Deep Neck Infection Based on Clinical and Computed Tomography Findings—Preliminary Data and a Pilot Study. Diagnostics, 12(8), 1943. https://doi.org/10.3390/diagnostics12081943






