An Experimental and Virtual Approach to Hip Revision Prostheses
Abstract
:1. Introduction
- -
- The models can be used in various real or virtual tests and experiments;
- -
- The virtual models of the revision components can be attached to the bone components, and various “in vitro” tests can be obtained in the virtual environment;
- -
- The prosthetic components are generally parameterized in virtual environments, so they can be adapted to different anthropological dimensions.
2. Material and Method
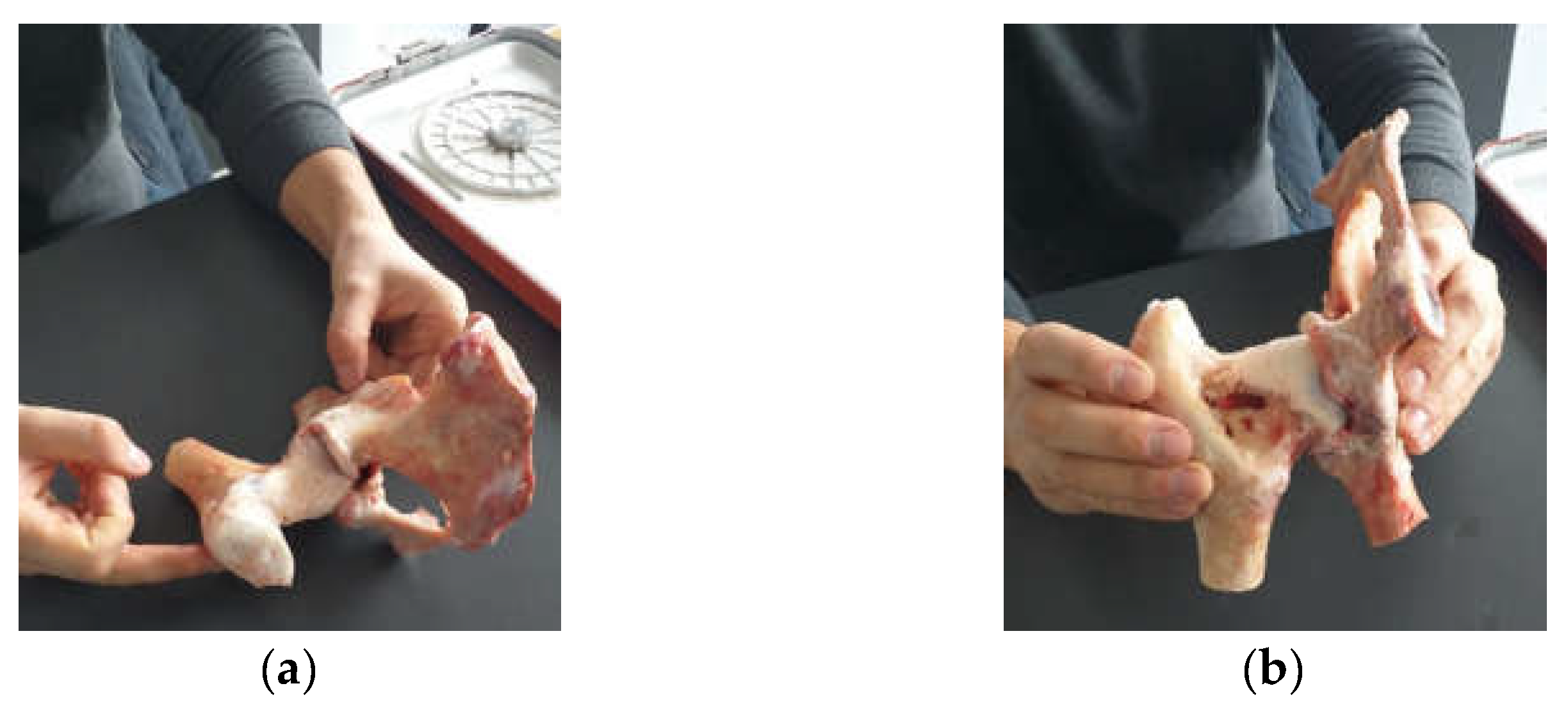
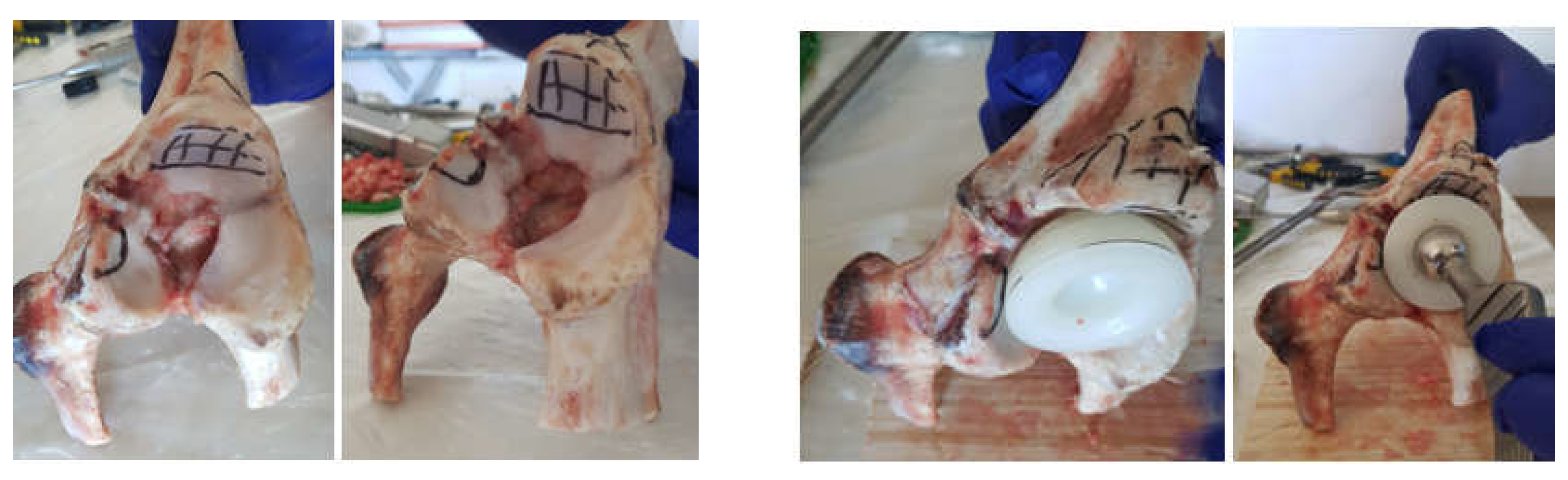
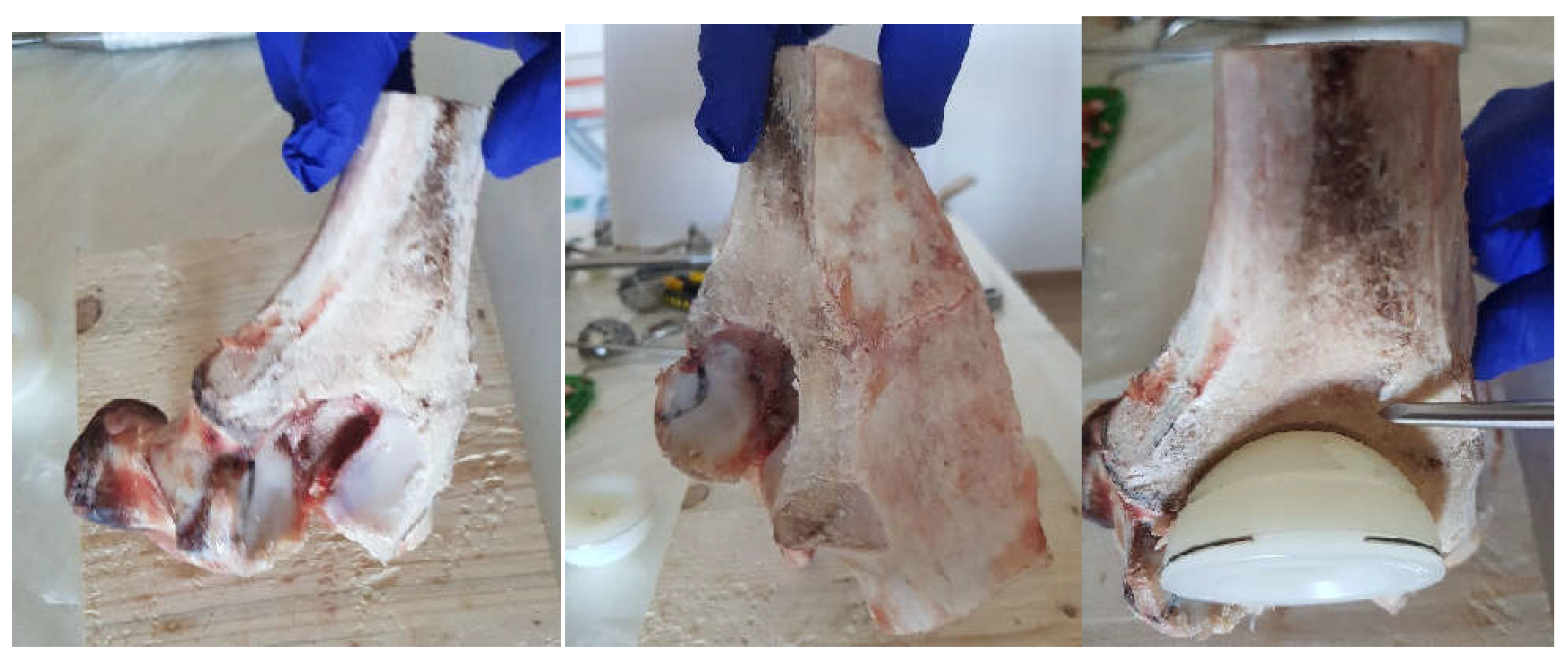
2.1. Material and Method for Performing Orthopedic Hip Revisions
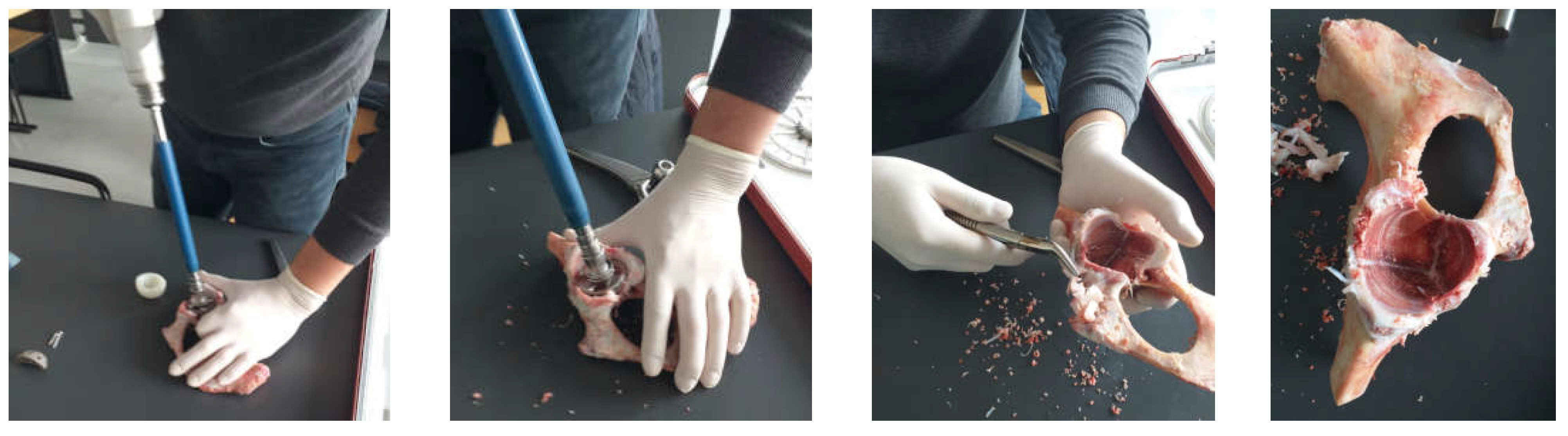
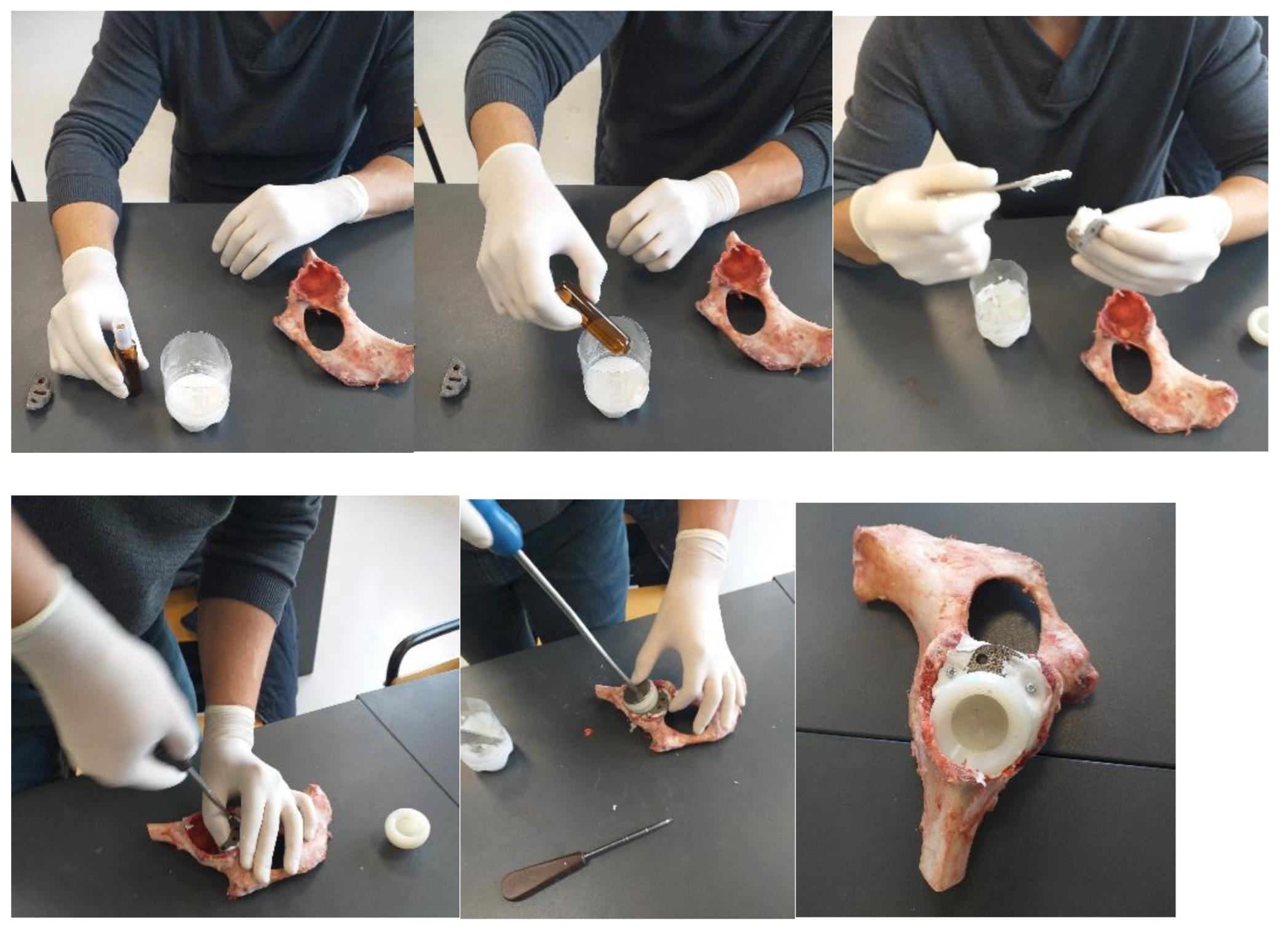
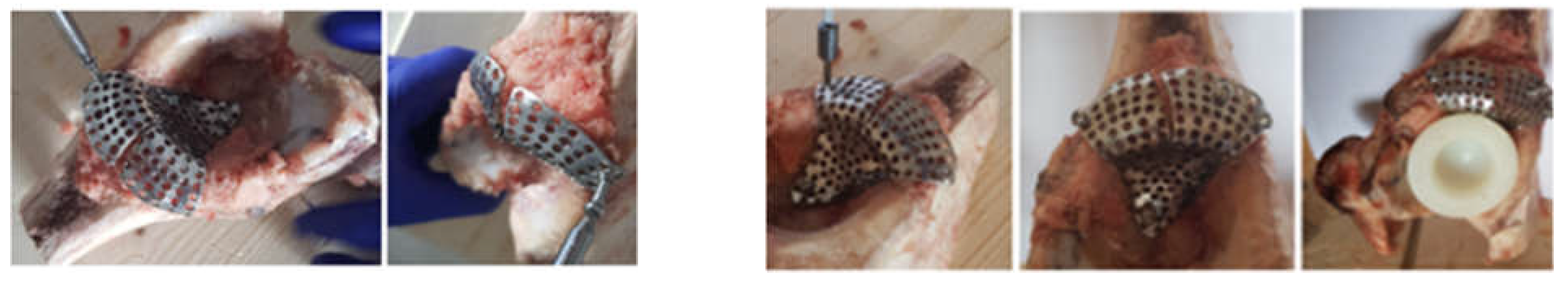
2.2. Material and Method for Revision Orthopaedical Prosthesis with Titanium Augment
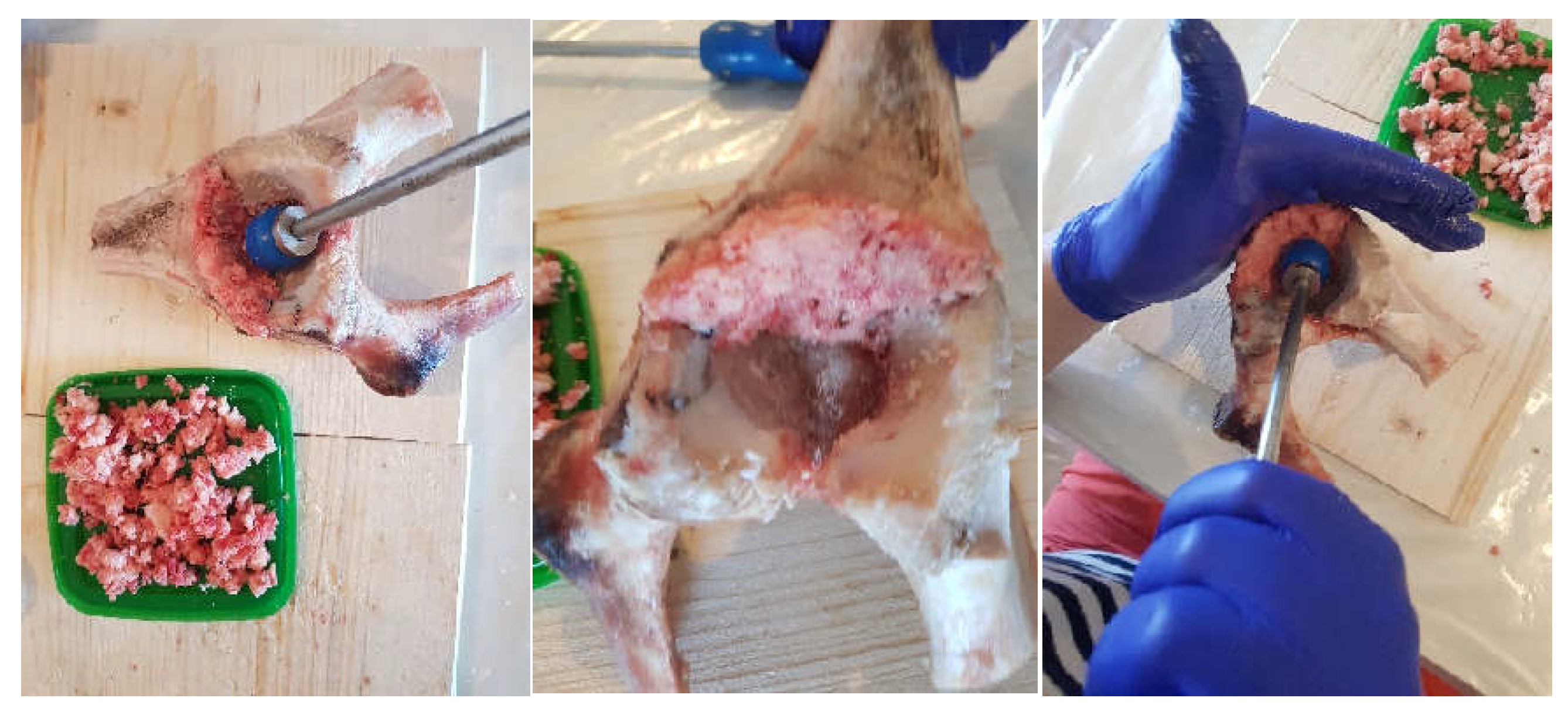
2.3. Material and Method Fororthopaedical Prosthesis with Morcellated Bone Graft and Reconstructive Mesh
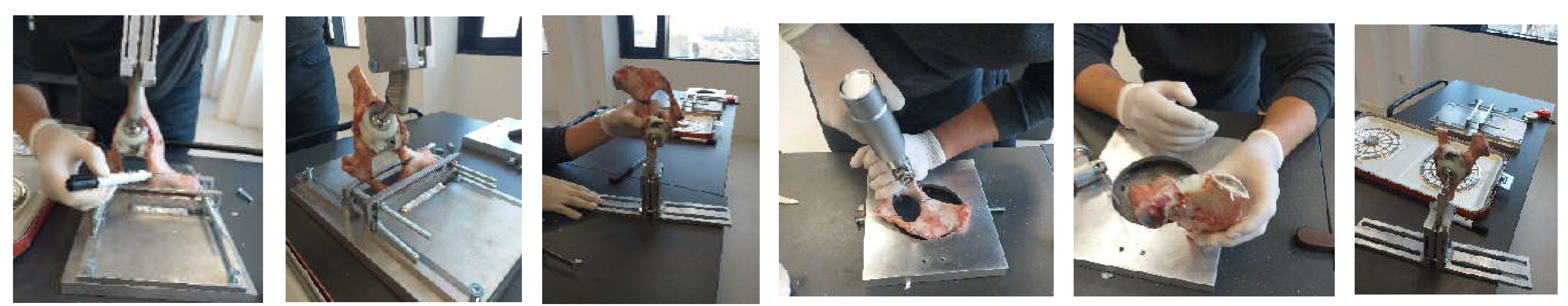
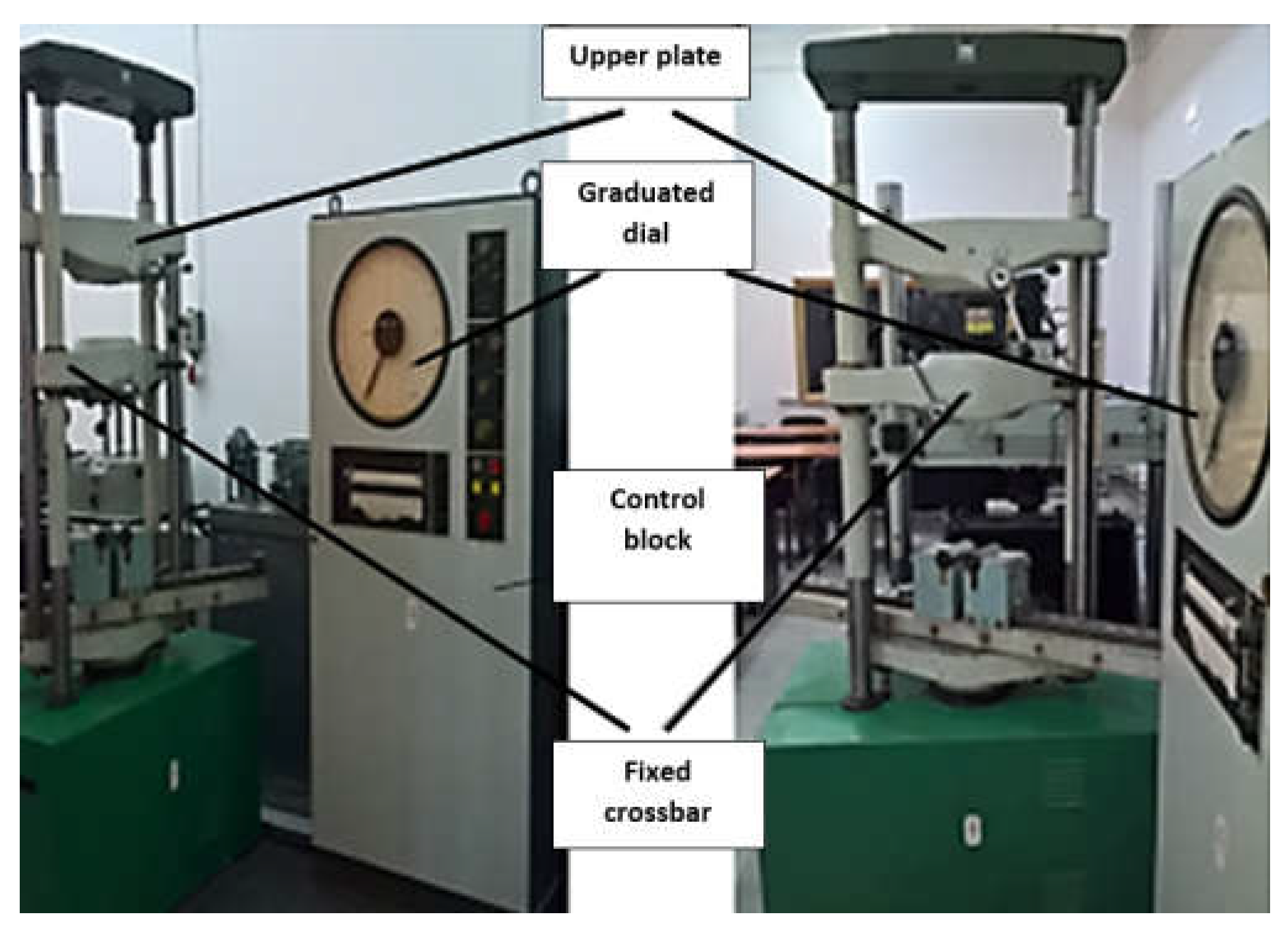
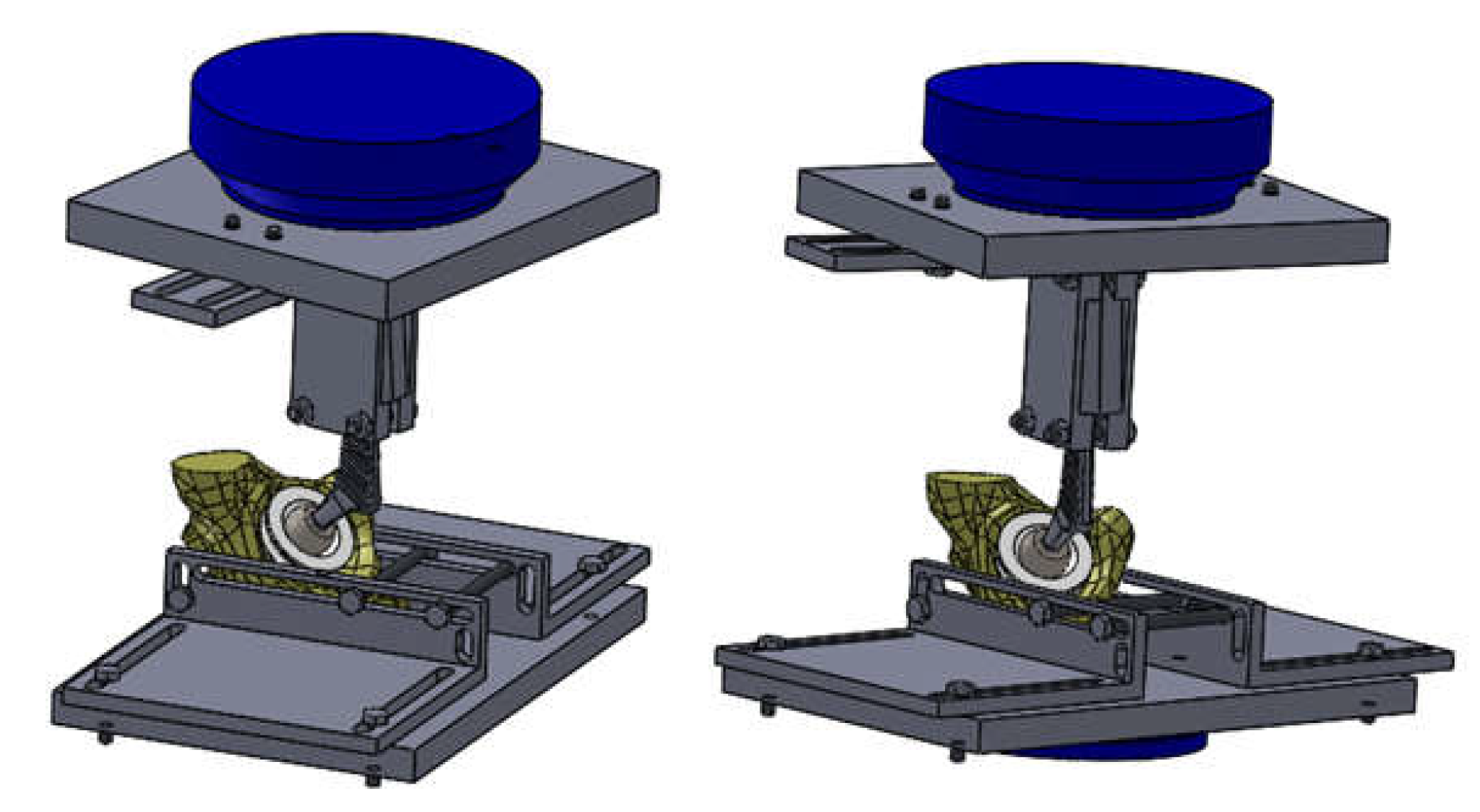
2.4. Material and Method for Experimental Testing of the Orthopedic Hip Joint Revision Prosthesis
- -
- Methods and techniques of experimental research;
- -
- Methods and principles of materials strength;
- -
- Methods of rigid solids theory and theory of material failure.
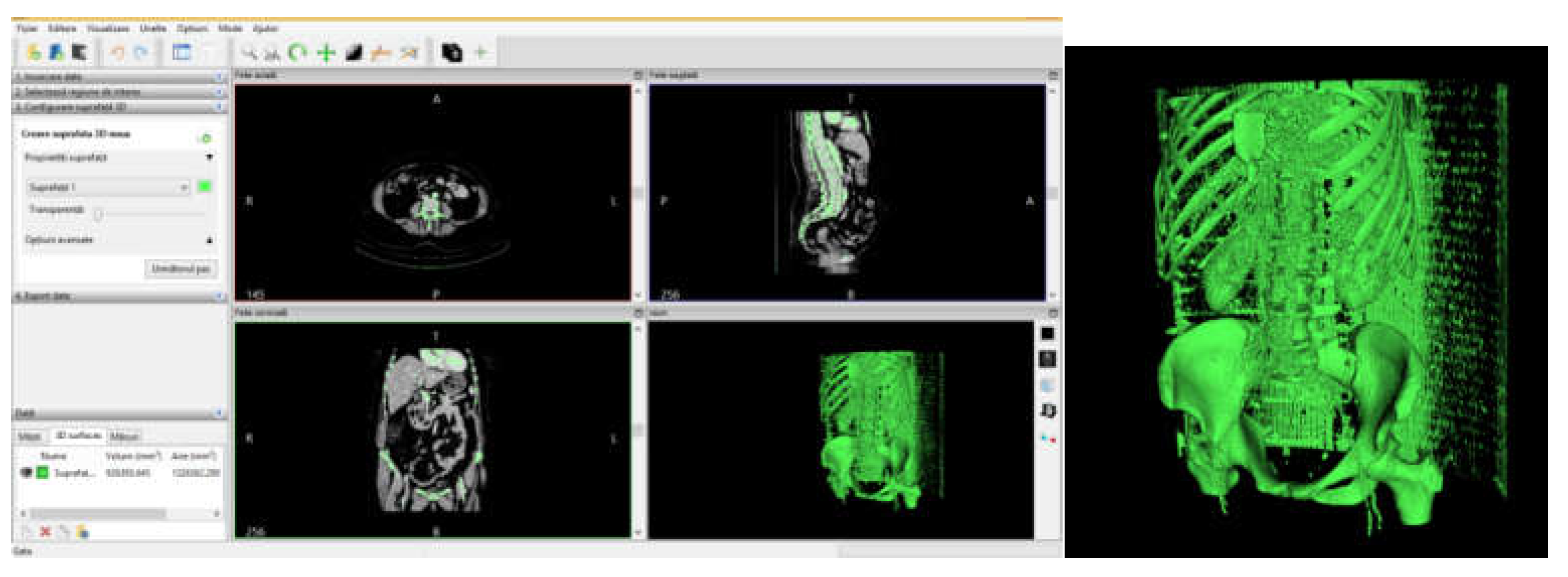
2.5. Material and Method for Virtual Testing of the Intact and Prosthetic Hip Joint under Normal Human Gait Loading
- -
- The normally intact joint of the human hip;
- -
- Joint protected with titanium augment;
- -
- Joint protected with morcellated graft and reconstructive mesh.
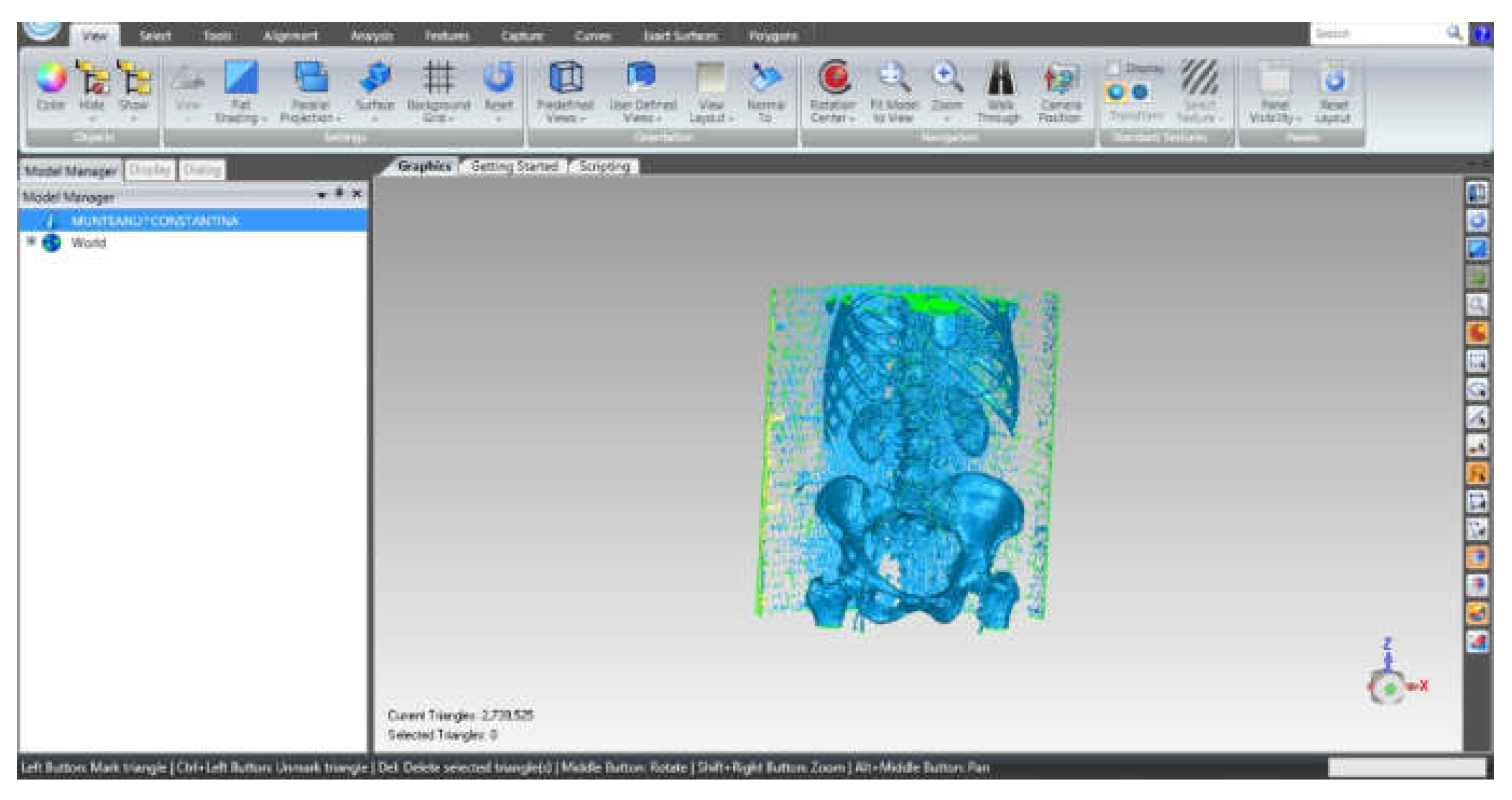
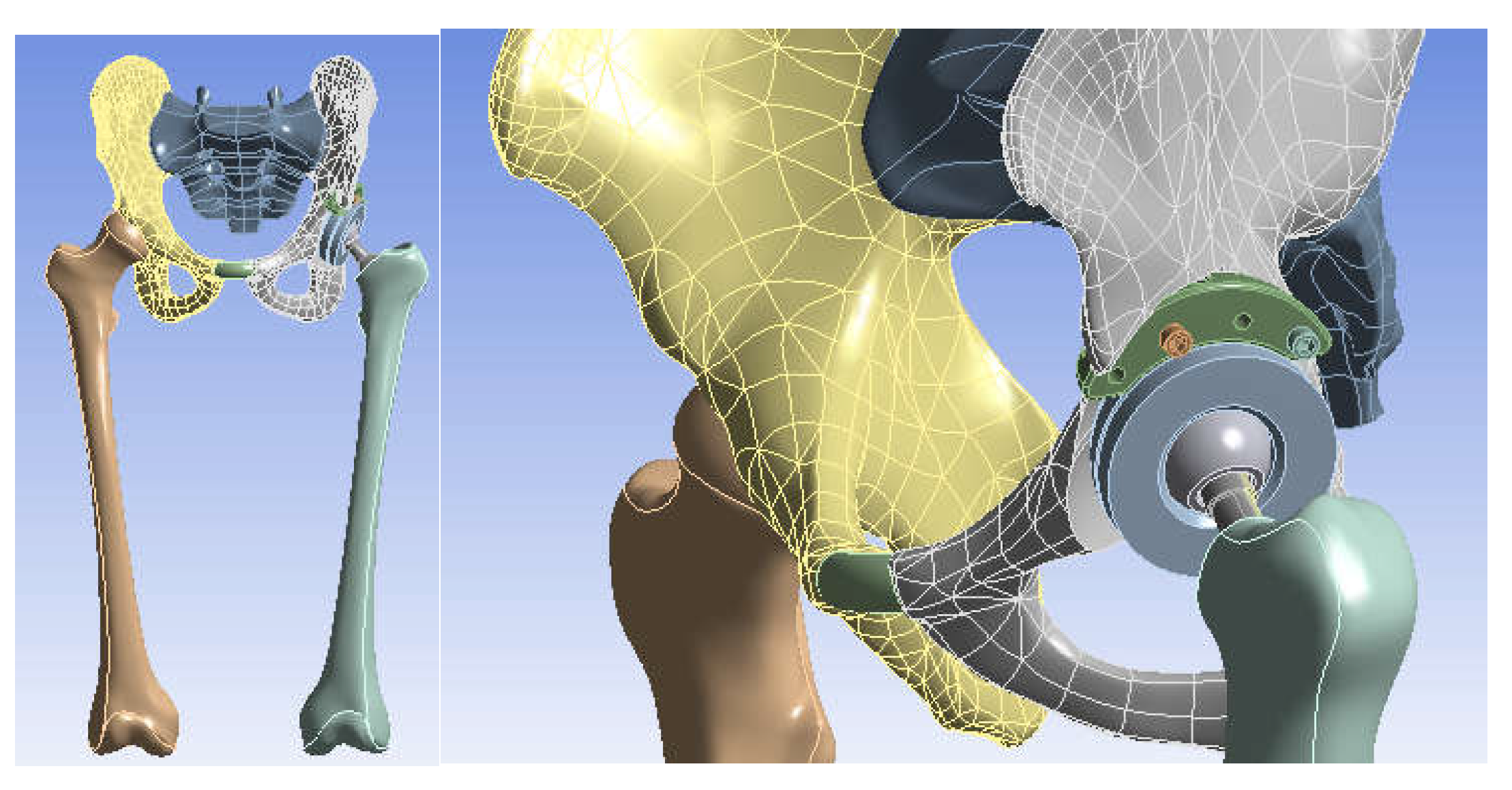
2.6. Material and Method for Virtual Testing of Normal Hip Joint and Orthopedic Hip Joint Revision Prosthesis
- SolidWorks, which is a computer-aided design program used in engineering, which allows the generation of multibody models, in this case, for modelling the three systems to be studied using the finite element method;
- Ansys Workbench, which is a finite element analysis program that allows the study of the behaviour of different mechanical or biomechanical systems and that can show displacement, strain, and stress maps that occur in orthopaedical prostheses that have hip revision prostheses.
- Knowing that orthopaedic cement, used in these orthopaedic prostheses, provides stability and fixation of the components, it was not geometrically modelled, and its behaviour was replaced with the use of bonded elements that practically provide cohesion of the elements between themselves [26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41];
- It is known that, during the human gait, the variation of the force occurring in the hip joint varies between values of 0 N and about 2300 N. We have considered that it is sufficient to use an equivalent force that has a linear variation between the values of 800 N (characteristic of the orthostatic position) and 2300 N (maximum value of the force during normal human gait [26,27,28,29,30,31,39,42]);
3. Results
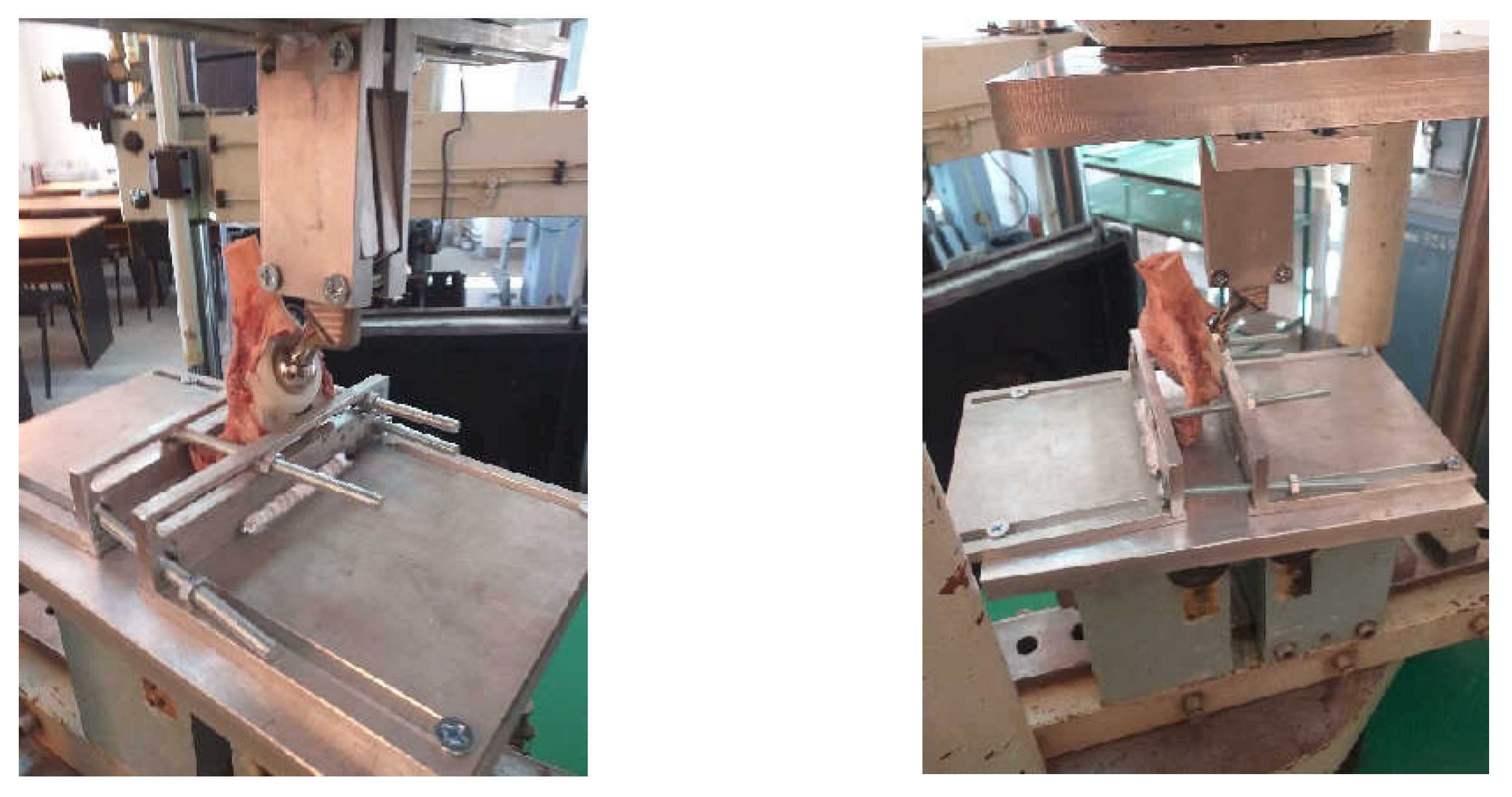
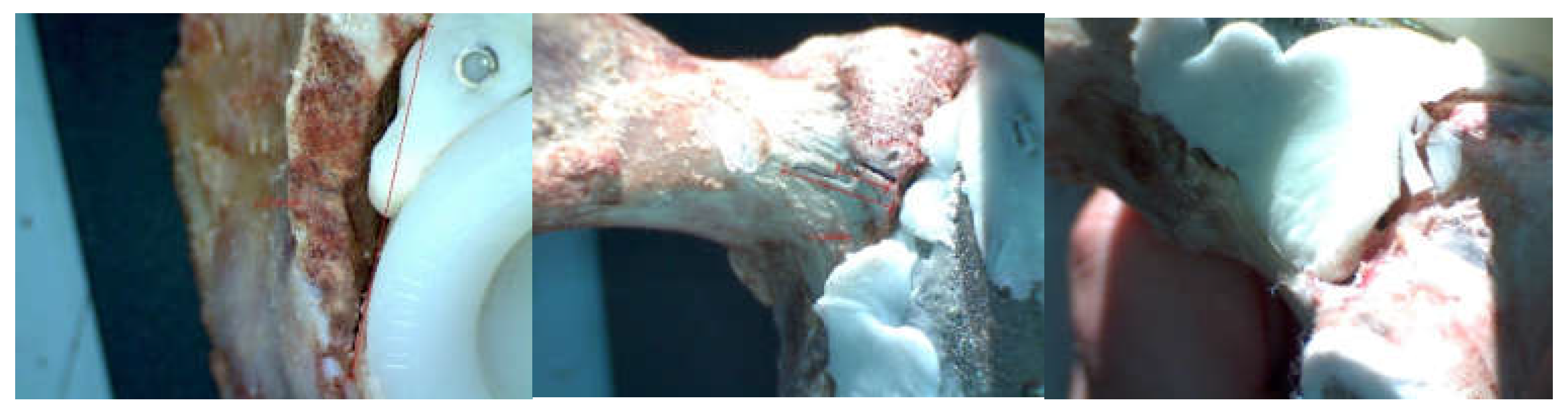
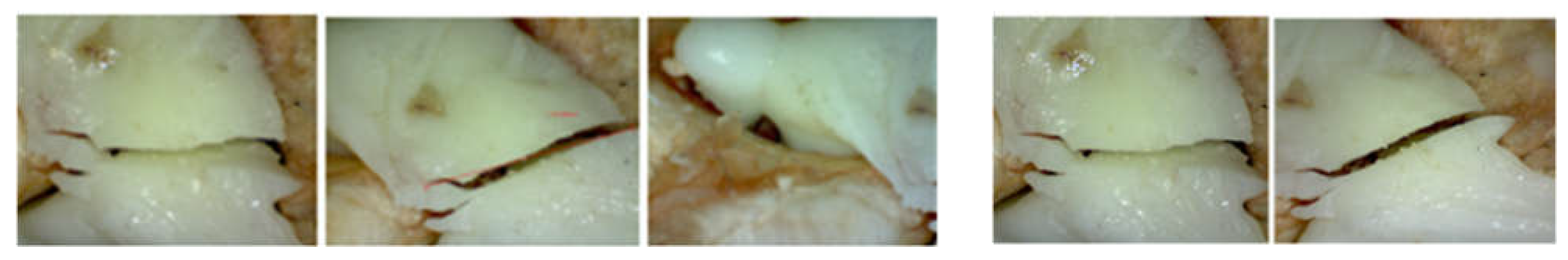
3.1. Experimental Testing of Orthopaedic Revision Prosthesis with Titanium Augment
3.2. Experimental Testing of Orthopaedic Revision Prosthesis with Morcellated Graft and Reconstructive Mesh
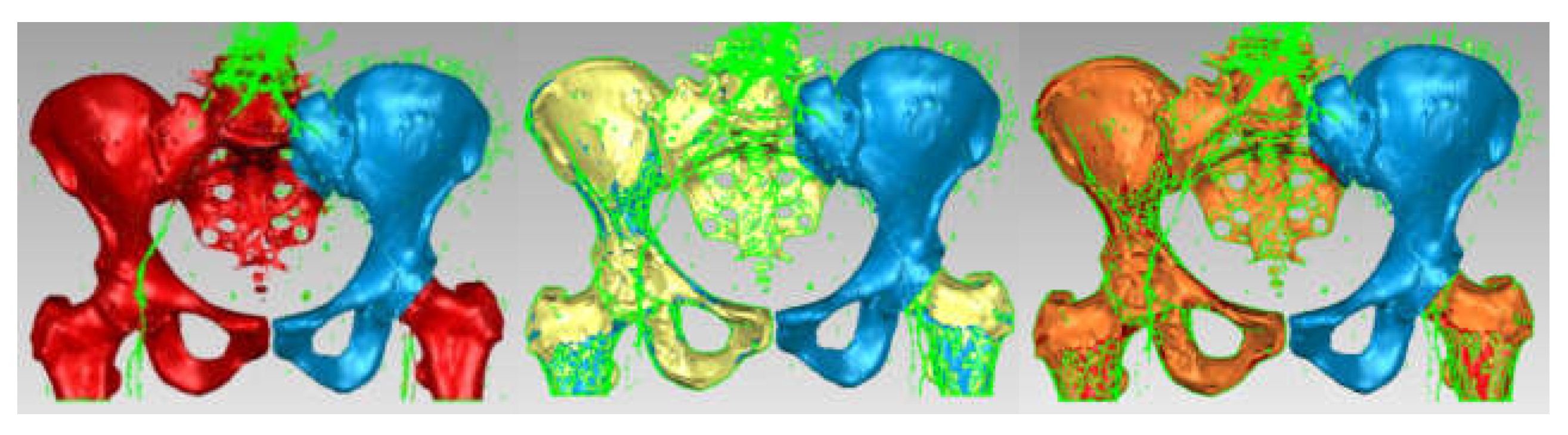
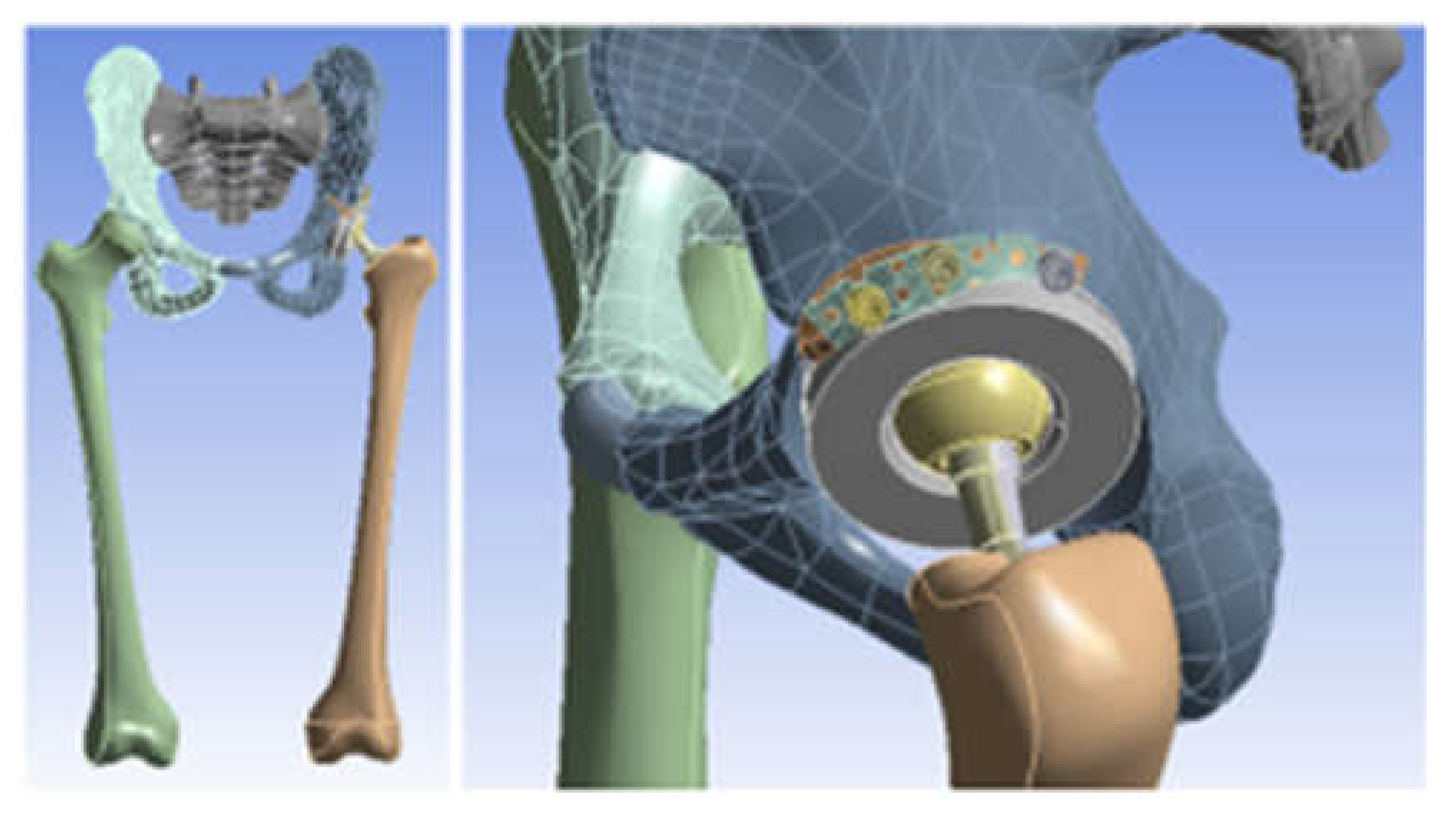
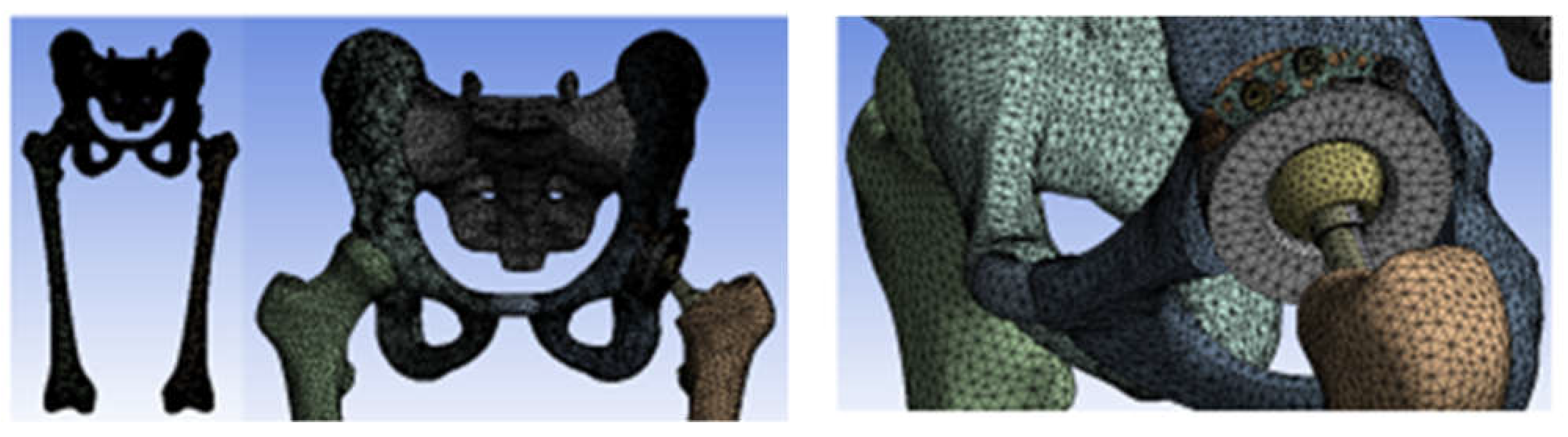
3.3. Models of the Intact and Prosthetic Hip Joint
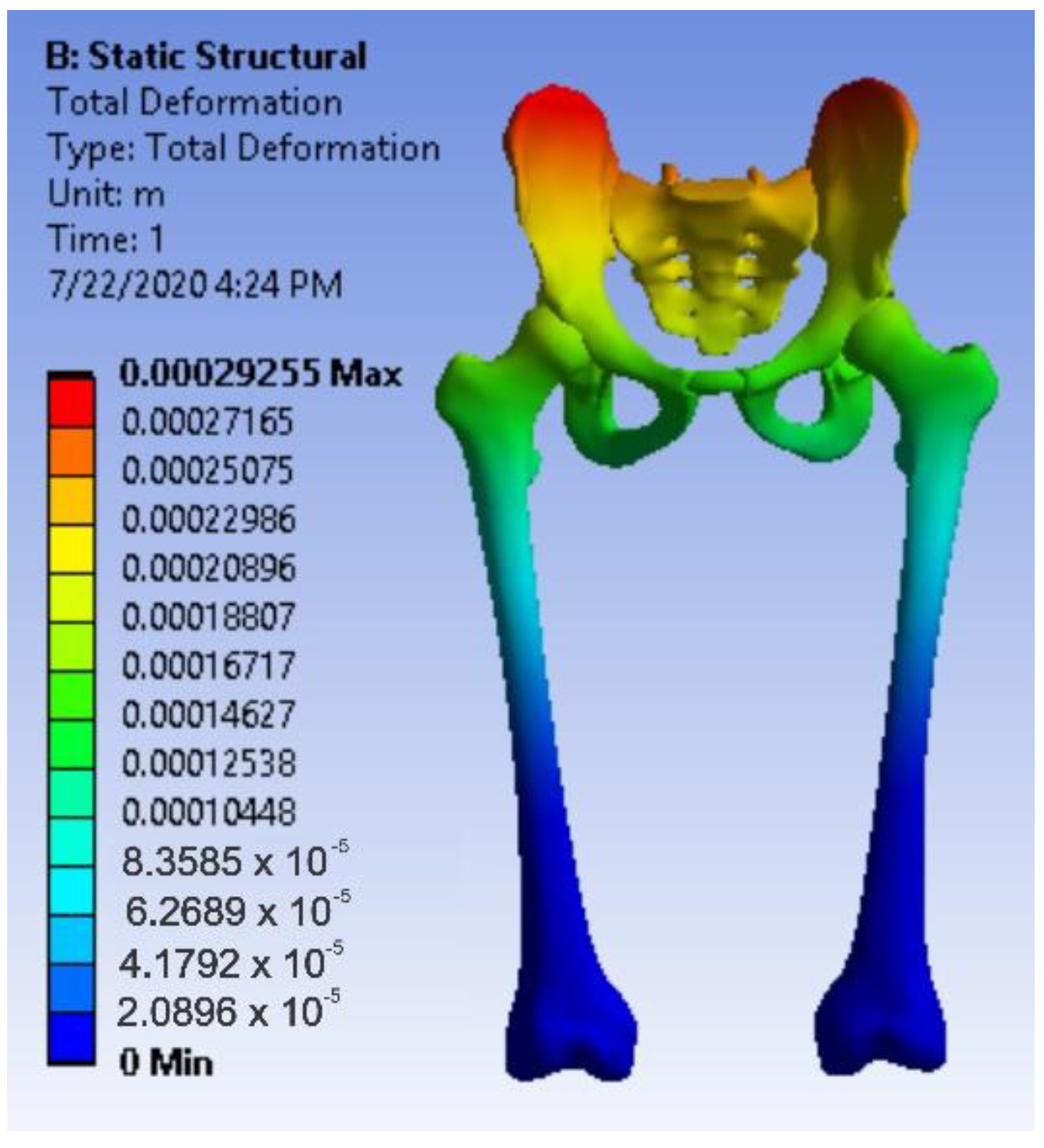
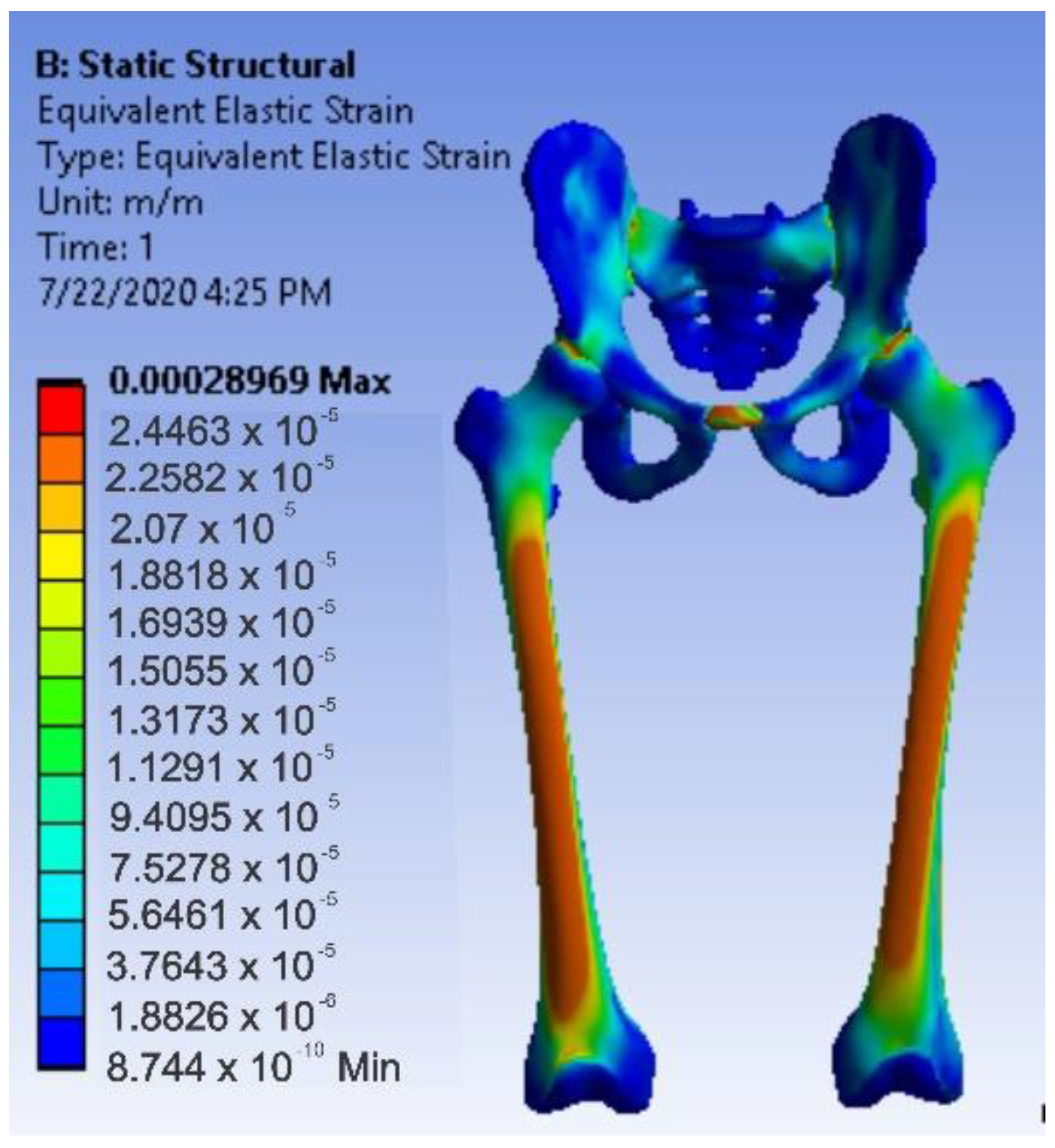
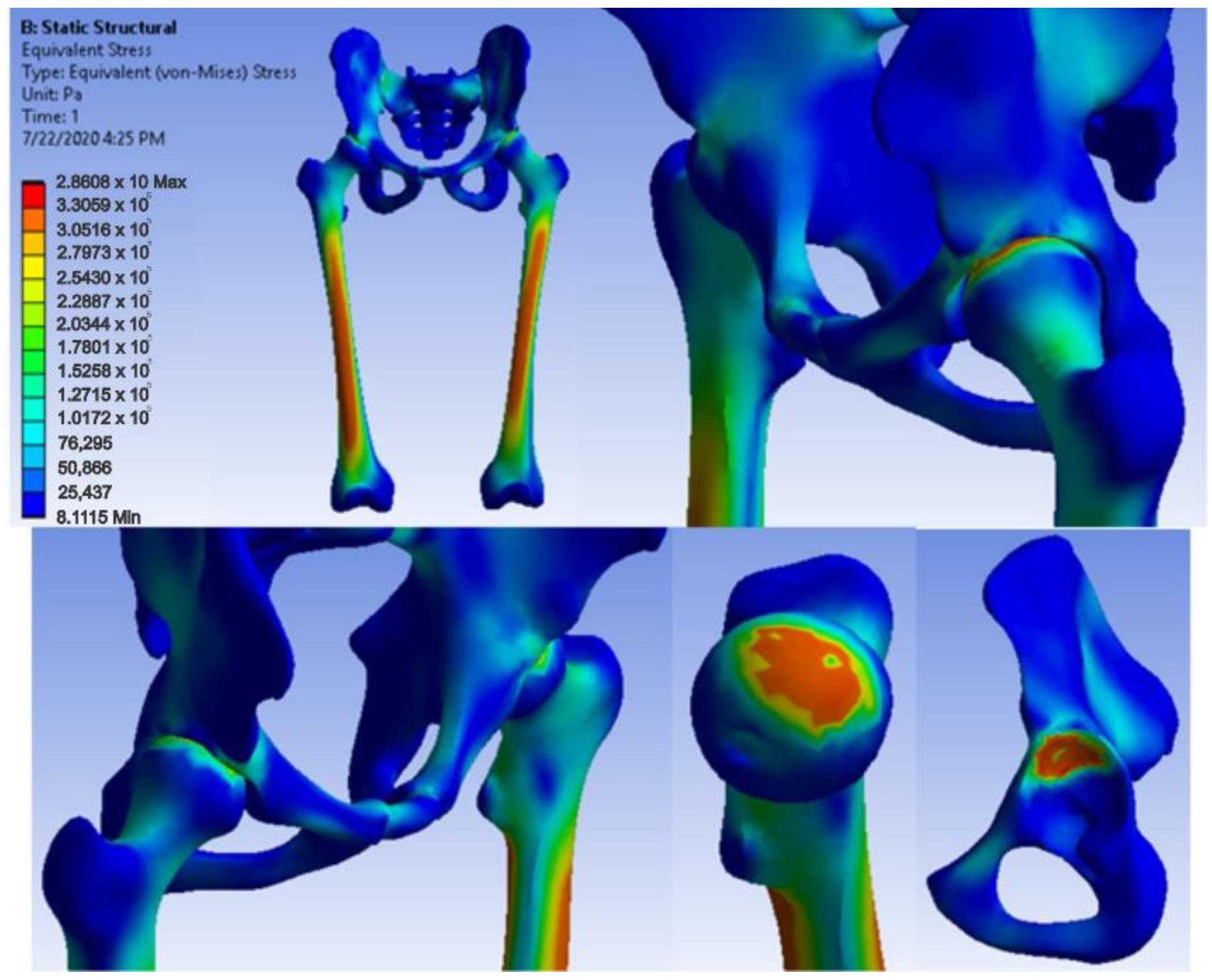
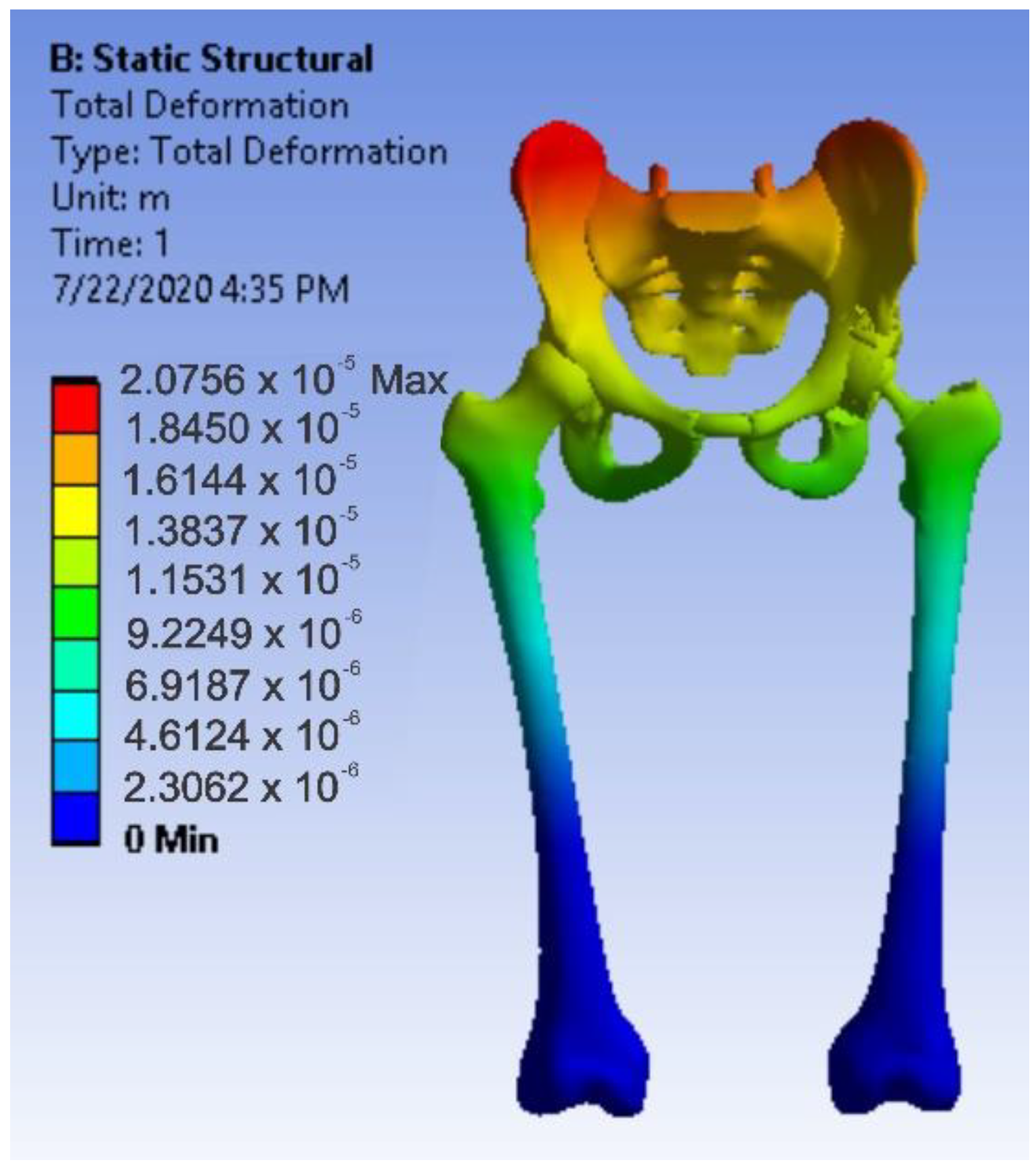
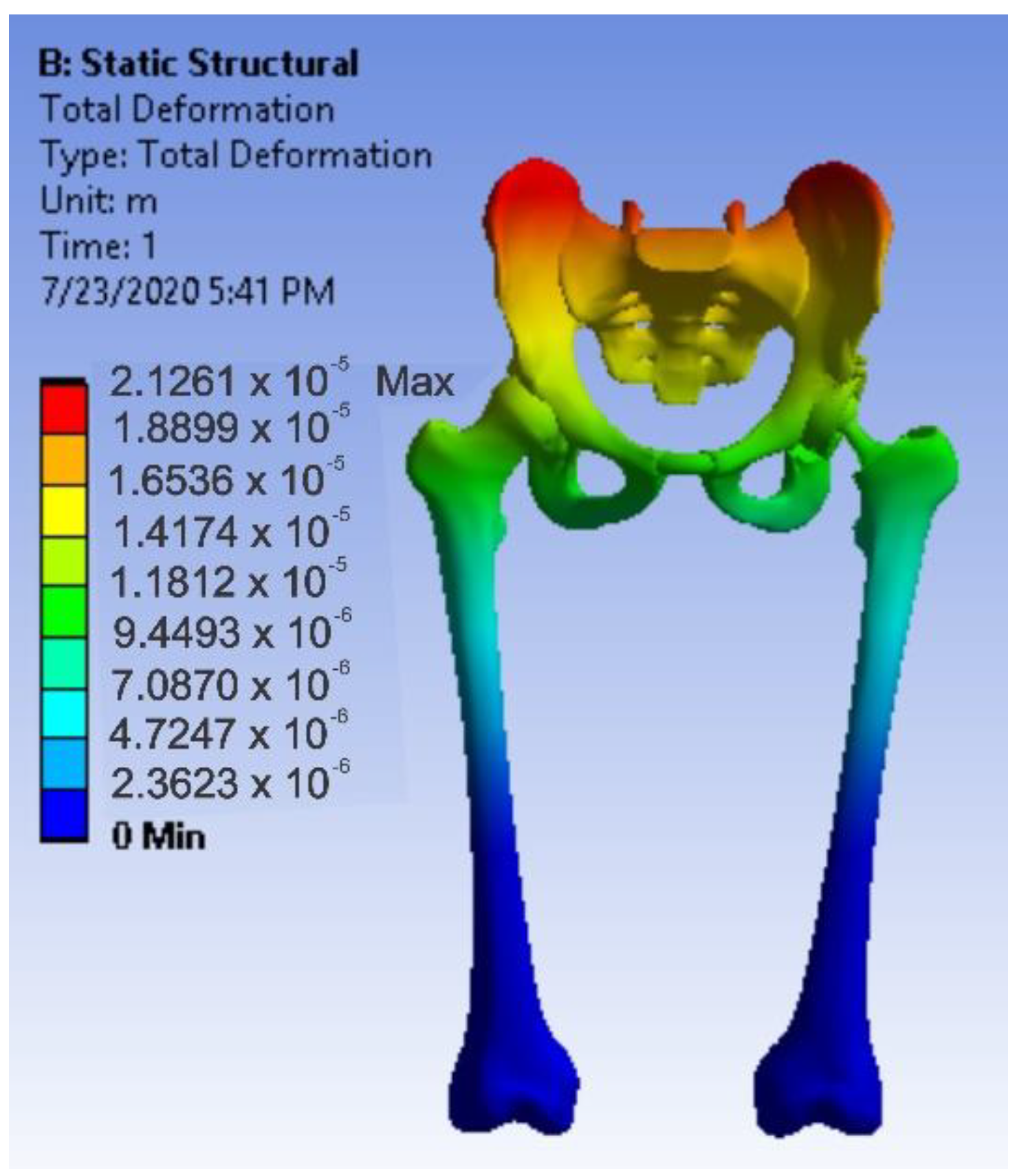
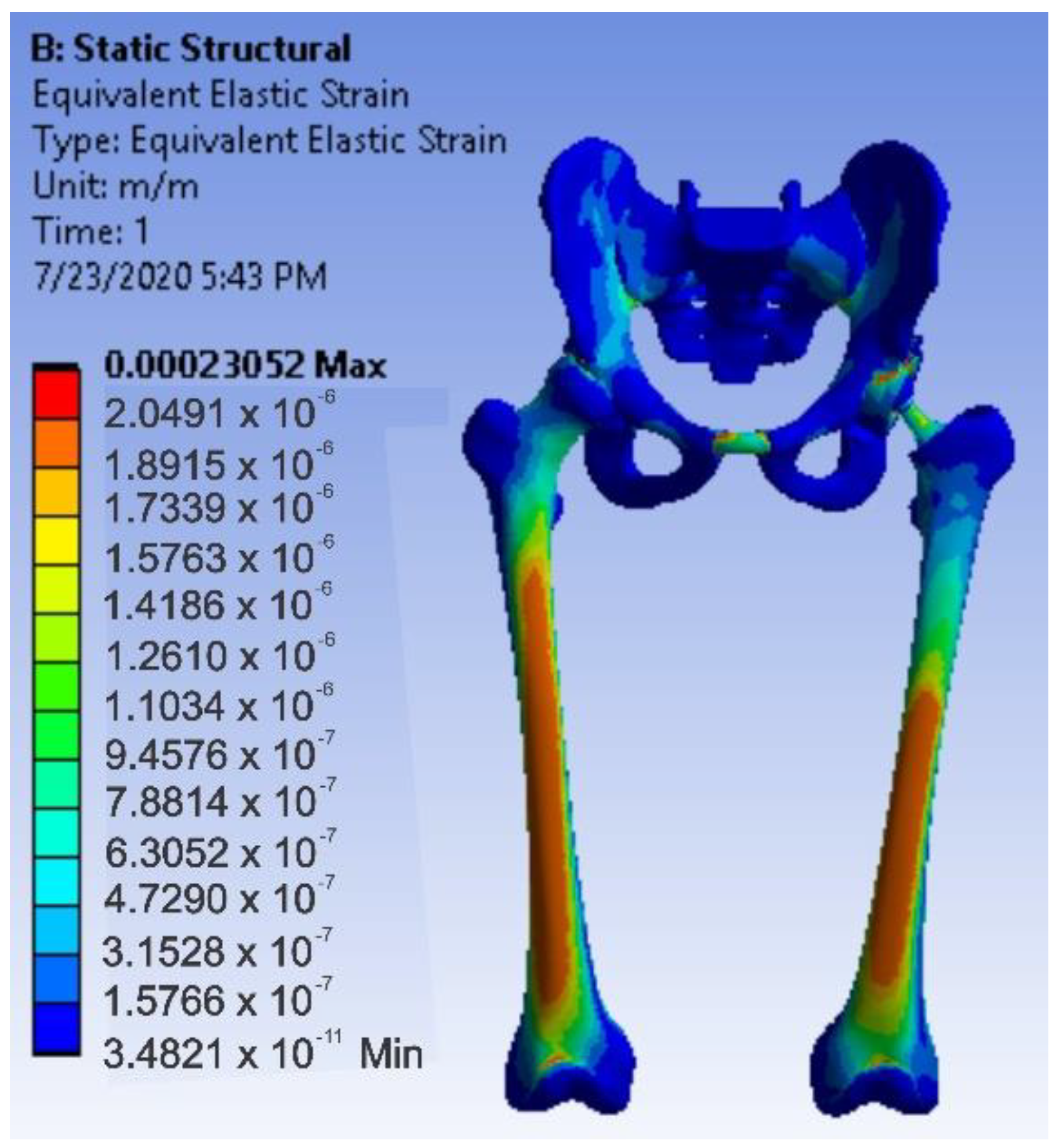
3.4. Virtual Testing of the Normal (Intact) Hip Joint Subjected to Normal Gait Loading
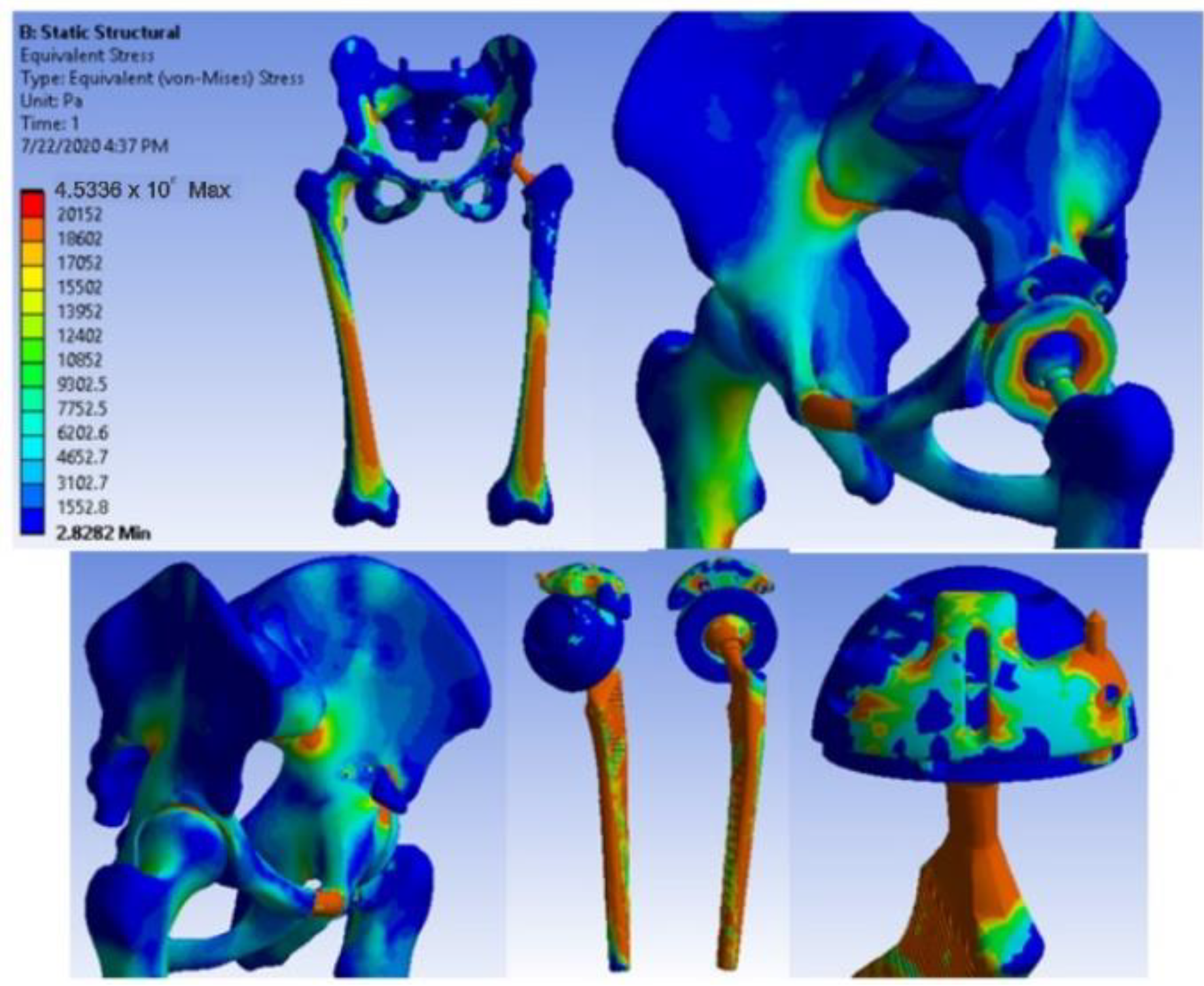
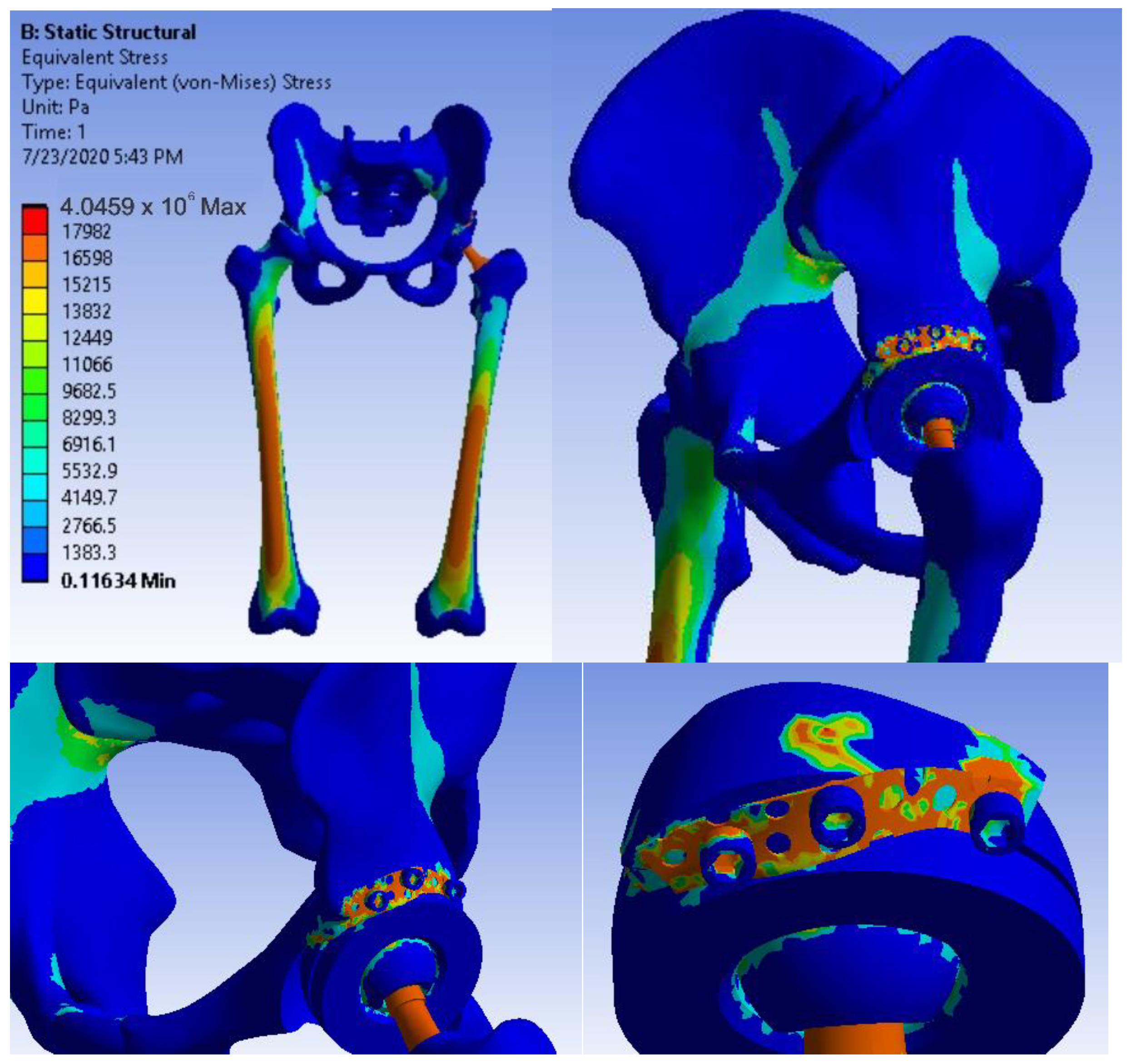
3.5. Virtual Testing of the Hip Joint with Titanium Augment Revision Prosthesis Subjected to Normal Gait Loading
3.6. Virtual Hip Joint Testing with Revision Prosthesis with Morcellated Graft and Reconstructive Mesh Subjected to Normal Gait Loading
4. Discussions
- The components of the prostheses and revision systems are parameterized so that they can be adapted to different anthropological dimensions;
- Different “in vitro” analyses can be obtained by attaching these prosthetic revision systems to the virtual bone components;
- These models can be used in various real or virtual tests and experiments.
- Very complicated biological systems can be modelled and simulated by using CAD and FEM methods;
- The virtual models proposed by this research study have been experimentally validated;
- Finite element method analysis, coupled with virtual re-construction from CT or MRI images and reverse engineering methods, pave the way for the innovation of orthopaedic systems customized for each patient.
5. Conclusions
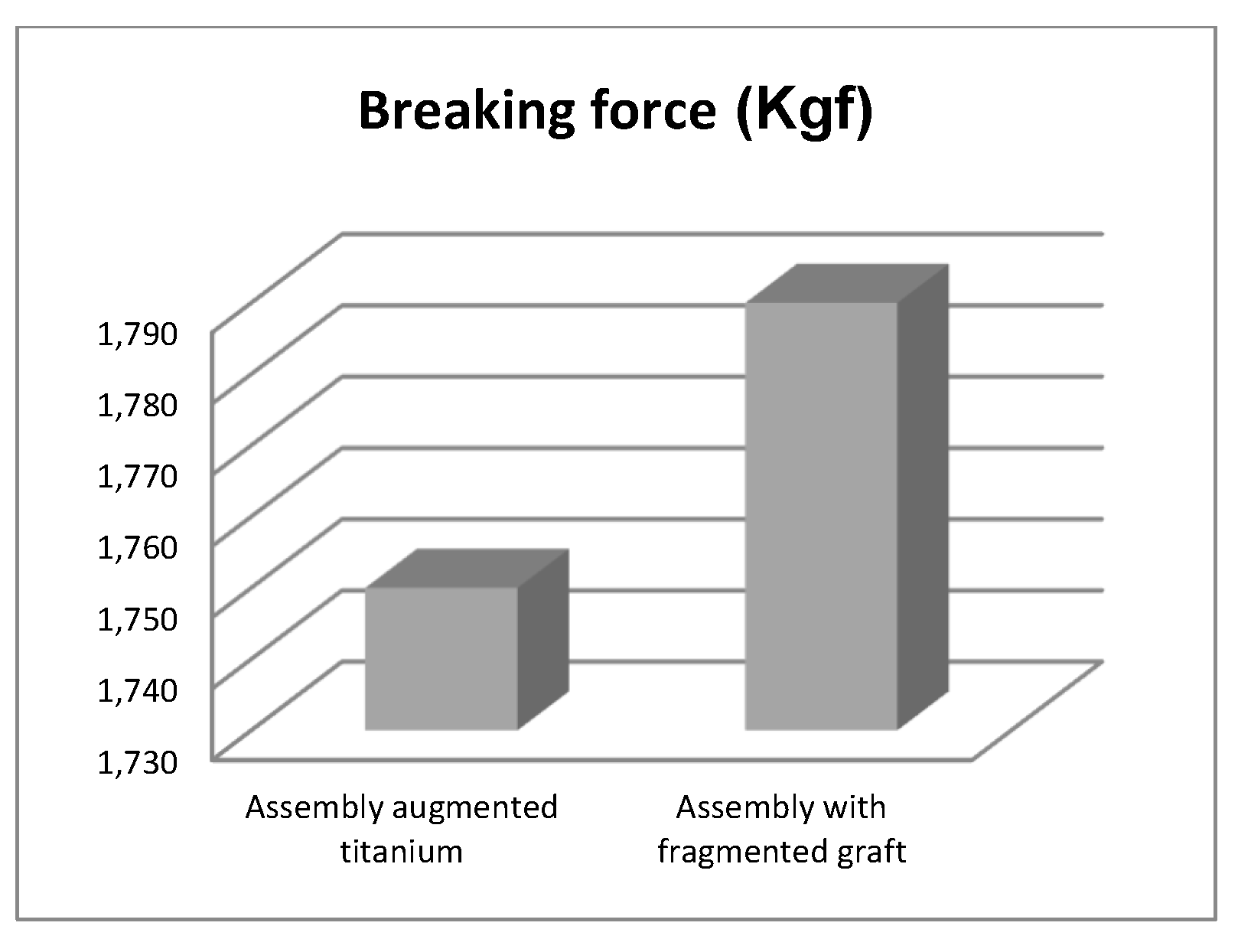
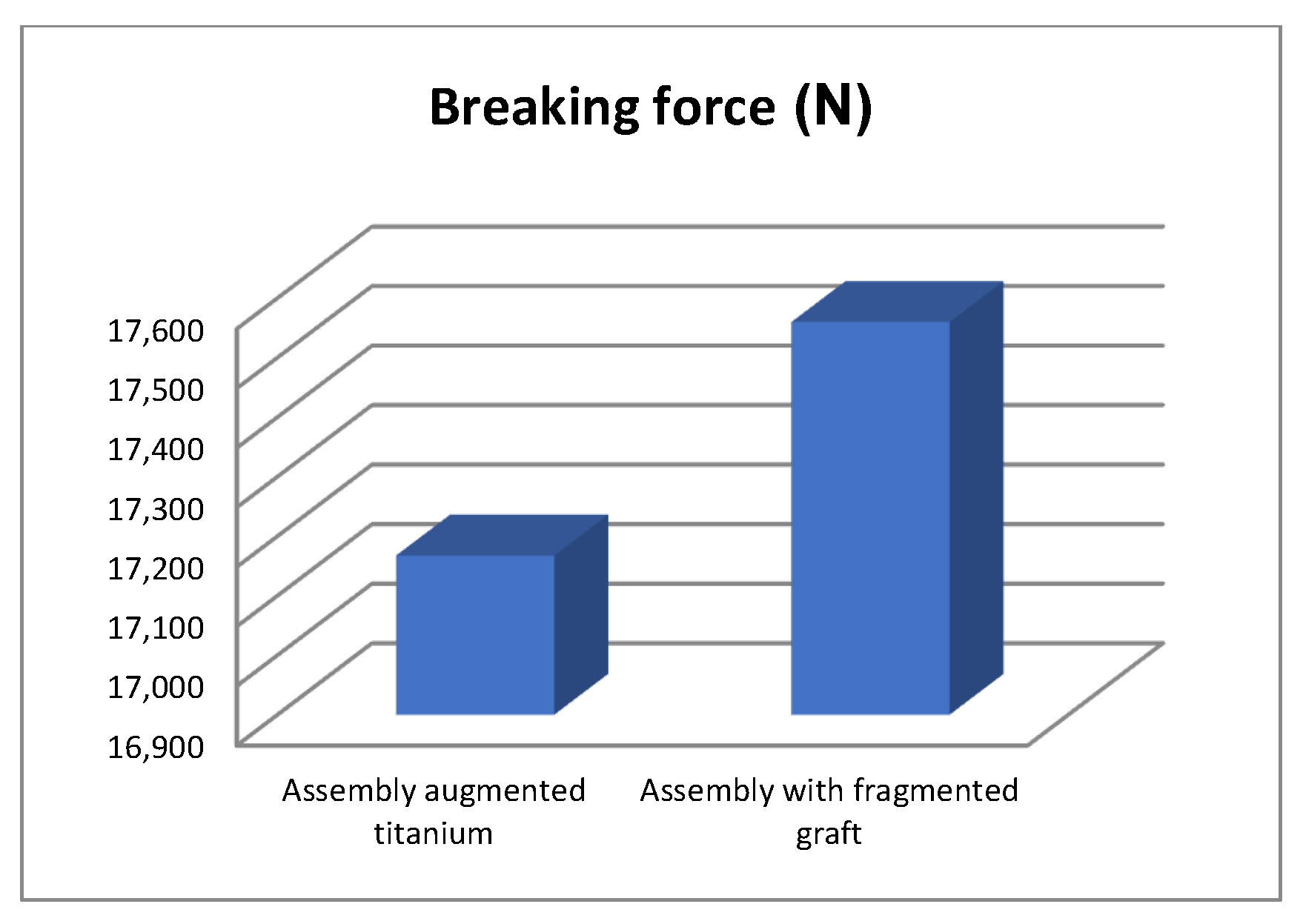
- The morcellated graft and reconstructive mesh prosthesis was stronger, failing at a force value of 1790 Kgf or 17,560 N;
- Studying the values of the forces obtained at the mechanical failure of the two orthopaedic prostheses, it was found that there is a minimum difference of 40 Kgf between the two values.
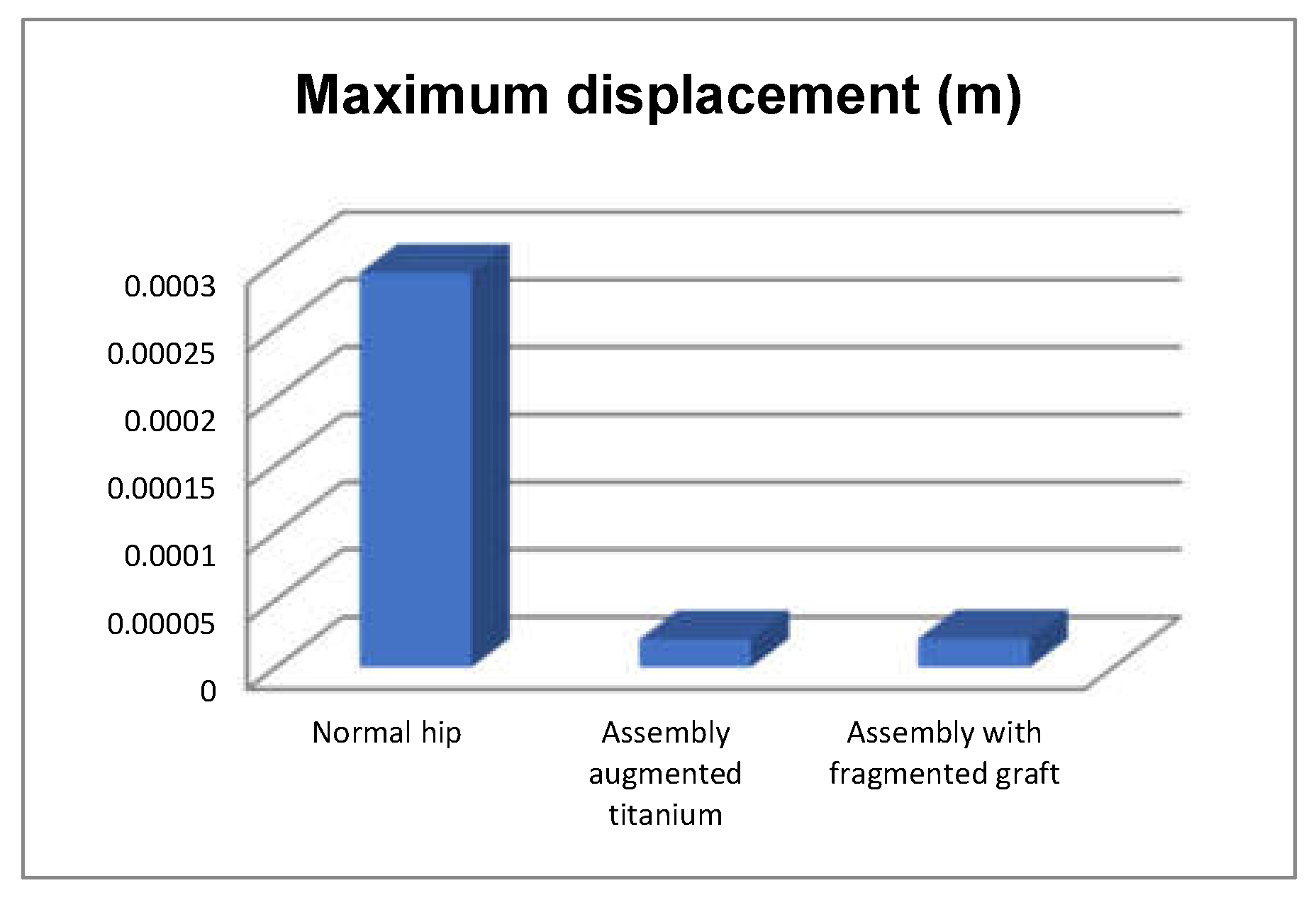
- The highest displacement values were obtained in the case of the normal hip joint and the lowest in the case of the titanium augment prosthesis;
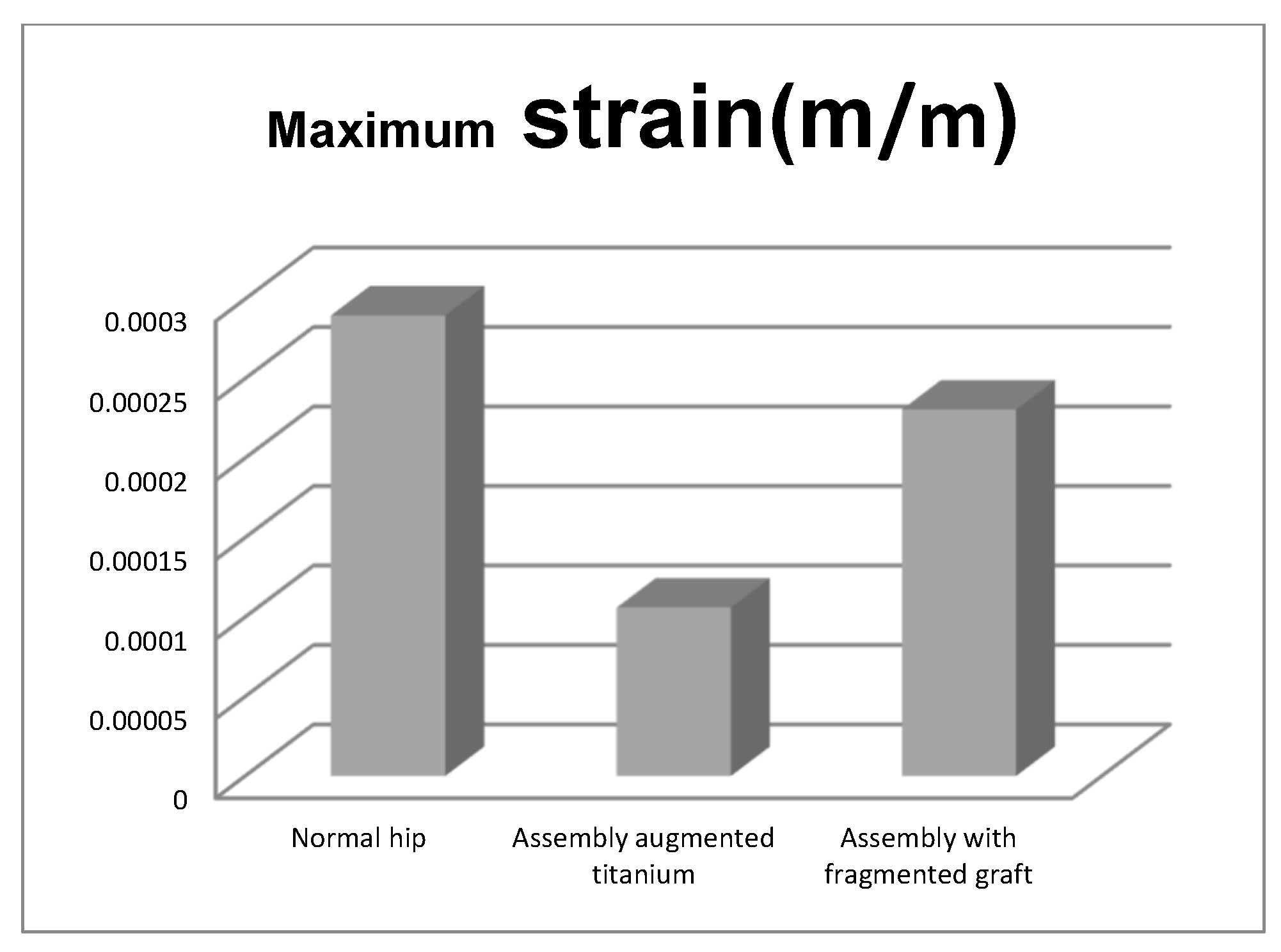
- The maximum strain was obtained in the situation of the normal intact hip joint and the lowest in the situation of the titanium augment;
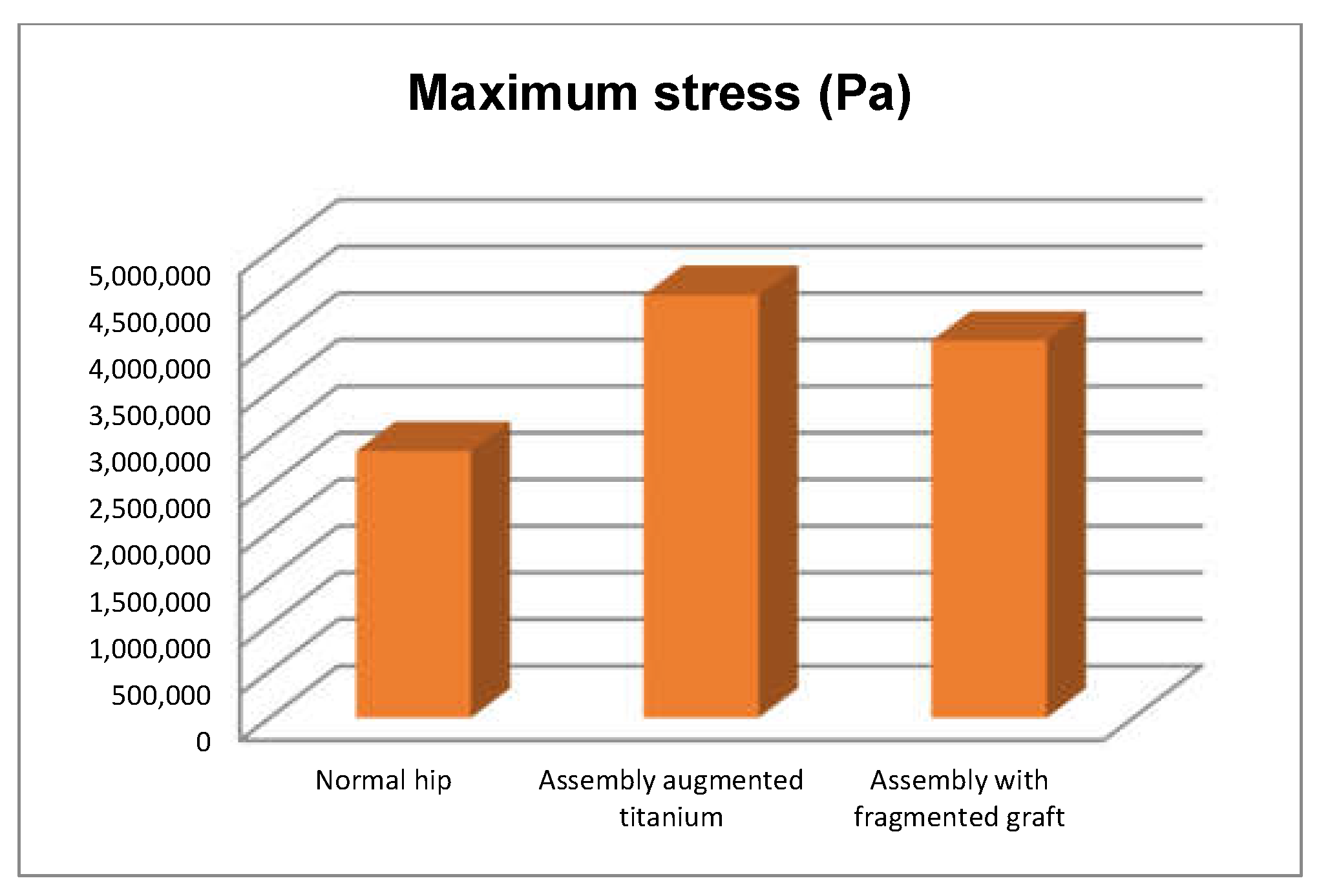
- The highest stress values were observed for the titanium augment prosthesis and the lowest for the intact hip joint;
- The highest values of displacement and strain indicate that the normal intact hip joint is more elastic than the prosthetic hip;
- Analysing the most stressed areas and surfaces, it was observed that the maximum stresses are found on the prosthesis components, and the bone in contact is less stressed;
- If the comparative diagrams of the failure forces and maximum stresses in the two orthopaedic revision prostheses are analysed, it is found that the titanium augment prosthesis is the most stressed and consequently failed mechanically at the lowest force, which validates the two virtual and experimental studies.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ulrich, S.D.; Seyler, T.M.; Bennett, D.; Delanois, R.E.; Saleh, K.J.; Thongtrangan, I.; Kuskowski, M.; Cheng, E.Y.; Sharkey, P.F.; Parvizi, J.; et al. Total hip arthroplasties: What are the reasons for revision? Int. Orthop. 2008, 32, 597–604. [Google Scholar] [CrossRef] [PubMed]
- Yang-Bo, L.; Pan, H.; Chen, L.; Ye, H.; Wu, C.; Wu, P.; Chen, L. Total hip revision with custom-made spacer and prosthesis: A case report. World J. Clin. Cases 2021, 9, 7605–7613. [Google Scholar]
- Lucchini, S.; Castagnini, F.; Giardina, F.; Tentoni, F.; Traina, F. Cementless ceramic-on-ceramic total hip arthroplasty in post-traumatic osteoarthritis after acetabular fracture: Long-term results. Arch. Orthop. Trauma Surg. 2021, 141, 683–691. [Google Scholar] [CrossRef]
- Philippon, M.J.; Schenker, M.L.; Briggs, K.K.; Kuppersmith, D.A.; Maxwell, R.B.; Stubbs, A.J. Revision Hip Arthroscopy. Am. J. Sports Med. 2007, 35, 1918–1921. [Google Scholar] [CrossRef]
- Kurtz, S.; Ong, K.; Lau, E.; Mowat, F.; Halpern, M. Projections of Primary and Revision Hip and Knee Arthroplasty in the United States from 2005 to 2030. J. Bone Jt. Surg. 2007, 89, 780–785. [Google Scholar] [CrossRef]
- JCrowe, F.; Sculco, T.P.; Kahn, B. Revision Total Hip Arthroplasty: Hospital Cost and Reimbursement Analysis. Clin. Orthop. Relat. Res. 2003, 413, 175–182. [Google Scholar]
- JLuttjeboer, S.; Bénard, M.R.; Defoort, K.C.; van Hellemondt, G.G.; Wymenga, A.B. Revision Total Knee Arthroplasty for Instability—Outcome for Different Types of Instability and Implants. J. Arthroplast. 2016, 31, 2672–2676. [Google Scholar] [CrossRef] [PubMed]
- Ong, K.L.; Mowat, F.S.; Chan, N.; Lau, E.; Halpern, M.T.; Kurtz, S.M. Economic burden of revision hip and knee arthroplasty in medicare enrollees. Clin. Orthop. Relat. Res. 2006, 446, 22–28. [Google Scholar] [CrossRef] [PubMed]
- Ionescu, M.; Baciu, C.L.; Buzescu, G. Postero-External Access Path on the Hip, 1st ed.; Chirurgia Publishing House: Bucharest, Romania, 1962. (In Romanian) [Google Scholar]
- Weber, B.G. Total hip replacement revision surgery: Surgical technique and experience. Hip 1981, 1, 3–14. [Google Scholar]
- Witvoet, J. Technique de Pose d’une Prothese Totale de Hanche a Cotyle Visse, Encyl. Med. Chir, 1st ed.; Techniques; Ortopedie Publishing House: Paris, France, 1990. [Google Scholar]
- Callaghan, J.J.W.; Rosenberg, G.A.; Rubash, E.H. Blood conservation (Perioperative Considerations). In The Adult Hip, 1st ed.; Lippincott-Raven Publishing House: New York, NY, USA, 1998. [Google Scholar]
- Zaharia, C. Elements of Locomotor System Pathology, 1st ed.; Padeica Publishing House: Bucharest, Romania, 1994. (In Romanian) [Google Scholar]
- Harris, W.H.; Sledge, C.B. Medical progress: Total hip and total knee replacement. N. Engl. J. Med. 1990, 323, 725–731. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, A.M.; Raab, A.; Miller, J.E. Metal/cement interface strength in cemented stem fixation. J. Orthop. Res. 1984, 2, 105–118. [Google Scholar] [CrossRef] [PubMed]
- Murloy, R.D.; Harris, W.H. The effect of improved cementing techniques on component loosening in total hip replacement. J. Bone Jt. Surg. Br. 1990, 72, 757–760. [Google Scholar]
- Harris, W.H.; Schiller, A.L.; Scholler, J.M.; Freiberg, R.A.; Scott, R. Extensive localizated bone resorbtion in the femur following total hip replacement. J. Bone Jt. Surg. Am. 1976, 58, 612–618. [Google Scholar] [CrossRef]
- Lange, H.-E.; Bader, R.; Kluess, D. Endurance testing and finite element simulation of a modified hip stem for integration of an energy harvesting system. Proc. Inst. Mech. Eng. Part H J. Eng. Med. 2021, 1, 985–992. [Google Scholar] [CrossRef] [PubMed]
- Ciliberti, F.K.; Cesarelli, G.; Guerrini, L.; Gunnarsson, A.E.; Forni, R.; Aubonnet, R.; Recenti, M.; Jacob, D.; Jónsson, H.J.; Cangiano, V.; et al. The role of bone mineral density and cartilage volume to predict knee cartilage degeneration. Eur. J. Transl. Myol. 2022, 32, 10678. [Google Scholar] [CrossRef] [PubMed]
- Recenti, M.; Ricciardi, C.; Edmunds, K.; Jacob, D.; Gambacorta, M.; Gargiulo, P. Testing soft tissue radiodensity parameters interplay with age and self-reported physical activity. Eur. J. Transl. Myol. 2021, 31, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Latessa, I.; Ricciardi, C.; Jacob, D.; Jónsson, H., Jr.; Gambacorta, M.; Improta, G.; Gargiulo, P. Health technology assessment through Six Sigma Methodology to assess cemented and uncemented protheses in total hip arthroplasty. Eur. J. Transl. Myol. 2021, 31, 9651. [Google Scholar] [CrossRef] [PubMed]
- Ricciardi, C.; Jónsson, H.J.; Jacob, D.; Improta, G.; Recenti, M.; Gíslason, M.K.; Cesarelli, G.; Esposito, L.; Minutolo, V.; Bifulco, P.; et al. Improving Prosthetic Selection and Predicting BMD from Biometric Measurements in Patients Receiving Total Hip Arthroplasty. Diagnostics 2020, 10, 815. [Google Scholar] [CrossRef]
- Zajc, J.; Predan, J.; Gubeljak, N.; Moličnik, A.; Fokter, S.K. Modular femoral neck failure after revision of a total hip arthroplasty: A finite element analysis. Eur. J. Orthop. Surg. Traumatol. 2019, 29, 717–723. [Google Scholar] [CrossRef]
- Poumarat, G.; Squire, P. Comparison of mechanical properties of human, bovine bone and a new processed bone xenograft. Biomaterials 1993, 14, 337–340. [Google Scholar] [CrossRef]
- Odgaard, A.; Linde, F. The underestimation of Young’s modulus in compressive testing of cancellous bone specimens. J. Biomech. 1991, 24, 691–698. [Google Scholar] [CrossRef]
- Popa, D.; Tarnita, D.; Iordachita, I. Study method for human knee applicable to humanoid robots. In Proceedings of the 14th International Workshop on Robotics in Alpe-Adria-Danube Region, RAAD, Bucharest, Romania, 26–28 May 2005; Volume 5, pp. 26–28. [Google Scholar]
- DPopa; Tarnita, D.N.; Tarnita, D.; Grecu, D. The generation of the three-dimensional model of the human knee joint. Rom. J. Morph. Embr. 2005, 46, 3–6. [Google Scholar]
- Popa, D.L.; Duta, A.; Tutunea, D.; Gherghina, G.; Buciu, G.; Calin, D.C. Virtual Methods Applied to Human Bones and Joints Re-Construction Used for Orthopedic Systems. Appl. Mech. Mater. 2016, 822, 160–165. [Google Scholar] [CrossRef]
- Călin, D.C.; Popa, D.L.; Grecu, A.F.; Grecu, D.C.; Nemeș, R.N.; Buciu, G. About the Experimental and Virtual Analysis of Orthopedic Implant Systems for the Revision of the Hip Prosthesis with Morcellated Bone Graft and Reconstruction Net. Curr. Health Sci. J. 2021, 47, 249–255. [Google Scholar] [PubMed]
- Calin, D.C.; Tarnita, D.; Popa, D.L.; Rosca, A.; Tarnita, D.N. The 3D Virtual Model of a Classical Hip Joint Prosthesis. Appl. Mech. Mater. 2016, 823, 161–166. [Google Scholar] [CrossRef]
- Calin, D.C.; Tarnita, D.; Popa, D.L.; Calafeteanu, D.; Tarnita, D.N. Virtual Model and Simulation of the Normal and Affected Human Hip Joint. Appl. Mech. Mater. 2016, 823, 167–172. [Google Scholar] [CrossRef]
- Tarnita, D.; Boborelu, C.; Popa, D.L.; Malciu, R.; Tarnita, D.N. Virtual Modeling and Numerical Simulations of the Latitude Prosthesis-Human Elbow Assembly. In Proceedings of the SIAR International Congress of Automotive and Transport Engineering: Science and Management of Automotive and Transportation Engineering, Craiova, Romania, 23–25 October 2019; pp. 706–712. [Google Scholar]
- Tudora, A.; Ontica, V.; Tarnita, D.N.; Popa, D.L.; Calin, D.C.; Oncescu, A.; Capitanescu, B.; Albulescu, D. Virtual human hip joint obtained from CT images and FEM test. JIDEG 2019, 14, 195–198. [Google Scholar]
- Kim, B.C.; Lee, J.; Kwon, K.-Y. Method for automatically generating a two-dimensional triangular mesh of a bone from a CT image considering its density heterogeneity. J. Mech. Sci. Technol. 2020, 34, 2941–2952. [Google Scholar] [CrossRef]
- Anguiano-Sanchez, J.; Martinez-Romero, O.; Siller, H.R.; Diaz-Elizondo, J.A.; Flores-Villalba, E.; Rodriguez, C.A. Influence of PEEK Coating on Hip Implant Stress Shielding: A Finite Element Analysis. Comput. Math. Methods Med. 2016, 1, 6183679. [Google Scholar] [CrossRef] [PubMed]
- Amirouche, F.; Solitro, G.F.; Walia, A.; Gonzalez, M.; Bobko, A. Segmental acetabular rim defects, bone loss, oversizing, and press fit cup in total hip arthroplasty evaluated with a probabilistic finite element analysis. Int. Orthop. 2017, 41, 1527–1533. [Google Scholar] [CrossRef] [PubMed]
- Xin, Y.-Z.; Cho, H.-M.; Yang, S.-J. Initial stability analysis of cementless hemiarthroplasty using a rectangular cross-sectional stem for type A2 intertrochanteric fractures. J. Mech. Sci. Technol. 2015, 29, 5043–5047. [Google Scholar] [CrossRef]
- Huiskes, R.; Chao, E.Y.S. A Survey of Finite Element Analysis in Orthopedic Biomechanics: The First Decade. J. Biomech. 1983, 16, 385–409. [Google Scholar] [CrossRef]
- Vaduva, R.C.; Petrovici, I.L.; Tenovici, M.C.; Tarnita, D.N.; Popa, D.L.; Rogoveanu, O.; Capitanescu, B. A Three-Dimensional Virtual System Used to Analyze the Normal and Prosthetic Joint of the Hip. Adv. Eng. Forum 2019, 34, 165–174. [Google Scholar] [CrossRef]
- Mihalko, W.M.; Beaudoin, A.J.; Cardea, J.A.; Krause, W.R. Finite-element modelling of femoral shaft fracture fixation techniques post total hip arthroplasty. J. Biomech. 1992, 25, 469–476. [Google Scholar] [CrossRef]
- Chiang, F.-T.; Hung, J.-P. Investigation of the fracture characteristics of the interfacial bond between bone and cement: Experimental and finite element approaches. J. Mech. Sci. Technol. 2010, 24, 1235–1244. [Google Scholar] [CrossRef]
- Li, K.; Tang, W. Load displacement relationship model and measurement of deep groove ball bearing and 4-point contact ball bearing. J. Mech. Sci. Technol. 2021, 35, 3045–3058. [Google Scholar] [CrossRef]
- Kawanabe, K.; Akiyama, H.; Goto, K.; Maeno, S.; Nakamura, T. Load Dispersion Effects of Acetabular Reinforcement Devices Used in Revision Total Hip Arthroplasty. J. Arthroplast. 2011, 26, 1061–1066. [Google Scholar] [CrossRef]
- Borovkov, A.I.; Maslov, L.B.; Zhmaylo, M.A.; Zelinskiy, I.A.; Voinov, I.B.; Keresten, I.A.; Mamchits, D.V.; Tikhilov, R.M.; Kovalenko, A.N.; Bilyk, S.S.; et al. Finite element stress analysis of a total hip replacement in two-legged standing. Russ. J. Biomech. 2018, 22, 382–400. [Google Scholar]
- Hsu, M.L.; Chang, C.L. Application of finite element analysis in dentistry. Finite Elem. Anal. 2010, 5, 43–66. [Google Scholar]
- Cicciù, M.; Cervino, G.; Bramanti, E.; Lauritano, F.; LoGudice, G.; Scappaticci, L.; Rapparini, A.; Guglielmino, E.; Risitano, G. FEM Analysis of Mandibular Prosthetic Overdenture Supported by Dental Implants: Evaluation of Different Retention Methods. Comput. Math. Methods Med. 2015, 1, 943839. [Google Scholar] [CrossRef]
- Keulemans, F.; Shinya, A.; Lassila, L.V.J.; Vallittu, P.K.; Kleverlaan, C.J.; Feilzer, A.J.; DeMoor, R.J.G. Three-Dimensional Finite Element Analysis of Anterior Two-Unit Cantilever Resin-Bonded Fixed Dental Prostheses. Sci. World J. 2015, 1, 864389. [Google Scholar] [CrossRef] [PubMed]
- Benazzi, S.; Nguyen, H.N.; Kullmer, O.; Kupczik, K. Dynamic Modelling of Tooth Deformation Using Occlusal Kinematics and Finite Element Analysis. PLoS ONE 2016, 11, e0152663. [Google Scholar] [CrossRef]
- Baciu, C.C. Reconstruction of the Anterior Cruciate Ligament Using a Synthetic-Biological Mixed Graft—Experimental Model on the Study Animal. Ph.D. Thesis, University of Medicine and Pharmacy Carol Davila Bucharest, Doctoral School of Medicine, Bucharest, Romania, 2019. (In Romanian). [Google Scholar]
- Laflamme, M.; Lamontagne, J.; Guidoin, R. Anterior cruciate ligament prostheses using biotextiles, Biotextiles as Medical Implants. M.W.K.S.G. Guidoin 2013, 1, 590–639. [Google Scholar]
- Ambrosio, L.; Gloria, A.; Causa, F. Composite materials for replacement of ligaments and tendons. Biomed. Compos. 2010, 1, 234–254. [Google Scholar]
- Woo, S.L.-Y.; Abramowitch, S.D.; Loh, J.C.; Musahl, V.; Wang, J.H.-C. Ligament healing: Present status and the future of functional tissue engineering. Funct. Tissue Eng. 2003, 1, 17–20. [Google Scholar]
- Kirkendall, D.T.; Garrett, J. Muscle, tendon, and ligament: Structure, function, and physiology. In Orthopaedics; Fitzgerald, R.H., Kaufer, H., Malkani, A.L., Eds.; Mosby: St. Louis, MO, USA, 2002; Volume 1, pp. 177–178. [Google Scholar]
- Ciunel, S.; Duta, A.; Popa, D.L.; Popa-Mitroi, G.; Dumitru, V.C. The Behavior of the Virtual Human Head-Neck System during the Main Movements. Appl. Mech. Mater. 2014, 657, 780–784. [Google Scholar] [CrossRef]
- BEl-Zayat, F.; Heyse, T.J.; Fanciullacci, N.; Labey, L.; Fuchs-Winkelmann, S.; Innocenti, B. Fixation techniques and stem dimensions in hinged total knee arthroplasty: A finite element study. Arch. Orthop. Trauma Surg. 2016, 136, 1741–1752. [Google Scholar] [CrossRef] [PubMed]
- Petrescu, S.M.S.; Tuculina, M.J.; Popa, D.L.; Duta, A.; Sălan, A.I.; Georgescu, R.V.; Diaconu, O.A.; Turcu, A.A.; Nicola, A.G.; Dascălu, I.T. Modeling and simulating an orthodontic system using virtual methods. Diagnostics 2022, 12, 1296. [Google Scholar] [CrossRef] [PubMed]
- Țenovici, M.C.; Tarnița, D.N.; Popa, D.L.; Vaduva, R.C.; Ciobanu, M.O.; Petrovici, I.L. Contributions to the Study on the Effects of Incorrect Implantation of Knee Prostheses Depending on the Degree of Varus/Valgus. Curr. Health Sci. J. 2022, 48, 57–62. [Google Scholar]
- Rodrigues, G.; Dias, A.; Ribeiro, D. Relationship between Isometric Hip Torque with Three Kinematic Tests in Soccer Players. Phys. Act. Health 2020, 4, 142–149. [Google Scholar] [CrossRef]
- Levadnyi, I.; Awrejcewicz, J.; Zhang, Y.; Gu, Y. Comparison of femur strain under different loading scenarios: Experimental testing. Proc. Inst. Mech. Eng. Part H J. Eng. Med. 2021, 235, 17–27. [Google Scholar] [CrossRef] [PubMed]
- Liang, F.-Y.; Gao, F.; Cao, J.; Law, S.-W.; Liao, W.-H. Gait Synergy Analysis and Modeling on Amputees and Stroke Patients for Lower Limb Assistive Devices. Sensors 2022, 22, 4814. [Google Scholar] [CrossRef] [PubMed]







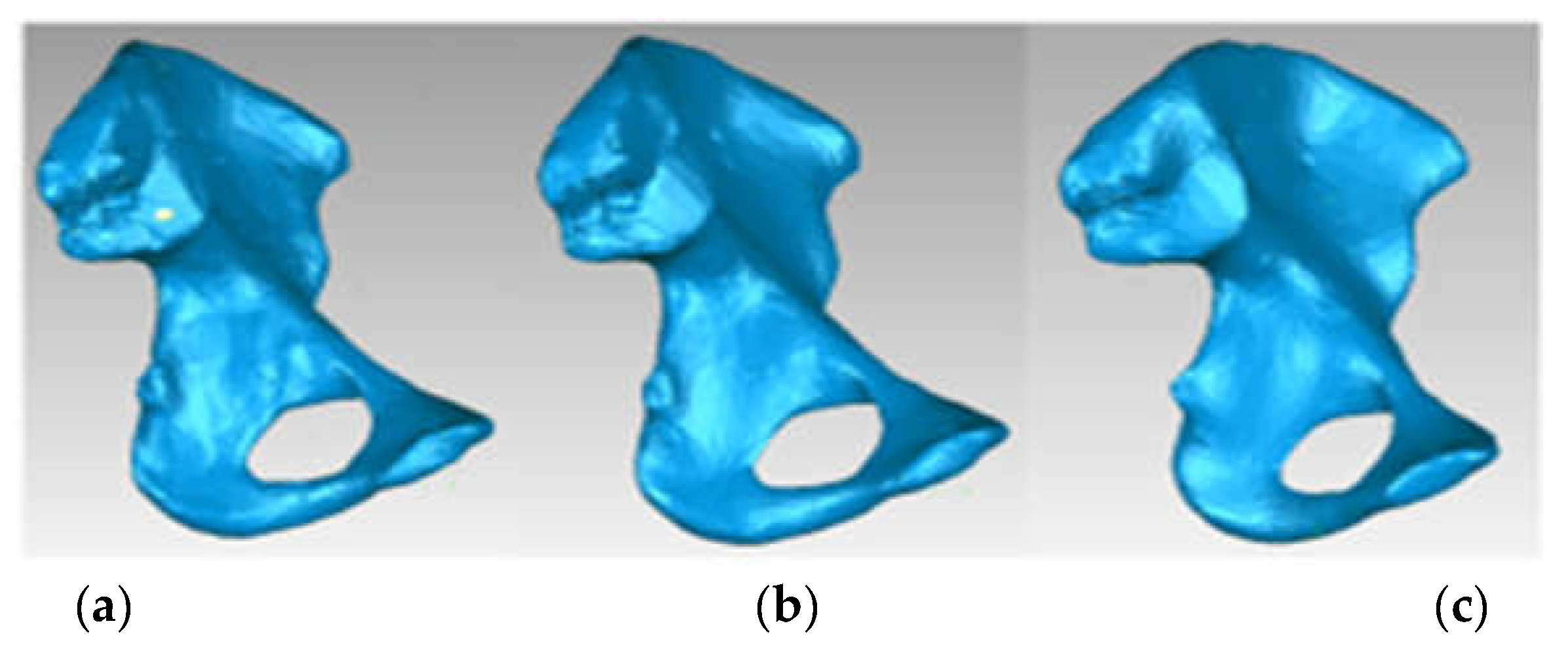
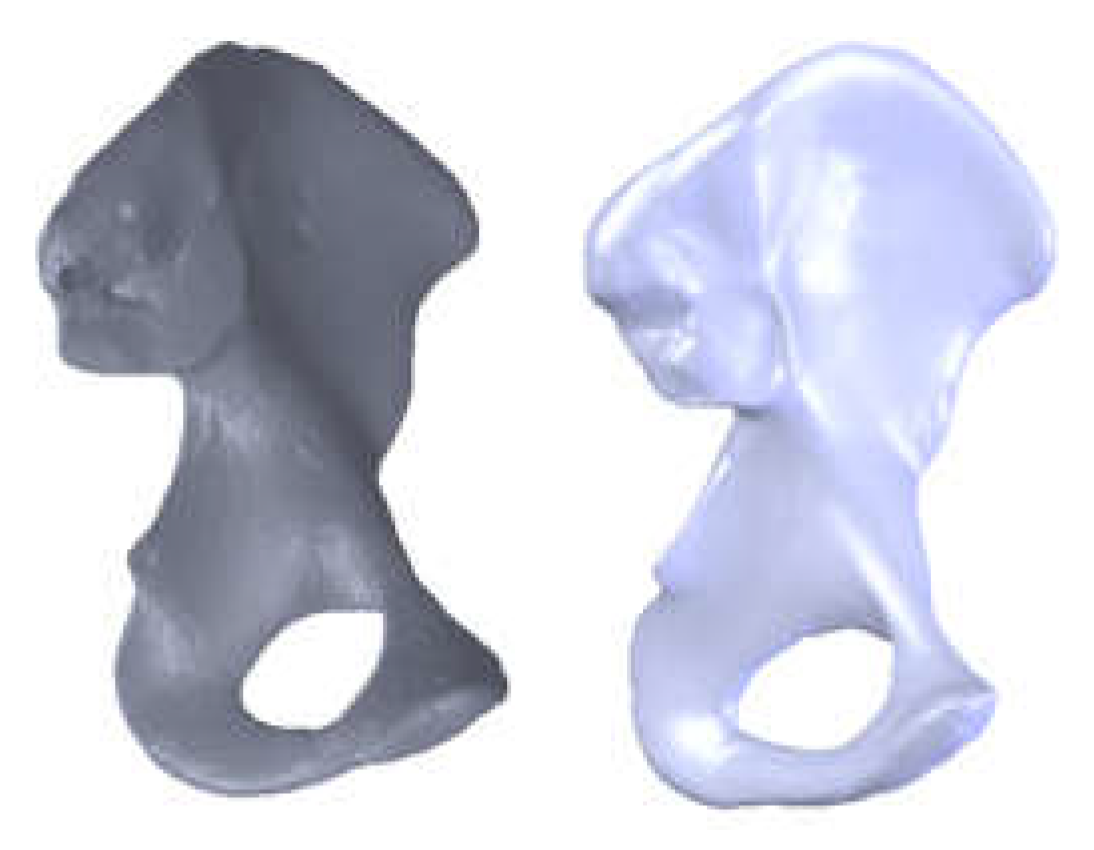
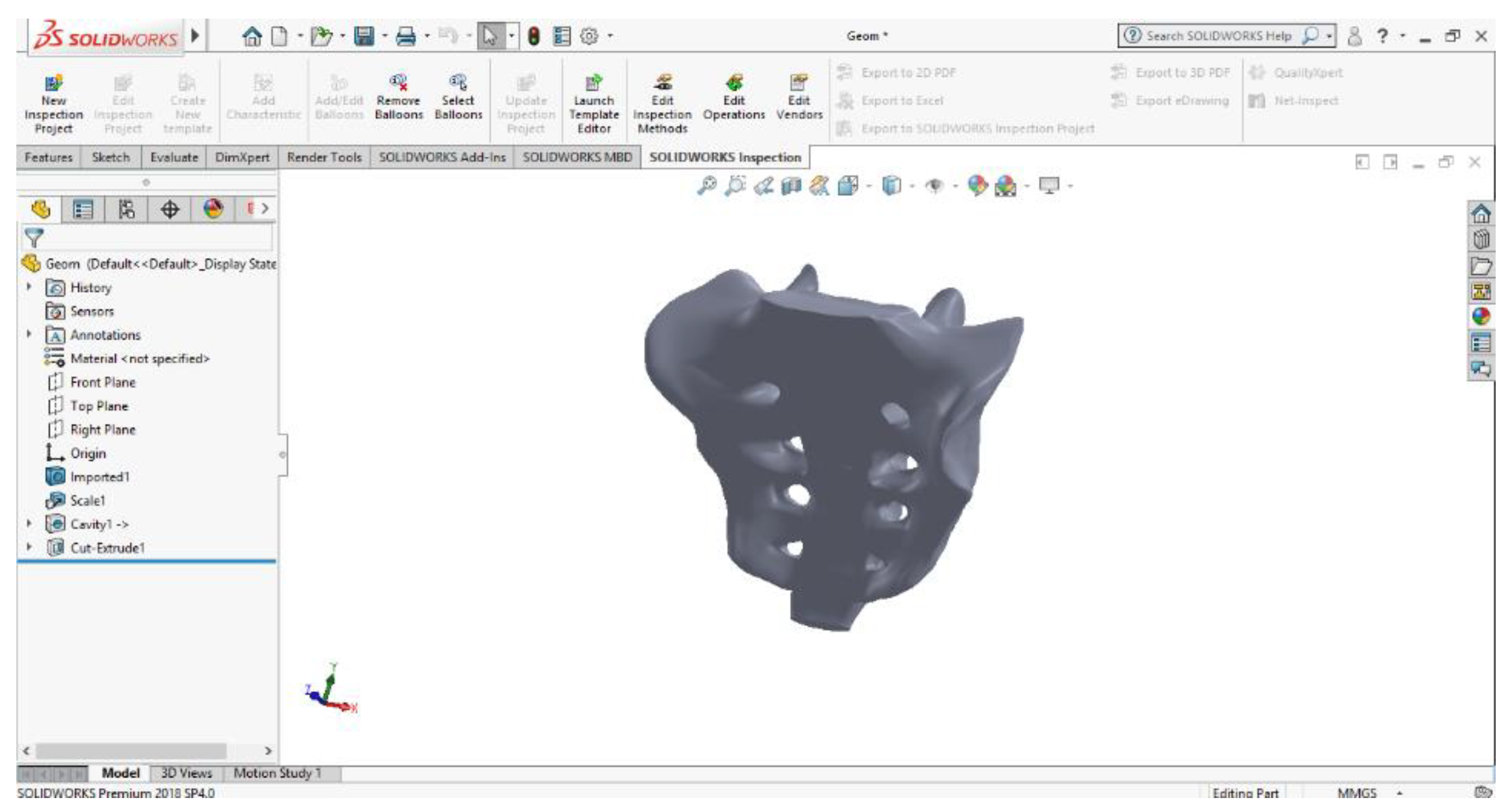

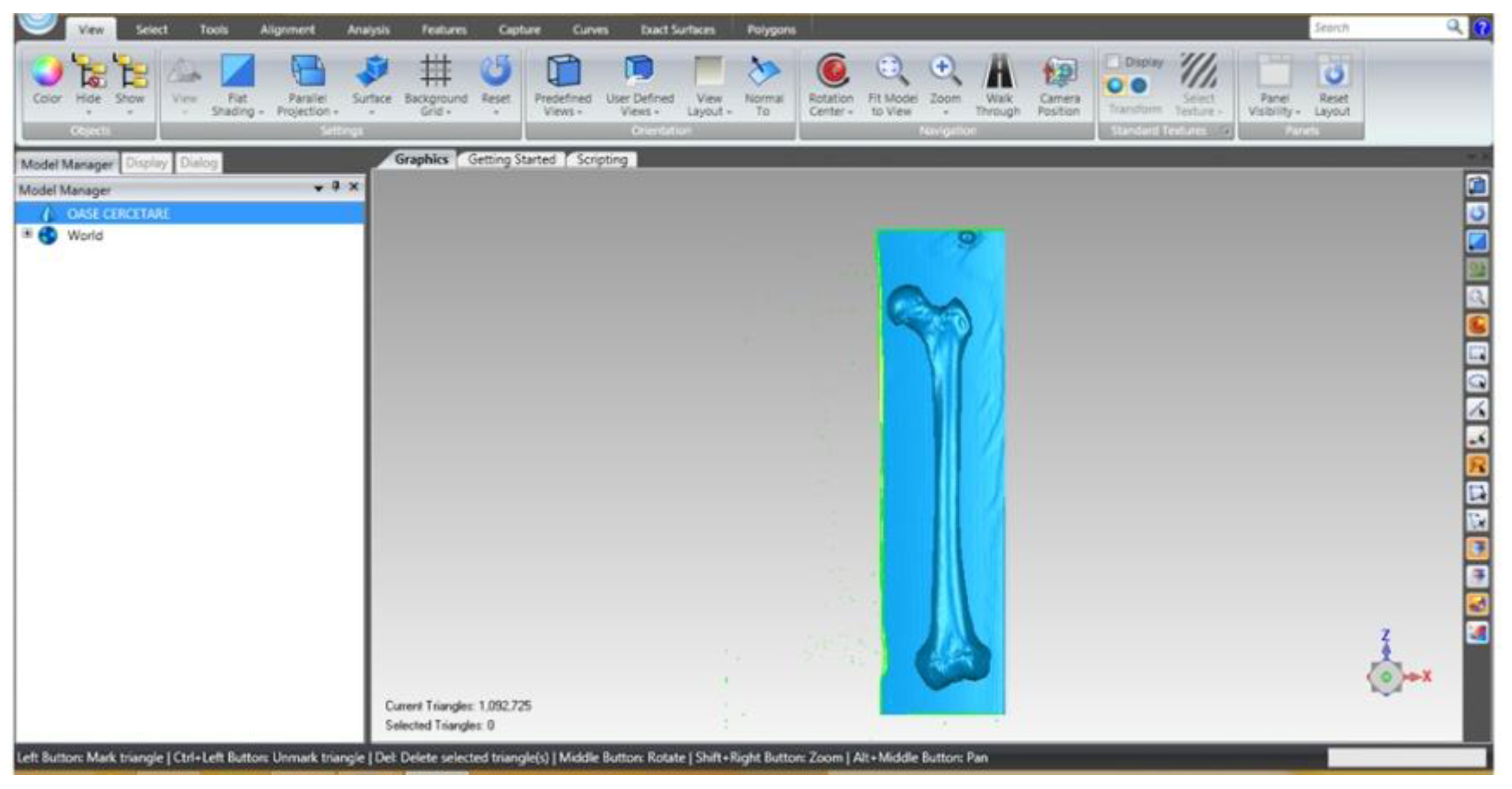
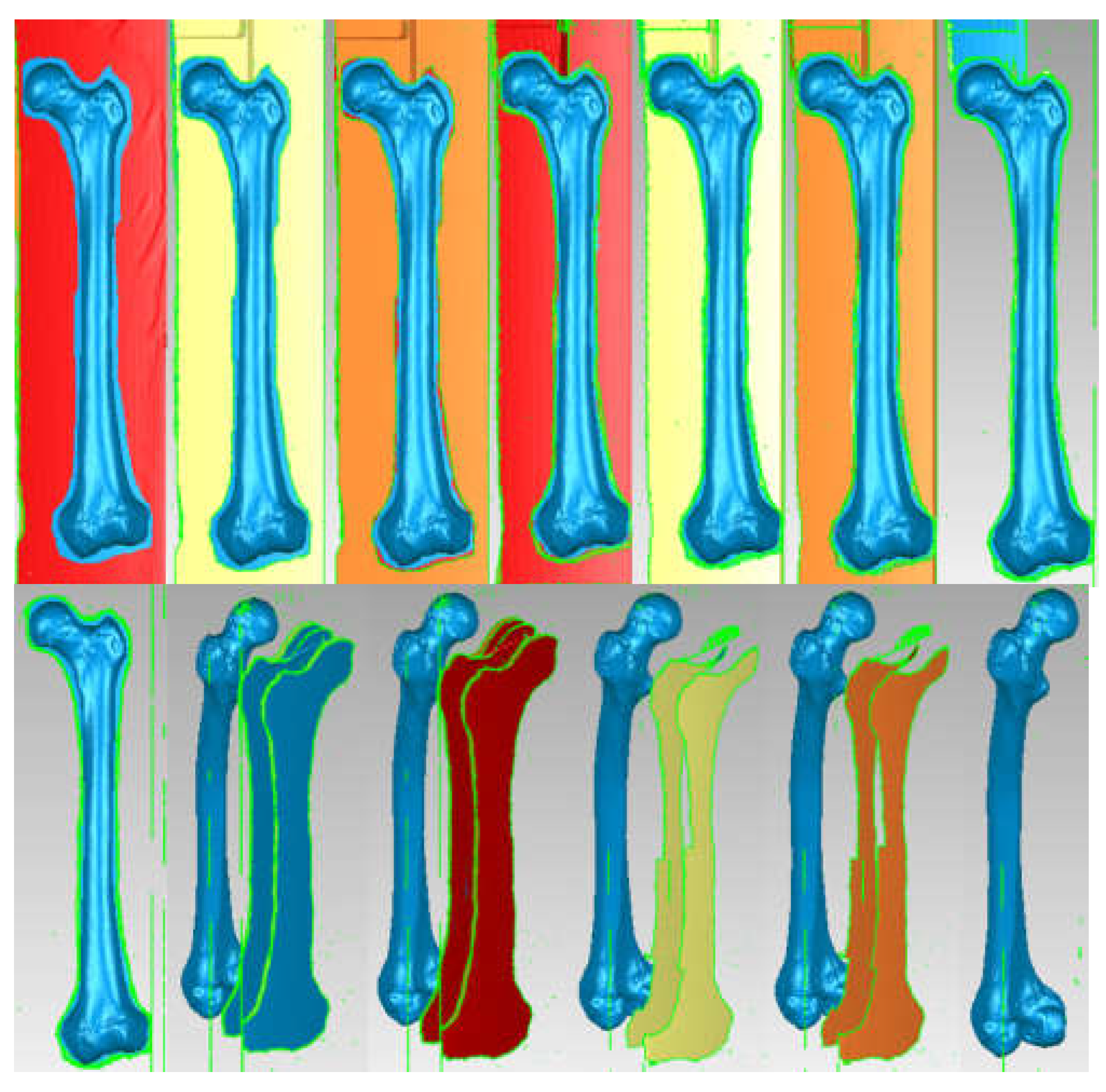

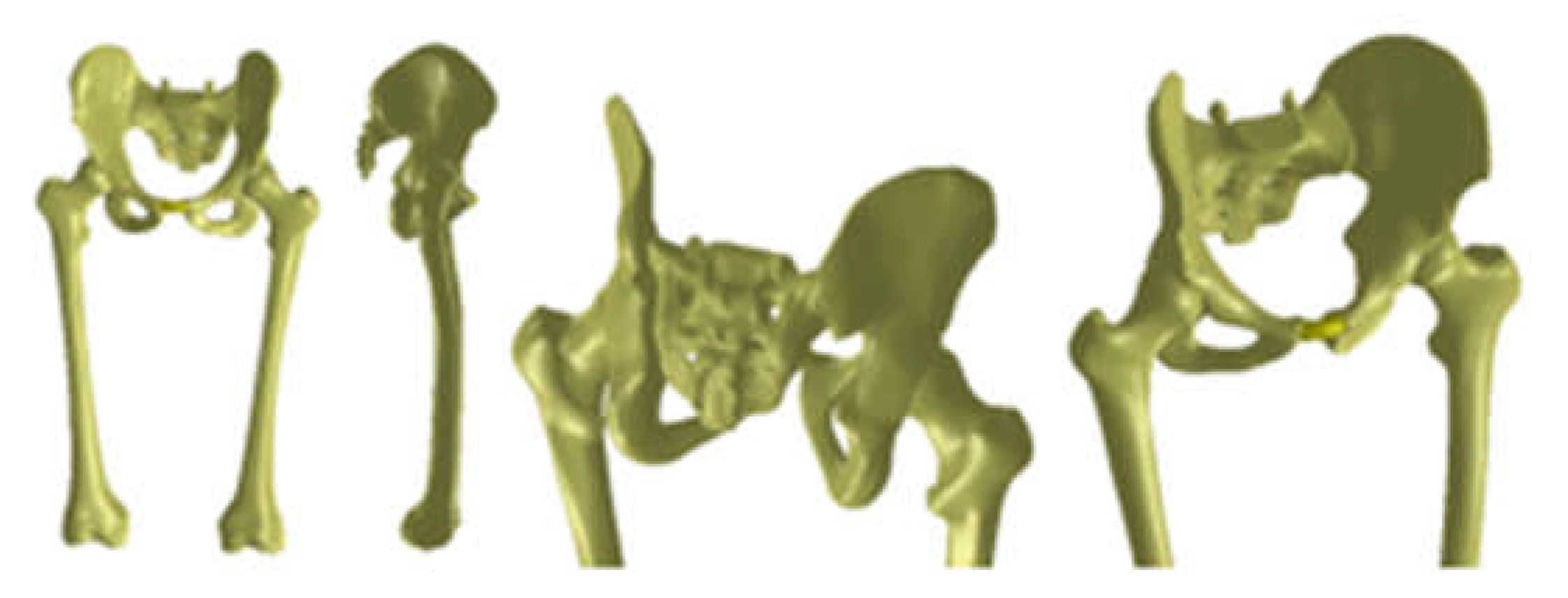
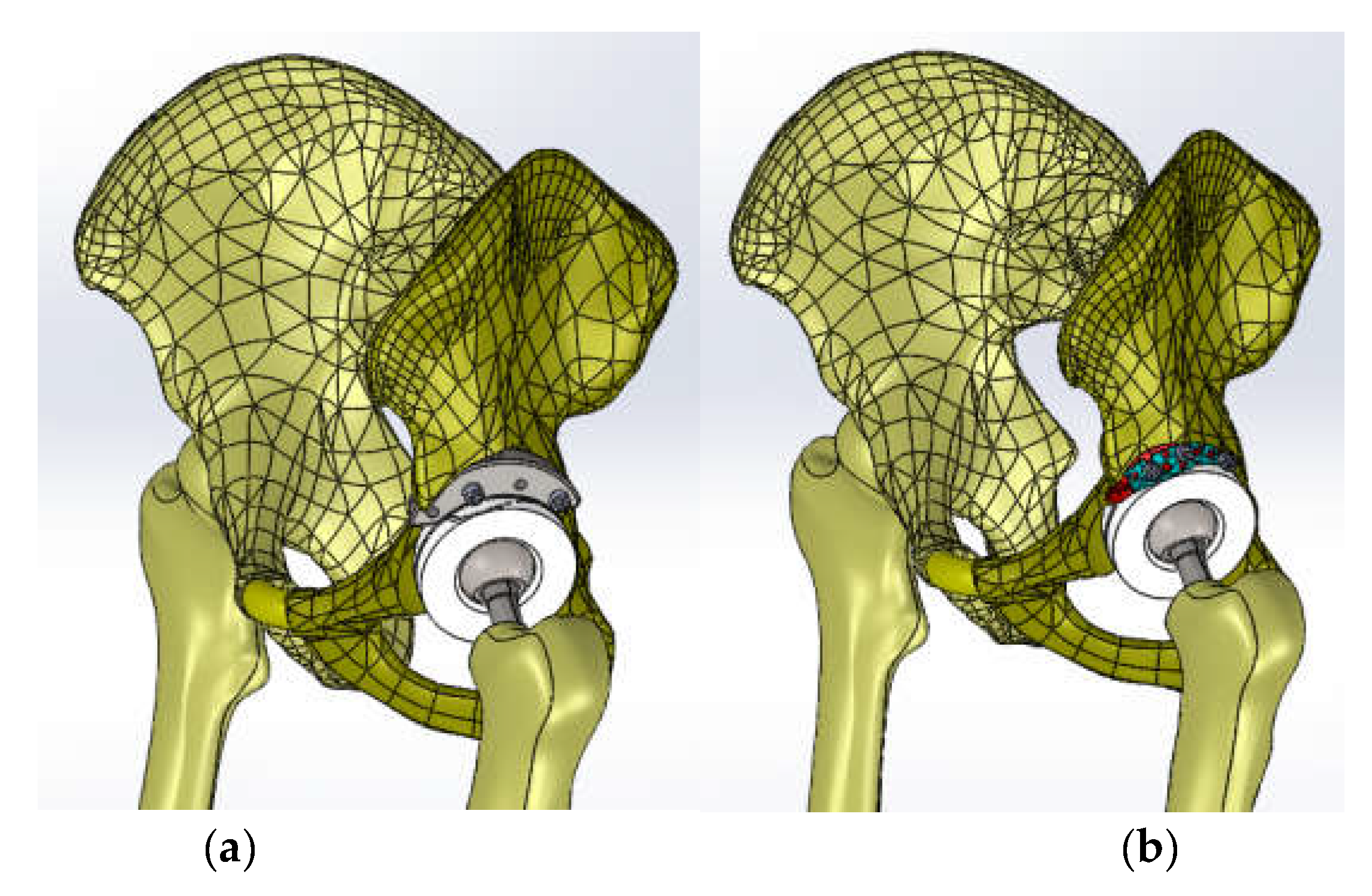
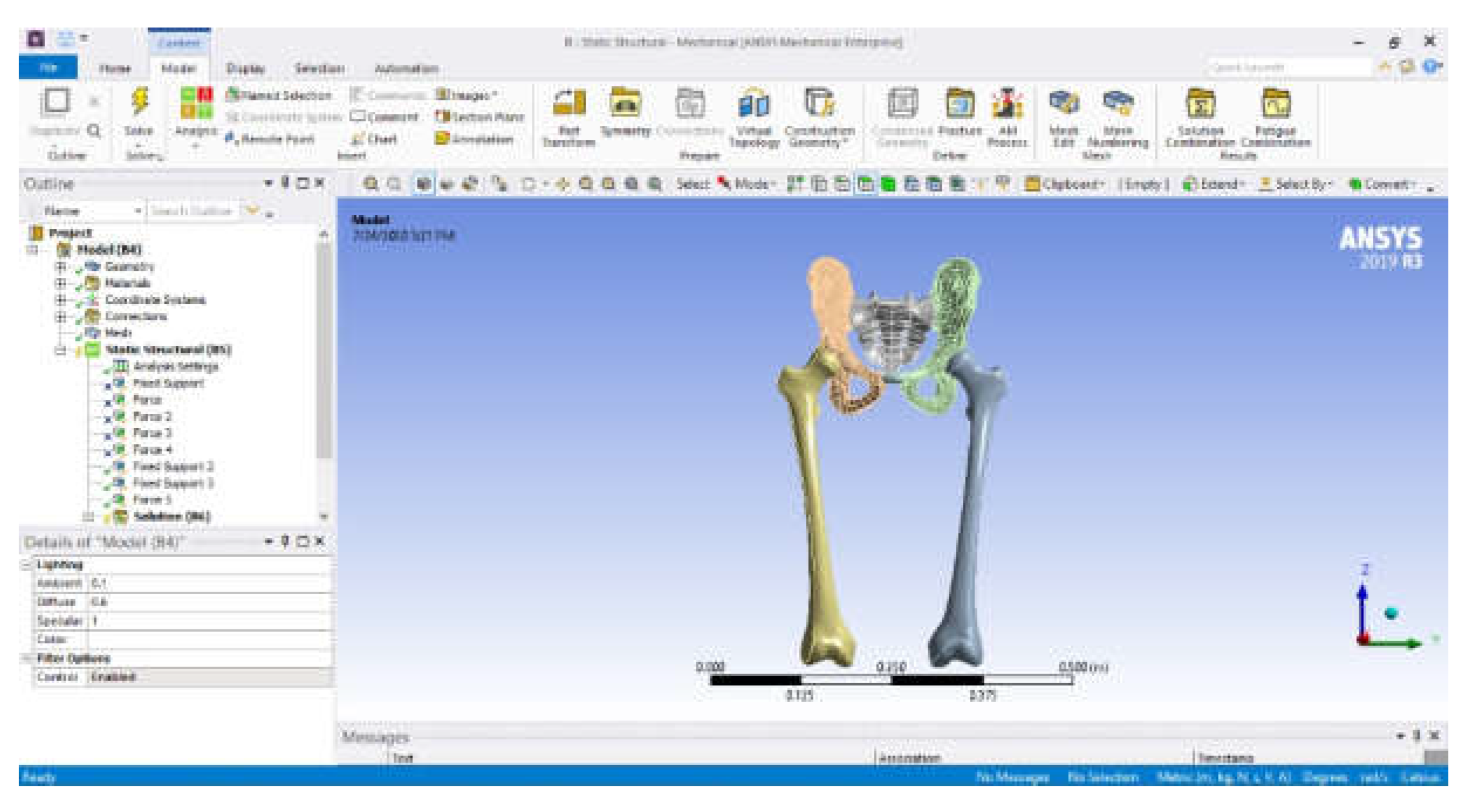
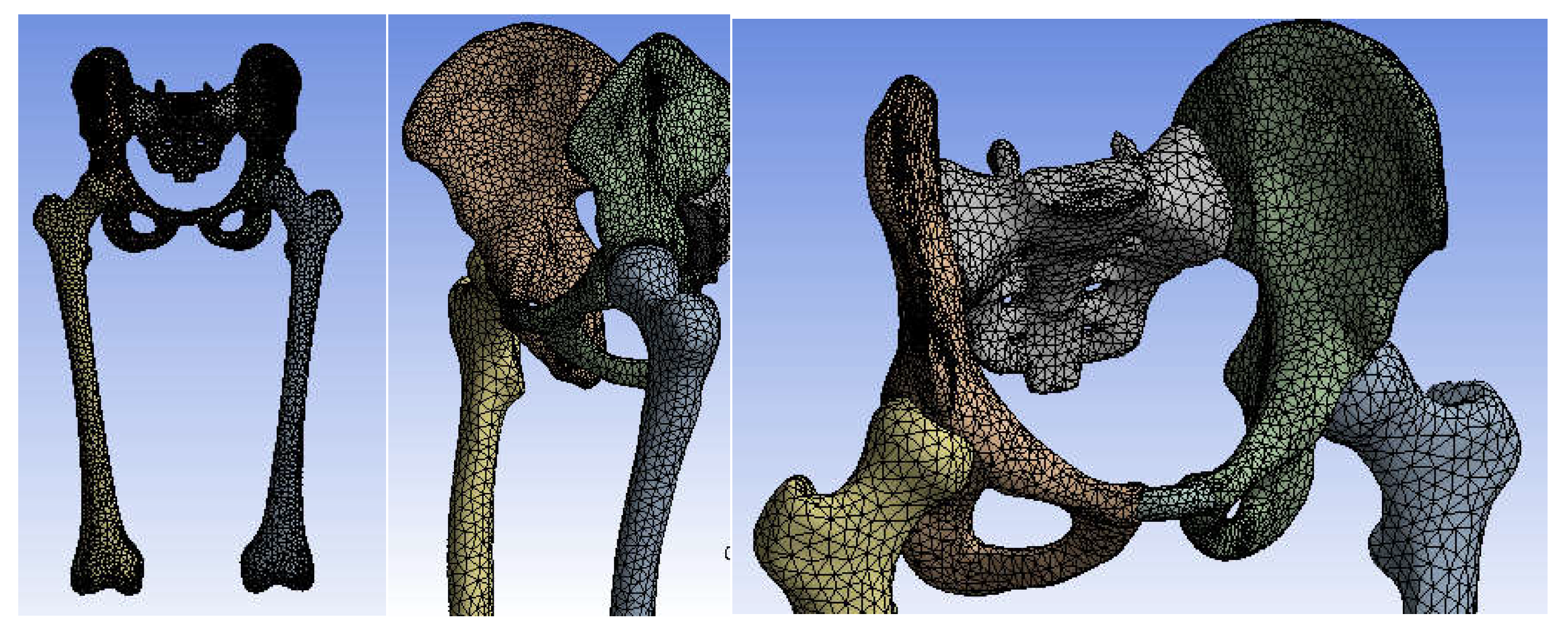
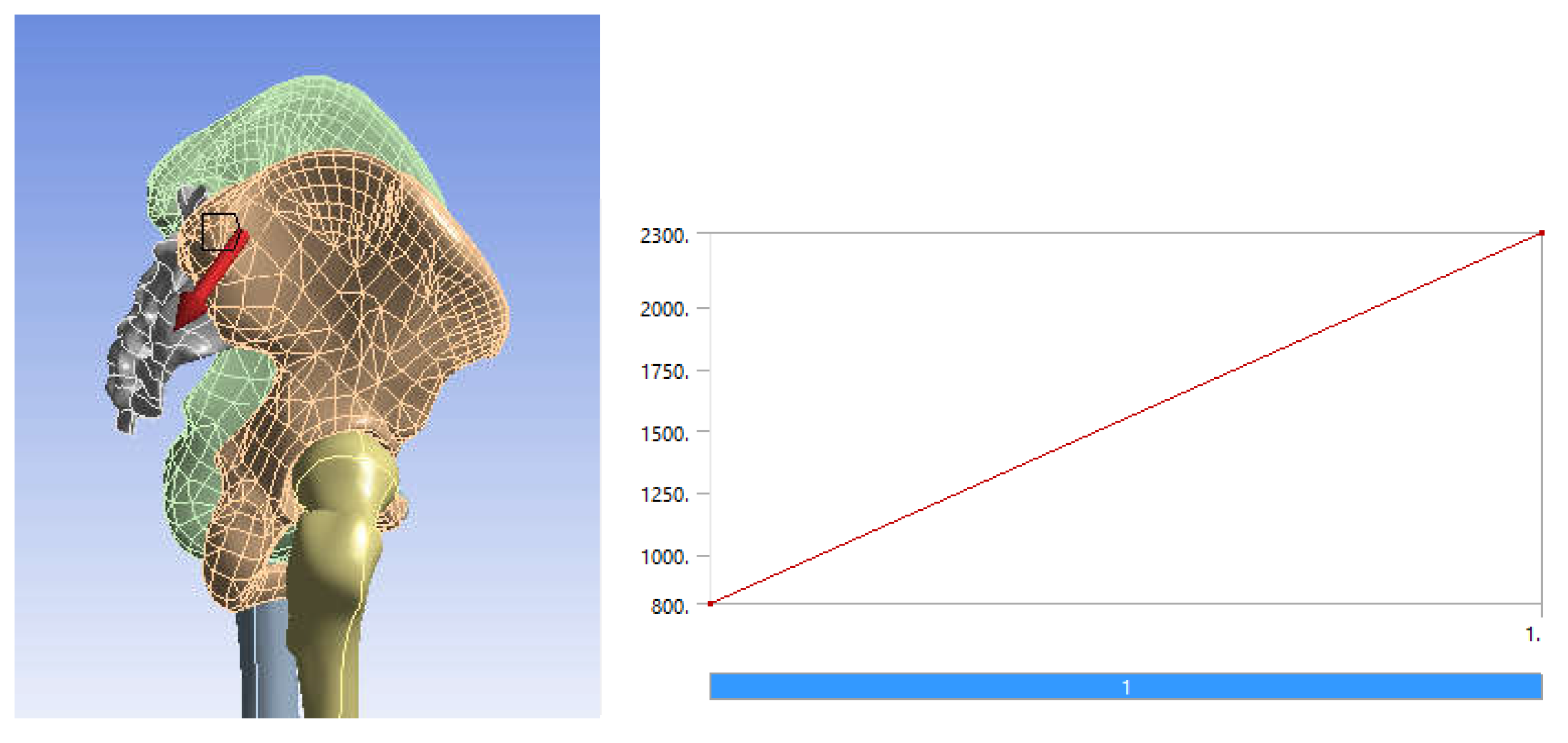


| Component | Material | Density (Kg/m3) | Young’s Modulus (GPa) | Poisson Ratio |
|---|---|---|---|---|
| Femur, pelvis, sacrum | Bone | 1400 | 1 × 1010 | 0.3 |
| Pubic symphysis | Ligament | 955 | 1.2 × 109 | 0.42 |
| Component | Material | Density (Kg/m3) | Young’s Modulus (GPa) | Poisson Ratio |
|---|---|---|---|---|
| Femur, pelvis, sacrum | Bone | 1400 | 1 × 1010 | 0.3 |
| Pubic symphysis | Ligament | 955 | 1.2 × 109 | 0.42 |
| Augment | Titanium alloy | 4620 | 9.6 × 1010 | 0.36 |
| Polyethylene cup | Polyethylene | 950 | 1.1 × 109 | 0.42 |
| Spherical metal head | Stainless steel | 7750 | 1.93 × 1011 | 0.31 |
| Orthopaedic screw | Stainless steel | 7750 | 1.93 × 1011 | 0.31 |
| Femoral stem | Stainless steel | 7750 | 1.93 × 1011 | 0.31 |
| Component | Material | Density (Kg/m3) | Young’s Modulus (GPa) | Poisson Ratio |
|---|---|---|---|---|
| Femur, pelvis, sacrum | Bone | 1400 | 1 × 1010 | 0.3 |
| Morcellated bone graft | Trabecular bone | 2140 | 1.76 × 1010 | 0.25 |
| Pubic symphysis | Ligament | 955 | 1.2 × 109 | 0.42 |
| Reconstruction mesh | Stainless steel | 7750 | 1.93 × 1011 | 0.31 |
| Polyethylene cup | Polyethylene | 950 | 1.1 × 109 | 0.42 |
| Spherical metal head | Stainless steel | 7750 | 1.93 × 1011 | 0.31 |
| Orthopaedic screw | Stainless steel | 7750 | 1.93 × 1011 | 0.31 |
| Femoral stem | Stainless steel | 7750 | 1.93 × 1011 | 0.31 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Duta, A.; Popa, D.-L.; Vintila, D.D.; Buciu, G.; Dina, N.A.; Ionescu, A.; Berceanu, M.C.; Calin, D.C. An Experimental and Virtual Approach to Hip Revision Prostheses. Diagnostics 2022, 12, 1952. https://doi.org/10.3390/diagnostics12081952
Duta A, Popa D-L, Vintila DD, Buciu G, Dina NA, Ionescu A, Berceanu MC, Calin DC. An Experimental and Virtual Approach to Hip Revision Prostheses. Diagnostics. 2022; 12(8):1952. https://doi.org/10.3390/diagnostics12081952
Chicago/Turabian StyleDuta, Alina, Dragos-Laurentiu Popa, Daniela Doina Vintila, Gabriel Buciu, Nicolae Adrian Dina, Adriana Ionescu, Mihaela Corina Berceanu, and Daniel Cosmin Calin. 2022. "An Experimental and Virtual Approach to Hip Revision Prostheses" Diagnostics 12, no. 8: 1952. https://doi.org/10.3390/diagnostics12081952
APA StyleDuta, A., Popa, D.-L., Vintila, D. D., Buciu, G., Dina, N. A., Ionescu, A., Berceanu, M. C., & Calin, D. C. (2022). An Experimental and Virtual Approach to Hip Revision Prostheses. Diagnostics, 12(8), 1952. https://doi.org/10.3390/diagnostics12081952






