Retinal and Choroidal Thinning—A Predictor of Coronary Artery Occlusion?
Abstract
:1. Introduction
2. Materials and Methods
Statistical Analysis
3. Results
Logistic Regression Model for Cardiovascular Disease
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Virani, S.S.; Alonso, A.; Benjamin, E.J.; Bittencourt, M.S.; Callaway, C.W.; Carson, A.P.; Chamberlain, A.M.; Chang, A.R.; Cheng, S.; Delling, F.N.; et al. Heart Disease and Stroke Statistics—2020 Update: A Report from the American Heart Association. Circulation 2020, 141, e139–e596. [Google Scholar] [CrossRef] [PubMed]
- Sim, R.; Cheung, G.; Ting, D.; Wong, E.; Wong, T.Y.; Yeo, I.; Wong, C.W. Retinal microvascular signs in COVID-19. Br. J. Ophthalmol. 2021, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Sun, C.; Ladores, C.; Hong, J.; Nguyen, D.Q.; Chua, J.; Ting, D.; Schmetterer, L.; Wong, T.Y.; Cheng, C.-Y.; Tan, A.C.S. Systemic hypertension associated retinal microvascular changes can be detected with optical coherence tomography angiography. Sci. Rep. 2020, 10, 9580. [Google Scholar] [CrossRef] [PubMed]
- Wagner, S.K.; Fu, D.J.; Faes, L.; Liu, X.; Huemer, J.; Khalid, H.; Ferraz, D.; Korot, E.; Kelly, C.; Balaskas, K.; et al. Insights into Systemic Disease through Retinal Imaging-Based Oculomics. Transl. Vis. Sci. Technol. 2020, 9, 6, Erratum in Transl. Vis. Sci. Technol. 2021, 10, 13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matsuoka, S.; Kaneko, H.; Okada, A.; Itoh, H.; Suzuki, Y.; Fujiu, K.; Michihata, N.; Jo, T.; Takeda, N.; Morita, H.; et al. Association of retinal atherosclerosis assessed using Keith-Wagener-Barker system with incident heart failure and other atherosclerotic cardiovascular disease: Analysis of 319,501 individuals from the general population. Atherosclerosis 2022, 348, 68–74. [Google Scholar] [CrossRef]
- Chua, J.; Le, T.; Sim, Y.C.; Chye, H.Y.; Tan, B.; Yao, X.; Wong, D.; Ang, B.W.Y.; Toh, D.; Lim, H.; et al. Relationship of Quantitative Retinal Capillary Network and Myocardial Remodeling in Systemic Hypertension. J. Am. Heart Assoc. 2022, 11, e024226. [Google Scholar] [CrossRef]
- Zhong, P.; Li, Z.; Lin, Y.; Peng, Q.; Huang, M.; Jiang, L.; Li, C.; Kuang, Y.; Cui, S.; Yu, D.; et al. Retinal microvasculature impairments in patients with coronary artery disease: An optical coherence tomography angiography study. Acta Ophthalmol. 2021, 100, 225–233. [Google Scholar] [CrossRef]
- Poplin, R.; Varadarajan, A.V.; Blumer, K.; Liu, Y.; McConnell, M.V.; Corrado, G.S.; Peng, L.; Webster, D.R. Prediction of cardiovascular risk factors from retinal fundus photographs via deep learning. Nat. Biomed. Eng. 2018, 2, 158–164. [Google Scholar] [CrossRef]
- Graham, I.; Atar, D.; Borch-Johnsen, K.; Boysen, G.; Burell, G.; Cifkova, R.; Dallongeville, J.; de Backer, G.; Ebrahim, S.; Gjelsvik, B.; et al. European Society of Cardiology (ESC) Committee for Practice Guidelines (CPG). European guidelines on cardiovascular disease preven-tion in clinical practice: Executive summary: Fourth Joint Task Force of the European Society of Cardiology and Other Societies on Cardiovascular Disease Prevention in Clinical Practice (Constituted by representatives of nine societies and by invited ex-perts). Eur. Heart J. 2007, 28, 2375–2414. [Google Scholar] [CrossRef] [Green Version]
- Weiter, J.J.; Zuckerman, R. The influence of the photoreceptor-RPE complex on the inner retina. An explanation for the beneficial effects of photocoagulation. Ophthalmology 1980, 87, 1133–1139. [Google Scholar] [CrossRef]
- Pournaras, C.J.; Rungger-Brändle, E.; Riva, C.E.; Hardarson, S.H.; Stefansson, E. Regulation of retinal blood flow in health and disease. Prog. Retin. Eye Res. 2008, 27, 284–330. [Google Scholar] [CrossRef] [PubMed]
- Madamanchi, N.R.; Vendrov, A.; Runge, M.S. Oxidative Stress and Vascular Disease. Arteroscler. Thromb. Vasc. Biol. 2005, 25, 29–38. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hanssen, H.; Streese, L.; Vilser, W. Retinal vessel diameters and function in cardiovascular risk and disease. Prog. Retin. Eye Res. 2022, 101095. [Google Scholar] [CrossRef]
- Luo, X.; Shen, Y.-M.; Jiang, M.-N.; Lou, X.-F.; Shen, Y. Ocular Blood Flow Autoregulation Mechanisms and Methods. J. Ophthalmol. 2015, 2015, 864871. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Palkovits, S.; Lasta, M.; Told, R.; Schmidl, D.; Boltz, A.; Napora, K.J.; Werkmeister, R.M.; Popa-Cherecheanu, A.; Garhöfer, G.; Schmetterer, L. Retinal Oxygen Metabolism during Normoxia and Hyperoxia in Healthy Subjects. Investig. Opthalmol. Vis. Sci. 2014, 55, 4707–4713. [Google Scholar] [CrossRef]
- Lansman, J.B.; Hallam, T.J.; Rink, T.J. Single stretch-activated ion channels in vascular endothelial cells as mechanotransducers? Nature 1987, 325, 811–813. [Google Scholar] [CrossRef]
- Luksch, A.; Garhöfer, G.; Imhof, A.; Polak, K.; Polska, E.; Dorner, G.T.; Anzenhofer, S.; Wolzt, M.; Schmetterer, L. Effect of inhalation of different mixtures of O2 and CO2 on retinal blood flow. Br. J. Ophthalmol. 2002, 86, 1143–1147. [Google Scholar] [CrossRef] [Green Version]
- Keeter, W.C.; Ma, S.; Stahr, N.; Moriarty, A.K.; Galkina, E.V. Atherosclerosis and multi-organ-associated pathologies. Semin. Immunopathol. 2022, 44, 363–374. [Google Scholar] [CrossRef]
- Mushenkova, N.V.; Summerhill, V.I.; Zhang, D.; Romanenko, E.B.; Grechko, A.V.; Orekhov, A.N. Current Advances in the Diagnostic Imaging of Atherosclerosis: Insights into the Pathophysiology of Vulnerable Plaque. Int. J. Mol. Sci. 2020, 21, 2992. [Google Scholar] [CrossRef] [Green Version]
- Farrehi, P.M.; Bernstein, S.J.; Rasak, M.; Dabbous, S.A.; Stomel, R.J.; Eagle, K.A.; Rubenfire, M. Frequency of negative coronary arterio-graphic findings in patients with chest pain is related to community practice patterns. Am. J. Manag. Care 2002, 8, 643. [Google Scholar]
- Tonino, P.A.; Fearon, W.F.; de Bruyne, B.; Oldroyd, K.G.; Leesar, M.A.; Lee, P.N.V.; MacCarthy, P.A.; Veer, M.V.; Pijls, N.H. Angiographic Versus Functional Severity of Coronary Artery Stenoses in the FAME Study: Fractional Flow Reserve Versus Angiography in Multivessel Evaluation. J. Am. Coll. Cardiol. 2010, 55, 2816–2821. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cheng, L.; Barlis, P.; Gibson, J.; Colville, D.; Hutchinson, A.F.; Gleeson, G.; Lamoureux, E.; VanGaal, W.; Savige, J. Microvascular retinopathy and angiographically-demonstrated coronary artery disease: A cross-sectional, observational study. PLoS ONE 2018, 13, e0192350. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liew, G.; Mitchell, P.; Chiha, J.; Plant, A.J.H.; White, A.; Joachim, N.; Wang, S.; Burlutsky, G.; Kovoor, P.; Thiagalingam, A.; et al. Retinal microvascular changes in microvascular angina: Findings from the Australian Heart Eye Study. Microcirculation 2019, 26, e12536. [Google Scholar] [CrossRef] [PubMed]
- Liew, G.; Wang, J.J. Manifestaciones vasculares retinianas: ¿Reflejan el estado del corazón? Rev. Esp. Cardiol. 2011, 64, 515–521. [Google Scholar] [CrossRef] [PubMed]
- Flammer, J.; Konieczka, K.; Bruno, R.M.; Virdis, A.; Flammer, A.; Taddei, S. The eye and the heart. Eur. Heart J. 2013, 34, 1270–1278. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.J.; Liew, G.; Klein, R.; Rochtchina, E.; Knudtson, M.D.; Klein, B.E.; Wong, T.Y.; Burlutsky, G.; Mitchell, P. Retinal vessel diameter and cardiovascular mortality: Pooled data analysis from two older populations. Eur. Heart J. 2007, 28, 1984–1992. [Google Scholar] [CrossRef] [PubMed]
- Aumann, S.; Donner, S.; Fischer, J.; Müller, F. Optical Coherence Tomography (OCT): Principle and Technical Realization. In High Resolution Imaging in Microscopy and Ophthalmology: New Frontiers in Biomedical Optics; Bille, J.F., Ed.; Springer: Cham, Switzerland, 2019; Chapter 3. [Google Scholar]
- Ahmad, M.; Kaszubski, P.A.; Cobbs, L.; Reynolds, H.; Smith, R.T. Choroidal thickness in patients with coronary artery disease. PLoS ONE 2017, 12, e0175691. [Google Scholar] [CrossRef] [Green Version]
- De Carlo, T.E.; Romano, A.; Waheed, N.K.; Duker, J.S. A review of optical coherence tomography angiography (OCTA). Int. J. Retin. Vitr. 2015, 1, 5. [Google Scholar] [CrossRef] [Green Version]
- Chua, J.; Chin, C.W.L.; Hong, J.; Chee, M.L.; Le, T.-T.; Ting, D.S.W.; Wong, T.Y.; Schmetterer, L. Impact of hypertension on retinal capillary microvasculature using optical coherence tomographic angiography. J. Hypertens. 2019, 37, 572–580. [Google Scholar] [CrossRef]
- Lee, W.H.; Park, J.-H.; Won, Y.; Lee, M.-W.; Shin, Y.-I.; Jo, Y.-J.; Kim, J.-Y. Retinal Microvascular Change in Hypertension as measured by Optical Coherence Tomography Angiography. Sci. Rep. 2019, 9, 156. [Google Scholar] [CrossRef] [Green Version]
- Arnould, L.; Guenancia, C.; Azemar, A.; Alan, G.; Pitois, S.; Bichat, F.; Zeller, M.; Gabrielle, P.-H.; Bron, A.M.; Creuzot-Garcher, C.; et al. The EYE-MI Pilot Study: A Prospective Acute Coronary Syndrome Cohort Evaluated With Retinal Optical Coherence Tomography Angiography. Investig. Opthalmol. Vis. Sci. 2018, 59, 4299–4306. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.H.; Kim, S.E.; Kim, S.H.; Choi, B.W.; Rim, T.H.; Byeon, S.H.; Kim, S.S. Relationship between Coronary Artery Calcification and Central Chorioretinal Thickness in Patients with Subclinical Atherosclerosis. Ophthalmologica 2021, 244, 18–26. [Google Scholar] [CrossRef] [PubMed]
- Yoon, J.; Kang, H.J.; Lee, J.Y.; Kim, J.-G.; Yoon, Y.H.; Jung, C.H.; Kim, Y.J. Associations Between the Macular Microvasculatures and Subclinical Atherosclerosis in Patients With Type 2 Diabetes: An Optical Coherence Tomography Angiography Study. Front. Med. 2022, 9, 843176. [Google Scholar] [CrossRef] [PubMed]
- Viladés, E.; Palomar, A.P.-D.; Cegoñino, J.; Obis, J.; Satue, M.; Orduna, E.; Pablo, L.E.; Ciprés, M.; Garcia-Martin, E. Physiological changes in retinal layers thicknesses measured with swept source optical coherence tomography. PLoS ONE 2020, 15, e0240441. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.A.; Hashim, M.J.; Mustafa, H.; Baniyas, M.Y.; Al Suwaidi, S.K.B.M.; Alkatheeri, R.; Alblooshi, F.M.K.; Almatrooshi, M.E.A.H.; Alzaabi, M.E.H.; Al Darmaki, R.S.; et al. Global Epidemiology of Ischemic Heart Disease: Results from the Global Burden of Disease Study. Cureus 2020, 12, e9349. [Google Scholar] [CrossRef] [PubMed]
- Aschauer, J.; Aschauer, S.; Pollreisz, A.; Datlinger, F.; Gatterer, C.; Mylonas, G.; Egner, B.; Hofer, D.; Steiner, I.; Hengstenberg, C.; et al. Identification of Subclinical Microvascular Biomarkers in Coronary Heart Disease in Retinal Imaging. Transl. Vis. Sci. Technol. 2021, 10, 24. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Jiang, J.; Zhang, Y.; Qian, Y.W.; Zhang, J.F.; Wang, Z.L. Retinal and choroidal vascular changes in coronary heart disease: An optical coherence tomography angiography study. Biomed. Opt. Express 2019, 10, 1532–1544. [Google Scholar] [CrossRef]
- Cheng, C.; Daskalakis, C.; Falkner, B. Original Research: Capillary rarefaction in treated and untreated hypertensive subjects. Ther. Adv. Cardiovasc. Dis. 2008, 2, 79–88. [Google Scholar] [CrossRef] [Green Version]
- Ziegler, T.; Abdel Rahman, F.; Jurisch, V.; Kupatt, C. Atherosclerosis and the Capillary Network; Pathophysiology and Potential Therapeutic Strategies. Cells 2019, 9, 50. [Google Scholar] [CrossRef] [Green Version]
- Arnould, L.; Seydou, A.; Gabrielle, P.-H.; Guenancia, C.; Tzourio, C.; Bourredjem, A.; El Alami, Y.; Daien, V.; Binquet, C.; Bron, A.M.; et al. Subfoveal Choroidal Thickness, Cardiovascular History, and Risk Factors in the Elderly: The Montrachet Study. Investig. Opthalmol. Vis. Sci. 2019, 60, 2431–2437. [Google Scholar] [CrossRef] [Green Version]
- Kocamaz, M.; Karadağ, O.; Onder, S.E. Comparison of choroidal thicknesses in patients with coronary artery disease and patients at risk of coronary artery disease. Int. Ophthalmol. 2021, 41, 2117–2124. [Google Scholar] [CrossRef] [PubMed]
- Aydin, E.; Kazanci, L.; Yilmaz, M.B.; Akcay, F.A.; Bayata, S. Analysis of central macular thickness and choroidal thickness changes in patients with cardiovascular risk factors. Eye 2020, 34, 2068–2075. [Google Scholar] [CrossRef] [PubMed]
- Mittal, S.; Waghamare, S.; Mittal, S.; Pathania, M.; Samanta, R.; Kumawat, D.; Gupta, N. Comparison of choroidal thickness in systemic hypertensive subjects with healthy individuals by spectral domain optical coherence tomography. Indian J. Ophthalmol. 2021, 69, 1183–1188. [Google Scholar] [CrossRef] [PubMed]
- Ding, X.; Li, J.; Zeng, J.; Ma, W.; Liu, R.; Li, T.; Yu, S.; Tang, S. Choroidal Thickness in Healthy Chinese Subjects. Investig. Opthalmol. Vis. Sci. 2011, 52, 9555–9560. [Google Scholar] [CrossRef] [Green Version]
- Zhong, P.; Qin, J.; Li, Z.; Jiang, L.; Peng, Q.; Huang, M.; Lin, Y.; Liu, B.; Li, C.; Wu, Q.; et al. Development and Validation of Retinal Vasculature Nomogram in Suspected Angina Due to Coronary Artery Disease. J. Atheroscler. Thromb. 2022, 29, 579–596. [Google Scholar] [CrossRef]
- Monteiro-Henriques, I.; Rocha-Sousa, A.; Barbosa-Breda, J. Optical coherence tomography angiography changes in cardiovascular systemic diseases and risk factors: A Review. Acta Ophthalmol. 2022, 100, e1–e15. [Google Scholar] [CrossRef]
- Cortes, V.A.; Busso, D.; Maiz, A.; Arteaga, A.; Nervi, F.; Rigotti, A. Physiological and pathological implications of cholesterol. Front. Biosci. 2014, 19, 416–428. [Google Scholar] [CrossRef] [Green Version]
- Curcio, C.A. Soft Drusen in Age-Related Macular Degeneration: Biology and Targeting Via the Oil Spill Strategies. Investig. Opthalmol. Vis. Sci. 2018, 59, AMD160–AMD181. [Google Scholar] [CrossRef] [Green Version]
- Tserentsoodol, N.; Sztein, J.M.; Campos, M.; Gordiyenko, N.V.; Fariss, R.N.; Lee, J.W.; Fliesler, S.J.; Rodriguez, I.R. Uptake of cholesterol by the retina occurs primarily via a low density lipoprotein receptor-mediated process. Mol. Vis. 2006, 12, 1306–1318. [Google Scholar]
- Shi, R.; Lu, Y.; Liu, D.; Guo, Z. Association of serum apolipoprotein B with retinal neurovascular structural alterations in patients with type 2 diabetes: An optical coherence tomography angiography study. Geol. Rundsch. 2021, 58, 1673–1681. [Google Scholar] [CrossRef]
- Mullins, R.F.; Russell, S.R.; Anderson, D.H.; Hageman, G.S. Drusen associated with aging and age-related macular degeneration contain proteins common to extracellular deposits associated with atherosclerosis, elastosis, amyloidosis, and dense deposit disease. FASEB J. 2000, 14, 835. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Feinberg, M.W. Vascular Endothelial Senescence: Pathobiological Insights, Emerging Long Noncoding RNA Targets, Challenges and Therapeutic Opportunities. Front. Physiol. 2021, 12, 693067. [Google Scholar] [CrossRef]
- Ramírez, R.; Ceprian, N.; Figuer, A.; Valera, G.; Bodega, G.; Alique, M.; Carracedo, J. Endothelial Senescence and the Chronic Vascular Diseases: Challenges and Therapeutic Opportunities in Atherosclerosis. J. Pers. Med. 2022, 12, 215. [Google Scholar] [CrossRef] [PubMed]
- Zechariah, A.; ElAli, A.; Hagemann, N.; Jin, F.; Doeppner, T.R.; Helfrich, I.; Mies, G.; Hermann, D.M. Hyperlipidemia Attenuates Vascular Endothelial Growth Factor–Induced Angiogenesis, Impairs Cerebral Blood Flow, and Disturbs Stroke Recovery via Decreased Pericyte Coverage of Brain Endothelial Cells. Arterioscler. Thromb. Vasc. Biol. 2013, 33, 1561–1567. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Radice, G.L. N-Cadherin-Mediated Adhesion and Signaling from Development to Disease. Prog. Mol. Biol. Transl. Sci. 2013, 116, 263–289. [Google Scholar] [CrossRef]
- Abdolrahimzadeh, S.; Parisi, F.; Scavella, V.; Recupero, S.M. Optical Coherence Tomography Evidence on The Correlation of Choroidal Thickness and Age with Vascularized Retinal Layers in Normal Eyes. Retina 2016, 36, 2329–2338. [Google Scholar] [CrossRef]
- Flint, A.J.; Rexrode, K.M.; Hu, F.B.; Glynn, R.J.; Caspard, H.; Manson, J.E.; Willett, W.C.; Rimm, E.B. Body mass index, waist circumference, and risk of coronary heart disease: A prospective study among men and women. Obes. Res. Clin. Pract. 2010, 4, e171–e181. [Google Scholar] [CrossRef] [Green Version]
- Ross, R.; Neeland, I.J.; Yamashita, S.; Shai, I.; Seidell, J.; Magni, P.; Santos, R.D.; Arsenault, B.; Cuevas, A.; Hu, F.B.; et al. Waist circumference as a vital sign in clinical practice: A Consensus Statement from the IAS and ICCR Working Group on Visceral Obesity. Nat. Rev. Endocrinol. 2020, 16, 177–189. [Google Scholar] [CrossRef]
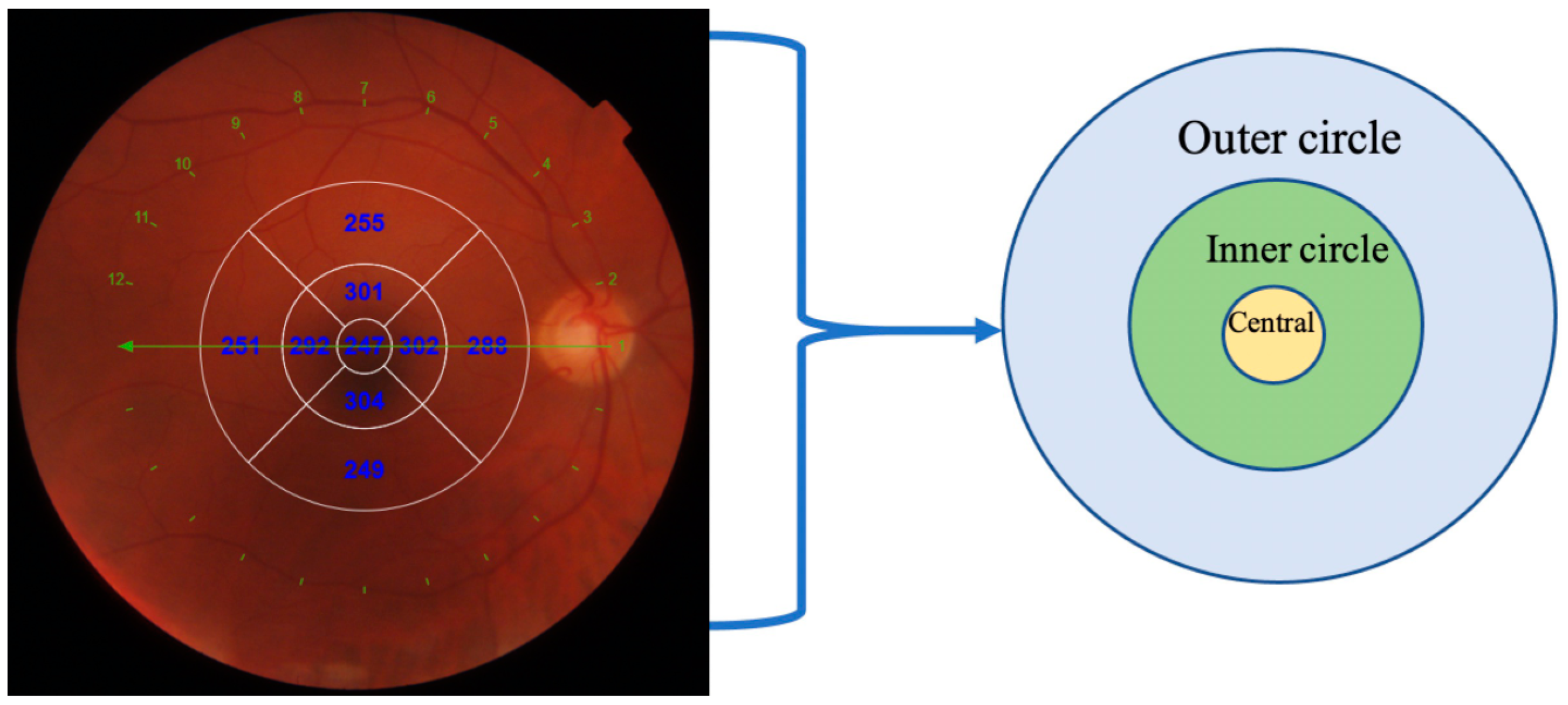

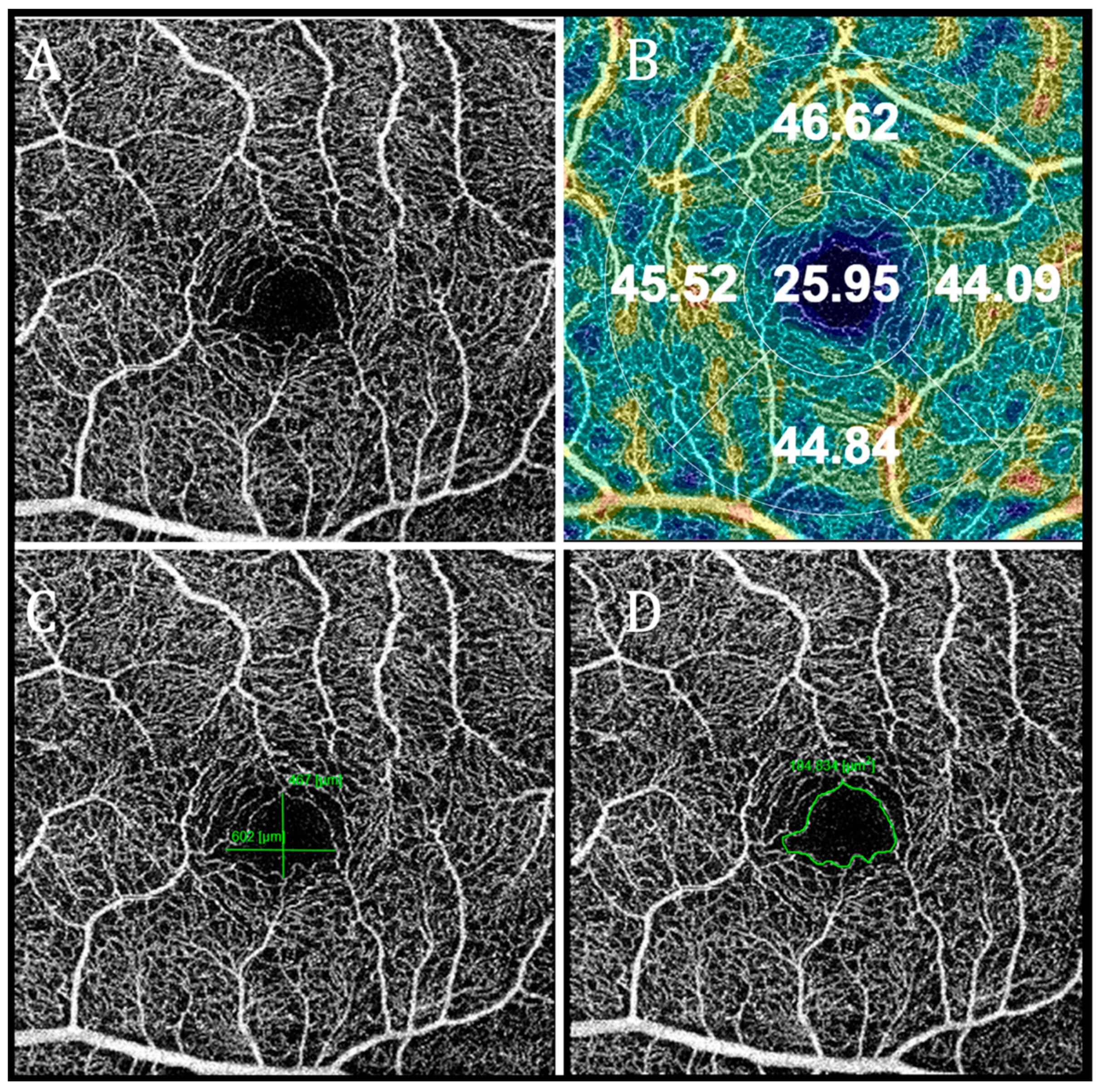
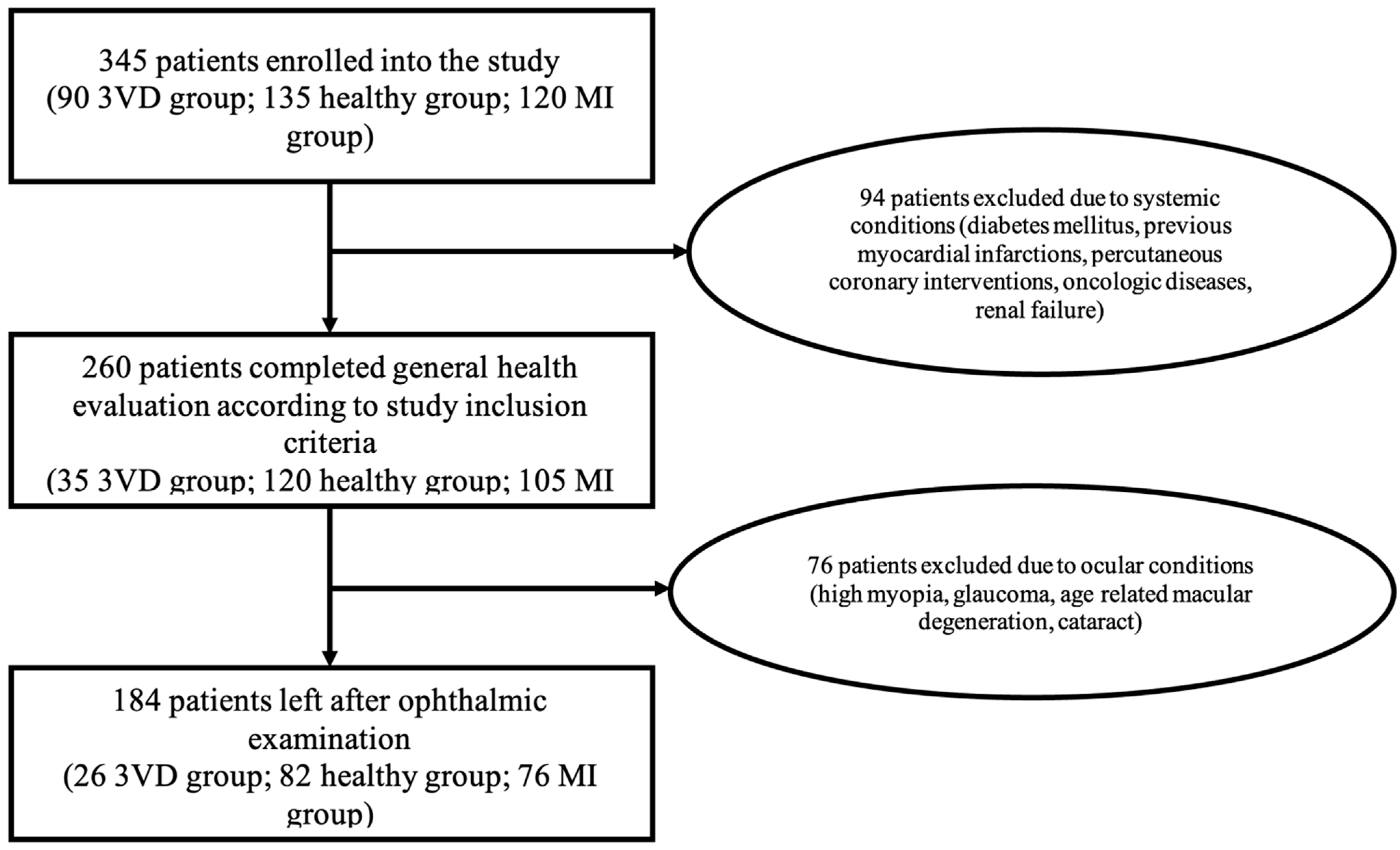
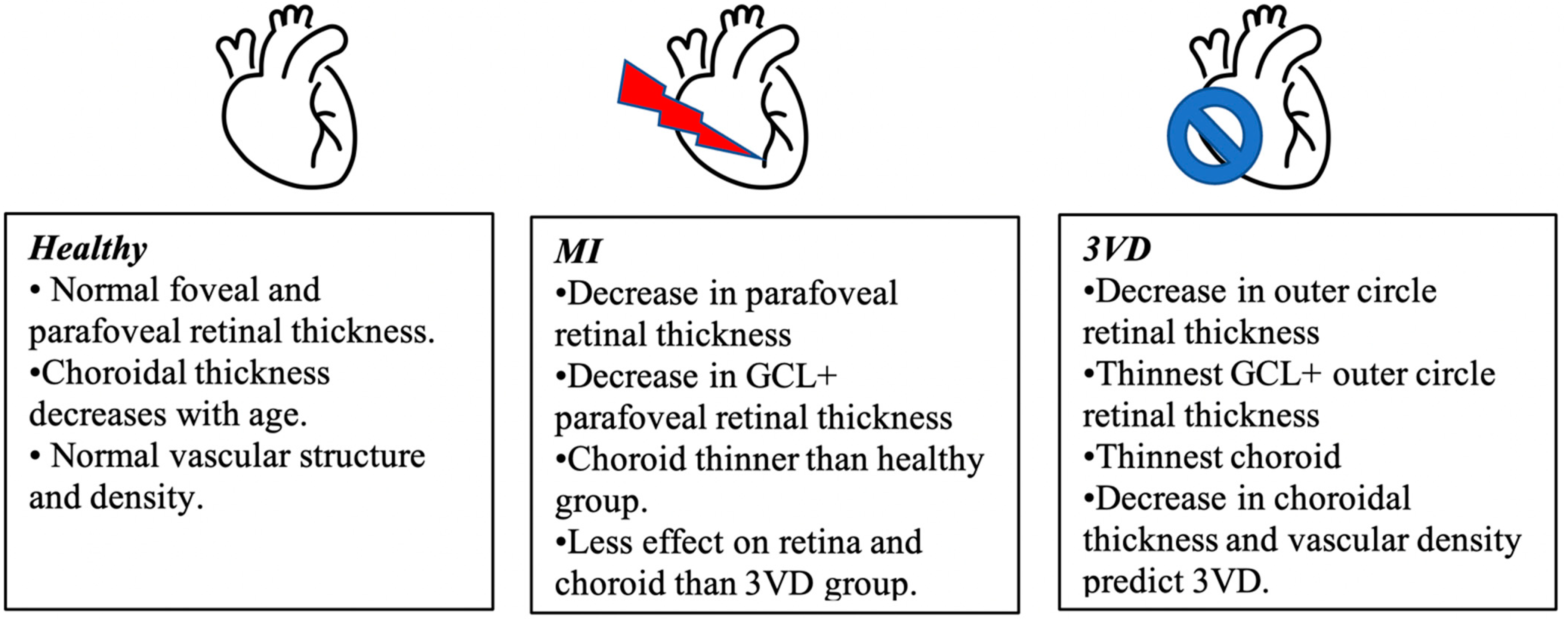
| Variable | Healthy | Myocardial Infarction Group | 3 Vessel Disease Group |
|---|---|---|---|
| Age in years | |||
| Median (range) | 61.22 (44.51–77.19) 1 | 61.76 (37.71–78.81) | 64.83 (50.14–80.45) 1 |
| Sex: | |||
| Male, 112 (60.9%) | 46 (56%) | 48 (63%) | 18 (69%) |
| Female, 72 (39.1%) | 36 (44%) | 28 (37%) | 8 (31%) |
| Systolic blood pressure (mmHg) | |||
| Median (range) | 130 (100–180) | 140 (80–250) | 137.50 (120–160) |
| Diastolic blood pressure (mmHg) | |||
| Median (range) * | 80 (60–102) 2 | 85 (60–140) 2 | 80 (70–100) |
| Body mass index (kg/m2) | |||
| Median (range) º | 29.74 (23.03–45.91) 3 | 28.41 (20.55–39.79) 3 | 28.82 (22.07–36.78) |
| Waist circumference (cm) | |||
| Median (range) | 104 (81–132) | 98 (70–133) | 101 (76–131) |
| Smoking (in pack years) | |||
| Median (range) • | 12.13 (0.1–52.0) 4 | 26.75 (0.25–110) 4 | 23.5 (1.0–50.0) |
| Alcohol consumption (standard alcohol unit) | |||
| Median (range) | 0.75 (0.0–20.0) | 0.5 (0.0–32.0) | 0.0 (0.0–21.0) |
| Left ventricular dimensions at end of diastole (mm) | |||
| Median (range) | 49.0 (40.0–73.0) | 48.0 (37.0–61.0) | 48.5 (37.0–59.0) |
| Left ventricular posterior wall thickness at end of diastole (mm) | |||
| Median (range) | 11.0 (8.0–14.0) | 10.0 (8.0–13.8) | 10.0 (8.0–13.0) |
| Left ventricular ejection fraction (%) | |||
| Median (range) • | 55.0 (25.0–70.0) 5 | 45.0 (20.0–55.0) 5,6 | 55.0 (40.0–60.0) 6 |
| Interventricular septum at end diastole (mm) | |||
| Median (range) | 11.0 (8.0–15.5) | 11.3 (7.5–17.3) | 11.0 (9.5–16.0) |
| Myocardial mass index (g/m2) | |||
| Median (range) | 97.80 (70.12–182.7) | 95.42 (47.91–176.06) | 91.56 (65.05–150.83) |
| Variable | Myocardial Infarction | Three Vessel Disease | ||||
|---|---|---|---|---|---|---|
| Odds Ratio | 95% CI | p-Value | Odds Ratio | 95% CI | p-Value | |
| Body mass index | 0.899 | 0.832–0.971 | 0.007 | No significance | ||
| Waist circumference | 0.973 | 0.948–0.999 | 0.041 | No significance | ||
| Left ventricular dimensions at end of diastole (mm) | 0.934 | 0.875–0.996 | 0.038 | No significance | ||
| Total cholesterol | 1.366 | 1.026 1.818 | 0.033 | No significance | ||
| LDL cholesterol | 1.503 | 1.051–2.149 | 0.026 | No significance | ||
| Atherogenic coefficient | 1.523 | 1.128–2.055 | 0.006 | No significance | ||
| Age | No significance | 1.064 | 1.008–1.123 | 0.024 | ||
| Creatinine | No significance | 1.034 | 1.00–1.070 | 0.047 | ||
| Retinal thickness | ||||||
| Outer circle | 0.975 | 0.953–0.998 | 0.036 | No significance | ||
| RNFL layer | ||||||
| Inner circle | No significance | 1.082 | 1.004–1.166 | 0.039 | ||
| GCL+ layer | ||||||
| Inner circle | 0.952 | 0.916–0.989 | 0.012 | 0.929 | 0.878–0.983 | 0.011 |
| Outer circle | No significance | 0.865 | 0.794–0.941 | 0.001 | ||
| Choroidal thickness | ||||||
| Central | No significance | 0.993 | 0.986–1.00 | 0.041 | ||
| Inner circle | No significance | 0.989 | 0.982–0.997 | 0.005 | ||
| Outer circle | No significance | 0.987 | 0.978–0.995 | 0.002 | ||
| Vascular density—Superficial capillary plexus (FAZ 6 × 6) | ||||||
| Central | No significance | 0.872 | 0.773–0.985 | 0.027 | ||
| Circle | No significance | 0.735 | 0.548–0.984 | 0.038 | ||
| Vascular density—Deep capillary plexus (FAZ 3 × 3) | ||||||
| Circle | No significance | 0.773 | 0.602–0.992 | 0.043 | ||
| Variable | Odds Ratio | 95% CI | p-Value |
|---|---|---|---|
| Choroid outer circle | 0.979 | 0.966–0.992 | 0.002 |
| Vascular density—superficial capillary plexus (FAZ 6 × 6) central | 0.819 | 0.699–0.959 | 0.013 |
| Creatinine | 1.041 | 1.00–1.084 | 0.048 |
| Authors | Journal | Year of Publication | No. of Participants | Results |
|---|---|---|---|---|
| Presented study | Choroidal thickness and central vascular density in SCP are significant predictors of three vessel disease. Decreased outer retinal thickness in MI and 3VD groups. | |||
| Zhong et al. [7] | Acta ophthalmologica | 2022 | 410 participants | Decreased VD in SCP and DCP decreased total retinal thickness in coronary artery disease. |
| Aschauer et al. [37] | Transaltional Vision Science and Technology | 2021 | 45 participants | A trend of decreased vascular density (p > 0.05) in coronary heart disease. |
| Wang et al. [38] | Biomedical optics express | 2019 | 316 participants | Decreased retinal thickness, density and flow area, except fovea, and more intensive vessel density in outer retina in coronary heart disease patients. Retinal and choroidal microvasculature changes related to coronary artery and branch stenosis. |
| Arnould et al. [22] | Investigative ophthalmology and visual science | 2018 | 275 participants | Decreased retinal vascular density (inner vessel density), association between inner vessel density and the GRACE and REACH score. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Matulevičiūtė, I.; Sidaraitė, A.; Tatarūnas, V.; Veikutienė, A.; Dobilienė, O.; Žaliūnienė, D. Retinal and Choroidal Thinning—A Predictor of Coronary Artery Occlusion? Diagnostics 2022, 12, 2016. https://doi.org/10.3390/diagnostics12082016
Matulevičiūtė I, Sidaraitė A, Tatarūnas V, Veikutienė A, Dobilienė O, Žaliūnienė D. Retinal and Choroidal Thinning—A Predictor of Coronary Artery Occlusion? Diagnostics. 2022; 12(8):2016. https://doi.org/10.3390/diagnostics12082016
Chicago/Turabian StyleMatulevičiūtė, Indrė, Agnė Sidaraitė, Vacis Tatarūnas, Audronė Veikutienė, Olivija Dobilienė, and Dalia Žaliūnienė. 2022. "Retinal and Choroidal Thinning—A Predictor of Coronary Artery Occlusion?" Diagnostics 12, no. 8: 2016. https://doi.org/10.3390/diagnostics12082016
APA StyleMatulevičiūtė, I., Sidaraitė, A., Tatarūnas, V., Veikutienė, A., Dobilienė, O., & Žaliūnienė, D. (2022). Retinal and Choroidal Thinning—A Predictor of Coronary Artery Occlusion? Diagnostics, 12(8), 2016. https://doi.org/10.3390/diagnostics12082016





