The Urinary Microbiome: Role in Bladder Cancer and Treatment
Abstract
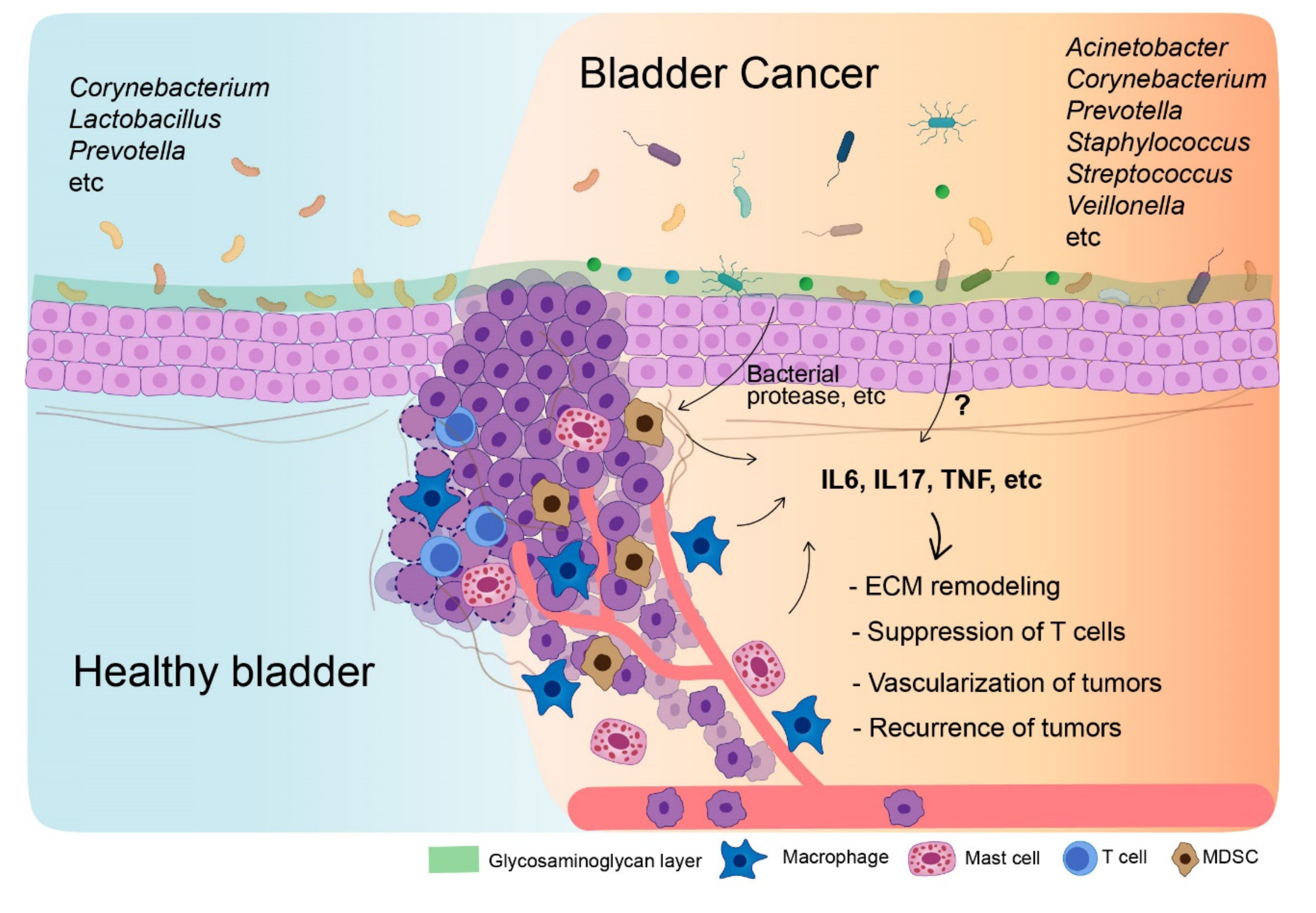
:1. Introduction
2. Differences in Urinary Microbiome Sample Collection Methods
3. Urinary Microbiome in Healthy Individuals
4. Urinary Microbiome in Bladder Cancer Patients
5. Tumor Microenvironment in Bladder Cancer
5.1. Macrophages
5.2. Myeloid-Derived Suppressor Cells
5.3. Tregs
5.4. Mast Cells
6. Microbiome-Mediated Cytokines for Tumor Microenvironment Establishment
6.1. IL6
6.2. Tumor Necrosis Factor
6.3. Interleukin-17
7. Urinary Microbiome and BCG Responsiveness
8. Urinary Microbiome as Bladder Cancer Therapy
9. Microbiome and Immune Checkpoint Immunotherapy
10. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2020. CA Cancer J. Clin. 2020, 70, 7–30. [Google Scholar] [CrossRef] [PubMed]
- Lenis, A.T.; Lec, P.M.; Chamie, K.; Mshs, M.D. Bladder Cancer: A Review. JAMA 2020, 324, 1980–1991. [Google Scholar] [CrossRef] [PubMed]
- Inamura, K. Bladder Cancer: New Insights into Its Molecular Pathology. Cancers 2018, 10, 100. [Google Scholar] [CrossRef] [PubMed]
- Berdik, C. Unlocking bladder cancer. Nature 2017, 551, S34–S35. [Google Scholar] [CrossRef]
- Andolfi, C.; Bloodworth, J.C.; Papachristos, A.; Sweis, R.F. The Urinary Microbiome and Bladder Cancer: Susceptibility and Immune Responsiveness. Bladder Cancer 2020, 6, 225–235. [Google Scholar] [CrossRef]
- National Cancer Institute. Cancer Stat Facts: Bladder Cancer. Available online: https://seer.cancer.gov/statfacts/html/urinb.html (accessed on 4 July 2022).
- Mostafa, M.H.; Sheweita, S.A.; O’Connor, P.J. Relationship between schistosomiasis and bladder cancer. Clin. Microbiol. Rev. 1999, 12, 97–111. [Google Scholar] [CrossRef]
- Bucevic Popovic, V.; Situm, M.; Chow, C.T.; Chan, L.S.; Roje, B.; Terzic, J. The urinary microbiome associated with bladder cancer. Sci. Rep. 2018, 8, 12157. [Google Scholar] [CrossRef]
- Lewis, D.A.; Brown, R.; Williams, J.; White, P.; Jacobson, S.K.; Marchesi, J.R.; Drake, M.J. The human urinary microbiome; bacterial DNA in voided urine of asymptomatic adults. Front. Cell Infect. Microbiol. 2013, 3, 41. [Google Scholar] [CrossRef]
- Fouts, D.E.; Pieper, R.; Szpakowski, S.; Pohl, H.; Knoblach, S.; Suh, M.J.; Huang, S.T.; Ljungberg, I.; Sprague, B.M.; Lucas, S.K.; et al. Integrated next-generation sequencing of 16S rDNA and metaproteomics differentiate the healthy urine microbiome from asymptomatic bacteriuria in neuropathic bladder associated with spinal cord injury. J. Transl. Med. 2012, 10, 174. [Google Scholar] [CrossRef]
- Pederzoli, F.; Ferrarese, R.; Amato, V.; Locatelli, I.; Alchera, E.; Luciano, R.; Nebuloni, M.; Briganti, A.; Gallina, A.; Colombo, R.; et al. Sex-Specific Alterations in the Urinary and Tissue Microbiome in Therapy-Naive Urothelial Bladder Cancer Patients. Eur. Urol. Oncol. 2020, 3, 784–788. [Google Scholar] [CrossRef]
- Alfano, M.; Canducci, F.; Nebuloni, M.; Clementi, M.; Montorsi, F.; Salonia, A. The interplay of extracellular matrix and microbiome in urothelial bladder cancer. Nat. Rev. Urol. 2016, 13, 77–90. [Google Scholar] [CrossRef]
- Kiraly, O.; Gong, G.; Olipitz, W.; Muthupalani, S.; Engelward, B.P. Inflammation-induced cell proliferation potentiates DNA damage-induced mutations in vivo. PLoS Genet. 2015, 11, e1004901. [Google Scholar] [CrossRef]
- Wu, P.; Zhang, G.; Zhao, J.; Chen, J.; Chen, Y.; Huang, W.; Zhong, J.; Zeng, J. Profiling the Urinary Microbiota in Male Patients With Bladder Cancer in China. Front. Cell Infect. Microbiol. 2018, 8, 167. [Google Scholar] [CrossRef]
- Takahashi, T.; Kushiro, A.; Nomoto, K.; Uchida, K.; Morotomi, M.; Yokokura, T.; Akaza, H. Antitumor effects of the intravesical instillation of heat killed cells of the Lactobacillus casei strain Shirota on the murine orthotopic bladder tumor MBT-2. J. Urol. 2001, 166, 2506–2511. [Google Scholar] [CrossRef]
- Seow, S.W.; Cai, S.; Rahmat, J.N.; Bay, B.H.; Lee, Y.K.; Chan, Y.H.; Mahendran, R. Lactobacillus rhamnosus GG induces tumor regression in mice bearing orthotopic bladder tumors. Cancer Sci. 2010, 101, 751–758. [Google Scholar] [CrossRef]
- Naito, S.; Koga, H.; Yamaguchi, A.; Fujimoto, N.; Hasui, Y.; Kuramoto, H.; Iguchi, A.; Kinukawa, N.; Kyushu University Urological Oncology Group. Prevention of recurrence with epirubicin and Lactobacillus casei after transurethral resection of bladder cancer. J. Urol. 2008, 179, 485–490. [Google Scholar] [CrossRef]
- Perez-Carrasco, V.; Soriano-Lerma, A.; Soriano, M.; Gutierrez-Fernandez, J.; Garcia-Salcedo, J.A. Urinary Microbiome: Yin and Yang of the Urinary Tract. Front. Cell Infect. Microbiol. 2021, 11, 617002. [Google Scholar] [CrossRef]
- Eliacik, K.; Kanik, A.; Yavascan, O.; Alparslan, C.; Kocyigit, C.; Aksu, N.; Bakiler, A.R. A Comparison of Bladder Catheterization and Suprapubic Aspiration Methods for Urine Sample Collection From Infants With a Suspected Urinary Tract Infection. Clin. Pediatr. 2016, 55, 819–824. [Google Scholar] [CrossRef]
- Gajdács, M.; Ábrók, M.; Lázár, A.; Burián, K. Microbiology of urine samples obtained through suprapubic bladder aspiration: A 10-year epidemiological snapshot. Dev. Health Sci. DHS 2019, 2, 76–78. [Google Scholar] [CrossRef]
- Badiee, Z.; Sadeghnia, A.; Zarean, N. Suprapubic Bladder Aspiration or Urethral Catheterization: Which is More Painful in Uncircumcised Male Newborns? Int. J. Prev. Med. 2014, 5, 1125–1130. [Google Scholar]
- Liu, F.; Liu, A.; Lu, X.; Zhang, Z.; Xue, Y.; Xu, J.; Zeng, S.; Xiong, Q.; Tan, H.; He, X.; et al. Dysbiosis signatures of the microbial profile in tissue from bladder cancer. Cancer Med. 2019, 8, 6904–6914. [Google Scholar] [CrossRef] [Green Version]
- Mansour, B.; Monyok, A.; Makra, N.; Gajdacs, M.; Vadnay, I.; Ligeti, B.; Juhasz, J.; Szabo, D.; Ostorhazi, E. Bladder cancer-related microbiota: Examining differences in urine and tissue samples. Sci. Rep. 2020, 10, 11042. [Google Scholar] [CrossRef]
- Pohl, H.G.; Groah, S.L.; Perez-Losada, M.; Ljungberg, I.; Sprague, B.M.; Chandal, N.; Caldovic, L.; Hsieh, M. The Urine Microbiome of Healthy Men and Women Differs by Urine Collection Method. Int. Neurourol. J. 2020, 24, 41–51. [Google Scholar] [CrossRef]
- He, S.; Li, H.; Yu, Z.; Zhang, F.; Liang, S.; Liu, H.; Chen, H.; Lu, M. The Gut Microbiome and Sex Hormone-Related Diseases. Front. Microbiol. 2021, 12, 711137. [Google Scholar] [CrossRef]
- Kim, Y.S.; Unno, T.; Kim, B.Y.; Park, M.S. Sex Differences in Gut Microbiota. World J. Mens Health 2020, 38, 48–60. [Google Scholar] [CrossRef]
- Fransen, F.; van Beek, A.A.; Borghuis, T.; Meijer, B.; Hugenholtz, F.; van der Gaast-de Jongh, C.; Savelkoul, H.F.; de Jonge, M.I.; Faas, M.M.; Boekschoten, M.V.; et al. The Impact of Gut Microbiota on Gender-Specific Differences in Immunity. Front. Immunol. 2017, 8, 754. [Google Scholar] [CrossRef]
- Modena, B.D.; Milam, R.; Harrison, F.; Cheeseman, J.A.; Abecassis, M.M.; Friedewald, J.J.; Kirk, A.D.; Salomon, D.R. Changes in Urinary Microbiome Populations Correlate in Kidney Transplants With Interstitial Fibrosis and Tubular Atrophy Documented in Early Surveillance Biopsies. Am. J. Transplant. 2017, 17, 712–723. [Google Scholar] [CrossRef]
- Siddiqui, H.; Nederbragt, A.J.; Lagesen, K.; Jeansson, S.L.; Jakobsen, K.S. Assessing diversity of the female urine microbiota by high throughput sequencing of 16S rDNA amplicons. BMC Microbiol. 2011, 11, 244. [Google Scholar] [CrossRef] [PubMed]
- Pearce, M.M.; Hilt, E.E.; Rosenfeld, A.B.; Zilliox, M.J.; Thomas-White, K.; Fok, C.; Kliethermes, S.; Schreckenberger, P.C.; Brubaker, L.; Gai, X.; et al. The female urinary microbiome: A comparison of women with and without urgency urinary incontinence. mBio 2014, 5, e01283-4. [Google Scholar] [CrossRef] [PubMed]
- Song, C.H.; Kim, Y.H.; Naskar, M.; Hayes, B.W.; Abraham, M.A.; Noh, J.H.; Suk, G.; Kim, M.J.; Cho, K.S.; Shin, M.; et al. Lactobacillus crispatus Limits Bladder Uropathogenic E. coli Infection by Triggering a Host Type I Interferon Response. Proc. Natl. Acad. Sci. USA 2022, 119, e2117904119. [Google Scholar] [CrossRef]
- Price, T.K.; Hilt, E.E.; Thomas-White, K.; Mueller, E.R.; Wolfe, A.J.; Brubaker, L. The urobiome of continent adult women: A cross-sectional study. BJOG 2020, 127, 193–201. [Google Scholar] [CrossRef]
- Curtiss, N.; Balachandran, A.; Krska, L.; Peppiatt-Wildman, C.; Wildman, S.; Duckett, J. A case controlled study examining the bladder microbiome in women with Overactive Bladder (OAB) and healthy controls. Eur. J. Obstet. Gynecol. Reprod. Biol. 2017, 214, 31–35. [Google Scholar] [CrossRef]
- Pearce, M.M.; Zilliox, M.J.; Rosenfeld, A.B.; Thomas-White, K.J.; Richter, H.E.; Nager, C.W.; Visco, A.G.; Nygaard, I.E.; Barber, M.D.; Schaffer, J.; et al. The female urinary microbiome in urgency urinary incontinence. Am. J. Obstet. Gynecol. 2015, 213, 347.e1–55.e11. [Google Scholar] [CrossRef]
- Thomas-White, K.J.; Kliethermes, S.; Rickey, L.; Lukacz, E.S.; Richter, H.E.; Moalli, P.; Zimmern, P.; Norton, P.; Kusek, J.W.; Wolfe, A.J.; et al. Evaluation of the urinary microbiota of women with uncomplicated stress urinary incontinence. Am. J. Obstet. Gynecol. 2017, 216, 55.e1–55.e16. [Google Scholar] [CrossRef]
- Xie, J.; Huang, J.S.; Huang, X.J.; Peng, J.M.; Yu, Z.; Yuan, Y.Q.; Xiao, K.F.; Guo, J.N. Profiling the urinary microbiome in men with calcium-based kidney stones. BMC Microbiol. 2020, 20, 41. [Google Scholar] [CrossRef]
- Brubaker, L.; Wolfe, A.J. The female urinary microbiota, urinary health and common urinary disorders. Ann. Transl. Med. 2017, 5, 34. [Google Scholar] [CrossRef]
- Choi, H.-W.; Lee, K.-W.; Kim, Y.-H. The Microbiome’s Function in Disorders of the Urinary Bladder. Appl. Microbiol. 2021, 1, 445–459. [Google Scholar] [CrossRef]
- Zeng, J.; Zhang, G.; Chen, C.; Li, K.; Wen, Y.; Zhao, J.; Wu, P. Alterations in Urobiome in Patients with Bladder Cancer and Implications for Clinical Outcome: A Single-Institution Study. Front. Cell Infect. Microbiol. 2020, 10, 555508. [Google Scholar] [CrossRef]
- Chipollini, J.; Wright, J.R.; Nwanosike, H.; Kepler, C.Y.; Batai, K.; Lee, B.R.; Spiess, P.E.; Stewart, D.B.; Lamendella, R. Characterization of urinary microbiome in patients with bladder cancer: Results from a single-institution, feasibility study. Urol. Oncol. 2020, 38, 615–621. [Google Scholar] [CrossRef]
- Hussein, A.A.; Elsayed, A.S.; Durrani, M.; Jing, Z.; Iqbal, U.; Gomez, E.C.; Singh, P.K.; Liu, S.; Smith, G.; Tang, L.; et al. Investigating the association between the urinary microbiome and bladder cancer: An exploratory study. Urol. Oncol. 2021, 39, 370.e9–370.e19. [Google Scholar] [CrossRef]
- Oresta, B.; Braga, D.; Lazzeri, M.; Frego, N.; Saita, A.; Faccani, C.; Fasulo, V.; Colombo, P.; Guazzoni, G.; Hurle, R.; et al. The Microbiome of Catheter Collected Urine in Males with Bladder Cancer According to Disease Stage. J. Urol. 2021, 205, 86–93. [Google Scholar] [CrossRef] [PubMed]
- Ketter, P.M.; Yu, J.J.; Guentzel, M.N.; May, H.C.; Gupta, R.; Eppinger, M.; Klose, K.E.; Seshu, J.; Chambers, J.P.; Cap, A.P.; et al. Acinetobacter baumannii Gastrointestinal Colonization Is Facilitated by Secretory IgA Which Is Reductively Dissociated by Bacterial Thioredoxin A. mBio 2018, 9, e01298-18. [Google Scholar] [CrossRef] [PubMed]
- Dijkshoorn, L.; Nemec, A.; Seifert, H. An increasing threat in hospitals: Multidrug-resistant Acinetobacter baumannii. Nat. Rev. Microbiol. 2007, 5, 939–951. [Google Scholar] [CrossRef] [PubMed]
- Gevers, D.; Kugathasan, S.; Denson, L.A.; Vazquez-Baeza, Y.; Van Treuren, W.; Ren, B.; Schwager, E.; Knights, D.; Song, S.J.; Yassour, M.; et al. The treatment-naive microbiome in new-onset Crohn’s disease. Cell Host Microbe 2014, 15, 382–392. [Google Scholar] [CrossRef]
- Bonnet, M.; Buc, E.; Sauvanet, P.; Darcha, C.; Dubois, D.; Pereira, B.; Dechelotte, P.; Bonnet, R.; Pezet, D.; Darfeuille-Michaud, A. Colonization of the human gut by E. coli and colorectal cancer risk. Clin. Cancer Res. 2014, 20, 859–867. [Google Scholar] [CrossRef]
- Di Giacinto, C.; Marinaro, M.; Sanchez, M.; Strober, W.; Boirivant, M. Probiotics ameliorate recurrent Th1-mediated murine colitis by inducing IL-10 and IL-10-dependent TGF-beta-bearing regulatory cells. J. Immunol. 2005, 174, 3237–3246. [Google Scholar] [CrossRef]
- Burrello, C.; Garavaglia, F.; Cribiu, F.M.; Ercoli, G.; Lopez, G.; Troisi, J.; Colucci, A.; Guglietta, S.; Carloni, S.; Guglielmetti, S.; et al. Therapeutic faecal microbiota transplantation controls intestinal inflammation through IL10 secretion by immune cells. Nat. Commun. 2018, 9, 5184. [Google Scholar] [CrossRef]
- Noon, A.P.; Albertsen, P.C.; Thomas, F.; Rosario, D.J.; Catto, J.W. Competing mortality in patients diagnosed with bladder cancer: Evidence of undertreatment in the elderly and female patients. Br. J. Cancer 2013, 108, 1534–1540. [Google Scholar] [CrossRef]
- De Jong, J.J.; Boormans, J.L.; van Rhijn, B.W.G.; Seiler, R.; Boorjian, S.A.; Konety, B.; Bivalacqua, T.J.; Wheeler, T.; Svatek, R.S.; Douglas, J.; et al. Distribution of Molecular Subtypes in Muscle-invasive Bladder Cancer Is Driven by Sex-specific Differences. Eur. Urol. Oncol. 2020, 3, 420–423. [Google Scholar] [CrossRef]
- Goto, T.; Miyamoto, H. The Role of Estrogen Receptors in Urothelial Cancer. Front. Endocrinol. 2021, 12, 643870. [Google Scholar] [CrossRef]
- Vollmer, P.; Walev, I.; Rose-John, S.; Bhakdi, S. Novel pathogenic mechanism of microbial metalloproteinases: Liberation of membrane-anchored molecules in biologically active form exemplified by studies with the human interleukin-6 receptor. Infect. Immun. 1996, 64, 3646–3651. [Google Scholar] [CrossRef] [Green Version]
- Horvat, R.T.; Parmely, M.J. Pseudomonas aeruginosa alkaline protease degrades human gamma interferon and inhibits its bioactivity. Infect. Immun. 1988, 56, 2925–2932. [Google Scholar] [CrossRef]
- Mullberg, J.; Durie, F.H.; Otten-Evans, C.; Alderson, M.R.; Rose-John, S.; Cosman, D.; Black, R.A.; Mohler, K.M. A metalloprotease inhibitor blocks shedding of the IL-6 receptor and the p60 TNF receptor. J. Immunol. 1995, 155, 5198–5205. [Google Scholar]
- Kayagaki, N.; Kawasaki, A.; Ebata, T.; Ohmoto, H.; Ikeda, S.; Inoue, S.; Yoshino, K.; Okumura, K.; Yagita, H. Metalloproteinase-mediated release of human Fas ligand. J. Exp. Med. 1995, 182, 1777–1783. [Google Scholar] [CrossRef]
- Roviello, G.; Catalano, M.; Santi, R.; Palmieri, V.E.; Vannini, G.; Galli, I.C.; Buttitta, E.; Villari, D.; Rossi, V.; Nesi, G. Immune Checkpoint Inhibitors in Urothelial Bladder Cancer: State of the Art and Future Perspectives. Cancers 2021, 13, 4411. [Google Scholar] [CrossRef]
- Wahlin, S.; Nodin, B.; Leandersson, K.; Boman, K.; Jirstrom, K. Clinical impact of T cells, B cells and the PD-1/PD-L1 pathway in muscle invasive bladder cancer: A comparative study of transurethral resection and cystectomy specimens. Oncoimmunology 2019, 8, e1644108. [Google Scholar] [CrossRef]
- Kalathil, S.G.; Thanavala, Y. High immunosuppressive burden in cancer patients: A major hurdle for cancer immunotherapy. Cancer Immunol. Immunother. 2016, 65, 813–819. [Google Scholar] [CrossRef]
- Labani-Motlagh, A.; Ashja-Mahdavi, M.; Loskog, A. The Tumor Microenvironment: A Milieu Hindering and Obstructing Antitumor Immune Responses. Front. Immunol. 2020, 11, 940. [Google Scholar] [CrossRef]
- Lin, Y.; Xu, J.; Lan, H. Tumor-associated macrophages in tumor metastasis: Biological roles and clinical therapeutic applications. J. Hematol. Oncol. 2019, 12, 76. [Google Scholar] [CrossRef]
- Grivennikov, S.I.; Wang, K.; Mucida, D.; Stewart, C.A.; Schnabl, B.; Jauch, D.; Taniguchi, K.; Yu, G.Y.; Osterreicher, C.H.; Hung, K.E.; et al. Adenoma-linked barrier defects and microbial products drive IL-23/IL-17-mediated tumour growth. Nature 2012, 491, 254–258. [Google Scholar] [CrossRef]
- Kong, L.; Zhou, Y.; Bu, H.; Lv, T.; Shi, Y.; Yang, J. Deletion of interleukin-6 in monocytes/macrophages suppresses the initiation of hepatocellular carcinoma in mice. J. Exp. Clin. Cancer Res. 2016, 35, 131. [Google Scholar] [CrossRef]
- Ravi, J.; Elbaz, M.; Wani, N.A.; Nasser, M.W.; Ganju, R.K. Cannabinoid receptor-2 agonist inhibits macrophage induced EMT in non-small cell lung cancer by downregulation of EGFR pathway. Mol. Carcinog. 2016, 55, 2063–2076. [Google Scholar] [CrossRef]
- Lima, L.; Oliveira, D.; Tavares, A.; Amaro, T.; Cruz, R.; Oliveira, M.J.; Ferreira, J.A.; Santos, L. The predominance of M2-polarized macrophages in the stroma of low-hypoxic bladder tumors is associated with BCG immunotherapy failure. Urol. Oncol. 2014, 32, 449–457. [Google Scholar] [CrossRef]
- Tian, Y.F.; Tang, K.; Guan, W.; Yang, T.; Xu, H.; Zhuang, Q.Y.; Ye, Z.Q. OK-432 Suppresses Proliferation and Metastasis by Tumor Associated Macrophages in Bladder Cancer. Asian Pac. J. Cancer Prev. 2015, 16, 4537–4542. [Google Scholar] [CrossRef]
- Chen, C.; He, W.; Huang, J.; Wang, B.; Li, H.; Cai, Q.; Su, F.; Bi, J.; Liu, H.; Zhang, B.; et al. LNMAT1 promotes lymphatic metastasis of bladder cancer via CCL2 dependent macrophage recruitment. Nat. Commun. 2018, 9, 3826. [Google Scholar] [CrossRef]
- Sjodahl, G.; Lovgren, K.; Lauss, M.; Chebil, G.; Patschan, O.; Gudjonsson, S.; Mansson, W.; Ferno, M.; Leandersson, K.; Lindgren, D.; et al. Infiltration of CD3(+) and CD68(+) cells in bladder cancer is subtype specific and affects the outcome of patients with muscle-invasive tumors. Urol. Oncol. 2014, 32, 791–797. [Google Scholar] [CrossRef]
- Wang, B.; Liu, H.; Dong, X.; Wu, S.; Zeng, H.; Liu, Z.; Wan, D.; Dong, W.; He, W.; Chen, X.; et al. High CD204+ tumor-infiltrating macrophage density predicts a poor prognosis in patients with urothelial cell carcinoma of the bladder. Oncotarget 2015, 6, 20204–20214. [Google Scholar] [CrossRef] [PubMed]
- Takeuchi, H.; Tanaka, M.; Tanaka, A.; Tsunemi, A.; Yamamoto, H. Predominance of M2-polarized macrophages in bladder cancer affects angiogenesis, tumor grade and invasiveness. Oncol. Lett. 2016, 11, 3403–3408. [Google Scholar] [CrossRef]
- Hanada, T.; Nakagawa, M.; Emoto, A.; Nomura, T.; Nasu, N.; Nomura, Y. Prognostic value of tumor-associated macrophage count in human bladder cancer. Int. J. Urol. 2000, 7, 263–269. [Google Scholar] [CrossRef]
- Xue, Y.; Tong, L.; LiuAnwei Liu, F.; Liu, A.; Zeng, S.; Xiong, Q.; Yang, Z.; He, X.; Sun, Y.; Xu, C. Tumorinfiltrating M2 macrophages driven by specific genomic alterations are associated with prognosis in bladder cancer. Oncol. Rep. 2019, 42, 581–594. [Google Scholar] [CrossRef]
- Suriano, F.; Santini, D.; Perrone, G.; Amato, M.; Vincenzi, B.; Tonini, G.; Muda, A.; Boggia, S.; Buscarini, M.; Pantano, F. Tumor associated macrophages polarization dictates the efficacy of BCG instillation in non-muscle invasive urothelial bladder cancer. J. Exp. Clin. Cancer Res. 2013, 32, 87. [Google Scholar] [CrossRef] [PubMed]
- Ayari, C.; LaRue, H.; Hovington, H.; Decobert, M.; Harel, F.; Bergeron, A.; Tetu, B.; Lacombe, L.; Fradet, Y. Bladder tumor infiltrating mature dendritic cells and macrophages as predictors of response to bacillus Calmette-Guerin immunotherapy. Eur. Urol. 2009, 55, 1386–1395. [Google Scholar] [CrossRef] [PubMed]
- Rong, Z.; Li, L.; Fei, F.; Luo, L.; Qu, Y. Combined treatment of glibenclamide and CoCl2 decreases MMP9 expression and inhibits growth in highly metastatic breast cancer. J. Exp. Clin. Cancer Res. 2013, 32, 32. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.; Shen, W.; Zhang, Y.; Liu, M.; Zhang, L.; Liu, Q.; Lu, H.H.; Bo, J. Accumulation of myeloid-derived suppressor cells (MDSCs) induced by low levels of IL-6 correlates with poor prognosis in bladder cancer. Oncotarget 2017, 8, 38378–38388. [Google Scholar] [CrossRef]
- Gabrilovich, D.I.; Bronte, V.; Chen, S.H.; Colombo, M.P.; Ochoa, A.; Ostrand-Rosenberg, S.; Schreiber, H. The terminology issue for myeloid-derived suppressor cells. Cancer Res. 2007, 67, 425. [Google Scholar] [CrossRef]
- Lim, H.X.; Kim, T.S.; Poh, C.L. Understanding the Differentiation, Expansion, Recruitment and Suppressive Activities of Myeloid-Derived Suppressor Cells in Cancers. Int. J. Mol. Sci. 2020, 21, 3599. [Google Scholar] [CrossRef]
- Eruslanov, E.; Neuberger, M.; Daurkin, I.; Perrin, G.Q.; Algood, C.; Dahm, P.; Rosser, C.; Vieweg, J.; Gilbert, S.M.; Kusmartsev, S. Circulating and tumor-infiltrating myeloid cell subsets in patients with bladder cancer. Int. J. Cancer 2012, 130, 1109–1119. [Google Scholar] [CrossRef]
- Yuan, X.K.; Zhao, X.K.; Xia, Y.C.; Zhu, X.; Xiao, P. Increased circulating immunosuppressive CD14(+)HLA-DR(-/low) cells correlate with clinical cancer stage and pathological grade in patients with bladder carcinoma. J. Int. Med. Res. 2011, 39, 1381–1391. [Google Scholar] [CrossRef]
- Prima, V.; Kaliberova, L.N.; Kaliberov, S.; Curiel, D.T.; Kusmartsev, S. COX2/mPGES1/PGE2 pathway regulates PD-L1 expression in tumor-associated macrophages and myeloid-derived suppressor cells. Proc. Natl. Acad. Sci. USA 2017, 114, 1117–1122. [Google Scholar] [CrossRef]
- Sinha, P.; Clements, V.K.; Fulton, A.M.; Ostrand-Rosenberg, S. Prostaglandin E2 promotes tumor progression by inducing myeloid-derived suppressor cells. Cancer Res. 2007, 67, 4507–4513. [Google Scholar] [CrossRef]
- Chevalier, M.F.; Trabanelli, S.; Racle, J.; Salome, B.; Cesson, V.; Gharbi, D.; Bohner, P.; Domingos-Pereira, S.; Dartiguenave, F.; Fritschi, A.S.; et al. ILC2-modulated T cell-to-MDSC balance is associated with bladder cancer recurrence. J. Clin. Invest. 2017, 127, 2916–2929. [Google Scholar] [CrossRef]
- Zhang, H.; Ye, Y.L.; Li, M.X.; Ye, S.B.; Huang, W.R.; Cai, T.T.; He, J.; Peng, J.Y.; Duan, T.H.; Cui, J.; et al. CXCL2/MIF-CXCR2 signaling promotes the recruitment of myeloid-derived suppressor cells and is correlated with prognosis in bladder cancer. Oncogene 2017, 36, 2095–2104. [Google Scholar] [CrossRef]
- Hao, Z.; Li, R.; Wang, Y.; Li, S.; Hong, Z.; Han, Z. Landscape of Myeloid-derived Suppressor Cell in Tumor Immunotherapy. Biomark Res. 2021, 9, 77. [Google Scholar] [CrossRef]
- Diaz-Montero, C.M.; Salem, M.L.; Nishimura, M.I.; Garrett-Mayer, E.; Cole, D.J.; Montero, A.J. Increased circulating myeloid-derived suppressor cells correlate with clinical cancer stage, metastatic tumor burden, and doxorubicin-cyclophosphamide chemotherapy. Cancer Immunol. Immunother. 2009, 58, 49–59. [Google Scholar] [CrossRef]
- Solito, S.; Falisi, E.; Diaz-Montero, C.M.; Doni, A.; Pinton, L.; Rosato, A.; Francescato, S.; Basso, G.; Zanovello, P.; Onicescu, G.; et al. A human promyelocytic-like population is responsible for the immune suppression mediated by myeloid-derived suppressor cells. Blood 2011, 118, 2254–2265. [Google Scholar] [CrossRef]
- Raychaudhuri, B.; Rayman, P.; Ireland, J.; Ko, J.; Rini, B.; Borden, E.C.; Garcia, J.; Vogelbaum, M.A.; Finke, J. Myeloid-derived suppressor cell accumulation and function in patients with newly diagnosed glioblastoma. Neuro. Oncol. 2011, 13, 591–599. [Google Scholar] [CrossRef]
- Brandau, S.; Trellakis, S.; Bruderek, K.; Schmaltz, D.; Steller, G.; Elian, M.; Suttmann, H.; Schenck, M.; Welling, J.; Zabel, P.; et al. Myeloid-derived suppressor cells in the peripheral blood of cancer patients contain a subset of immature neutrophils with impaired migratory properties. J. Leukoc. Biol. 2011, 89, 311–317. [Google Scholar] [CrossRef]
- Kanamori, M.; Nakatsukasa, H.; Okada, M.; Lu, Q.; Yoshimura, A. Induced Regulatory T Cells: Their Development, Stability, and Applications. Trends Immunol. 2016, 37, 803–811. [Google Scholar] [CrossRef]
- Loskog, A.; Ninalga, C.; Paul-Wetterberg, G.; de la Torre, M.; Malmstrom, P.U.; Totterman, T.H. Human bladder carcinoma is dominated by T-regulatory cells and Th1 inhibitory cytokines. J. Urol. 2007, 177, 353–358. [Google Scholar] [CrossRef]
- Huang, X.; Pan, T.; Yan, L.; Jin, T.; Zhang, R.; Chen, B.; Feng, J.; Duan, T.; Xiang, Y.; Zhang, M.; et al. The inflammatory microenvironment and the urinary microbiome in the initiation and progression of bladder cancer. Genes Dis. 2021, 8, 781–797. [Google Scholar] [CrossRef]
- Miyake, M.; Tatsumi, Y.; Gotoh, D.; Ohnishi, S.; Owari, T.; Iida, K.; Ohnishi, K.; Hori, S.; Morizawa, Y.; Itami, Y.; et al. Regulatory T Cells and Tumor-Associated Macrophages in the Tumor Microenvironment in Non-Muscle Invasive Bladder Cancer Treated with Intravesical Bacille Calmette-Guerin: A Long-Term Follow-Up Study of a Japanese Cohort. Int. J. Mol. Sci. 2017, 18, 2186. [Google Scholar] [CrossRef] [PubMed]
- Murai, R.; Itoh, Y.; Kageyama, S.; Nakayama, M.; Ishigaki, H.; Teramoto, K.; Narita, M.; Yoshida, T.; Tomita, K.; Kobayashi, K.I.; et al. Prediction of intravesical recurrence of non-muscle-invasive bladder cancer by evaluation of intratumoral Foxp3+ T cells in the primary transurethral resection of bladder tumor specimens. PLoS ONE 2018, 13, e0204745. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Zhou, Q.; Zeng, H.; Zhang, H.; Liu, Z.; Shao, J.; Wang, Z.; Xiong, Y.; Wang, J.; Bai, Q.; et al. CCR8 blockade primes anti-tumor immunity through intratumoral regulatory T cells destabilization in muscle-invasive bladder cancer. Cancer Immunol. Immunother. 2020, 69, 1855–1867. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.N.; Zhang, H.; Zhang, L.; Cai, T.T.; Huang, D.J.; He, J.; Ni, H.H.; Zhou, F.J.; Zhang, X.S.; Li, J. Sphingosine 1 phosphate receptor-1 (S1P1) promotes tumor-associated regulatory T cell expansion: Leading to poor survival in bladder cancer. Cell Death Dis. 2019, 10, 50. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Zhu, Y.; Xu, L.; Zhang, J.; Xie, H.; Fu, H.; Zhou, Q.; Chang, Y.; Dai, B.; Xu, J. Tumor stroma-infiltrating mast cells predict prognosis and adjuvant chemotherapeutic benefits in patients with muscle invasive bladder cancer. Oncoimmunology 2018, 7, e1474317. [Google Scholar] [CrossRef] [PubMed]
- Moradi Tabriz, H.; Obohat, M.; Vahedifard, F.; Eftekharjavadi, A. Survey of Mast Cell Density in Transitional Cell Carcinoma. Iran. J. Pathol. 2021, 16, 119–127. [Google Scholar] [CrossRef]
- Sari, A.; Calli, A.; Cakalagaoglu, F.; Altinboga, A.A.; Bal, K. Association of mast cells with microvessel density in urothelial carcinomas of the urinary bladder. Ann. Diagn. Pathol. 2012, 16, 1–6. [Google Scholar] [CrossRef]
- Kim, J.H.; Kang, Y.J.; Kim, D.S.; Lee, C.H.; Jeon, Y.S.; Lee, N.K.; Oh, M.H. The relationship between mast cell density and tumour grade in transitional cell carcinoma of the bladder. J. Int. Med. Res. 2011, 39, 1675–1681. [Google Scholar] [CrossRef]
- Rao, Q.; Chen, Y.; Yeh, C.R.; Ding, J.; Li, L.; Chang, C.; Yeh, S. Recruited mast cells in the tumor microenvironment enhance bladder cancer metastasis via modulation of ERbeta/CCL2/CCR2 EMT/MMP9 signals. Oncotarget 2016, 7, 7842–7855. [Google Scholar] [CrossRef]
- Fu, J.; Wang, Y. Identification of a Novel Pyroptosis-Related Gene Signature for Predicting Prognosis in Bladder Cancer. Cancer Invest. 2022, 40, 134–150. [Google Scholar] [CrossRef]
- Dowell, A.C.; Cobby, E.; Wen, K.; Devall, A.J.; During, V.; Anderson, J.; James, N.D.; Cheng, K.K.; Zeegers, M.P.; Bryan, R.T.; et al. Interleukin-17-positive mast cells influence outcomes from BCG for patients with CIS: Data from a comprehensive characterisation of the immune microenvironment of urothelial bladder cancer. PLoS ONE 2017, 12, e0184841. [Google Scholar] [CrossRef]
- Liu, Y.; Wu, Y.; Zhang, P.; Xu, C.; Liu, Z.; He, C.; Liu, Y.; Kang, Z. CXCL12 and CD3E as Indicators for Tumor Microenvironment Modulation in Bladder Cancer and Their Correlations With Immune Infiltration and Molecular Subtypes. Front. Oncol. 2021, 11, 636870. [Google Scholar] [CrossRef]
- Hicks, R.M.; Ismail, M.M.; Walters, C.L.; Beecham, P.T.; Rabie, M.F.; El Alamy, M.A. Association of bacteriuria and urinary nitrosamine formation with Schistosoma haematobium infection in the Qalyub area of Egypt. Trans. R Soc. Trop. Med. Hyg. 1982, 76, 519–527. [Google Scholar] [CrossRef]
- Lin, W.W.; Karin, M. A cytokine-mediated link between innate immunity, inflammation, and cancer. J. Clin. Invest. 2007, 117, 1175–1183. [Google Scholar] [CrossRef]
- Heo, G.; Lee, Y.; Im, E. Interplay between the Gut Microbiota and Inflammatory Mediators in the Development of Colorectal Cancer. Cancers 2021, 13, 734. [Google Scholar] [CrossRef]
- Stonehill, W.H.; Dmochowski, R.R.; Patterson, A.L.; Cox, C.E. Risk factors for bladder tumors in spinal cord injury patients. J. Urol. 1996, 155, 1248–1250. [Google Scholar] [CrossRef]
- Locke, J.R.; Hill, D.E.; Walzer, Y. Incidence of squamous cell carcinoma in patients with long-term catheter drainage. J. Urol. 1985, 133, 1034–1035. [Google Scholar] [CrossRef]
- Chen, M.F.; Lin, P.Y.; Wu, C.F.; Chen, W.C.; Wu, C.T. IL-6 expression regulates tumorigenicity and correlates with prognosis in bladder cancer. PLoS ONE 2013, 8, e61901. [Google Scholar] [CrossRef]
- Cardillo, M.R.; Sale, P.; Di Silverio, F. Heat shock protein-90, IL-6 and IL-10 in bladder cancer. Anticancer Res. 2000, 20, 4579–4583. [Google Scholar]
- Kumari, N.; Agrawal, U.; Mishra, A.K.; Kumar, A.; Vasudeva, P.; Mohanty, N.K.; Saxena, S. Predictive role of serum and urinary cytokines in invasion and recurrence of bladder cancer. Tumour. Biol. 2017, 39, 1010428317697552. [Google Scholar] [CrossRef]
- Chiang, Y.T.; Ho, C.H.; Hu, S.W.; Yang, T.Y.; Sung, C.W.; Wang, Y.H.; Wu, C.C. Association between the rs1800795G>C polymorphism in the promoter of interleukin-6 gene and bladder cancer. Int. J. Clin. Exp. Pathol. 2018, 11, 3598–3604. [Google Scholar]
- Ebadi, N.; Jahed, M.; Mivehchi, M.; Majidizadeh, T.; Asgary, M.; Hosseini, S.A. Interleukin-12 and interleukin-6 gene polymorphisms and risk of bladder cancer in the Iranian population. Asian Pac. J. Cancer Prev. 2014, 15, 7869–7873. [Google Scholar] [CrossRef]
- Rossi, J.F.; Lu, Z.Y.; Jourdan, M.; Klein, B. Interleukin-6 as a therapeutic target. Clin. Cancer Res. 2015, 21, 1248–1257. [Google Scholar] [CrossRef]
- Zheng, Z.; Zheng, X.; Zhu, Y.; Yao, Z.; Zhao, W.; Zhu, Y.; Sun, F.; Mu, X.; Wang, Y.; He, W.; et al. IL-6 Promotes the Proliferation and Immunosuppressive Function of Myeloid-Derived Suppressor Cells via the MAPK Signaling Pathway in Bladder Cancer. Biomed. Res. Int. 2021, 2021, 5535578. [Google Scholar] [CrossRef]
- Gatta, L.B.; Melocchi, L.; Bugatti, M.; Missale, F.; Lonardi, S.; Zanetti, B.; Cristinelli, L.; Belotti, S.; Simeone, C.; Ronca, R.; et al. Hyper-Activation of STAT3 Sustains Progression of Non-Papillary Basal-Type Bladder Cancer via FOSL1 Regulome. Cancers 2019, 11, 1219. [Google Scholar] [CrossRef]
- Goulet, C.R.; Champagne, A.; Bernard, G.; Vandal, D.; Chabaud, S.; Pouliot, F.; Bolduc, S. Cancer-associated fibroblasts induce epithelial-mesenchymal transition of bladder cancer cells through paracrine IL-6 signalling. BMC Cancer 2019, 19, 137. [Google Scholar] [CrossRef]
- Trikha, M.; Corringham, R.; Klein, B.; Rossi, J.F. Targeted anti-interleukin-6 monoclonal antibody therapy for cancer: A review of the rationale and clinical evidence. Clin. Cancer Res. 2003, 9, 4653–4665. [Google Scholar]
- Segain, J.P.; Raingeard de la Bletiere, D.; Bourreille, A.; Leray, V.; Gervois, N.; Rosales, C.; Ferrier, L.; Bonnet, C.; Blottiere, H.M.; Galmiche, J.P. Butyrate inhibits inflammatory responses through NFkappaB inhibition: Implications for Crohn’s disease. Gut 2000, 47, 397–403. [Google Scholar] [CrossRef]
- Chang, P.V.; Hao, L.; Offermanns, S.; Medzhitov, R. The microbial metabolite butyrate regulates intestinal macrophage function via histone deacetylase inhibition. Proc. Natl. Acad. Sci. USA 2014, 111, 2247–2252. [Google Scholar] [CrossRef]
- Adegbola, S.O.; Sahnan, K.; Warusavitarne, J.; Hart, A.; Tozer, P. Anti-TNF Therapy in Crohn’s Disease. Int. J. Mol. Sci. 2018, 19, 2244. [Google Scholar] [CrossRef]
- Okamoto, M.; Oyasu, R. Transformation in vitro of a nontumorigenic rat urothelial cell line by tumor necrosis factor-alpha. Lab. Invest. 1997, 77, 139–144. [Google Scholar] [PubMed]
- Raziuddin, S.; Masihuzzaman, M.; Shetty, S.; Ibrahim, A. Tumor necrosis factor alpha production in schistosomiasis with carcinoma of urinary bladder. J. Clin. Immunol. 1993, 13, 23–29. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; Liu, L.; Li, J.; Huang, J.; Xie, J.H.; Menard, L.; Shi, Y.; Zhao, X.; Xie, S.; Zang, W.; et al. Systematic characterization of the tumor microenvironment in Chinese patients with hepatocellular carcinoma highlights intratumoral B cells as a potential immunotherapy target. Oncol. Rep. 2022, 47, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.J.; Kim, W.J.; Moon, S.K. Cordycepin suppresses TNF-alpha-induced invasion, migration and matrix metalloproteinase-9 expression in human bladder cancer cells. Phytother. Res. 2010, 24, 1755–1761. [Google Scholar] [CrossRef]
- Marsh, H.P.; Haldar, N.A.; Bunce, M.; Marshall, S.E.; le Monier, K.; Winsey, S.L.; Christodoulos, K.; Cranston, D.; Welsh, K.I.; Harris, A.L. Polymorphisms in tumour necrosis factor (TNF) are associated with risk of bladder cancer and grade of tumour at presentation. Br. J. Cancer 2003, 89, 1096–1101. [Google Scholar] [CrossRef] [Green Version]
- Marshall, S.E.; Bordea, C.; Haldar, N.A.; Mullighan, C.G.; Wojnarowska, F.; Morris, P.J.; Welsh, K.I. Glutathione S-transferase polymorphisms and skin cancer after renal transplantation. Kidney Int. 2000, 58, 2186–2193. [Google Scholar] [CrossRef]
- Balkwill, F.; Mantovani, A. Inflammation and cancer: Back to Virchow? Lancet 2001, 357, 539–545. [Google Scholar] [CrossRef]
- Atarashi, K.; Suda, W.; Luo, C.; Kawaguchi, T.; Motoo, I.; Narushima, S.; Kiguchi, Y.; Yasuma, K.; Watanabe, E.; Tanoue, T.; et al. Ectopic colonization of oral bacteria in the intestine drives TH1 cell induction and inflammation. Science 2017, 358, 359–365. [Google Scholar] [CrossRef]
- Yang, Y.; Weng, W.; Peng, J.; Hong, L.; Yang, L.; Toiyama, Y.; Gao, R.; Liu, M.; Yin, M.; Pan, C.; et al. Fusobacterium nucleatum Increases Proliferation of Colorectal Cancer Cells and Tumor Development in Mice by Activating Toll-Like Receptor 4 Signaling to Nuclear Factor-kappaB, and Up-regulating Expression of MicroRNA-21. Gastroenterology 2017, 152, 851–866.e24. [Google Scholar] [CrossRef]
- Shi, C.; Yang, Y.; Xia, Y.; Okugawa, Y.; Yang, J.; Liang, Y.; Chen, H.; Zhang, P.; Wang, F.; Han, H.; et al. Novel evidence for an oncogenic role of microRNA-21 in colitis-associated colorectal cancer. Gut 2016, 65, 1470–1481. [Google Scholar] [CrossRef]
- Jia, Y.P.; Wang, K.; Zhang, Z.J.; Tong, Y.N.; Han, D.; Hu, C.Y.; Li, Q.; Xiang, Y.; Mao, X.H.; Tang, B. TLR2/TLR4 activation induces Tregs and suppresses intestinal inflammation caused by Fusobacterium nucleatum in vivo. PLoS ONE 2017, 12, e0186179. [Google Scholar] [CrossRef]
- Yan, F.; Cao, H.; Cover, T.L.; Whitehead, R.; Washington, M.K.; Polk, D.B. Soluble proteins produced by probiotic bacteria regulate intestinal epithelial cell survival and growth. Gastroenterology 2007, 132, 562–575. [Google Scholar] [CrossRef]
- Dutta, D.; Lim, S.H. Bidirectional interaction between intestinal microbiome and cancer: Opportunities for therapeutic interventions. Biomark Res. 2020, 8, 31. [Google Scholar] [CrossRef]
- Mo, S.; Ru, H.; Huang, M.; Cheng, L.; Mo, X.; Yan, L. Oral-Intestinal Microbiota in Colorectal Cancer: Inflammation and Immunosuppression. J. Inflamm. Res. 2022, 15, 747–759. [Google Scholar] [CrossRef]
- Hayden, M.S.; Ghosh, S. NF-kappaB in immunobiology. Cell Res. 2011, 21, 223–244. [Google Scholar] [CrossRef] [Green Version]
- Kantor, A.F.; Hartge, P.; Hoover, R.N.; Narayana, A.S.; Sullivan, J.W.; Fraumeni, J.F., Jr. Urinary tract infection and risk of bladder cancer. Am. J. Epidemiol. 1984, 119, 510–515. [Google Scholar] [CrossRef]
- Moseley, T.A.; Haudenschild, D.R.; Rose, L.; Reddi, A.H. Interleukin-17 family and IL-17 receptors. Cytokine Growth Factor Rev. 2003, 14, 155–174. [Google Scholar] [CrossRef]
- Yang, B.; Kang, H.; Fung, A.; Zhao, H.; Wang, T.; Ma, D. The role of interleukin 17 in tumour proliferation, angiogenesis, and metastasis. Mediat. Inflamm. 2014, 2014, 623759. [Google Scholar] [CrossRef]
- Liu, J.; Wang, L.; Wang, T.; Wang, J. Expression of IL-23R and IL-17 and the pathology and prognosis of urinary bladder carcinoma. Oncol. Lett. 2018, 16, 4325–4330. [Google Scholar] [CrossRef]
- Liu, J.; Duan, Y.; Cheng, X.; Chen, X.; Xie, W.; Long, H.; Lin, Z.; Zhu, B. IL-17 is associated with poor prognosis and promotes angiogenesis via stimulating VEGF production of cancer cells in colorectal carcinoma. Biochem. Biophys. Res. Commun. 2011, 407, 348–354. [Google Scholar] [CrossRef]
- Ibrahim, S.; Girault, A.; Ohresser, M.; Lereclus, E.; Paintaud, G.; Lecomte, T.; Raoul, W. Monoclonal Antibodies Targeting the IL-17/IL-17RA Axis: An Opportunity to Improve the Efficiency of Anti-VEGF Therapy in Fighting Metastatic Colorectal Cancer? Clin. Colorectal Cancer 2018, 17, e109–e113. [Google Scholar] [CrossRef] [PubMed]
- El-Gedamy, M.; El-Khayat, Z.; Abol-Enein, H.; El-Said, A.; El-Nahrery, E. Rs-1884444 G/T variant in IL-23 receptor is likely to modify risk of bladder urothelial carcinoma by regulating IL-23/IL-17 inflammatory pathway. Cytokine 2021, 138, 155355. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Yi, T.; Kortylewski, M.; Pardoll, D.M.; Zeng, D.; Yu, H. IL-17 can promote tumor growth through an IL-6–Stat3 signaling pathway. J. Exp. Med. 2009, 206, 1457–1464. [Google Scholar] [CrossRef] [PubMed]
- El-Gedamy, M.; El-Khayat, Z.; Abol-Enein, H.; El-Said, A.; El-Nahrery, E. Rs-10889677 variant in interleukin-23 receptor may contribute to creating an inflammatory milieu more susceptible to bladder tumourigenesis: Report and meta-analysis. Immunogenetics 2021, 73, 207–226. [Google Scholar] [CrossRef]
- Wu, S.; Rhee, K.J.; Albesiano, E.; Rabizadeh, S.; Wu, X.; Yen, H.R.; Huso, D.L.; Brancati, F.L.; Wick, E.; McAllister, F.; et al. A human colonic commensal promotes colon tumorigenesis via activation of T helper type 17 T cell responses. Nat. Med. 2009, 15, 1016–1022. [Google Scholar] [CrossRef]
- Toprak, N.U.; Yagci, A.; Gulluoglu, B.M.; Akin, M.L.; Demirkalem, P.; Celenk, T.; Soyletir, G. A possible role of Bacteroides fragilis enterotoxin in the aetiology of colorectal cancer. Clin. Microbiol. Infect. 2006, 12, 782–786. [Google Scholar] [CrossRef]
- Huang, J.Y.; Lee, S.M.; Mazmanian, S.K. The human commensal Bacteroides fragilis binds intestinal mucin. Anaerobe 2011, 17, 137–141. [Google Scholar] [CrossRef]
- Calcinotto, A.; Brevi, A.; Chesi, M.; Ferrarese, R.; Garcia Perez, L.; Grioni, M.; Kumar, S.; Garbitt, V.M.; Sharik, M.E.; Henderson, K.J.; et al. Microbiota-driven interleukin-17-producing cells and eosinophils synergize to accelerate multiple myeloma progression. Nat. Commun. 2018, 9, 4832. [Google Scholar] [CrossRef]
- Dupraz, L.; Magniez, A.; Rolhion, N.; Richard, M.L.; Da Costa, G.; Touch, S.; Mayeur, C.; Planchais, J.; Agus, A.; Danne, C.; et al. Gut microbiota-derived short-chain fatty acids regulate IL-17 production by mouse and human intestinal gammadelta T cells. Cell Rep. 2021, 36, 109332. [Google Scholar] [CrossRef]
- Gregg, J.R.; Dahm, P.; Chang, S.S. Guideline-based management of non-muscle invasive bladder cancer. Indian J. Urol. 2015, 31, 320–326. [Google Scholar] [CrossRef]
- Sylvester, R.J.; van der Meijden, A.P.; Witjes, J.A.; Kurth, K. Bacillus calmette-guerin versus chemotherapy for the intravesical treatment of patients with carcinoma in situ of the bladder: A meta-analysis of the published results of randomized clinical trials. J. Urol. 2005, 174, 86–91; discussion 91-2. [Google Scholar] [CrossRef]
- Brausi, M.; Oddens, J.; Sylvester, R.; Bono, A.; van de Beek, C.; van Andel, G.; Gontero, P.; Turkeri, L.; Marreaud, S.; Collette, S.; et al. Side effects of Bacillus Calmette-Guerin (BCG) in the treatment of intermediate- and high-risk Ta, T1 papillary carcinoma of the bladder: Results of the EORTC genito-urinary cancers group randomised phase 3 study comparing one-third dose with full dose and 1 year with 3 years of maintenance BCG. Eur. Urol. 2014, 65, 69–76. [Google Scholar] [CrossRef]
- Macleod, L.C.; Ngo, T.C.; Gonzalgo, M.L. Complications of intravesical bacillus calmette-guerin. Can. Urol. Assoc. J. 2014, 8, e540–e544. [Google Scholar] [CrossRef]
- Zhao, W.; Schorey, J.S.; Bong-Mastek, M.; Ritchey, J.; Brown, E.J.; Ratliff, T.L. Role of a bacillus Calmette-Guerin fibronectin attachment protein in BCG-induced antitumor activity. Int. J. Cancer 2000, 86, 83–88. [Google Scholar] [CrossRef]
- Bevers, R.F.; Kurth, K.H.; Schamhart, D.H. Role of urothelial cells in BCG immunotherapy for superficial bladder cancer. Br. J. Cancer 2004, 91, 607–612. [Google Scholar] [CrossRef] [Green Version]
- Redelman-Sidi, G.; Iyer, G.; Solit, D.B.; Glickman, M.S. Oncogenic activation of Pak1-dependent pathway of macropinocytosis determines BCG entry into bladder cancer cells. Cancer Res. 2013, 73, 1156–1167. [Google Scholar] [CrossRef]
- Huang, G.; Redelman-Sidi, G.; Rosen, N.; Glickman, M.S.; Jiang, X. Inhibition of mycobacterial infection by the tumor suppressor PTEN. J. Biol. Chem. 2012, 287, 23196–23202. [Google Scholar] [CrossRef]
- Redelman-Sidi, G.; Glickman, M.S.; Bochner, B.H. The mechanism of action of BCG therapy for bladder cancer—A current perspective. Nat. Rev. Urol. 2014, 11, 153–162. [Google Scholar] [CrossRef]
- De Reijke, T.M.; Vos, P.C.; de Boer, E.C.; Bevers, R.F.; de Muinck Keizer, W.H.; Kurth, K.H.; Schamhart, D.H. Cytokine production by the human bladder carcinoma cell line T24 in the presence of bacillus Calmette-Guerin (BCG). Urol. Res. 1993, 21, 349–352. [Google Scholar] [CrossRef]
- Zhang, Y.; Khoo, H.E.; Esuvaranathan, K. Effects of bacillus Calmette-Guerin and interferon alpha-2B on cytokine production in human bladder cancer cell lines. J. Urol. 1999, 161, 977–983. [Google Scholar] [CrossRef]
- Luo, Y.; Knudson, M.J. Mycobacterium bovis bacillus Calmette-Guerin-induced macrophage cytotoxicity against bladder cancer cells. Clin. Dev. Immunol. 2010, 2010, 357591. [Google Scholar] [CrossRef]
- Mitropoulos, D.N. Novel insights into the mechanism of action of intravesical immunomodulators. In Vivo 2005, 19, 611–621. [Google Scholar]
- Zuiverloon, T.C.; Nieuweboer, A.J.; Vekony, H.; Kirkels, W.J.; Bangma, C.H.; Zwarthoff, E.C. Markers predicting response to bacillus Calmette-Guerin immunotherapy in high-risk bladder cancer patients: A systematic review. Eur. Urol. 2012, 61, 128–145. [Google Scholar] [CrossRef]
- Luo, Y. Blocking IL-10 enhances bacillus Calmette-Guerin induced T helper Type 1 immune responses and anti-bladder cancer immunity. Oncoimmunology 2012, 1, 1183–1185. [Google Scholar] [CrossRef]
- Lombardo, K.A.; Obradovic, A.; Singh, A.K.; Liu, J.L.; Joice, G.; Kates, M.; Bishai, W.; McConkey, D.; Chaux, A.; Eich, M.L.; et al. BCG invokes superior STING-mediated innate immune response over radiotherapy in a carcinogen murine model of urothelial cancer. J. Pathol. 2022, 256, 223–234. [Google Scholar] [CrossRef]
- Han, J.; Gu, X.; Li, Y.; Wu, Q. Mechanisms of BCG in the treatment of bladder cancer-current understanding and the prospect. Biomed. Pharmacother. 2020, 129, 110393. [Google Scholar] [CrossRef]
- Sandes, E.; Lodillinsky, C.; Cwirenbaum, R.; Arguelles, C.; Casabe, A.; Eijan, A.M. Cathepsin B is involved in the apoptosis intrinsic pathway induced by Bacillus Calmette-Guerin in transitional cancer cell lines. Int. J. Mol. Med. 2007, 20, 823–828. [Google Scholar] [CrossRef]
- See, W.A.; Zhang, G.; Chen, F.; Cao, Y.; Langenstroer, P.; Sandlow, J. Bacille-Calmette Guerin induces caspase-independent cell death in urothelial carcinoma cells together with release of the necrosis-associated chemokine high molecular group box protein 1. BJU Int. 2009, 103, 1714–1720. [Google Scholar] [CrossRef]
- Zhang, G.; Chen, F.; Cao, Y.; Johnson, B.; See, W.A. HMGB1 release by urothelial carcinoma cells is required for the in vivo antitumor response to Bacillus Calmette-Guerin. J. Urol. 2013, 189, 1541–1546. [Google Scholar] [CrossRef]
- Knorr, J.; Adler, A.; Agudelo, J.; Ericson, K.; Murthy, P.; Campbell, R.; Bajic, P.; Almassi, N.; Weight, C.; Haber, G.-P.; et al. Pd42-04 Tumor Microbiome Associated with Bcg Response in Non-Muscle Invasive Bladder Cancer. J. Urol. 2021, 206, e725–e726. [Google Scholar] [CrossRef]
- Sweis, R.F.; Golan, S.; Barashi, N.; Hill, E.; Andolfi, C.; Werntz, R.P.; Bloodworth, J.; Steinberg, G.D. Association of the commensal urinary microbiome with response to Bacillus Calmette-Guérin (BCG) immunotherapy in nonmuscle invasive bladder cancer. J. Clin. Oncol. 2019, 37, 423. [Google Scholar] [CrossRef]
- Seow, S.W.; Rahmat, J.N.; Mohamed, A.A.; Mahendran, R.; Lee, Y.K.; Bay, B.H. Lactobacillus species is more cytotoxic to human bladder cancer cells than Mycobacterium bovis (bacillus Calmette-Guerin). J. Urol. 2002, 168, 2236–2239. [Google Scholar] [CrossRef]
- Knorr, J.; Werneburg, G.; Adler, A.; Agudelo, J.; Murthy, P.; Campbell, R.; Bajic, P.; Almassi, N.; Weight, C.; Haber, G.-P.; et al. Pd12-01 Bladder Tumor Microbiome May Augment Response to Bcg in Non-Muscle Invasive Bladder Cancer. J. Urol. 2022, 207, e195. [Google Scholar] [CrossRef]
- Bieri, U.; Scharl, M.; Sigg, S.; Szczerba, B.M.; Morsy, Y.; Ruschoff, J.H.; Schraml, P.H.; Krauthammer, M.; Hefermehl, L.J.; Eberli, D.; et al. Prospective observational study of the role of the microbiome in BCG responsiveness prediction (SILENT-EMPIRE): A study protocol. BMJ Open 2022, 12, e061421. [Google Scholar] [CrossRef] [PubMed]
- Taheri, F.; Moazamian, E.; Mahdavi, M. Lactobacillus acidophilus Cytotoxicity Effect and Apoptosis in Human Bladder Carcinoma Cells: An In Vitro Study. Immunoregulation 2021, 3, 127–134. [Google Scholar] [CrossRef]
- Cairns, C.M.; Gordon, J.R.; Li, F.; Baca-Estrada, M.E.; Moyana, T.; Xiang, J. Lymphotactin expression by engineered myeloma cells drives tumor regression: Mediation by CD4+ and CD8+ T cells and neutrophils expressing XCR1 receptor. J. Immunol. 2001, 167, 57–65. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hedrick, J.A.; Saylor, V.; Figueroa, D.; Mizoue, L.; Xu, Y.; Menon, S.; Abrams, J.; Handel, T.; Zlotnik, A. Lymphotactin is produced by NK cells and attracts both NK cells and T cells in vivo. J. Immunol. 1997, 158, 1533–1540. [Google Scholar] [PubMed]
- Schwartz, J.C.; Zhang, X.; Fedorov, A.A.; Nathenson, S.G.; Almo, S.C. Structural basis for co-stimulation by the human CTLA-4/B7-2 complex. Nature 2001, 410, 604–608. [Google Scholar] [CrossRef]
- Sui, X.; Ma, J.; Han, W.; Wang, X.; Fang, Y.; Li, D.; Pan, H.; Zhang, L. The anticancer immune response of anti-PD-1/PD-L1 and the genetic determinants of response to anti-PD-1/PD-L1 antibodies in cancer patients. Oncotarget 2015, 6, 19393–19404. [Google Scholar] [CrossRef]
- Galsky, M.D.; Wang, H.; Hahn, N.M.; Twardowski, P.; Pal, S.K.; Albany, C.; Fleming, M.T.; Starodub, A.; Hauke, R.J.; Yu, M.; et al. Phase 2 Trial of Gemcitabine, Cisplatin, plus Ipilimumab in Patients with Metastatic Urothelial Cancer and Impact of DNA Damage Response Gene Mutations on Outcomes. Eur. Urol. 2018, 73, 751–759. [Google Scholar] [CrossRef]
- Sui, X.; Lei, L.; Chen, L.; Xie, T.; Li, X. Inflammatory microenvironment in the initiation and progression of bladder cancer. Oncotarget 2017, 8, 93279–93294. [Google Scholar] [CrossRef]
- Farina, M.S.; Lundgren, K.T.; Bellmunt, J. Immunotherapy in Urothelial Cancer: Recent Results and Future Perspectives. Drugs 2017, 77, 1077–1089. [Google Scholar] [CrossRef]
- Routy, B.; Le Chatelier, E.; Derosa, L.; Duong, C.P.M.; Alou, M.T.; Daillere, R.; Fluckiger, A.; Messaoudene, M.; Rauber, C.; Roberti, M.P.; et al. Gut microbiome influences efficacy of PD-1-based immunotherapy against epithelial tumors. Science 2018, 359, 91–97. [Google Scholar] [CrossRef]
- Gollwitzer, E.S.; Saglani, S.; Trompette, A.; Yadava, K.; Sherburn, R.; McCoy, K.D.; Nicod, L.P.; Lloyd, C.M.; Marsland, B.J. Lung microbiota promotes tolerance to allergens in neonates via PD-L1. Nat. Med. 2014, 20, 642–647. [Google Scholar] [CrossRef]
- Groeger, S.; Jarzina, F.; Mamat, U.; Meyle, J. Induction of B7-H1 receptor by bacterial cells fractions of Porphyromonas gingivalis on human oral epithelial cells: B7-H1 induction by Porphyromonas gingivalis fractions. Immunobiology 2017, 222, 137–147. [Google Scholar] [CrossRef]
- Matson, V.; Fessler, J.; Bao, R.; Chongsuwat, T.; Zha, Y.; Alegre, M.L.; Luke, J.J.; Gajewski, T.F. The commensal microbiome is associated with anti-PD-1 efficacy in metastatic melanoma patients. Science 2018, 359, 104–108. [Google Scholar] [CrossRef] [Green Version]
- Fukumoto, K.; Kikuchi, E.; Mikami, S.; Hayakawa, N.; Matsumoto, K.; Niwa, N.; Oya, M. Clinical Role of Programmed Cell Death-1 Expression in Patients with Non-muscle-invasive Bladder Cancer Recurring After Initial Bacillus Calmette-Guerin Therapy. Ann. Surg. Oncol. 2018, 25, 2484–2491. [Google Scholar] [CrossRef]
- Bidnur, S.; Savdie, R.; Black, P.C. Inhibiting Immune Checkpoints for the Treatment of Bladder Cancer. Bladder Cancer 2016, 2, 15–25. [Google Scholar] [CrossRef]
- Warren, R.L.; Freeman, D.J.; Pleasance, S.; Watson, P.; Moore, R.A.; Cochrane, K.; Allen-Vercoe, E.; Holt, R.A. Co-occurrence of anaerobic bacteria in colorectal carcinomas. Microbiome 2013, 1, 16. [Google Scholar] [CrossRef]
- Torres, P.J.; Fletcher, E.M.; Gibbons, S.M.; Bouvet, M.; Doran, K.S.; Kelley, S.T. Characterization of the salivary microbiome in patients with pancreatic cancer. PeerJ 2015, 3, e1373. [Google Scholar] [CrossRef]
- Castano-Rodriguez, N.; Goh, K.L.; Fock, K.M.; Mitchell, H.M.; Kaakoush, N.O. Dysbiosis of the microbiome in gastric carcinogenesis. Sci. Rep. 2017, 7, 15957. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Huang, Z.; Huang, P.; Li, K.; Zeng, J.; Wen, Y.; Li, B.; Zhao, J.; Wu, P. Urogenital Microbiota:Potentially Important Determinant of PD-L1 Expression in Male Patients with Non-muscle Invasive Bladder Cancer. BMC Microbiol. 2022, 22, 7. [Google Scholar] [CrossRef] [PubMed]
- Kaur, J.; Choi, W.; Geynisman, D.M.; Plimack, E.R.; Ghatalia, P. Role of immunotherapy in localized muscle invasive urothelial cancer. Ther. Adv. Med. Oncol. 2021, 13, 17588359211045858. [Google Scholar] [CrossRef] [PubMed]
- Barone, B.; Calogero, A.; Scafuri, L.; Ferro, M.; Lucarelli, G.; Di Zazzo, E.; Sicignano, E.; Falcone, A.; Romano, L.; De Luca, L.; et al. Immune Checkpoint Inhibitors as a Neoadjuvant/Adjuvant Treatment of Muscle-Invasive Bladder Cancer: A Systematic Review. Cancers 2022, 14, 2545. [Google Scholar] [CrossRef] [PubMed]
- Raggi, D.; Bandini, M.; Pederzoli, F.; Giannatempo, P.; Marandino, L.; Basile, G.; Gallina, A.; Briganti, A.; Montorsi, F.; Necchi, A. Concomitant antibiotics (ATBs) use and survival outcomes in patients (pts) with muscle-invasive bladder cancer (MIBC) treated with neoadjuvant pembrolizumab (PURE-01 study). J. Clin. Oncol. 2021, 39, 449. [Google Scholar] [CrossRef]
| Type of BC | Enriched Genera | Sex | Urine Collection | Reference |
|---|---|---|---|---|
| Healthy subjects only | Staphylococcus, Streptococcus, Lactobacillus | F | Midstream urine | Curtiss et al. [33] |
| Healthy subjects only | Genera of the phyla Firmicutes, Actinobacteria, Bacteroidetes | F, M | Midstream urine | Lewis et al. [9] |
| Healthy subjects only | Lactobacillus, Streptococcus, Gardnerella, Escherichia | F | Transurethral catheter | Price et al. [32] |
| Healthy subjects only | M: Enterococcus, Proteus, Klebsiella F: Lactobacillus | F, M | Midstream urine, transurethral catheter | Fouts et al. [10] |
| Healthy subjects only | Lactobacillus, Prevotella, Garnderella | F | Midstream urine | Siddiqui et al. [29] |
| Healthy subjects only | Lactobacillus, Staphylococcus | F | Transurethral catheter | Pearce et al. [30] |
| Healthy subjects only | Lactobacillus, Gardnerella, Gardnerella/Prevotella | F | Transurethral catheter | Pearce et al. [34] |
| Healthy subjects only | Lactobacillus, Gardnerella, Corynebacterium | F | Midstream urine, transurethral catheter | Thomas-White et al. [35] |
| Study on microbiome in men with kidney stones | Prevotella, Lactobacillus | M | Transurethral catheter | Xie et al. [36] |
| Type of BC | Enriched Genera | Sex | Description | Urine Collection | Reference |
|---|---|---|---|---|---|
| Mixed | Fusobacterium, Actinobaculum, Facklamia, Campylobacter | M | No difference in alpha diversity | Midstream urine | Bucevic Popovic et al. [8] |
| Mixed | Acinetobacter, Anaerococcus, Rubrobacter | M | Increased bacterial richness in cancer, significant beta diversity | Midstream urine | Wu et al. [14] |
| Recurrent NMIBC | Staphylococcus, Streptococcus, Prevotella | M | Increased bacterial richness in cancer, no differences in Shannon and Simpson indices, species diversity higher in recurrence group, more OTUs in cancer | Midstream urine | Zeng et al. [39] |
| MIBC, NMIBC | Bacteroides, Akkermansia, Klebsiella | M, F | No relationship found between microbiota in NMIBC and MIBC | Transurethral resection (tissue) | Mansour et al. [23] |
| MIBC, NMIBC | Lactobacillus, Corynebacterium, Streptococcus | M, F | No relationship found between microbiota in NMIBC and MIBC | Transurethral resectoscopy (urine) | Mansour et al. [23] |
| MIBC | Bacteroides, Faecalibacterium | M, F | Decreased bacterial richness in cancer | Midstream urine | Chipollini et al. [40] |
| Mixed | Brucellaceae, Acinetobacter, E.Shigella, Proteobacteria | M, F | Study on noncancerous vs. cancerous tissue;lower Shannon diversity in cancerous tissue, lower degrees of species richness and diversity in cancer | Tissue collected intraoperatively | Liu et al. [22] |
| Mixed | Actinomyces, Achromobacter, Brevibacterium phyla: Actinobacteria, Proteobacteria | M, F | No difference in alpha diversity but higher beta diversity | Midstream urine, Transurethral catheter | Hussein et al. [41] |
| Mixed | Veillonella, Corynebacterium | M | Significantly higher evenness parameter in BC group | Transurethral catheter | Oresta et al. [42] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Friedrich, V.; Choi, H.W. The Urinary Microbiome: Role in Bladder Cancer and Treatment. Diagnostics 2022, 12, 2068. https://doi.org/10.3390/diagnostics12092068
Friedrich V, Choi HW. The Urinary Microbiome: Role in Bladder Cancer and Treatment. Diagnostics. 2022; 12(9):2068. https://doi.org/10.3390/diagnostics12092068
Chicago/Turabian StyleFriedrich, Veronika, and Hae Woong Choi. 2022. "The Urinary Microbiome: Role in Bladder Cancer and Treatment" Diagnostics 12, no. 9: 2068. https://doi.org/10.3390/diagnostics12092068
APA StyleFriedrich, V., & Choi, H. W. (2022). The Urinary Microbiome: Role in Bladder Cancer and Treatment. Diagnostics, 12(9), 2068. https://doi.org/10.3390/diagnostics12092068






