Factors Predicting 150 and 200 Microgram Adenosine Requirement during Four Increasing Doses of Intracoronary Adenosine Bolus Fractional Flow Reserve Assessment
Abstract
:1. Introduction
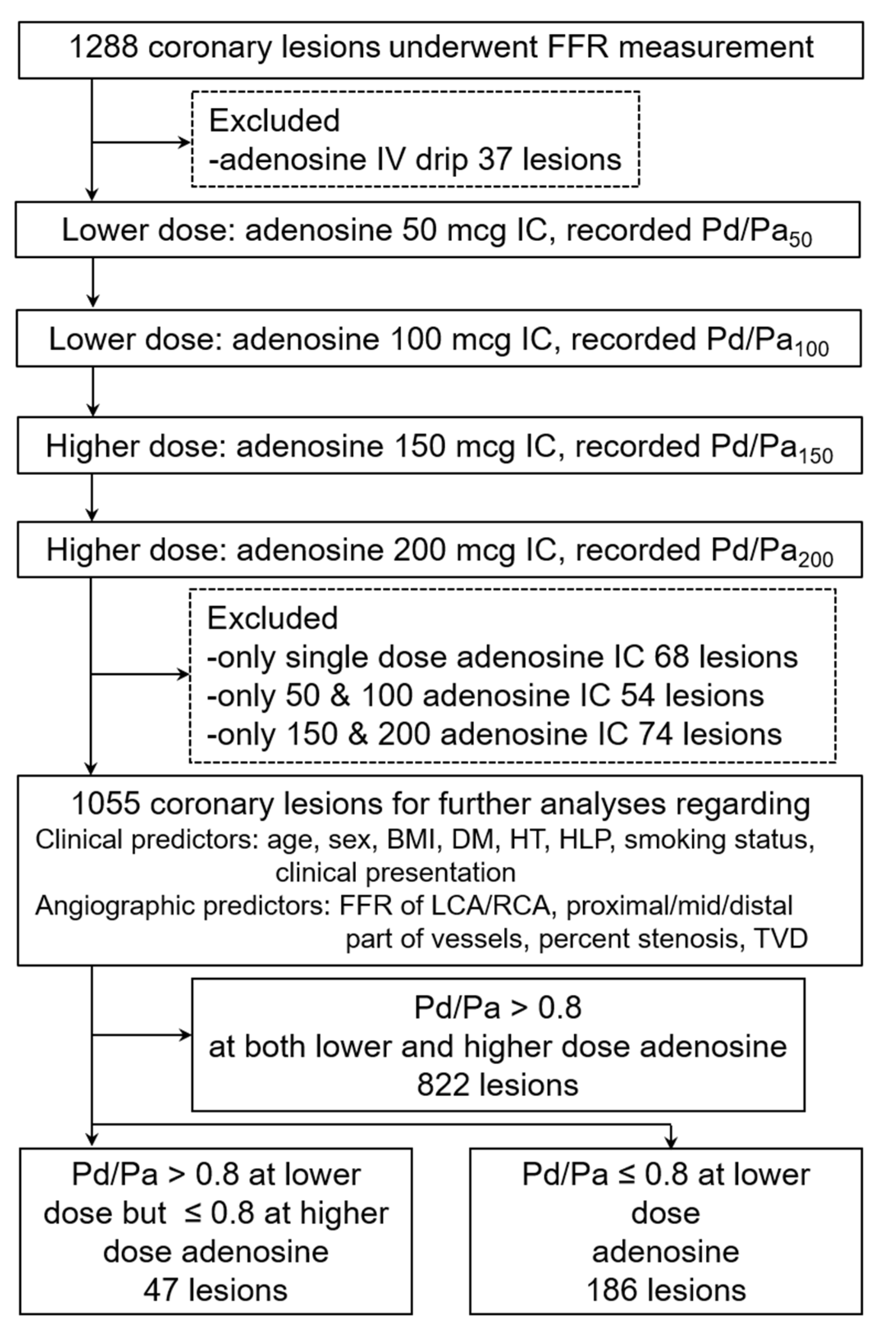
2. Materials and Methods
2.1. Study Design and Setting
2.2. Patient Population
- Age more than 18 years old.
- Underwent FFR measurement in the left or right coronary artery with complete four increasing intracoronary adenosine bolus protocol (50, 100, 150, 200 mcg). In case of receiving less than four doses (incomplete protocol), the patient needed to receive at least one adenosine bolus of the lower dose (either 50 or 100 mcg) plus at least one adenosine bolus of the higher dose (either 150 or 200 mcg).
- Underwent FFR measurement with intravenous adenosine infusion protocol.
- Patients with aorto-ostial (within 3 mm) lesions of left main or right coronary artery, diffuse coronary lesions, or culprit lesions of unstable angina or acute NSTEMI.
- Patients within the first four days of acute ST-segment elevation myocardial infarction.
2.3. Fractional Flow Reserve (FFR) Measurement
2.4. Visual Estimation (VE) of Coronary Stenotic Lesions
2.5. Study Size Estimation
2.6. Data Collection and Predictors
2.7. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pijls, N.H.J.; de Bruyne, B.; Peels, K.; van der Voort, P.H.; Bonnier, H.J.R.M.; Bartunek, J.; Koolen, J.J. Measurement of Fractional Flow Reserve to Assess the Functional Severity of Coronary-Artery Stenoses. N. Engl. J. Med. 1996, 334, 1703–1708. [Google Scholar] [CrossRef] [PubMed]
- Pijls, N.H.J.; Sels, J.-W.E. Functional Measurement of Coronary Stenosis. J. Am. Coll. Cardiol. 2012, 59, 1045–1057. [Google Scholar] [CrossRef] [PubMed]
- Task Force on Myocardial Revascularization of the European Society of Cardiology (ESC); The European Association for Cardio-Thoracic Surgery (EACTS); European Association for Percutaneous Cardiovascular Interventions (EAPCI); Kolh, P.; Wijns, W.; Danchin, N.; Di Mario, C.; Falk, V.; Folliguet, T.; Garg, S.; et al. Guidelines on myocardial revascularization. Eur. J. Cardio-Thoracic Surg. 2010, 38, S1–S52. [Google Scholar] [CrossRef] [PubMed]
- Levine, G.N.; Bates, E.R.; Blankenship, J.C.; Bailey, S.R.; Bittl, J.A.; Cercek, B.; Chambers, C.E.; Ellis, S.G.; Guyton, R.A.; Hollenberg, S.M.; et al. 2011 ACCF/AHA/SCAI Guideline for Percutaneous Coronary Intervention: A Report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines and the Society for Cardiovascular Angiography and Interventions. J. Am. Coll. Cardiol. 2011, 58, e44–e122. [Google Scholar] [CrossRef]
- Lotfi, A.; Jeremias, A.; Fearon, W.F.; Feldman, M.D.; Mehran, R.; Messenger, J.C.; Grines, C.L.; Dean, L.S.; Kern, M.J.; Klein, L.W. Expert consensus statement on the use of fractional flow reserve, intravascular ultrasound, and optical coherence tomography. Catheter. Cardiovasc. Interv. 2014, 83, 509–518. [Google Scholar] [CrossRef] [PubMed]
- Neumann, F.-J.; Sousa-Uva, M.; Ahlsson, A.; Alfonso, F.; Banning, A.P.; Benedetto, U.; Byrne, R.A.; Collet, J.-P.; Falk, V.; Head, S.J.; et al. 2018 ESC/EACTS Guidelines on myocardial revascularization. Eur. Heart J. 2019, 40, 87–165, Erratum in Eur. Heart J. 2019, 40, 3096. [Google Scholar] [CrossRef] [PubMed]
- De Bruyne, B.; Pijls, N.H.J.; Barbato, E.; Bartunek, J.; Bech, J.-W.; Wijns, W.; Heyndrickx, G.R. Intracoronary and Intravenous Adenosine 5’-Triphosphate, Adenosine, Papaverine, and Contrast Medium to Assess Fractional Flow Reserve in Humans. Circulation 2003, 107, 1877–1883. [Google Scholar] [CrossRef]
- McGeoch, R.J.; Oldroyd, K.G. Pharmacological options for inducing maximal hyperaemia during studies of coronary physiology. Catheter. Cardiovasc. Interv. 2008, 71, 198–204. [Google Scholar] [CrossRef]
- Vranckx, P.; Cutlip, D.E.; McFadden, E.P.; Kern, M.J.; Mehran, R.; Muller, O. Coronary Pressure–Derived Fractional Flow Reserve Measurements. Circ. Cardiovasc. Interv. 2012, 5, 312–317. [Google Scholar] [CrossRef]
- Wilson, R.F.; Wyche, K.; Christensen, B.V.; Zimmer, S.; Laxson, D.D. Effects of adenosine on human coronary arterial circulation. Circulation 1990, 82, 1595–1606. [Google Scholar] [CrossRef] [Green Version]
- Jeremias, A.; Whitbourn, R.J.; Filardo, S.D.; Fitzgerald, P.J.; Cohen, D.J.; Tuzcu, E.M.; Anderson, W.D.; Abizaid, A.A.; Mintz, G.S.; Yeung, A.C.; et al. Adequacy of intracoronary versus intravenous adenosine-induced maximal coronary hyperemia for fractional flow reserve measurements. Am. Heart J. 2000, 140, 651–657. [Google Scholar] [CrossRef] [PubMed]
- Casella, G.; Rieber, J.; Schiele, T.M.; Stempfle, H.-U.; Siebert, U.; Leibig, M.; Theisen, K.; Buchmeier, U.; Klauss, V. A randomized comparison of 4 doses of intracoronary adenosine in the assessment of fractional flow reserve. Z. Kardio. 2003, 92, 627–632. [Google Scholar] [CrossRef]
- Lopez-Palop, R.; Saura, D.; Pinar, E.; Lozano, I.; Pérez-Lorente, F.; Picó, F.; Valdez, M. Adequate intracoronary adenosine doses to achieve maximum hyperaemia in coronary functional studies by pressure derived fractional flow reserve: A dose response study. Heart 2004, 90, 95–96. [Google Scholar] [CrossRef] [PubMed]
- Rioufol, G.; Caignault, J.-R.; Finet, G.; Staat, P.; Bonnefoy, E.; de Gevigney, G.; Rossi, R.; André-Fouët, X. 150 microgram intracoronary adenosine bolus for accurate fractional flow reserve assessment of angiographically intermediate coronary stenosis. EuroIntervention 2005, 1, 204–207. [Google Scholar] [PubMed]
- De Luca, G.; Venegoni, L.; Iorio, S.; Giuliani, L.; Marino, P. Effects of Increasing Doses of Intracoronary Adenosine on the Assessment of Fractional Flow Reserve. JACC: Cardiovasc. Interv. 2011, 4, 1079–1084. [Google Scholar] [CrossRef] [PubMed]
- Adjedj, J.; Toth, G.G.; Johnson, N.P.; Pellicano, M.; Ferrara, A.; Floré, V.; Di Gioia, G.; Barbato, E.; Muller, O.; De Bruyne, B. Intracoronary Adenosine: Dose-Response Relationship with Hyperemia. JACC: Cardiovasc. Interv. 2015, 8, 1422–1430. [Google Scholar] [CrossRef]
- Rigattieri, S.; Biondi Zoccai, G.; Sciahbasi, A.; Di Russo, C.; Cera, M.; Patrizi, R.; Fedele, S.; Berni, A.; Pugliese, F.R. Meta-Analysis of Head-to-Head Comparison of Intracoronary Versus Intravenous Adenosine for the Assessment of Fractional Flow Reserve. Am. J. Cardiol. 2017, 120, 563–568. [Google Scholar] [CrossRef]
- Gili, S.; Barbero, U.; Errigo, D.; De Luca, G.; Biondi-Zoccai, G.; Leone, A.M.; Iannaccone, M.; Montefusco, A.; Omedé, P.; Moretti, C.; et al. Intracoronary versus intravenous adenosine to assess fractional flow reserve: A systematic review and meta-analysis. J. Cardiovasc. Med. 2018, 19, 274–283. [Google Scholar] [CrossRef]
- Jong, C.-B.; Lu, T.-S.; Liu, P.Y.-T.; Hsieh, M.-Y.; Meng, S.-W.; Huang, C.-C.; Kao, H.-L.; Wu, C.-C. High dose escalation of intracoronary adenosine in the assessment of fractional flow reserve: A retrospective cohort study. PLoS ONE 2020, 15, e0240699. [Google Scholar] [CrossRef]
- Toth, G.G.; Johnson, N.P.; Jeremias, A.; Pellicano, M.; Vranckx, P.; Fearon, W.F.; Barbato, E.; Kern, M.J.; Pijls, N.H.J.; De Bruyne, B. Standardization of Fractional Flow Reserve Measurements. J. Am. Coll. Cardiol. 2016, 68, 742–753. [Google Scholar] [CrossRef]
- Braunwald, E. Unstable angina. A classification. Circulation 1989, 80, 410–414. [Google Scholar] [CrossRef] [PubMed]
- Thygesen, K.; Alpert, J.S.; Jaffe, A.S.; Chaitman, B.R.; Bax, J.J.; Morrow, D.A.; White, H.D.; The Executive Group on behalf of the Joint European Society of Cardiology (ESC)/American College of Cardiology (ACC)/American Heart Association (AHA)/World Heart Federation (WHF) Task Force for the Universal Definition of Myocardial Infarction. Fourth Universal Definition of Myocardial Infarction (2018). J. Am. Coll. Cardiol. 2018, 72, 2231–2264. [Google Scholar] [CrossRef] [PubMed]
- Fihn, S.D.; Gardin, J.M.; Abrams, J.; Berra, K.; Blankenship, J.C.; Dallas, A.P.; Douglas, P.S.; Foody, J.M.; Gerber, T.C.; Hinderliter, A.L.; et al. 2012 ACCF/AHA/ACP/AATS/PCNA/SCAI/STS Guideline for the diagnosis and management of patients with stable ischemic heart disease: A report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines, and the American College of Physicians, American Association for Thoracic Surgery, Preventive Cardiovascular Nurses Association, Society for Cardiovascular Angiography and Interventions, and Society of Thoracic Surgeons. J. Am. Coll. Cardiol. 2012, 60, e44–e164. [Google Scholar] [CrossRef] [PubMed]
- Walsh, R.; Fang, J.; Fuster, V.; O’Rourke, R. Hurst’s the Heart Manual of Cardiology, 13th ed.; McGraw-Hill Professional: New York, NY, USA, 2012. [Google Scholar]
- von Elm, E.; Altman, D.G.; Egger, M.; Pocock, S.J.; Gøtzsche, P.C.; Vandenbroucke, J.P.; STROBE Initiative. The strengthening the reporting of observational studies in epidemiology (STROBE) statement: Guidelines for reporting observational studies. Int. J. Surg. 2014, 12, 1495–1499. [Google Scholar] [CrossRef]
- Beard, J.R.; Officer, A.M.; Cassels, A.K. The World Report on Ageing and Health. Gerontologist 2016, 56 (Suppl. 2), S163–S166. [Google Scholar] [CrossRef]
- Hosmer, D.W.; Lemeshow, S.; Sturdivant, R.X. Applied Logistic Regression, 3rd ed.; John Wiley & Sons Inc.: Hoboken, NJ, USA, 2013; ISBN 978-0-470-58247-3. [Google Scholar]
- Kim, H.-S.; Tonino, P.A.L.; De Bruyne, B.; Yong, A.S.C.; Tremmel, J.A.; Pijls, N.H.J.; Fearon, W.F.; FAME Study Investigators. The Impact of Sex Differences on Fractional Flow Reserve–Guided Percutaneous Coronary Intervention: A FAME (Fractional Flow Reserve Versus Angiography for Multivessel Evaluation) Substudy. JACC: Cardiovasc. Interv. 2012, 5, 1037–1042. [Google Scholar] [CrossRef]
- Fineschi, M.; Guerrieri, G.; Orphal, D.; Palmerini, E.; Münzel, T.; Warnholtz, A.; Pierli, C.; Gori, T. The impact of gender on fractional flow reserve measurements. EuroIntervention 2013, 9, 360–366. [Google Scholar] [CrossRef]
- Pijls, N.H.J.; Fearon, W.F.; Tonino, P.A.L.; Siebert, U.; Ikeno, F.; Bornschein, B.; van’t Veer, M.; Klauss, V.; Manoharan, G.; Engstrøm, T.; et al. Fractional Flow Reserve Versus Angiography for Guiding Percutaneous Coronary Intervention in Patients With Multivessel Coronary Artery Disease: 2-Year Follow-Up of the FAME (Fractional Flow Reserve Versus Angiography for Multivessel Evaluation) Study. J. Am. Coll. Cardiol. 2010, 56, 177–184. [Google Scholar] [CrossRef]
- Lim, H.-S.; Tonino, P.A.L.; De Bruyne, B.; Yong, A.S.C.; Lee, B.-K.; Pijls, N.H.J.; Fearon, W.F. The impact of age on fractional flow reserve-guided percutaneous coronary intervention: A FAME (Fractional Flow Reserve versus Angiography for Multivessel Evaluation) trial substudy. Int. J. Cardiol. 2014, 177, 66–70. [Google Scholar] [CrossRef]
- Mejia-Renteria, H.; Faria, D.; Lee, J.M.; Lee, S.H.; Jung, J.-H.; Doh, J.-H.; Nam, C.-W.; Shin, E.-S.; Hoshino, M.; Sugiyama, T.; et al. Association between patient age, microcirculation, and coronary stenosis assessment with fractional flow reserve and instantaneous wave-free ratio. Catheter. Cardiovasc. Interv. 2022, 99, 1104–1114. [Google Scholar] [CrossRef]
- Miyazaki, T.; Ashikaga, T.; Ohigashi, H.; Komura, M.; Kobayashi, K.; Isobe, M. Impact of Smoking on Coronary Microcirculatory Resistance in Patients With Coronary Artery Disease. Int. Heart J. 2015, 56, 29–36. [Google Scholar] [CrossRef] [PubMed]
- Hadjiloizou, N.; Davies, J.E.; Malik, I.S.; Aguado-Sierra, J.; Willson, K.; Foale, R.A.; Parker, K.H.; Hughes, A.D.; Francis, D.P.; Mayet, J. Differences in cardiac microcirculatory wave patterns between the proximal left mainstem and proximal right coronary artery. Am. J. Physiol. Heart Circ. Physiol. 2008, 295, H1198–H1205. [Google Scholar] [CrossRef] [PubMed]
- Wada, K.; Fujii, K.; Horitani, K.; Kishimoto, H.; Hashimoto, K.; Shibutani, H.; Tsujimoto, S.; Matsumura, K.; Otagaki, M.; Morishita, S.; et al. Influence of different physiological hemodynamics on fractional flow reserve values in the left coronary artery and right coronary artery. Heart Vessel. 2021, 36, 1125–1131. [Google Scholar] [CrossRef] [PubMed]
- Layland, J.; Carrick, D.; Lee, M.; Oldroyd, K.; Berry, C. Adenosine: Physiology, pharmacology, and clinical applications. JACC: Cardiovasc. Interv. 2014, 7, 581–591. [Google Scholar] [CrossRef] [Green Version]



| Site of Coronary Lesions FFR Performed | n (n%) | |
|---|---|---|
| LMCA | 19 (1.8) | |
| LAD | total | 625 (59.2) |
| proximal | 299 (28.3) | |
| mid | 314 (29.8) | |
| distal | 12 (1.1) | |
| LCX | total | 149 (14.1) |
| proximal | 81 (7.7) | |
| mid | 62 (5.9) | |
| distal | 6 (0.6) | |
| RCA | total | 262 (24.8) |
| proximal | 92 (8.7) | |
| mid | 142 (13.5) | |
| distal | 28 (2.7) | |
| Diameter Stenosis | n (n%) |
|---|---|
| 30–40% | 71 (6.73) |
| 41–50% | 205 (19.43) |
| 51–60% | 318 (30.14) |
| 61–70% | 380 (36.02) |
| 71–80% | 80 (7.58) |
| 81–90% | 1 (0.09) |
| Characteristics | FFR ≤ 0.8 at Adenosine 150, 200 mcg (n = 47 Lesions) | FFR ≤ 0.8 at Adenosine 50, 100 mcg (n = 186 Lesions) | p-Value |
|---|---|---|---|
| Clinical, n (n%) | |||
| Age (years, mean ± SD) | 60.8 ± 10.6 | 63.0 ± 10.3 | 0.194 |
| Age group | 0.012 | ||
| Age < 65 | 36 (76.6) | 104 (55.9) | |
| Age ≥ 65 | 11 (23.4) | 82 (44.1) | |
| Male | 38 (80.9) | 125 (67.2) | 0.076 |
| BMI (kg/m2, mean ± SD) | 25.5 ± 4.4 | 25.1 ± 3.7 | 0.599 |
| BMI group | 0.414 | ||
| BMI < 25 | 20 (42.6) | 94 (50.5) | |
| BMI ≥ 25 | 27 (57.5) | 92 (49.5) | |
| DM | 16 (34.0) | 75 (40.3.7) | 0.505 |
| HT | 38 (80.8) | 165 (88.7) | 0.151 |
| HLP | 44 (93.6) | 185 (99.5) | 0.027 |
| Smoking | 22 (46.8) | 97 (52.1) | 0.519 |
| Presentation | 0.775 | ||
| CCS | 42 (89.4) | 170 (91.4) | |
| UA/NSTEMI within 3 months | 5 (10.6) | 16 (8.6) | |
| Angiographic, n (n%) | |||
| Baseline Pd/Pa (mean ± SD) | 0.93 ± 0.02 | 0.91 ± 0.05 | 0.095 |
| FFR (mean ± SD) | 0.78 ± 0.04 | 0.75 ± 0.05 | <0.001 |
| Left vs. Right FFR | 0.004 | ||
| FFR of LMCA/LAD/LCX | 32 (68.1) | 162 (87.1) | |
| FFR of RCA | 15 (31.9) | 24 (12.9) | |
| Part of vessels | 1.000 | ||
| Proximal lesion | 20 (42.6) | 79 (42.5) | |
| Mid/Distal lesion | 27 (57.4) | 107 (57.5) | |
| Percent stenosis (mean ± SD) | 66.6 ± 11.1 | 69.1 ± 9.2 | 0.114 |
| Percent stenosis group | 0.257 | ||
| <70% in non-LM, <50% in LM | 15 (31.9) | 43 (23.1) | |
| ≥70% in non-LM, ≥50% in LM | 32 (68.1) | 143 (76.9) | |
| TVD | 22 (46.8) | 76 (40.9) | 0.510 |
| Variables | Crude | Adjusted | ||
|---|---|---|---|---|
| OR (95% CI) | p-Value | OR (95% CI) | p-Value | |
| Age < 65 yrs | 2.58 (1.24–5.39) | 0.012 | 2.26 (0.98–5.22) | 0.056 |
| Male | 2.06 (0.94–4.54) | 0.073 | 3.18 (1.08–9.37) | 0.036 |
| BMI ≥ 25 kg/m2 | 1.38 (0.72–2.63) | 0.330 | 1.77 (0.80–3.94) | 0.161 |
| Non-DM | 1.31 (0.67–2.56) | 0.432 | 1.48 (0.69–3.16) | 0.315 |
| Non-HT | 1.86 (0.79–4.39) | 0.156 | 1.50 (0.57–3.96) | 0.413 |
| Non-HLP | 12.61 (1.27–124.79) | 0.030 | 23.85 (3.70–153.88) | 0.001 |
| Non-smoking | 1.24 (0.65–2.35) | 0.514 | 3.02 (1.20–7.62) | 0.019 |
| UA/NSTEMI within 3 months | 1.26 (0.44–3.66) | 0.664 | 1.69 (0.48–5.93) | 0.411 |
| FFR of RCA | 3.16 (1.49–6.70) | 0.003 | 4.35 (1.72–11.03) | 0.002 |
| Mid/Distal lesion | 1.00 (0.52–1.91) | 0.992 | 0.70 (0.33–1.46) | 0.340 |
| Non-sig. CAG | 1.56 (0.77–3.15) | 0.216 | 1.65 (0.70–3.87) | 0.253 |
| TVD | 1.27 (0.67–2.43) | 0.462 | 1.48 (0.71–3.10) | 0.300 |
| Predictors | Adjusted OR (95% CI) | p-Value | AuROC (95% CI) |
|---|---|---|---|
| Age < 65 yrs | 2.51 (1.13–5.58) | 0.024 | 0.60 (0.57–0.64) |
| Male | 3.04 (1.15–8.06) | 0.025 | 0.57 (0.54–0.60) |
| Non-smoking | 2.42 (1.05–5.61) | 0.039 | 0.53 (0.49–0.57) |
| FFR of RCA | 3.31 (1.44–7.62) | 0.005 | 0.60 (0.56–0.63) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chantadansuwan, T.; Patumanond, J.; Charernboon, T.; Piyayotai, D. Factors Predicting 150 and 200 Microgram Adenosine Requirement during Four Increasing Doses of Intracoronary Adenosine Bolus Fractional Flow Reserve Assessment. Diagnostics 2022, 12, 2076. https://doi.org/10.3390/diagnostics12092076
Chantadansuwan T, Patumanond J, Charernboon T, Piyayotai D. Factors Predicting 150 and 200 Microgram Adenosine Requirement during Four Increasing Doses of Intracoronary Adenosine Bolus Fractional Flow Reserve Assessment. Diagnostics. 2022; 12(9):2076. https://doi.org/10.3390/diagnostics12092076
Chicago/Turabian StyleChantadansuwan, Thamarath, Jayanton Patumanond, Thammanard Charernboon, and Dilok Piyayotai. 2022. "Factors Predicting 150 and 200 Microgram Adenosine Requirement during Four Increasing Doses of Intracoronary Adenosine Bolus Fractional Flow Reserve Assessment" Diagnostics 12, no. 9: 2076. https://doi.org/10.3390/diagnostics12092076






