Abstract
Background: Narrower retinal arterioles and wider retinal venules have been associated with the incidence of heart failure (HF). However, whether they are predictive of the prognosis of heart failure (HF) is unclear. We aimed to explore the role of retinal vessel calibers in predicting long-term clinical outcomes of HF. Methods: This is a prospective, single-center, observational study that surveyed patients in a tertiary referral hospital for the treatment of HF. Retinal vessel caliber was graded using retinal photography. The primary endpoint was the composite endpoint of HF rehospitalization and mortality at 12 months. Results: There were 55 patients with chronic HF included in the final analysis. At 12 months, the cumulative incidence of the primary endpoint, HF rehospitalization, and mortality tended to be higher with the widening of the central retinal venular equivalent (CRVE) (p for non-linearity = 0.059) and was significantly increased when CRVE reached a cut-off value (283 μm) (p = 0.011) following adjustment for age, sex, etiology of HF, and diabetes. No association between the central retinal arteriolar equivalent (CRAE) and arteriolar-to-venular caliber ratio (AVR) was found with the clinical outcome in both univariable and multivariable Cox regression. CRAE, CRVE, and AVR had no relationship with the concentration of the N-terminal pro-B-type natriuretic peptide. In addition, CRVE was not associated with cardiac diastolic and systolic function. Conclusions: When the retinal venular caliber widens to a certain point, the composite incidence of HF rehospitalization and mortality significantly increase, suggesting retinal vessel caliber imaging may provide insight into the development of HF.
1. Introduction
Heart failure (HF) has been recognized as one of most common causes of cardiovascular morbidity and mortality. Currently, there are 64.3 million HF patients worldwide, and the prevalence in the general adult population of developed countries is 1–2% and increases with age [1]. Despite significant progress in pharmacological and device therapy for HF, its long-term prognosis remains poor and its pathophysiology is incompletely understood.
Endothelial dysfunction is a pathophysiological mechanism in heart failure and represents a sensitive marker for disease severity and outcome prognosis [2]. Reduced blood flow and impairment of microcirculation play an important role in the systemic effects of heart failure on different organs [3]. Recently, retinal vessel analysis, a non-invasive and radiation-free method, has been widely explored for its potential application for HF prediction. Nagele et al. found an association between retinal microvascular abnormalities and the prevalence of HF of either ischaemic or non-ischaemic etiology, with a suggestion of a slightly downregulated function of retinal microcirculation [4]. In addition, data from the Atherosclerosis Risk in Communities (ARIC) Study suggested that wider retinal venular calibers have a predictive value for incident HF, which remained statistically significant following adjustment for traditional risk factors [5]. However, the prognostic value of retinal microcirculation for heart failure patients has been not defined. Therefore, we aimed to assess the association between retinal arteriolar and venule caliber with 12-month HF rehospitalization and mortality in heart failure patients.
2. Materials and Methods
2.1. Study Population
This was a prospective cohort clinical trial that evaluated the value of retinal vascular diameters in predicting the prognosis of chronic heart failure patients (ClinicalTrials.gov Identifier: ChiCTR1800018244). The study was conducted according to the tenets of the Declaration of Helsinki and the principles of good clinical practice guidelines [6]. The study protocol was approved by the local ethics committee of the Second Hospital of Tianjin Medical University (KY2018K063). All participants provided written informed consent.
Participants were recruited from October 2018 to June 2020 at the Second Hospital of Tianjin Medical University. The inclusion criteria for chronic heart failure accorded with the European Guidelines for the diagnosis and treatment of acute and chronic heart failure [7]: their left ventricular ejection fraction (LVEF) was lower than 40% and they had symptoms and/or signs of chronic heart failure (CHF) or their LVEF was ≥40% and their N-terminal pro-B-type natriuretic peptide (NT-proBNP) value was higher than 125 pg/mL with evidence of relevant structural heart disease such as left ventricular hypertrophy or atrial enlargement and/or diastolic dysfunction. Exclusion criteria included the following: (1) combined with other serious diseases, life expectancy was less than one year; (2) pregnancy or breastfeeding; (3) photosensitive epilepsy; (4) glaucoma, and (5) other significant eye pathologies and procedures such as blindness, inability to fixate (for instance, nystagmus), progressive diabetic retinopathy, or prior retinal laser coagulation.
2.2. Retinal Vessel Caliber Measurements
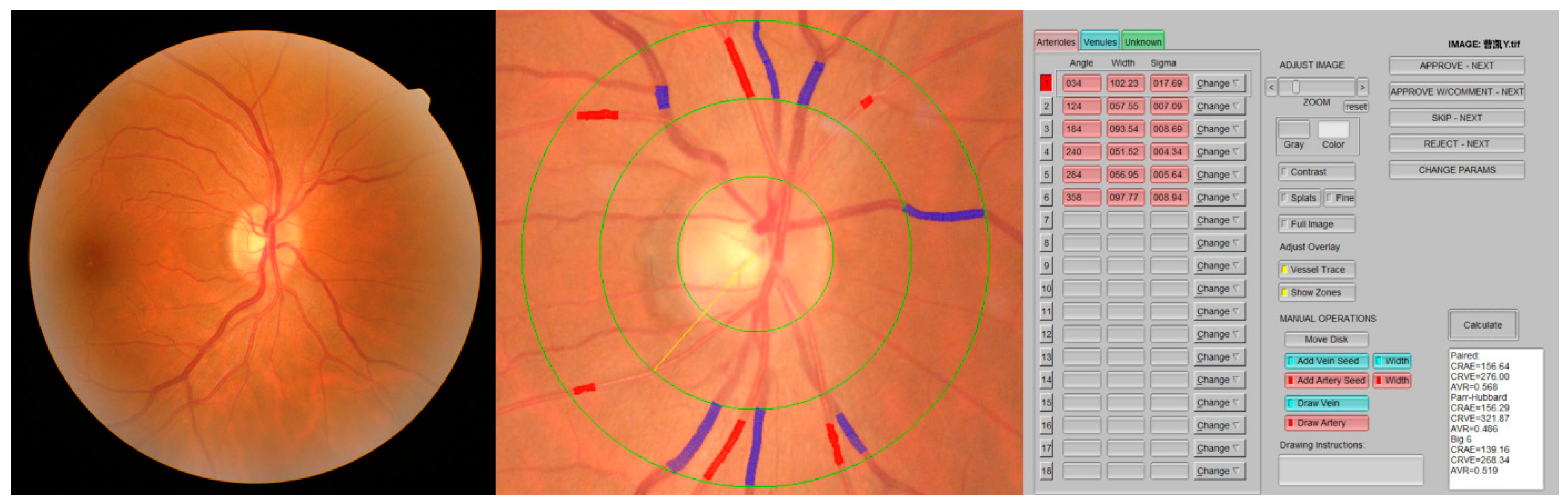
A 45-degree retinal photograph of a randomly selected eye of each participant was taken using a fundus camera (AFC-330; NIDEK Co., Ltd., Aichi, Japan) within 20–30 min after the application of two drops of 0.5% tropicamide solution. The photograph was centered on the region of the optic disc and was taken using an autofocus camera. At least 6 segments of retinal arteries and 6 segments of retinal vein diameters in an area of 0.5–1 optic disc diameter distant from the optic disc were added with the calculation of the central retinal artery and vein equivalent (CRAE and CRVE) using IVAN software [8]. With the use of formulas presented by Parr and Spears [9,10] and by Hubbard et al. [11], these measurements were combined into 3 summary indices, representing the average retinal arteriolar diameters (CRAE), retinal venular diameters (CRVE), and arteriole-to-venule ratio (AVR = CRAE/CRVE) (Figure 1).
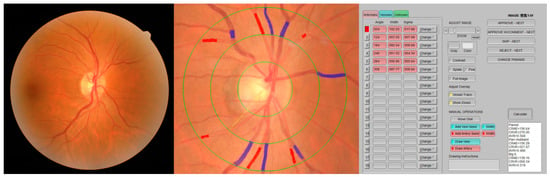
Figure 1.
Retinal vessel caliber measurements using IVAN software.
Each image was analyzed by well-trained researchers (S.G., S.Y., and K.H.), supervised and in the case of discrepancy, finally validated by the most experienced scientists (J.L. and L.S.) of the group to assure uniform and correct data acquisition.
2.3. Clinical, Biochemical, and Echocardiographic Data
Baseline data concerning demographic and clinical variables involving age, sex, hospital stay, etiology of HF, smoking history, comorbidities (such as hypertension, diabetes, coronary revascularization history, stroke, and atrial fibrillation), blood pressure, heart rate, biochemical results (in particular, NT-proBNP), and discharge medication were collected. Participants underwent transthoracic echocardiography performed by trained sonographers using Philips IE33 machines (Philips Ultrasound, Bothell, WA, USA). LVEF was measured based on the modified biplane Simpson’s method. The measurements of the left atrial diameter (LAD), interventricular septum thickness, left ventricular end-diastolic diameter, and left ventricular end-systolic diameter were from parasternal long-axis view. Early septal mitral annular velocities (e’) were measured using tissue Doppler imaging. Doppler measurements of mitral inflow were made from two-dimensional views.
2.4. Follow-Up and Outcomes
All patients with HF continued the standardized treatment for HF after discharge. Patients were followed up by clinical visits or telephone calls for 12 months. The primary endpoint was the composite endpoint of all-cause mortality and HF rehospitalization at 12 months. The follow-up time was calculated from discharge to all-cause mortality, first readmission, or termination of the study.
2.5. Statistical Analysis
Patients were divided into two groups based on the occurrence of the primary endpoint. Continuous variables were reported as the mean ± SD or median and interquartile range. Student’s-test was used for data with a normal distribution, and the Mann–Whitney test was used for data with a nonnormal distribution. Categorical variables were expressed as numbers and percentages and were compared using the Chi-square test or Fisher’s exact test. CRVE, CRAE, and AVR were analyzed as continuous variables.
Cox proportional hazards regression was used to calculate the hazard ratios (HR) among all patents. Multivariable models investigating the association between CRVE or CRAE and the primary endpoint were created, adjusting for age, sex, etiology of HF, and diabetes. Non-linearity was observed in the relationship between CRVE and the primary endpoints in restricted cubic spline regression analysis. According to the result of restricted cubic spline regression analysis, a cut-off value of CRVE was found to define the patients with higher risk. Log-rank tests for the Kaplan–Meier survival curves were performed according to subgroups divided by this cut-off value. Correlations between levels of CRAE, CRVE, AVR, and N-terminal pro-B-type natriuretic peptide (NT-proBNP) were assessed using Pearson’s r correlation. Linear and logistic regressions were applied to investigate the associations between echocardiographic structural/functional characteristics and CRVE at baseline.
All data were analyzed using SPSS statistical software (SPSS 25.0, SPSS Inc., Chicago, IL, USA) and R programming (version 4.1.2; R Foundation for Statistical Computing, Vienna, Austria). A p-value < 0.05 was considered statistically significant.
3. Results
Baseline Characteristics
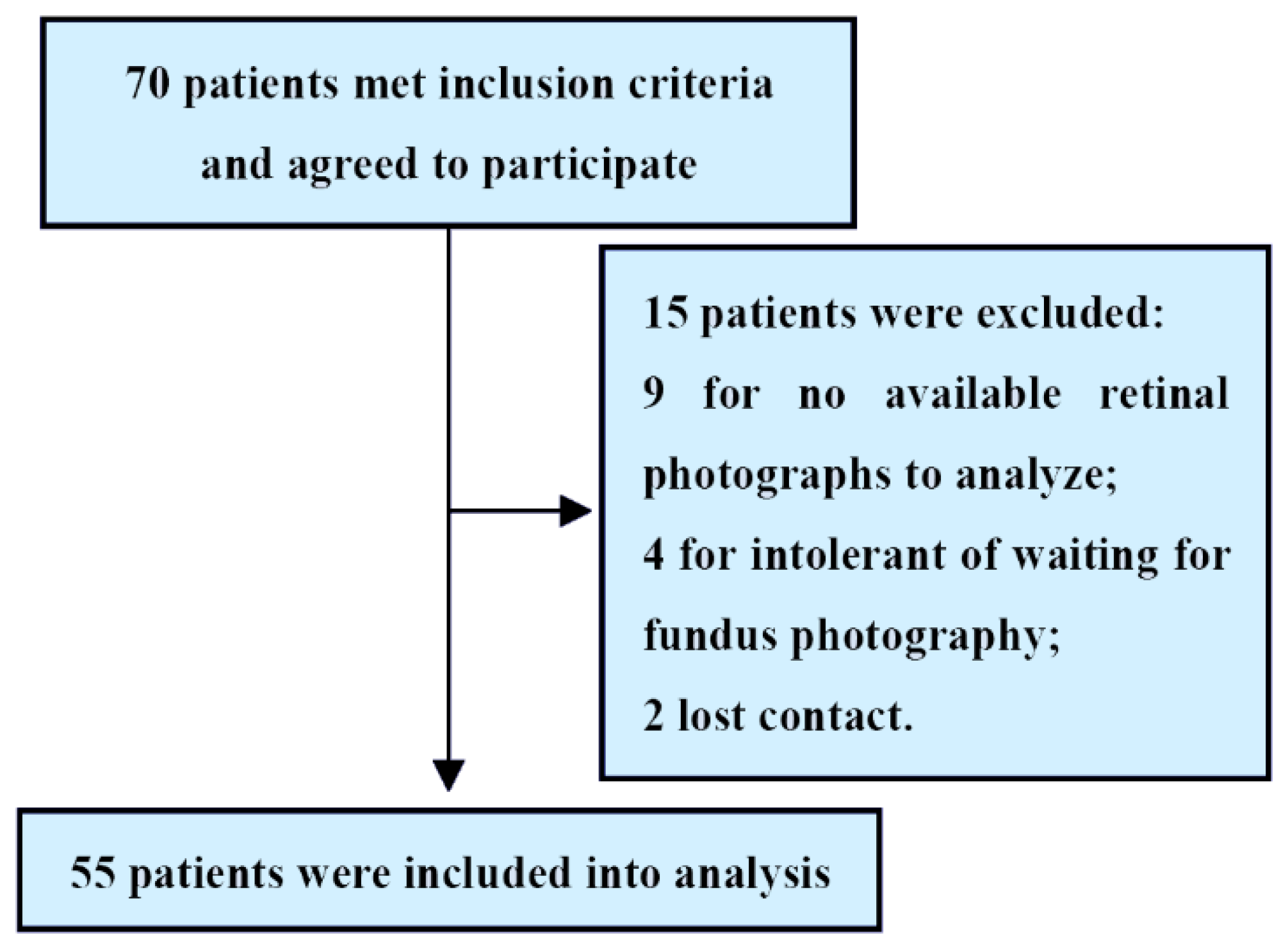
A total of 70 patients met the inclusion criteria. Of these, 15 patients were excluded because of a lack of available retinal photographs to analyze, intolerant of waiting for fundus photography or lost contact. Therefore, 55 patients with HF (mean age 65.6 ± 12.7 years; 79.9% men) were included in the final analysis (Figure 2).
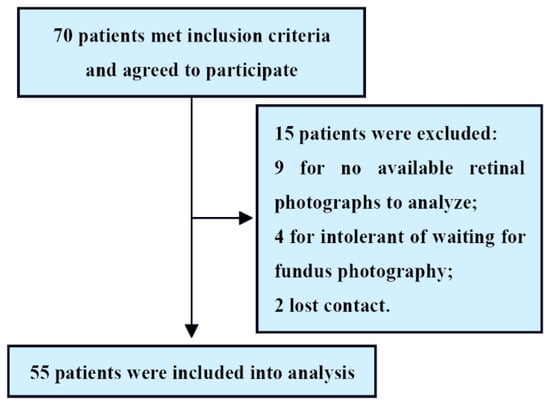
Figure 2.
Study flowchart.
At 12 months, the primary endpoint (HF rehospitalization and mortality) occurred in 22 (40%) patients, of whom 4 (7.3%) died and 18 (32.7%) were rehospitalized for HF. Compared to patients who did not have the primary endpoints, patients who developed the primary endpoints were more likely to have a revascularization history. No difference was found in age, sex, etiology of HF, medical history, laboratory parameters, and the prescribed medicines between the two groups. Otherwise, no difference was found between the two groups in CRAE, CRVE, and AVR (Table 1).

Table 1.
Demographic and baseline characteristics of HF patients with endpoints and without endpoints.
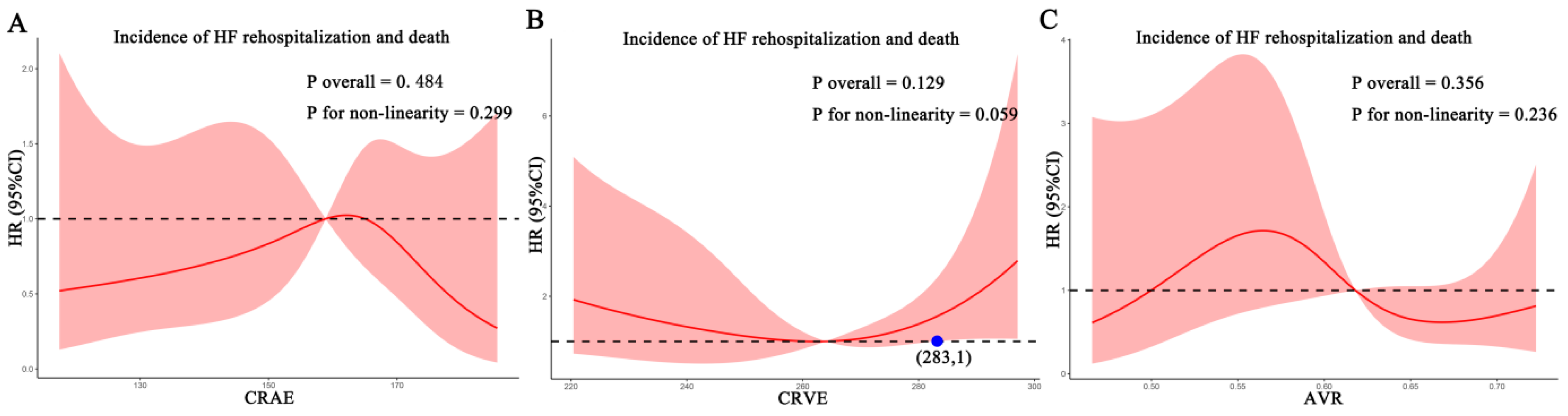
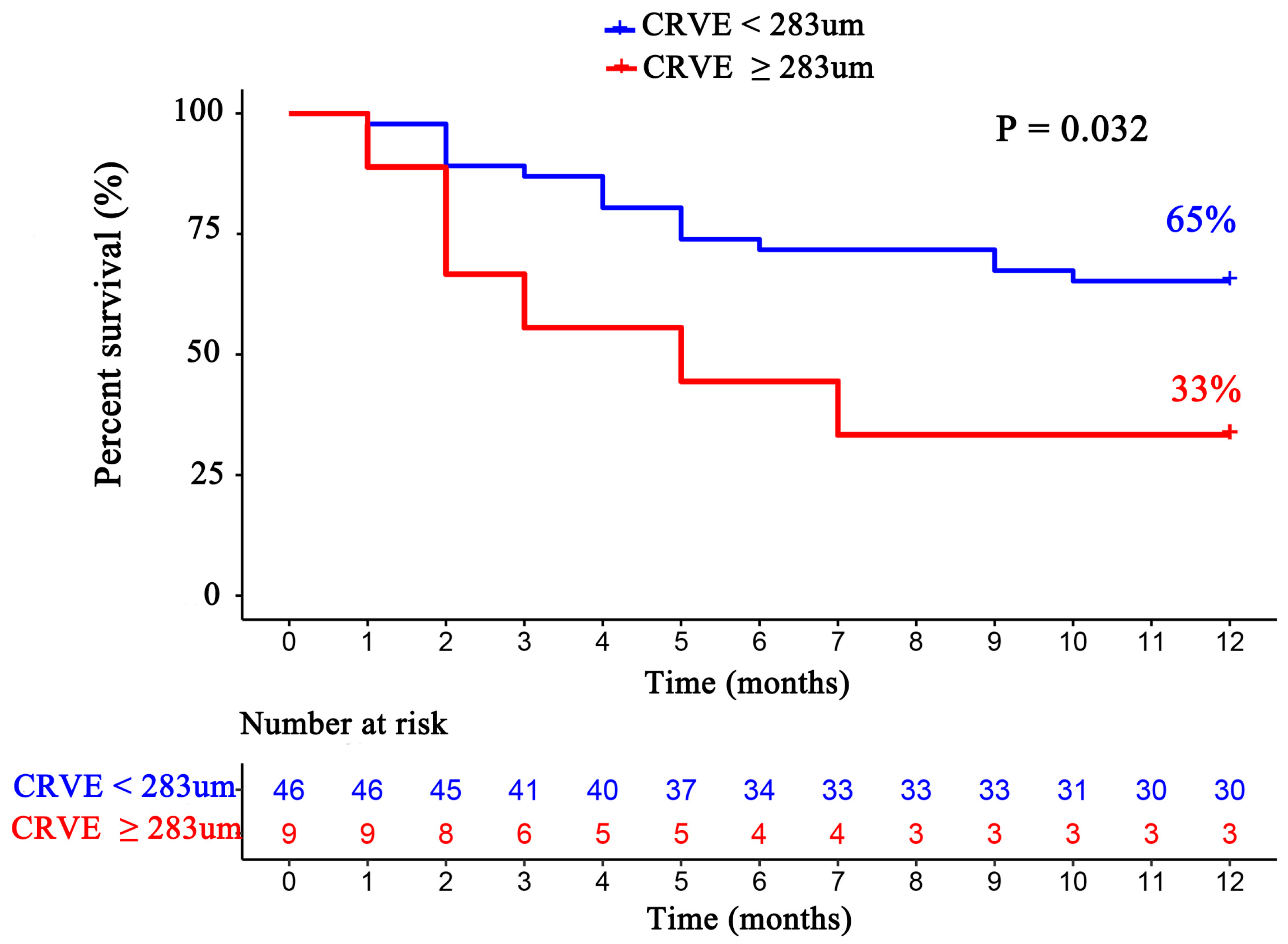
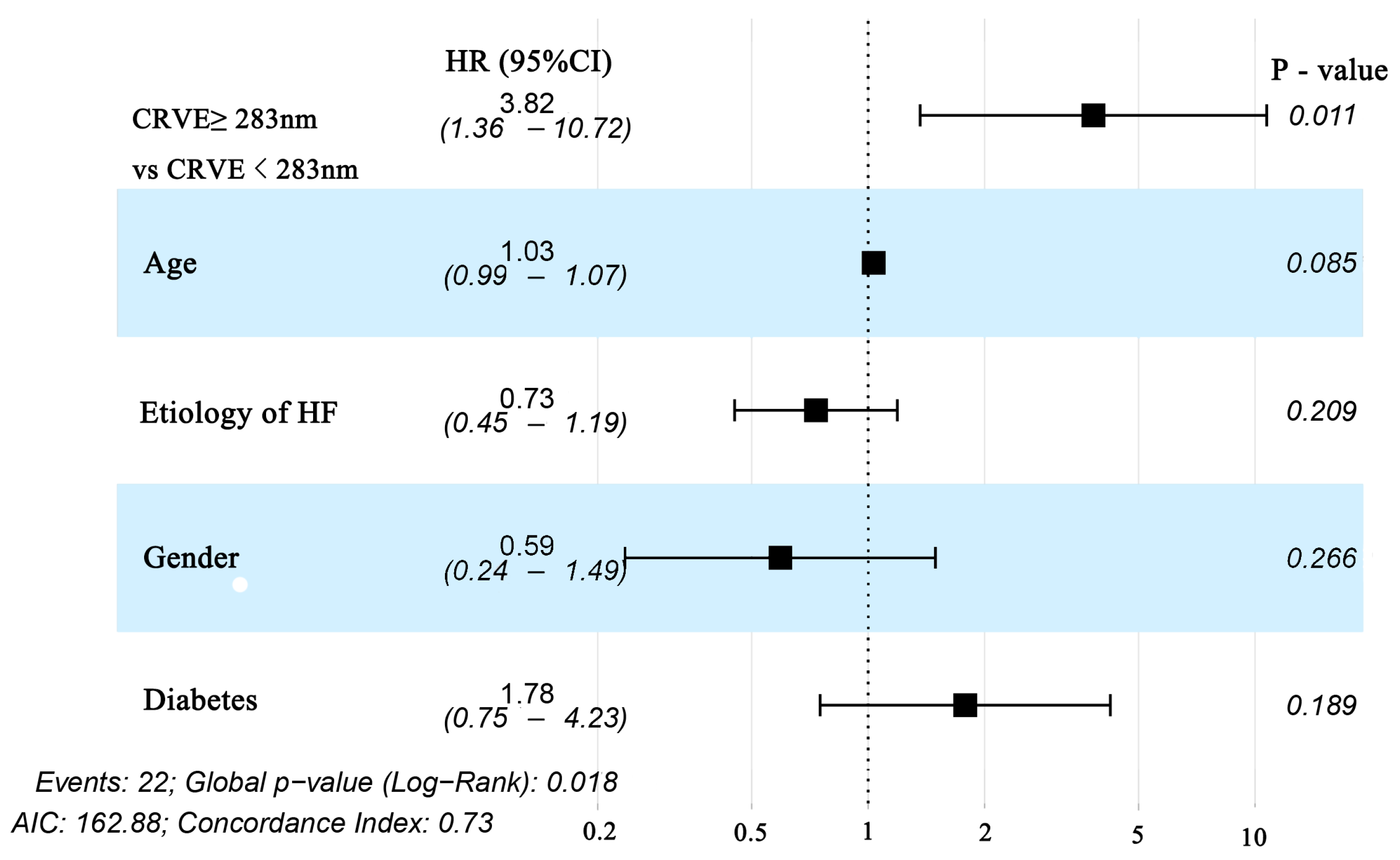
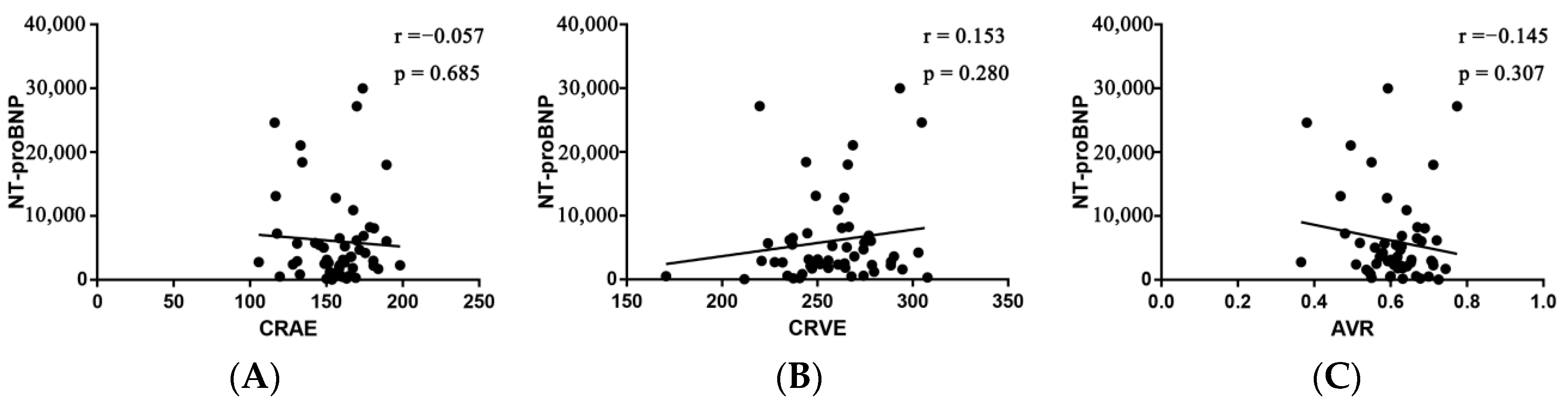
For each standard deviation change, CRAE, CRVE, and AVR were not associated with the risk of HF rehospitalization and mortality. After adjusting for age, sex, etiology of HF, and diabetes, the association remained insignificant (Table 2). However, the CRVE showed a trend of a higher risk of rehospitalization and mortality (p-value for overall trend = 0.129; p-value for non-linearity = 0.059), but no significant non-linear association was detected for CRAE (p-value for non-linearity = 0.229) and AVR (p-value for non-linearity = 0.236) (Figure 3). As shown in Figure 3B, a cut-off value (283 μm) of CRVE was found to have a prognostic value. Based on a comparison of the baseline characteristics (Supplemental Table S1), patients with CRVE ≥ 283 μm tended to be younger than patients with CRVE ≥ 283 μm. HF patients with CRVE ≥ 283 μm at baseline had a 2.7 times higher risk of HF rehospitalization and mortality than HF patients with CRVE < 283 μm (p = 0.032, Figure 4). Wider CRVE (≥283 μm) (hazard ratio [HR] 3.82, 95% confidence interval [CI] 1.36 to 10.72; p = 0.011), adjusted for age, sex, etiology of HF, and diabetes, was still significantly associated with HF rehospitalization and mortality (Figure 5). CRAE, CRVE, and AVR did not correlate with the NT-proBNP levels (Figure 6).

Table 2.
Associations of retinal vessel calibers (per one standard deviation change) with endpoints in HF participants at baseline.
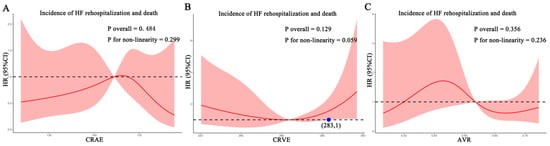
Figure 3.
Association of retinal vessel caliber with risk for HF rehospitalization and mortality in HF patients. (A) for CRAE; (B) for CRVE, (C) for AVR. AVR, arteriolar-to-venular caliber ratio; CI, confidence interval; CRAE, central retinal arteriolar equivalent; CRVE, central retinal venular equivalent; HF, heart failure. HR, hazard ratio.
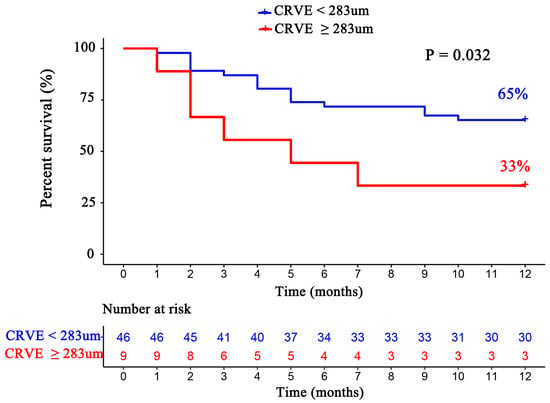
Figure 4.
The Kaplan–Meier survival curves according to subgroups divided by CRVE (CRVE ≥ 283 μm and CRVE < 283 μm). CRVE, central retinal venular equivalent.
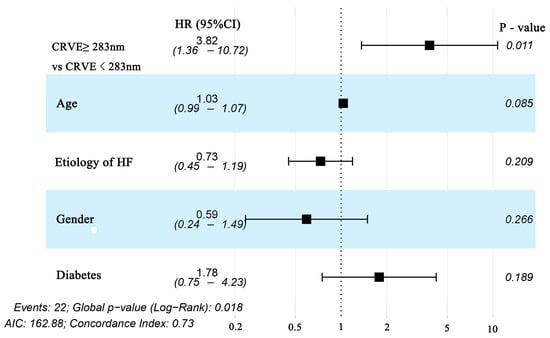
Figure 5.
Forest plot of subgroups divided by CRVE (CRVE ≥ 283 μm and CRVE < 283 μm) for Cox regression with multivariable adjustment for age, sex, etiology of HF, and diabetes for the prediction of the primary endpoint (HF rehospitalization and mortality at 12-month follow-up) in patients with heart failure. CRVE, central retinal venular equivalent; HF, heart failure.
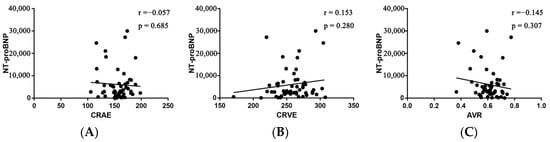
Figure 6.
Pearson’s correlation between NT-proBNP and CRAE (A), CRVE (B), and AVR (C). AVR, arteriolar-to-venular caliber ratio; CRAE, central retinal arteriolar equivalent; CRVE, central retinal venular equivalent; NT-proBNP, N-terminal probrain natriuretic peptide.
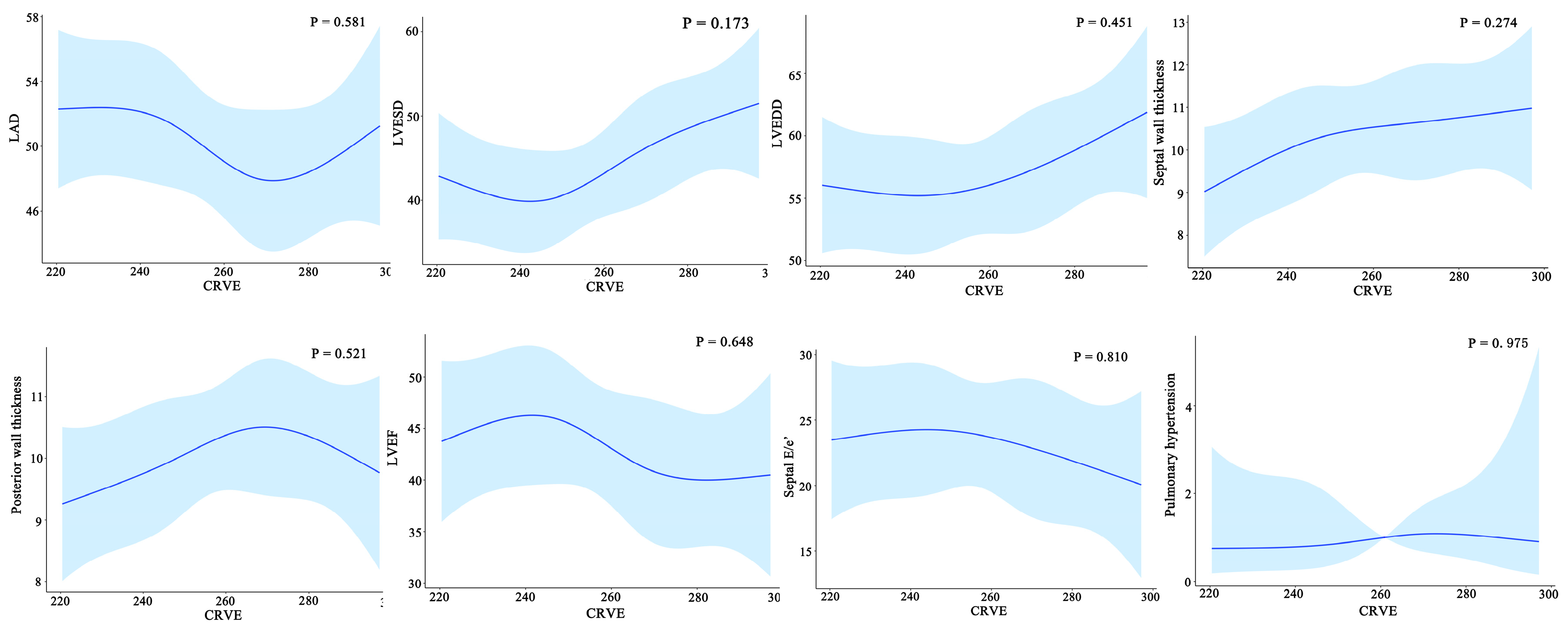
Furthermore, widening CRVE was not associated with cardiac structural characteristics such as left atria dimensions, left ventricular end-diastolic dimensions, left ventricular end-systolic dimensions, septal wall thickness, and posterior wall thickness and cardiac structural characteristics such as left ventricular ejection fraction, septal E/e’, and pulmonary arterial hypertension (Figure 7).
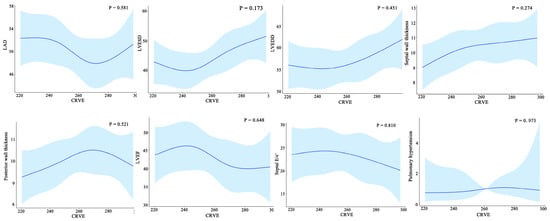
Figure 7.
Relationship between the cardiac structure/function and CRVE of HF patients. CRAE, central retinal arteriolar equivalent; HF, heart failure. LAD, left atrial diameter; LVESD, left ventricular end-systolic diameter; LVEDD, left ventricular end-diastolic diameter.
4. Discussion
In this study, we demonstrated that the caliber of retinal venules (CRVE) and narrower retinal arterioles (CRAE) were not associated with 12-month HF rehospitalization and mortality in HF patients, but widening of CRVE above the cut-off value of 283 μm was significantly associated with 12-month HF rehospitalization and mortality. To the best of our knowledge, this is the first study exploring the role of the retinal vascular caliber in the prognosis of HF patients.
In recent years, a growing body of evidence has suggested an association of various risk factors of HF with retinal microvascular signs, such as older age [12], hypertension [13], diabetes mellitus [14], coronary disease [15,16], stroke [17,18], and atrial fibrillation [19]. Retinal vessels are approximately the same size as the coronary and cerebral microvasculature and share embryologic, anatomic, and physiologic similarity. Therefore, retinal microvascular dysfunction may reflect subclinical pathological changes prior to the development of cardiovascular disease and diagnosis. The Atherosclerosis Risk in Communities (ARIC) in 2016 reported that narrower retinal arterioles and wider retinal venules conferred a long-term risk of coronary heart disease in females and ischemic stroke in both sexes [18], as confirmed by the meta-analysis conducted in 2020 [20]. There is also evidence demonstrating an increased risk of mortality in individuals with both narrower retinal arteriolar caliber and wider retinal venular caliber [18,21]. All evidence compelled investigators to further investigate the association between retinal vessel calibers and HF.
Currently, few studies have explored the role of retinal vessel calibers in HF; however, there are discrepancies among studies. In 2015, a cross-sectional study involving 1680 participants found a significant association between a wider caliber of retinal venules and the prevalence of heart failure [22]. Meanwhile, the ARIC study [18] published in 2016 did not find a significant association between retinal vessel caliber and heart failure, findings that are supported by a case-control study in 2018 [4]. Surprisingly, later evidence from the ARIC study group published in 2019 found that following adjustment for traditional risk factors, wider CRVE remained the statistically significant predictor of incident HF after a long-term follow up [5]. These findings therefore demonstrate that changes in retinal vessel caliber may only demonstrate a predictive value over longer periods. No association of wider CRVE and narrower CRAE with the prognosis of HF was found in our study, even after multivariable adjustment.
Despite all of these explorations of the relationship between retinal vessels and cardiovascular disease, there is no study on the role of retinal vessel calibers in the prognosis of HF, which may mainly be due to HF patients being usually frail and intolerant to conducting a time-consuming examination such as fundus photography. Our study builds upon these findings, showing that CRVE presented a trend of a higher risk of rehospitalization and mortality in non-linear analysis (p = 0.059), with a significant tendency when the CRVE widens beyond a certain value (p = 0.032). However, CRAE narrowing was not linearly or non-linearly associated with an increased risk of HF rehospitalization and mortality. This echoes the findings from ARIC in 2019 [5], demonstrating that CRVE has the potential value of predicting not only the incidence but also the prognosis of HF. A study with a large sample size is necessary to further explore the non-linear relationship between CRVE and the prognosis of HF.
In addition, no relation was found between the caliber of retinal veins and NT-proBNP, a well-recognized prognostic marker and indicator of elevated ventricular filling pressure among patients regardless of ejection fraction [23,24], indicating that a wider CRVE may serve as a supplementary not a replacement to estimate prognosis in HF patients.
Regarding cardiac structure and function, the ARIC study reported in 2019 that among individuals free of incident HF at baseline, a wider CRVE and narrower CRAE were significantly associated with an increased left ventricular size, a higher incidence of left ventricular hypertrophy, and greater abnormalities in diastolic and systolic functions, which notably differed by sex [5]. Our study found no relationship between CRVE and the cardiac structural and functional parameters among HF patients, suggesting that retinal microvascular abnormalities may subtend a continuum of left ventricular remodeling at a very early time before overt HF, but this cannot explain the different magnitudes of left ventricular remodeling.
Several limitations should be noted. First, the present study is a non-randomized, single-center analysis and is limited by its observational nature. However, we performed multivariable analysis, trying to balance the bias and mimic a randomized setting. Secondly, single-center experience with a limited sample size affects its wide application. Current trends observed in this study should be confirmed by future, larger prospective, adequately powered population studies. Third, due to the limited population size, subgroup analyses for HF subtypes, sex, and other factors were not performed and should be conducted in future larger scale studies.
5. Conclusions
In summary, the current study explored the association of retinal vessel calibers with clinical outcomes in HF patients. Wider retinal venous caliber was significantly associated with 12-month HF rehospitalization and mortality in HF patients.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/diagnostics12092078/s1, Table S1: Demographic and baseline characteristics of HF patients with CRVE ≥ 283 μm versus HF patients without endpoints.
Author Contributions
Conceptualization, L.S. and T.L.; Data curation, S.G., S.Y. and W.S.; Formal analysis, S.G., J.L. and K.H.; Funding acquisition, T.L.; Investigation, K.H.; Methodology, S.Y., W.S., G.T. and J.L.; Software, J.L. and K.H.; Supervision, K.C. and L.S.; Visualization, G.T.; Writing—original draft, S.G. and S.Y.; Writing—review & editing, K.C., L.S. and T.L. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by grants from the Tianjin Natural Science Foundation (20JCZDJC00340 and 20JCZXJC00130 to TL), the National Natural Science Foundation of China (81970270, 82170327 to TL), and the Tianjin Key Medical Discipline (Specialty) Construction Project (TJYXZDXK-029A).
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by the Ethics Committee of the Second Hospital of Tianjin Medical University (KY2018K063, 3 September 2018).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The data presented in this study are available on request from the corresponding author. The data are not publicly available due to patient privacy.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Groenewegen, A.; Rutten, F.H.; Mosterd, A.; Hoes, A.W. Epidemiology of heart failure. Eur. J. Heart Fail. 2020, 22, 1342–1356. [Google Scholar] [CrossRef] [PubMed]
- Streese, L.; Lona, G.; Wagner, J.; Knaier, R.; Schoch, R.; Kröpfl, J.M.; Gasser, B.A.; Schmidt-Trucksäss, A.; Hanssen, H. Microvascular endothelial dysfunction in heart failure patients: An indication for exercise treatment? Microvasc. Res. 2022, 142, 104345. [Google Scholar] [CrossRef] [PubMed]
- McMurray, J.J.; Pfeffer, M.A. Heart failure. Lancet 2005, 365, 1877–1889. [Google Scholar] [CrossRef]
- Nägele, M.P.; Barthelmes, J.; Ludovici, V.; Cantatore, S.; Von Eckardstein, A.; Enseleit, F.; Lüscher, T.F.; Ruschitzka, F.; Sudano, I.; Flammer, A.J. Retinal microvascular dysfunction in heart failure. Eur. Heart J. 2018, 39, 47–56. [Google Scholar] [CrossRef] [PubMed]
- Chandra, A.; Seidelmann, S.B.; Claggett, B.L.; Klein, B.E.; Klein, R.; Shah, A.M.; Solomon, S.D. The association of retinal vessel calibres with heart failure and long-term alterations in cardiac structure and function: The Atherosclerosis Risk in Communities (ARIC) Study. Eur. J. Heart Fail. 2019, 21, 1207–1215. [Google Scholar] [CrossRef] [PubMed]
- The Helsinki Declaration of the World Medical Association (WMA). Ethical principles of medical research involving human subjects. Pol. Merkur. Lek. Organ Pol. Tow. Lek. 2014, 36, 298–301. [Google Scholar]
- Ponikowski, P.; Voors, A.A.; Anker, S.D.; Bueno, H.; Cleland, J.G.; Coats, A.J.; Falk, V.; González-Juanatey, J.R.; Harjola, V.P.; Jankowska, E.A.; et al. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC). Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur. J. Heart Fail. 2016, 18, 891–975. [Google Scholar]
- Wong, T.Y.; Knudtson, M.D.; Klein, R.; Klein, B.E.; Meuer, S.M.; Hubbard, L.D. Computer-assisted measurement of retinal vessel diameters in the Beaver Dam Eye Study: Methodology, correlation between eyes, and effect of refractive errors. Ophthalmology 2004, 111, 1183–1190. [Google Scholar] [CrossRef]
- Parr, J.C.; Spears, G.F. Mathematic relationships between the width of a retinal artery and the widths of its branches. Am. J. Ophthalmol. 1974, 77, 478–483. [Google Scholar] [CrossRef]
- Parr, J.C.; Spears, G.F.S. General caliber of the retinal arteries expressed as the equivalent width of the central retinal artery. Am. J. Ophthalmol. 1974, 77, 472–477. [Google Scholar] [CrossRef]
- Hubbard, L.D.; Brothers, R.J.; King, W.N.; Clegg, L.X.; Klein, R.; Cooper, L.S.; Sharrett, A.; Davis, M.D.; Cai, J. Methods for evaluation of retinal microvascular abnormalities associated with hypertension/sclerosis in the atherosclerosis risk in communities study. Ophthalmology 1999, 106, 2269–2280. [Google Scholar] [CrossRef]
- Myers, C.E.; Klein, R.; Knudtson, M.D.; Lee, K.E.; Gangnon, R.; Wong, T.Y.; Klein, B.E. Determinants of Retinal Venular Diameter: The Beaver Dam Eye Study. Ophthalmology 2012, 119, 2563–2571. [Google Scholar] [CrossRef] [PubMed]
- Klein, R.; Myers, C.E.; Knudtson, M.D.; Lee, K.E.; Gangnon, R.; Wong, T.Y.; Klein, B.E. Relationship of blood pressure and other factors to serial retinal arteriolar diameter measurements over time: The beaver dam eye study. Arch. Ophthalmol. 2012, 130, 1019–1027. [Google Scholar] [CrossRef]
- Leontidis, G.; Al-Diri, B.; Hunter, A. Summarising the retinal vascular calibres in healthy, diabetic and diabetic retinopathy eyes. Comput. Biol. Med. 2016, 72, 65–74. [Google Scholar] [CrossRef] [PubMed]
- Cheng, L.; Barlis, P.; Gibson, J.; Colville, D.; Hutchinson, A.; Gleeson, G.; Lamoureux, E.; VanGaal, W.; Savige, J. Hypertensive and diabetic microvascular retinopathy, and angiographically-demonstrated coronary artery disease: A crosssectional, observational study. Nephrology 2016, 21, 63. [Google Scholar]
- McGeechan, K.; Liew, G.; Macaskill, P.; Irwig, L.; Klein, R.; Sharrett, A.R.; Klein, B.E.; Wang, J.J.; Chambless, L.E.; Wong, T.Y. Risk Prediction of Coronary Heart Disease Based on Retinal Vascular Caliber (from the Atherosclerosis Risk in Communities [ARIC] Study). Am. J. Cardiol. 2008, 102, 58–63. [Google Scholar] [CrossRef]
- Wu, H.-Q.; Wu, H.; Shi, L.-L.; Yu, L.-Y.; Wang, L.-Y.; Chen, Y.-L.; Geng, J.-S.; Shi, J.; Jiang, K.; Dong, J.-C. The association between retinal vasculature changes and stroke: A literature review and Meta-analysis. Int. J. Ophthalmol. 2017, 10, 109–114. [Google Scholar]
- Seidelmann, S.B.; Claggett, B.; Bravo, P.E.; Gupta, A.; Farhad, H.; Klein, B.E.; Klein, R.; Di Carli, M.; Solomon, S.D. Retinal Vessel Calibers in Predicting Long-Term Cardiovascular Outcomes: The Atherosclerosis Risk in Communities Study. Circulation 2016, 134, 1328–1338. [Google Scholar] [CrossRef]
- Gopinath, B.; Wang, S.B.; Liew, G.; Phan, K.; Joachim, N.; Burlutsky, G.; Thiagalingam, A.; Mitchell, P. Retinal Vascular Geometry and the Prevalence of Atrial Fibrillation and Heart Failure in a Clinic-Based Sample. Heart Lung Circ. 2018, 28, 1631–1637. [Google Scholar] [CrossRef]
- Guo, S.; Yin, S.; Tse, G.; Li, G.; Su, L.; Liu, T. Association Between Caliber of Retinal Vessels and Cardiovascular Disease: A Systematic Review and Meta-Analysis. Curr. Atheroscler. Rep. 2020, 22, 16. [Google Scholar] [CrossRef]
- Roy, M.S.; Klein, R.; Janal, M.N. Relationship of Retinal Vessel Caliber to Cardiovascular Disease and Mortality in African Americans with Type 1 Diabetes Mellitus. Arch. Ophthalmol. 2012, 130, 561–567. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Phan, K.; Mitchell, P.; Liew, G.; Plant, A.J.; Wang, S.B.; Au, C.; Chiha, J.; Kovoor, P.; Thiagalingam, A.; Burlutsky, G.; et al. Association between Retinal Arteriolar and Venule Calibre with Prevalent Heart Failure: A Cross-Sectional Study. PLoS ONE 2015, 10, e0144850. [Google Scholar]
- Iwanaga, Y.; Nishi, I.; Furuichi, S.; Noguchi, T.; Sase, K.; Kihara, Y.; Goto, Y.; Nonogi, H. B-Type Natriuretic Peptide Strongly Reflects Diastolic Wall Stress in Patients with Chronic Heart Failure: Comparison between Systolic and Diastolic Heart Failure. J. Am. Coll. Cardiol. 2006, 47, 742–748. [Google Scholar] [CrossRef] [PubMed]
- Van Veldhuisen, D.J.; Linssen, G.C.; Jaarsma, T.; van Gilst, W.H.; Hoes, A.W.; Tijssen, J.G.; Paulus, W.J.; Voors, A.A.; Hillege, H.L. B-type natriuretic peptide and prognosis in heart failure patients with preserved and reduced ejection fraction. J. Am. Coll. Cardiol. 2013, 61, 1498–1506. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).