Freely Available, Fully Automated AI-Based Analysis of Primary Tumour and Metastases of Prostate Cancer in Whole-Body [18F]-PSMA-1007 PET-CT
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patients and Imaging
2.2. Manual Segmentations for Training (Ground Truth)
2.3. AI Tool
2.4. Sampling
2.5. Training
2.6. Model Evaluation
- (1)
- Segment the suspected malignant lesions in the prostate and seminal vesicles in patients with prostate and in patients without a prostate (after prostatectomy) to segment any suspicious recurrence in the prostate bed and/or seminal vesicles.
- (2)
- To mark all suspected lymph node metastases, both pelvic and extra-pelvic, using the E-PSMA grading system as guidance [19]. Low uptake (equal to or lower than the background) in lymph nodes was generally considered non-pathologic while intense uptake (above liver) was marked pathologic. Intermediate uptake was generally considered pathologic when deviating from known patterns of unspecific uptake (such as low-intermediate uptake along the distal external iliac vessels or in the mediastinum). If highly suspicious lymph nodes with low uptake were found, for example, enlarged necrotic pelvic lymph nodes, the physicians were instructed to segment these as suspected lymph node metastases.
- (3)
- To mark uptakes in the bone that could be metastases but, in general, not low-grade uptake in, for example, the ribs and pelvic bones, which is a common unspecific finding in [18F]PSMA-1007.
2.7. Statistical Analysis
3. Results
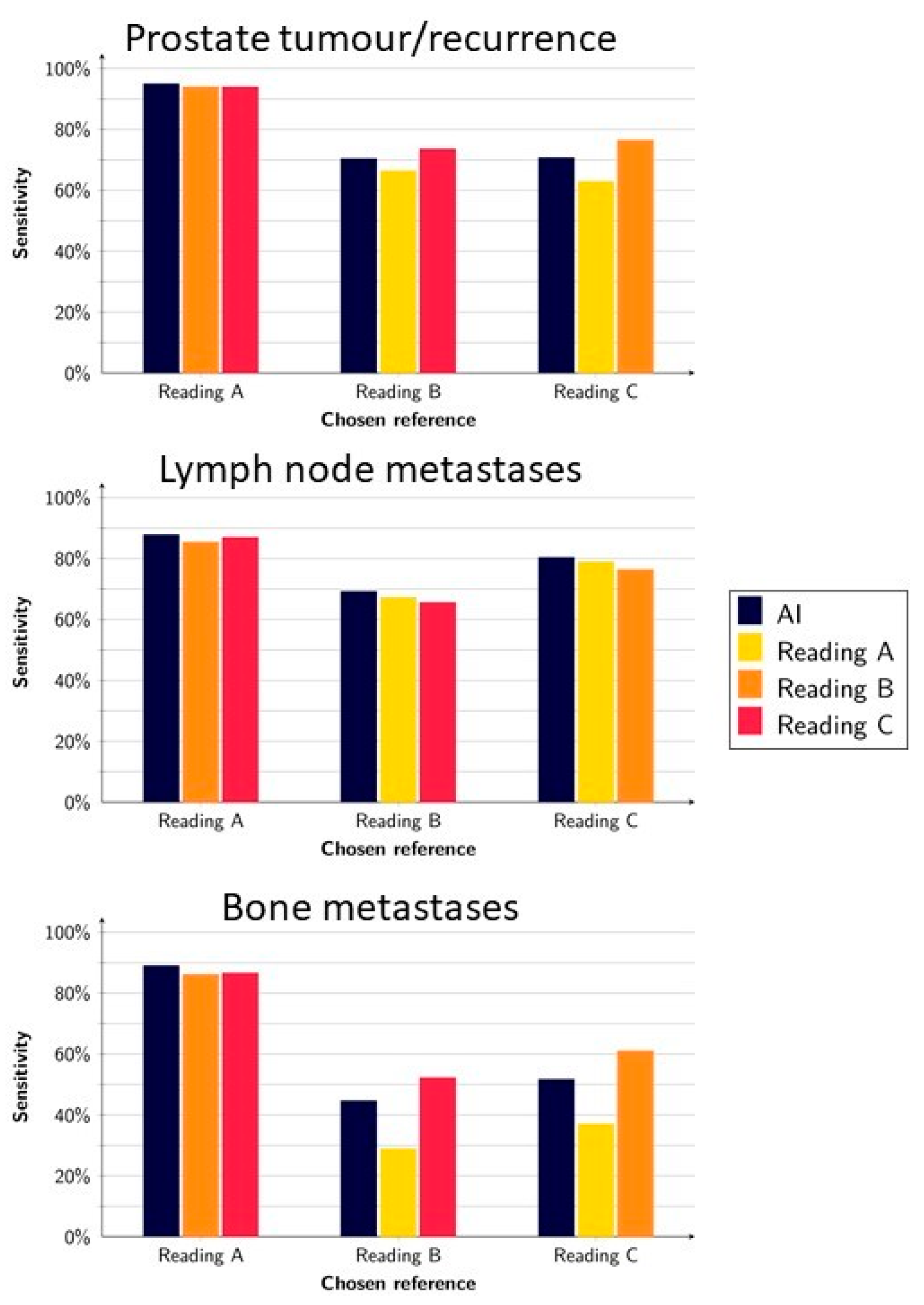
3.1. Detection of Suspected Tumours and Metastases
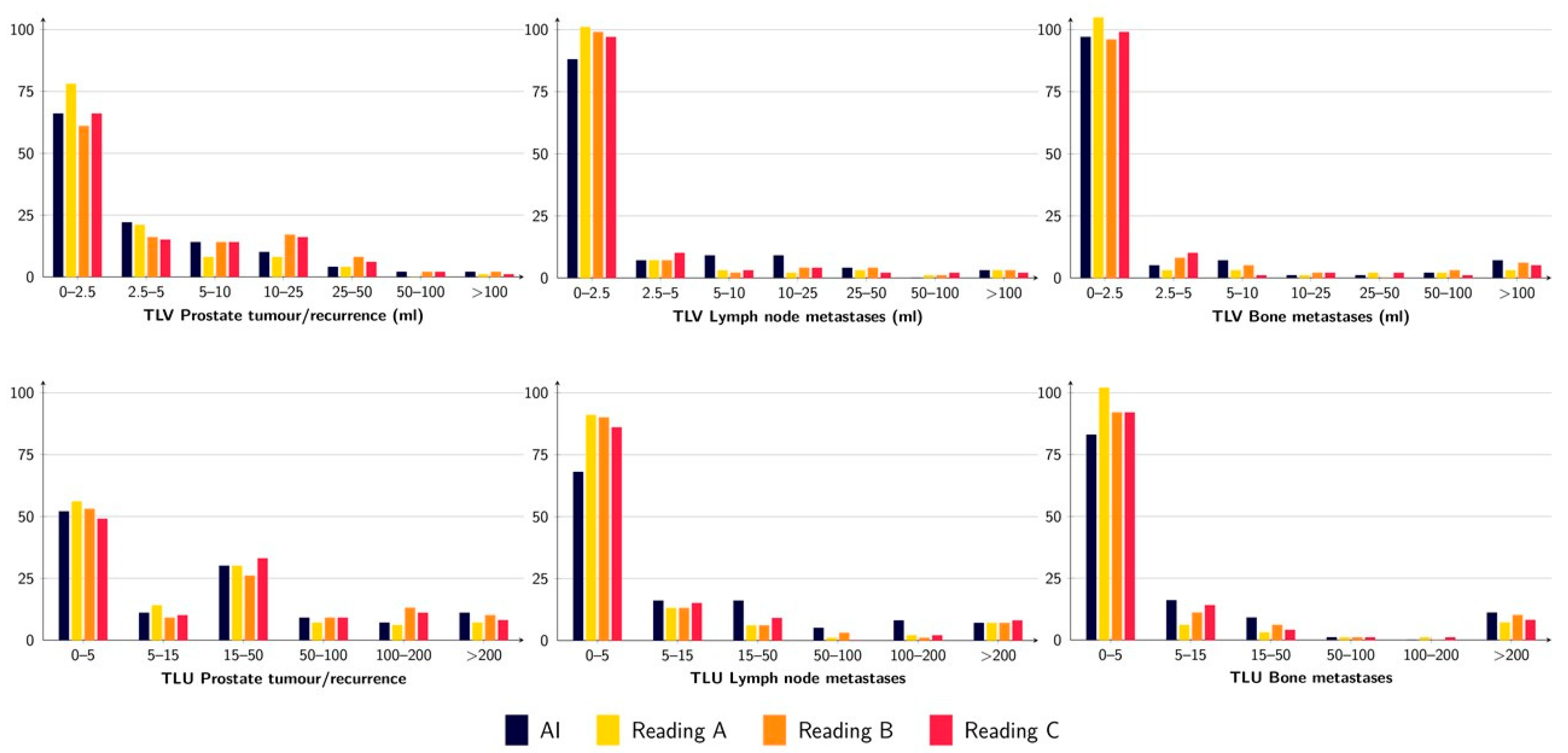
3.2. Quantification of Tumour Burden
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Culp, M.B.; Soerjomataram, I.; Efstathiou, J.A.; Bray, F.; Jemal, A. Recent Global Patterns in Prostate Cancer Incidence and Mortality Rates. Eur. Urol. 2020, 77, 38–52. [Google Scholar] [CrossRef]
- Troyer, J.K.; Beckett, M.L.; Wright, G.L., Jr. Detection and characterization of the prostate-specific membrane antigen (PSMA) in tissue extracts and body fluids. Int. J. Cancer 1995, 62, 552–558. [Google Scholar] [CrossRef]
- Maurer, T.; Gschwend, J.E.; Rauscher, I.; Souvatzoglou, M.; Haller, B.; Weirich, G.; Wester, H.J.; Heck, M.; Kubler, H.; Beer, A.J.; et al. Diagnostic Efficacy of (68)Gallium-PSMA Positron Emission Tomography Compared to Conventional Imaging for Lymph Node Staging of 130 Consecutive Patients with Intermediate to High Risk Prostate Cancer. J. Urol. 2016, 195, 1436–1443. [Google Scholar] [CrossRef]
- Herlemann, A.; Wenter, V.; Kretschmer, A.; Thierfelder, K.M.; Bartenstein, P.; Faber, C.; Gildehaus, F.J.; Stief, C.G.; Gratzke, C.; Fendler, W.P. (68)Ga-PSMA Positron Emission Tomography/Computed Tomography Provides Accurate Staging of Lymph Node Regions Prior to Lymph Node Dissection in Patients with Prostate Cancer. Eur. Urol. 2016, 70, 553–557. [Google Scholar] [CrossRef] [PubMed]
- Rowe, S.P.; Macura, K.J.; Mena, E.; Blackford, A.L.; Nadal, R.; Antonarakis, E.S.; Eisenberger, M.; Carducci, M.; Fan, H.; Dannals, R.F.; et al. PSMA-Based [(18)F]DCFPyL PET/CT Is Superior to Conventional Imaging for Lesion Detection in Patients with Metastatic Prostate Cancer. Mol. Imaging Biol. 2016, 18, 411–419. [Google Scholar] [CrossRef]
- Hofman, M.S.; Lawrentschuk, N.; Francis, R.J.; Tang, C.; Vela, I.; Thomas, P.; Rutherford, N.; Martin, J.M.; Frydenberg, M.; Shakher, R.; et al. Prostate-specific membrane antigen PET-CT in patients with high-risk prostate cancer before curative-intent surgery or radiotherapy (proPSMA): A prospective, randomised, multicentre study. Lancet 2020, 395, 1208–1216. [Google Scholar] [CrossRef]
- Awenat, S.; Piccardo, A.; Carvoeiras, P.; Signore, G.; Giovanella, L.; Prior, J.O.; Treglia, G. Diagnostic Role of (18)F-PSMA-1007 PET/CT in Prostate Cancer Staging: A Systematic Review. Diagnostics 2021, 11, 552. [Google Scholar] [CrossRef]
- Luining, W.I.; Meijer, D.; Dahele, M.R.; Vis, A.N.; Oprea-Lager, D.E. Nuclear Imaging for Bone Metastases in Prostate Cancer: The Emergence of Modern Techniques Using Novel Radiotracers. Diagnostics 2021, 11, 117. [Google Scholar] [CrossRef]
- Lenzo, N.P.; Meyrick, D.; Turner, J.H. Review of Gallium-68 PSMA PET/CT Imaging in the Management of Prostate Cancer. Diagnostics 2018, 8, 16. [Google Scholar] [CrossRef]
- Wibmer, A.G.; Morris, M.J.; Gonen, M.; Zheng, J.; Hricak, H.; Larson, S.; Scher, H.I.; Vargas, H.A. Quantification of Metastatic Prostate Cancer Whole-Body Tumor Burden with (18)F-FDG PET Parameters and Associations with Overall Survival After First-Line Abiraterone or Enzalutamide: A Single-Center Retrospective Cohort Study. J. Nucl. Med. 2021, 62, 1050–1056. [Google Scholar] [CrossRef]
- Seifert, R.; Herrmann, K.; Kleesiek, J.; Schafers, M.; Shah, V.; Xu, Z.; Chabin, G.; Grbic, S.; Spottiswoode, B.; Rahbar, K. Semiautomatically Quantified Tumor Volume Using (68)Ga-PSMA-11 PET as a Biomarker for Survival in Patients with Advanced Prostate Cancer. J. Nucl. Med. 2020, 61, 1786–1792. [Google Scholar] [CrossRef] [PubMed]
- Polymeri, E.; Sadik, M.; Kaboteh, R.; Borrelli, P.; Enqvist, O.; Ulen, J.; Ohlsson, M.; Tragardh, E.; Poulsen, M.H.; Simonsen, J.A.; et al. Deep learning-based quantification of PET/CT prostate gland uptake: Association with overall survival. Clin. Physiol. Funct. Imaging 2020, 40, 106–113. [Google Scholar] [CrossRef] [PubMed]
- Lindgren Belal, S.; Sadik, M.; Kaboteh, R.; Hasani, N.; Enqvist, O.; Svarm, L.; Kahl, F.; Simonsen, J.; Poulsen, M.H.; Ohlsson, M.; et al. 3D skeletal uptake of (18)F sodium fluoride in PET/CT images is associated with overall survival in patients with prostate cancer. EJNMMI Res. 2017, 7, 15. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, A.J.; Kaboteh, R.; Carducci, M.A.; Damber, J.E.; Stadler, W.M.; Hansen, M.; Edenbrandt, L.; Forsberg, G.; Nordle, O.; Pili, R.; et al. Assessment of the bone scan index in a randomized placebo-controlled trial of tasquinimod in men with metastatic castration-resistant prostate cancer (mCRPC). Urol. Oncol. 2014, 32, 1308–1316. [Google Scholar] [CrossRef]
- Bjoersdorff, M.; Oddstig, J.; Karindotter-Borgendahl, N.; Almquist, H.; Zackrisson, S.; Minarik, D.; Tragardh, E. Impact of penalizing factor in a block-sequential regularized expectation maximization reconstruction algorithm for (18)F-fluorocholine PET-CT regarding image quality and interpretation. EJNMMI Phys. 2019, 6, 5. [Google Scholar] [CrossRef] [PubMed]
- Tragardh, E.; Borrelli, P.; Kaboteh, R.; Gillberg, T.; Ulen, J.; Enqvist, O.; Edenbrandt, L. RECOMIA-a cloud-based platform for artificial intelligence research in nuclear medicine and radiology. EJNMMI Phys. 2020, 7, 51. [Google Scholar] [CrossRef]
- Çiçek, Ö.; Abdulkadir, A.; Lienkamp, S.S.; Brox, T.; Ronneberger, O. 3D U-Net: Learning Dense Volumetric Segmentation from Sparse Annotation; Springer: Cham, Switzerland, 2016; pp. 424–432. [Google Scholar]
- Kingma, D.; Ba, J. Adam: A method for stochastic optimization. arXiv 2014, arXiv:1412.6980. [Google Scholar] [CrossRef]
- Ceci, F.; Oprea-Lager, D.E.; Emmett, L.; Adam, J.A.; Bomanji, J.; Czernin, J.; Eiber, M.; Haberkorn, U.; Hofman, M.S.; Hope, T.A.; et al. E-PSMA: The EANM standardized reporting guidelines v1.0 for PSMA-PET. Eur. J. Nucl. Med. Mol. Imaging 2021, 48, 1626–1638. [Google Scholar] [CrossRef]
- Sadik, M.; Suurkula, M.; Hoglund, P.; Jarund, A.; Edenbrandt, L. Improved classifications of planar whole-body bone scans using a computer-assisted diagnosis system: A multicenter, multiple-reader, multiple-case study. J. Nucl. Med. 2009, 50, 368–375. [Google Scholar] [CrossRef]
- Has Simsek, D.; Kuyumcu, S.; Karadogan, S.; Oflas, M.; Isik, E.G.; Ozkan, Z.G.; Paksoy, N.; Ekmekcioglu, O.; Ekenel, M.; Sanli, Y. Can PSMA-based tumor burden predict response to docetaxel treatment in metastatic castration-resistant prostate cancer? Ann. Nucl. Med. 2021, 35, 680–690. [Google Scholar] [CrossRef]
- Gafita, A.; Rauscher, I.; Fendler, W.P.; Murthy, V.; Hui, W.; Armstrong, W.R.; Herrmann, K.; Weber, W.A.; Calais, J.; Eiber, M.; et al. Measuring response in metastatic castration-resistant prostate cancer using PSMA PET/CT: Comparison of RECIST 1.1, aPCWG3, aPERCIST, PPP, and RECIP 1.0 criteria. Eur. J. Nucl. Med. Mol. Imaging 2022. [Google Scholar] [CrossRef]
- Grunig, H.; Maurer, A.; Thali, Y.; Kovacs, Z.; Strobel, K.; Burger, I.A.; Muller, J. Focal unspecific bone uptake on [(18)F]-PSMA-1007 PET: A multicenter retrospective evaluation of the distribution, frequency, and quantitative parameters of a potential pitfall in prostate cancer imaging. Eur. J. Nucl. Med. Mol. Imaging 2021, 48, 4483–4494. [Google Scholar] [CrossRef]
- Vollnberg, B.; Alberts, I.; Genitsch, V.; Rominger, A.; Afshar-Oromieh, A. Assessment of malignancy and PSMA expression of uncertain bone foci in [(18)F]PSMA-1007 PET/CT for prostate cancer-a single-centre experience of PET-guided biopsies. Eur. J. Nucl. Med. Mol. Imaging 2022. [Google Scholar] [CrossRef]
- Borrelli, P.; Larsson, M.; Ulen, J.; Enqvist, O.; Tragardh, E.; Poulsen, M.H.; Mortensen, M.A.; Kjolhede, H.; Hoilund-Carlsen, P.F.; Edenbrandt, L. Artificial intelligence-based detection of lymph node metastases by PET/CT predicts prostate cancer-specific survival. Clin. Physiol. Funct. Imaging 2021, 41, 62–67. [Google Scholar] [CrossRef]
- Lindgren Belal, S.; Sadik, M.; Kaboteh, R.; Enqvist, O.; Ulen, J.; Poulsen, M.H.; Simonsen, J.; Hoilund-Carlsen, P.F.; Edenbrandt, L.; Tragardh, E. Deep learning for segmentation of 49 selected bones in CT scans: First step in automated PET/CT-based 3D quantification of skeletal metastases. Eur. J. Radiol. 2019, 113, 89–95. [Google Scholar] [CrossRef]
- Mortensen, M.A.; Borrelli, P.; Poulsen, M.H.; Gerke, O.; Enqvist, O.; Ulen, J.; Tragardh, E.; Constantinescu, C.; Edenbrandt, L.; Lund, L.; et al. Artificial intelligence-based versus manual assessment of prostate cancer in the prostate gland: A method comparison study. Clin. Physiol. Funct. Imaging 2019, 39, 399–406. [Google Scholar] [CrossRef]
- Tragardh, E.; Enqvist, O.; Ulen, J.; Hvittfeldt, E.; Garpered, S.; Belal, S.L.; Bjartell, A.; Edenbrandt, L. Freely available artificial intelligence for pelvic lymph node metastases in PSMA PET-CT that performs on par with nuclear medicine physicians. Eur. J. Nucl. Med. Mol. Imaging 2022. [Google Scholar] [CrossRef]
- Johnsson, K.; Brynolfsson, J.; Sahlstedt, H.; Nickols, N.G.; Rettig, M.; Probst, S.; Morris, M.J.; Bjartell, A.; Eiber, M.; Anand, A. Analytical performance of aPROMISE: Automated anatomic contextualization, detection, and quantification of [(18)F]DCFPyL (PSMA) imaging for standardized reporting. Eur. J. Nucl. Med. Mol. Imaging 2022, 49, 1041–1051. [Google Scholar] [CrossRef]
- Ferro, M.; de Cobelli, O.; Musi, G.; Del Giudice, F.; Carrieri, G.; Busetto, G.M.; Falagario, U.G.; Sciarra, A.; Maggi, M.; Crocetto, F.; et al. Radiomics in prostate cancer: An up-to-date review. Ther. Adv. Urol. 2022, 14, 17562872221109020. [Google Scholar] [CrossRef]
- Ferro, M.; de Cobelli, O.; Vartolomei, M.D.; Lucarelli, G.; Crocetto, F.; Barone, B.; Sciarra, A.; Del Giudice, F.; Muto, M.; Maggi, M.; et al. Prostate Cancer Radiogenomics-From Imaging to Molecular Characterization. Int. J. Mol. Sci. 2021, 22, 9971. [Google Scholar] [CrossRef]


| n = 120 Patients | AI vs. Reading | Reading vs. Reading |
|---|---|---|
| TP (n) | ||
| -Total | 93.7 (88–99) | 92.7 (83–107) |
| -Per patient | 0.8 (0.7–0.8) | 0.8 (0.7–0.9) |
| FP (n) | ||
| -Total | 62.3 (54–77) | 28.7 (6–52) |
| -Per patient | 0.5 (0.4–0.6) | 0.2 (0.05–0.4) |
| FN (n) | ||
| -Total | 27.7 (5–41) | 28.7 (6–52) |
| -Per patient | 0.2 (0.04–0.3) | 0.2 (0.05–0.4) |
| Sensitivity (%) | 78.7 (70.4–94.9) | 77.9 (62.9–93.9) |
| PPV (%) | 60.3 (55.0–63.9) | 78.3 (64.1–93.6) |
| n = 120 Patients | AI vs. Reading | Reading vs. Reading |
|---|---|---|
| TP (n) | ||
| -Total | 215.3 (209–221) | 208.7 (198–217) |
| -Per patient | 1.8 (1.7–1.8) | 1.7 (1.7–1.8) |
| FP (n) | ||
| -Total | 333.3 (331–335) | 65.7 (32–104) |
| -Per patient | 2.8 (2.8–2.8) | 0.5 (0.3–0.9) |
| FN (n) | ||
| -Total | 59.0 (30–93) | 65.7 (32–104) |
| -Per patient | 0.5 (0.3–0.8) | 0.5 (0.3–0.9) |
| Sensitivity (%) | 79.1 (69.2–87.8) | 77.9 (65.6–87.0) |
| PPV (%) | 39.2 (38.5–40.0) | 78.3 (66.9–87.1) |
| n = 120 Patients | AI vs. Reading | Reading vs. Reading |
|---|---|---|
| TP (n) | ||
| -Total | 236.7 (186–271) | 222.2 (175–317) |
| -Per patient | 2.0 (1.6–2.3) | 1.9 (1.5–2.6) |
| FP (n) | ||
| -Total | 357.7 (260–451) | 213.2 (28–432) |
| -Per patient | 3.0 (2.2–3.8) | 1.8 (0.2–3.6) |
| FN (n) | ||
| -Total | 198.7 (23–336) | 213.2 (29–432) |
| -Per patient | 1.7 (0.2–2.8) | 1.8 (0.2–3.6) |
| Sensitivity (%) | 61.8 (44.6–89.0) | 58.6 (28.8–86.6) |
| PPV (%) | 40.5 (29.2–51.0) | 58.7 (29.4–86.6) |
| AI | Reading A | Reading B | Reading C | |
|---|---|---|---|---|
| TLV Prostate tumour | 1.6 (0–491) | 1.2 (0–641) | 1.9 (0–2749) | 2.1 (0–640) |
| TLV Lymph nodes metastases | 0.8 (0–224) | 0 (0–207) | 0 (0–336) | 0 (0–174) |
| TLV Bone metastases | 0.5 (0–495) | 0 (0–559) | 0 (0–1398) | 0 (0–669) |
| TLU Prostate tumour | 11.3 (0–5903) | 6.4 (0–7291) | 12.4 (0–8007) | 12.8 (0–7326) |
| TLU Lymph nodes metastases | 4.0 (0–2810) | 0 (0–2433) | 0 (0–2382) | 0 (0–2390) |
| TLU Bone metastases | 1.5 (0–5199) | 0 (0–6763) | 0 (0–7389) | 0 (0–6570) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Trägårdh, E.; Enqvist, O.; Ulén, J.; Jögi, J.; Bitzén, U.; Hedeer, F.; Valind, K.; Garpered, S.; Hvittfeldt, E.; Borrelli, P.; et al. Freely Available, Fully Automated AI-Based Analysis of Primary Tumour and Metastases of Prostate Cancer in Whole-Body [18F]-PSMA-1007 PET-CT. Diagnostics 2022, 12, 2101. https://doi.org/10.3390/diagnostics12092101
Trägårdh E, Enqvist O, Ulén J, Jögi J, Bitzén U, Hedeer F, Valind K, Garpered S, Hvittfeldt E, Borrelli P, et al. Freely Available, Fully Automated AI-Based Analysis of Primary Tumour and Metastases of Prostate Cancer in Whole-Body [18F]-PSMA-1007 PET-CT. Diagnostics. 2022; 12(9):2101. https://doi.org/10.3390/diagnostics12092101
Chicago/Turabian StyleTrägårdh, Elin, Olof Enqvist, Johannes Ulén, Jonas Jögi, Ulrika Bitzén, Fredrik Hedeer, Kristian Valind, Sabine Garpered, Erland Hvittfeldt, Pablo Borrelli, and et al. 2022. "Freely Available, Fully Automated AI-Based Analysis of Primary Tumour and Metastases of Prostate Cancer in Whole-Body [18F]-PSMA-1007 PET-CT" Diagnostics 12, no. 9: 2101. https://doi.org/10.3390/diagnostics12092101
APA StyleTrägårdh, E., Enqvist, O., Ulén, J., Jögi, J., Bitzén, U., Hedeer, F., Valind, K., Garpered, S., Hvittfeldt, E., Borrelli, P., & Edenbrandt, L. (2022). Freely Available, Fully Automated AI-Based Analysis of Primary Tumour and Metastases of Prostate Cancer in Whole-Body [18F]-PSMA-1007 PET-CT. Diagnostics, 12(9), 2101. https://doi.org/10.3390/diagnostics12092101






