A Cationic Contrast Agent in X-ray Imaging of Articular Cartilage: Pre-Clinical Evaluation of Diffusion and Attenuation Properties
Abstract
:1. Introduction
2. Materials and Methods
2.1. MicroCT Acquisitions
2.1.1. Samples Preparation
2.1.2. Contrast Agent Synthesis and Solution Preparation
2.1.3. Calibration Curve Evaluation
2.1.4. Osteochondral Plugs Acquisition
2.1.5. Data Elaboration
2.1.6. Statistical Test
2.2. Clinical CT Acquisitions
2.2.1. Phantom Acquisition
2.2.2. Samples’ Preparation and Acquisition
2.2.3. Data Elaboration
2.2.4. Statistical Test
3. Results
3.1. MicroCT Acquisitions
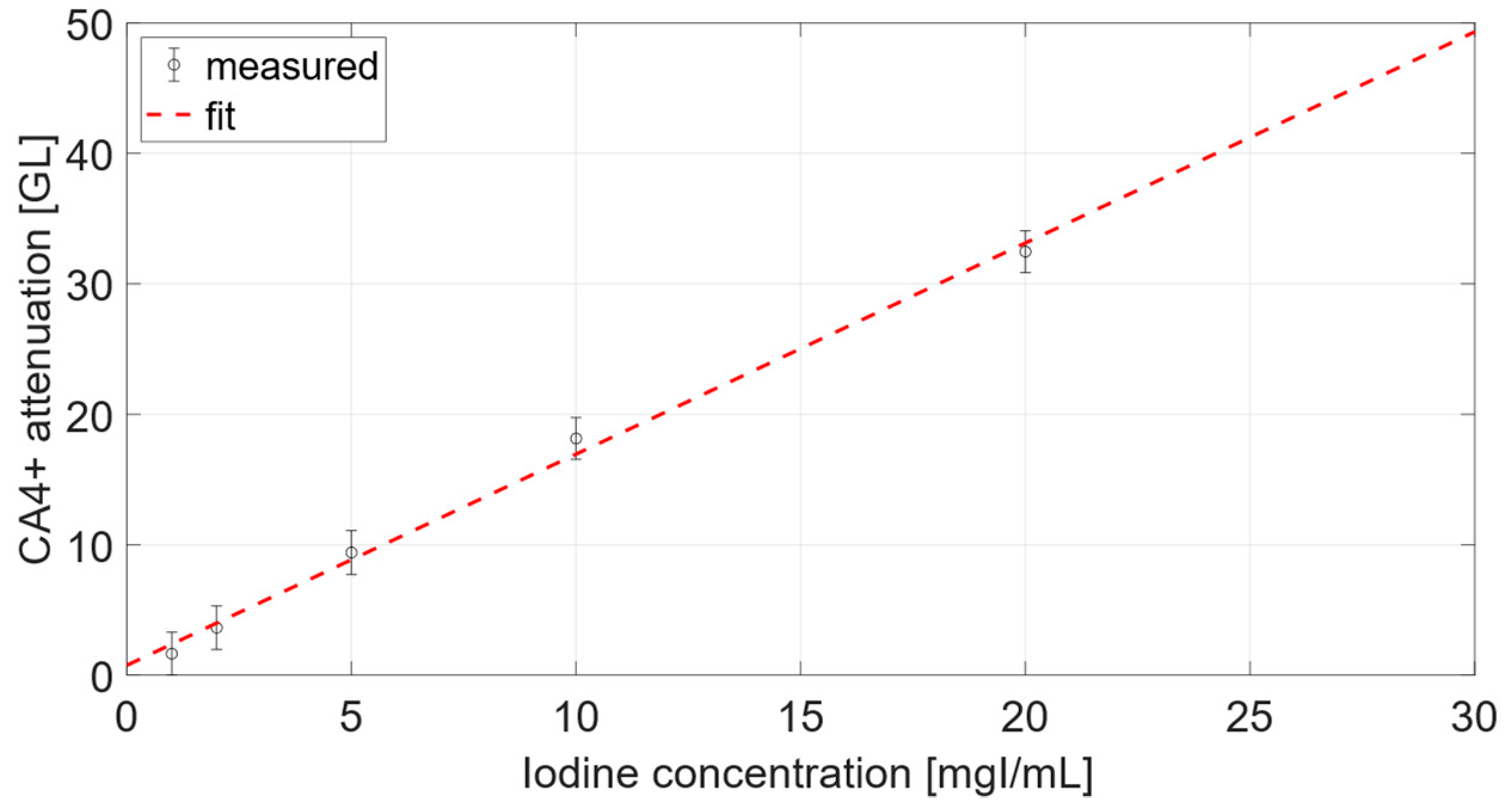
3.1.1. Calibration Curve
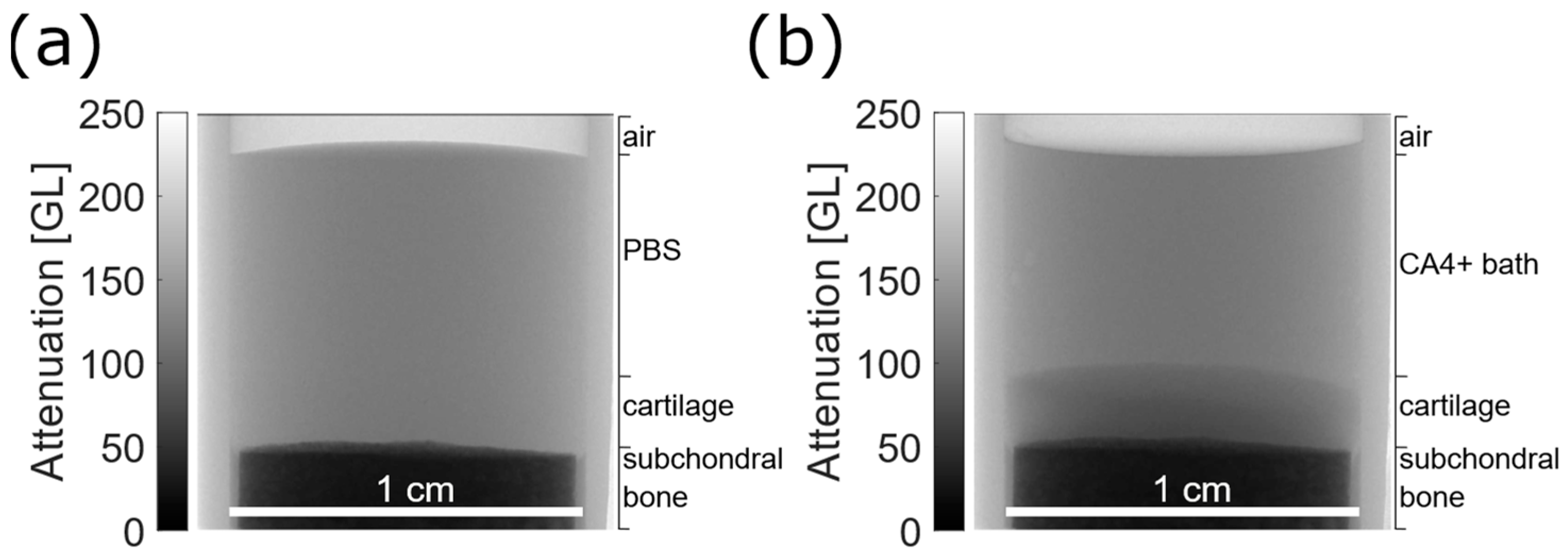
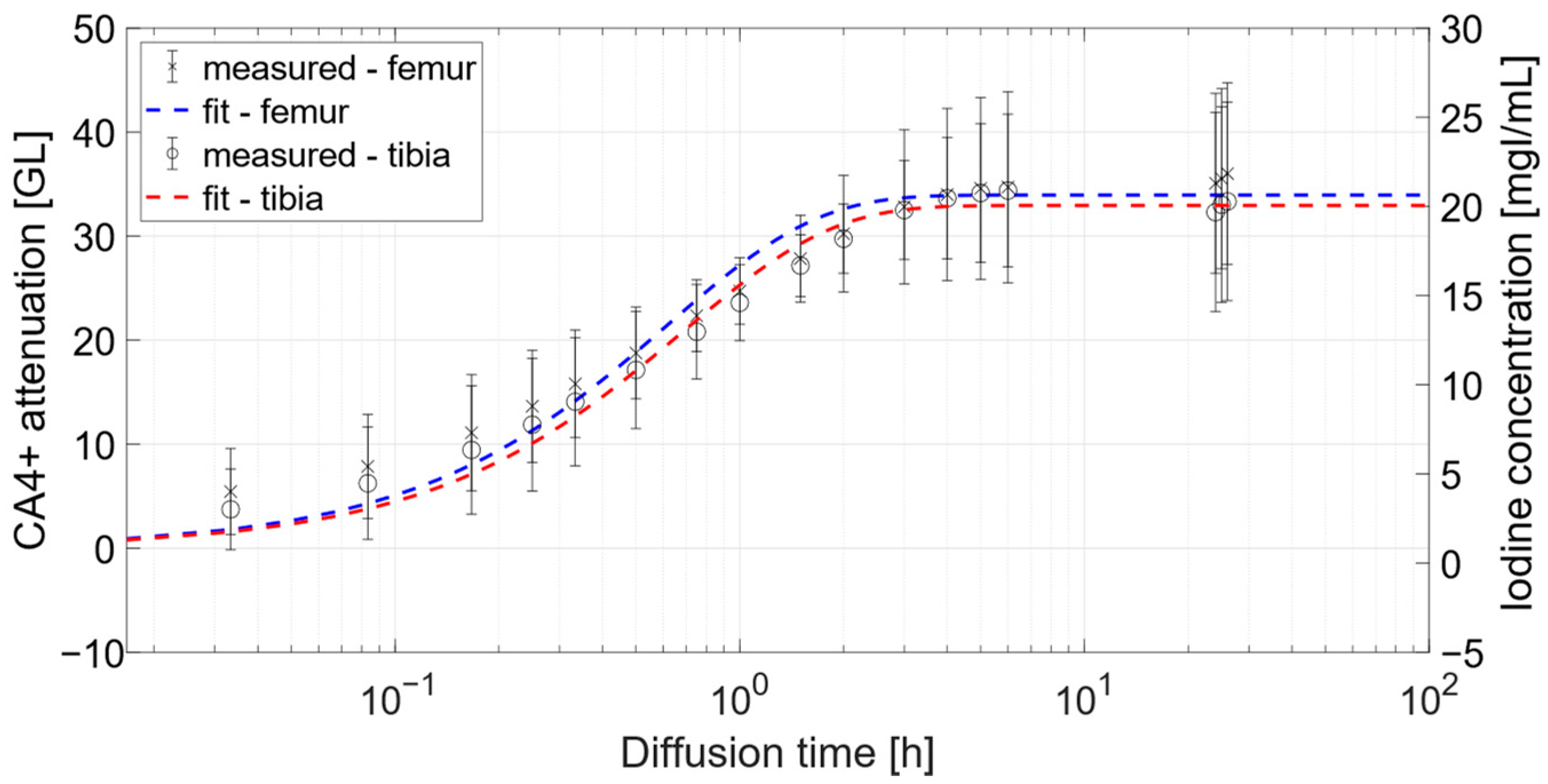
3.1.2. Osteochondral Plugs
3.2. Clinical CT Acquisitions
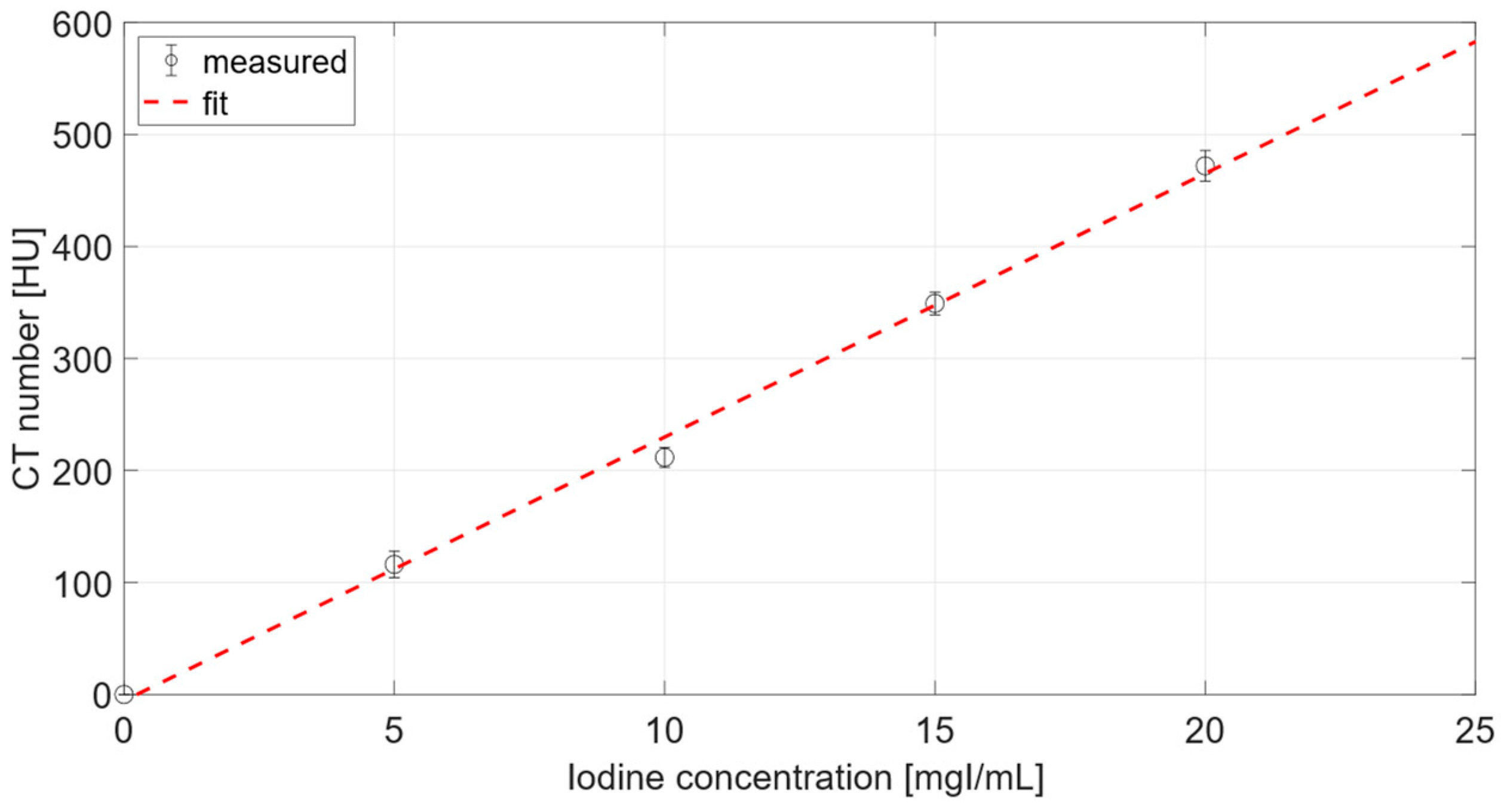
3.2.1. Calibration Curve
3.2.2. Attenuation Comparison
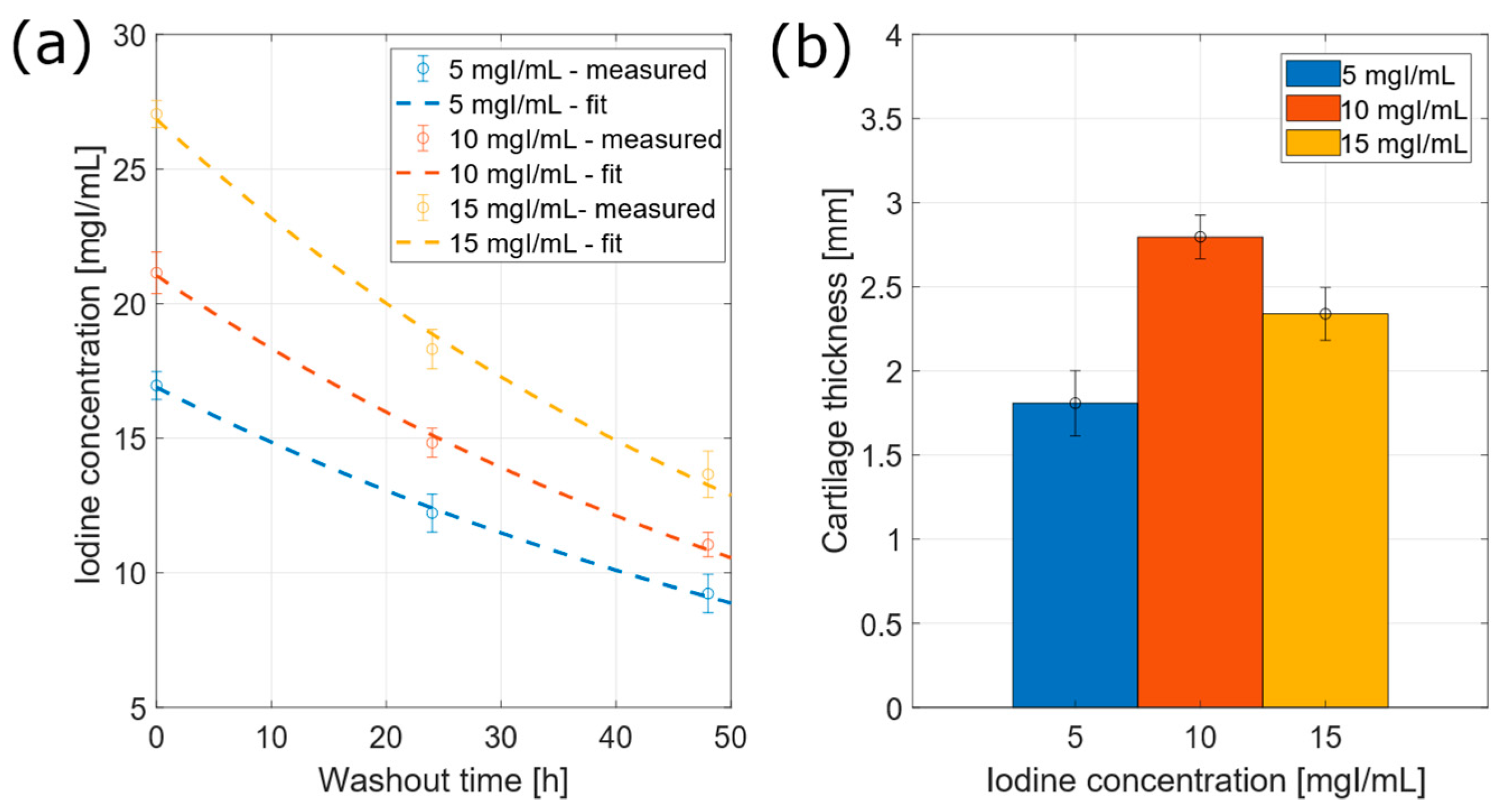
3.2.3. Iodine Concentration within Cartilage
3.3. Cartilage Volume and Thickness Estimation
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Mow, V.C.; Ratcliffe, A.; Poole, A.R. Cartilage and Diarthrodial Joints as Paradigms for Hierarchical Materials and Structures. Biomaterials 1992, 13, 67–97. [Google Scholar] [CrossRef]
- Little, C.B.; Hunter, D.J. Post-Traumatic Osteoarthritis: From Mouse Models to Clinical Trials. Nat. Rev. Rheumatol. 2013, 9, 485–497. [Google Scholar] [CrossRef] [PubMed]
- Kramer, W.C.; Hendricks, K.J.; Wang, J. Pathogenetic Mechanisms of Posttraumatic Osteoarthritis: Opportunities for Early Intervention. Int. J. Clin. Exp. Med. 2011, 4, 285–298. [Google Scholar]
- Palmer, A.J.R.; Brown, C.P.; McNally, E.G.; Price, A.J.; Tracey, I.; Jezzard, P.; Carr, A.J.; Glyn-Jones, S. Non-Invasive Imaging of Cartilage in Early Osteoarthritis. Bone Jt. J. 2013, 95-B, 738–746. [Google Scholar] [CrossRef]
- Taylor, C.; Carballido-Gamio, J.; Majumdar, S.; Li, X. Comparison of Quantitative Imaging of Cartilage for Osteoarthritis: T2, T1rho, DGEMRIC and Contrast-Enhanced Computed Tomography. Magn. Reson. Imaging 2009, 27, 779–784. [Google Scholar] [CrossRef] [PubMed]
- Hafner, T.; Schock, J.; Post, M.; Abrar, D.B.; Sewerin, P.; Linka, K.; Knobe, M.; Kuhl, C.; Truhn, D.; Nebelung, S. A Serial Multiparametric Quantitative Magnetic Resonance Imaging Study to Assess Proteoglycan Depletion of Human Articular Cartilage and Its Effects on Functionality. Sci. Rep. 2020, 10, 15106. [Google Scholar] [CrossRef]
- Lakin, B.A.; Snyder, B.D.; Grinstaff, M.W. Assessing Cartilage Biomechanical Properties: Techniques for Evaluating the Functional Performance of Cartilage in Health and Disease. Annu. Rev. Biomed. Eng. 2017, 19, 27–55. [Google Scholar] [CrossRef]
- Martel-Pelletier, J. Pathophysiology of Osteoarthritis. Osteoarthr. Cartil. 2004, 12, 31–33. [Google Scholar] [CrossRef]
- Aula, A.S.; Jurvelin, J.S.; Töyräs, J. Simultaneous Computed Tomography of Articular Cartilage and Subchondral Bone. Osteoarthr. Cartil. 2009, 17, 1583–1588. [Google Scholar] [CrossRef]
- Mankin, H.J.; Dorfman, H.; Lippiello, L.; Zarins, A. Biochemical and Metabolic Abnormalities in Articular Cartilage from Osteo-Arthritic Human Hips: II. Correlation of Morphology with Biochemical and Metabolic Data. JBJS 1971, 53, 523–537. [Google Scholar] [CrossRef]
- Palmer, A.W.; Guldberg, R.E.; Levenston, M.E. Analysis of Cartilage Matrix Fixed Charge Density and Three-Dimensional Morphology via Contrast-Enhanced Microcomputed Tomography. Proc. Natl. Acad. Sci. USA 2006, 103, 19255–19260. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Flynn, C.; Hurtig, M.; Linden, A.Z. Anionic Contrast–Enhanced MicroCT Imaging Correlates with Biochemical and Histological Evaluations of Osteoarthritic Articular Cartilage. Cartilage 2021, 13, 1388S–1397S. [Google Scholar] [CrossRef] [PubMed]
- Freedman, J.D.; Ellis, D.J.; Lusic, H.; Varma, G.; Grant, A.K.; Lakin, B.A.; Snyder, B.D.; Grinstaff, M.W. DGEMRIC and CECT Comparison of Cationic and Anionic Contrast Agents in Cadaveric Human Metacarpal Cartilage. J. Orthop. Res. Off. Publ. Orthop. Res. Soc. 2020, 38, 719–725. [Google Scholar] [CrossRef] [PubMed]
- Inerot, S.; Heinegård, D.; Audell, L.; Olsson, S.E. Articular-Cartilage Proteoglycans in Aging and Osteoarthritis. Biochem. J. 1978, 169, 143–156. [Google Scholar] [CrossRef]
- Bansal, P.N.; Joshi, N.S.; Entezari, V.; Grinstaff, M.W.; Snyder, B.D. Contrast Enhanced Computed Tomography Can Predict the Glycosaminoglycan Content and Biomechanical Properties of Articular Cartilage. Osteoarthr. Cartil. 2010, 18, 184–191. [Google Scholar] [CrossRef]
- Joshi, N.S.; Bansal, P.N.; Stewart, R.C.; Snyder, B.D.; Grinstaff, M.W. Effect of Contrast Agent Charge on Visualization of Articular Cartilage Using Computed Tomography: Exploiting Electrostatic Interactions for Improved Sensitivity. J. Am. Chem. Soc. 2009, 131, 13234–13235. [Google Scholar] [CrossRef]
- Bansal, P.N.; Joshi, N.S.; Entezari, V.; Malone, B.C.; Stewart, R.C.; Snyder, B.D.; Grinstaff, M.W. Cationic Contrast Agents Improve Quantification of Glycosaminoglycan (GAG) Content by Contrast Enhanced CT Imaging of Cartilage. J. Orthop. Res. 2011, 29, 704–709. [Google Scholar] [CrossRef]
- Stewart, R.C.; Patwa, A.N.; Lusic, H.; Freedman, J.D.; Wathier, M.; Snyder, B.D.; Guermazi, A.; Grinstaff, M.W. Synthesis and Preclinical Characterization of a Cationic Iodinated Imaging Contrast Agent (CA4+) and Its Use for Quantitative Computed Tomography of Ex Vivo Human Hip Cartilage. J. Med. Chem. 2017, 60, 5543–5555. [Google Scholar] [CrossRef]
- Bansal, P.N.; Stewart, R.C.; Entezari, V.; Snyder, B.D.; Grinstaff, M.W. Contrast Agent Electrostatic Attraction Rather than Repulsion to Glycosaminoglycans Affords a Greater Contrast Uptake Ratio and Improved Quantitative CT Imaging in Cartilage. Osteoarthr. Cartil. 2011, 19, 970–976. [Google Scholar] [CrossRef]
- Stewart, R.C.; Bansal, P.N.; Entezari, V.; Lusic, H.; Nazarian, R.M.; Snyder, B.D.; Grinstaff, M.W. Contrast-Enhanced CT with a High-Affinity Cationic Contrast Agent for Imaging Ex Vivo Bovine, Intact Ex Vivo Rabbit, and in Vivo Rabbit Cartilage. Radiology 2013, 266, 141–150. [Google Scholar] [CrossRef]
- Lakin, B.A.; Grasso, D.J.; Shah, S.S.; Stewart, R.C.; Bansal, P.N.; Freedman, J.D.; Grinstaff, M.W.; Snyder, B.D. Cationic Agent Contrast-Enhanced Computed Tomography Imaging of Cartilage Correlates with the Compressive Modulus and Coefficient of Friction. Osteoarthr. Cartil. 2013, 21, 60–68. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lakin, B.A.; Patel, H.; Holland, C.; Freedman, J.D.; Shelofsky, J.S.; Snyder, B.D.; Stok, K.S.; Grinstaff, M.W. Contrast-Enhanced CT Using a Cationic Contrast Agent Enables Non-Destructive Assessment of the Biochemical and Biomechanical Properties of Mouse Tibial Plateau Cartilage. J. Orthop. Res. 2016, 34, 1130–1138. [Google Scholar] [CrossRef] [PubMed]
- Stewart, R.C.; Nelson, B.B.; Kawcak, C.E.; Freedman, J.D.; Snyder, B.D.; Goodrich, L.R.; Grinstaff, M.W. Contrast-Enhanced Computed Tomography Scoring System for Distinguishing Early Osteoarthritis Disease States: A Feasibility Study. J. Orthop. Res. Off. Publ. Orthop. Res. Soc. 2019, 37, 2138–2148. [Google Scholar] [CrossRef] [PubMed]
- Karhula, S.S.; Finnilä, M.A.; Freedman, J.D.; Kauppinen, S.; Valkealahti, M.; Lehenkari, P.; Pritzker, K.P.H.; Nieminen, H.J.; Snyder, B.D.; Grinstaff, M.W.; et al. Micro-Scale Distribution of CA4+ in Ex Vivo Human Articular Cartilage Detected with Contrast-Enhanced Micro-Computed Tomography Imaging. Front. Phys. 2017, 5, 38. [Google Scholar] [CrossRef]
- Bhattarai, A.; Honkanen, J.T.J.; Myller, K.A.H.; Prakash, M.; Korhonen, M.; Saukko, A.E.A.; Virén, T.; Joukainen, A.; Patwa, A.N.; Kröger, H.; et al. Quantitative Dual Contrast CT Technique for Evaluation of Articular Cartilage Properties. Ann. Biomed. Eng. 2018, 46, 1038–1046. [Google Scholar] [CrossRef]
- Bhattarai, A.; Pouran, B.; Mäkelä, J.T.A.; Shaikh, R.; Honkanen, M.K.M.; Prakash, M.; Kröger, H.; Grinstaff, M.W.; Weinans, H.; Jurvelin, J.S.; et al. Dual Contrast in Computed Tomography Allows Earlier Characterization of Articular Cartilage over Single Contrast. J. Orthop. Res. 2020, 38, 2230–2238. [Google Scholar] [CrossRef]
- Bhattarai, A.; Mäkelä, J.T.A.; Pouran, B.; Kröger, H.; Weinans, H.; Grinstaff, M.W.; Töyräs, J.; Turunen, M.J. Effects of Human Articular Cartilage Constituents on Simultaneous Diffusion of Cationic and Nonionic Contrast Agents. J. Orthop. Res. 2021, 39, 771–779. [Google Scholar] [CrossRef]
- Honkanen, M.K.M.; Matikka, H.; Honkanen, J.T.J.; Bhattarai, A.; Grinstaff, M.W.; Joukainen, A.; Kröger, H.; Jurvelin, J.S.; Töyräs, J. Imaging of Proteoglycan and Water Contents in Human Articular Cartilage with Full-Body CT Using Dual Contrast Technique. J. Orthop. Res. 2019, 37, 1059–1070. [Google Scholar] [CrossRef]
- Paakkari, P.; Inkinen, S.I.; Honkanen, M.K.M.; Prakash, M.; Shaikh, R.; Nieminen, M.T.; Grinstaff, M.W.; Mäkelä, J.T.A.; Töyräs, J.; Honkanen, J.T.J. Quantitative Dual Contrast Photon-Counting Computed Tomography for Assessment of Articular Cartilage Health. Sci. Rep. 2021, 11, 5556. [Google Scholar] [CrossRef]
- Oh, D.J.; Lakin, B.A.; Stewart, R.C.; Wiewiorski, M.; Freedman, J.D.; Grinstaff, M.W.; Snyder, B.D. Contrast-Enhanced CT Imaging as a Non-Destructive Tool for Ex Vivo Examination of the Biochemical Content and Structure of the Human Meniscus. J. Orthop. Res. 2017, 35, 1018–1028. [Google Scholar] [CrossRef]
- Boos, M.A.; Grinstaff, M.W.; Lamandé, S.R.; Stok, K.S. Contrast-Enhanced Micro–Computed Tomography for 3D Visualization and Quantification of Glycosaminoglycans in Different Cartilage Types. Cartilage 2021, 13, 486S–494S. [Google Scholar] [CrossRef] [PubMed]
- Garcia, J.P.; Longoni, A.; Gawlitta, D.; Rosenberg, A.J.W.P.; Grinstaff, M.W.; Töyräs, J.; Weinans, H.; Creemers, L.B.; Pouran, B. Contrast Enhanced Computed Tomography for Real-Time Quantification of Glycosaminoglycans in Cartilage Tissue Engineered Constructs. Acta Biomater. 2019, 100, 202–212. [Google Scholar] [CrossRef] [PubMed]
- Saukko, A.E.A.; Turunen, M.J.; Honkanen, M.K.M.; Lovric, G.; Tiitu, V.; Honkanen, J.T.J.; Grinstaff, M.W.; Jurvelin, J.S.; Töyräs, J. Simultaneous Quantitation of Cationic and Non-Ionic Contrast Agents in Articular Cartilage Using Synchrotron MicroCT Imaging. Sci. Rep. 2019, 9, 7118. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Belev, G.; Stewart, R.C.; Grinstaff, M.W.; Snyder, B.D.; Wilson, D.R. Protocol Development for Synchrotron Contrast-Enhanced CT of Human Hip Cartilage. Med. Eng. Phys. 2019, 73, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Honkanen, M.K.M.; Saukko, A.E.A.; Turunen, M.J.; Shaikh, R.; Prakash, M.; Lovric, G.; Joukainen, A.; Kröger, H.; Grinstaff, M.W.; Töyräs, J. Synchrotron MicroCT Reveals the Potential of the Dual Contrast Technique for Quantitative Assessment of Human Articular Cartilage Composition. J. Orthop. Res. 2020, 38, 563–573. [Google Scholar] [CrossRef]
- Honkanen, M.K.M.; Saukko, A.E.A.; Turunen, M.J.; Xu, W.; Lovric, G.; Honkanen, J.T.J.; Grinstaff, M.W.; Lehto, V.-P.; Töyräs, J. Triple Contrast CT Method Enables Simultaneous Evaluation of Articular Cartilage Composition and Segmentation. Ann. Biomed. Eng. 2020, 48, 556–567. [Google Scholar] [CrossRef]
- Slavic, S.; Madhav, P.; Profio, M.; Crotty, D.; Nett, E.; Hsieh, J.; Liu, E. Technology White Paper: GSI Xtream on Revolution CT; GE Healthcare: Chicago, IL, USA, 2017; pp. 1–20. [Google Scholar]
- Király, K.; Lapveteläinen, T.; Arokoski, J.; Törrönen, K.; Módis, L.; Kiviranta, I.; Helminen, H.J. Application of Selected Cationic Dyes for the Semiquantitative Estimation of Glycosaminoglycans in Histological Sections of Articular Cartilage by Microspectrophotometry. Histochem. J. 1996, 28, 577–590. [Google Scholar] [CrossRef]
- Kiviranta, I.; Jurvelin, J.; Tammi, M.; Säämänen, A.M.; Helminen, H.J. Microspectrophotometric Quantitation of Glycosaminoglycans in Articular Cartilage Sections Stained with Safranin O. Histochemistry 1985, 82, 249–255. [Google Scholar] [CrossRef]
- Nieminen, M.T.; Rieppo, J.; Silvennoinen, J.; Töyräs, J.; Hakumäki, J.M.; Hyttinen, M.M.; Helminen, H.J.; Jurvelin, J.S. Spatial Assessment of Articular Cartilage Proteoglycans with Gd-DTPA-Enhanced T1 Imaging. Magn. Reson. Med. 2002, 48, 640–648. [Google Scholar] [CrossRef]
- Honkanen, J.T.J.; Turunen, M.J.; Freedman, J.D.; Saarakkala, S.; Grinstaff, M.W.; Ylärinne, J.H.; Jurvelin, J.S.; Töyräs, J. Cationic Contrast Agent Diffusion Differs Between Cartilage and Meniscus. Ann. Biomed. Eng. 2016, 44, 2913–2921. [Google Scholar] [CrossRef]
- Pedersen, D.R.; Goetz, J.E.; Kurriger, G.L.; Martin, J.A. Comparative Digital Cartilage Histology for Human and Common Osteoarthritis Models. Orthop. Res. Rev. 2013, 2013, 13–20. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kiviranta, P.; Lammentausta, E.; Töyräs, J.; Kiviranta, I.; Jurvelin, J.S. Indentation Diagnostics of Cartilage Degeneration. Osteoarthr. Cartil. 2008, 16, 796–804. [Google Scholar] [CrossRef] [PubMed]
- Alvarez, R.E.; Macovski, A. Energy-Selective Reconstructions in X-Ray Computerised Tomography. Phys. Med. Biol. 1976, 21, 733–744. [Google Scholar] [CrossRef]
- Zheng, S.; Xia, Y.; Bidthanapally, A.; Badar, F.; Ilsar, I.; Duvoisin, N. Damages to the Extracellular Matrix in Articular Cartilage Due to Cryopreservation by Microscopic Magnetic Resonance Imaging and Biochemistry. Magn. Reson. Imaging 2009, 27, 648–655. [Google Scholar] [CrossRef]
- Hunziker, E.B.; Lippuner, K.; Shintani, N. How Best to Preserve and Reveal the Structural Intricacies of Cartilaginous Tissue. Matrix Biol. 2014, 39, 33–43. [Google Scholar] [CrossRef] [PubMed]
- Qu, C.; Hirviniemi, M.; Tiitu, V.; Jurvelin, J.S.; Töyräs, J.; Lammi, M.J. Effects of Freeze–Thaw Cycle with and without Proteolysis Inhibitors and Cryopreservant on the Biochemical and Biomechanical Properties of Articular Cartilage. Cartilage 2014, 5, 97–106. [Google Scholar] [CrossRef]
- Pouran, B.; Arbabi, V.; Zadpoor, A.A.; Weinans, H. Isolated Effects of External Bath Osmolality, Solute Concentration, and Electrical Charge on Solute Transport across Articular Cartilage. Med. Eng. Phys. 2016, 38, 1399–1407. [Google Scholar] [CrossRef]
- Turunen, M.J.; Töyräs, J.; Lammi, M.J.; Jurvelin, J.S.; Korhonen, R.K. Hyperosmolaric Contrast Agents in Cartilage Tomography May Expose Cartilage to Overload-Induced Cell Death. J. Biomech. 2012, 45, 497–503. [Google Scholar] [CrossRef]
- Pouran, B.; Arbabi, V.; Bajpayee, A.G.; van Tiel, J.; Töyräs, J.; Jurvelin, J.S.; Malda, J.; Zadpoor, A.A.; Weinans, H. Multi-Scale Imaging Techniques to Investigate Solute Transport across Articular Cartilage. J. Biomech. 2018, 78, 10–20. [Google Scholar] [CrossRef]
- Cope, P.J.; Ourradi, K.; Li, Y.; Sharif, M. Models of Osteoarthritis: The Good, the Bad and the Promising. Osteoarthr. Cartil. 2019, 27, 230–239. [Google Scholar] [CrossRef]
- Whittier, D.E.; Boyd, S.K.; Burghardt, A.J.; Paccou, J.; Ghasem-Zadeh, A.; Chapurlat, R.; Engelke, K.; Bouxsein, M.L. Guidelines for the Assessment of Bone Density and Microarchitecture in Vivo Using High-Resolution Peripheral Quantitative Computed Tomography. Osteoporos. Int. 2020, 31, 1607–1627. [Google Scholar] [CrossRef] [PubMed]
- Keen, C.E.; Whittier, D.E.; Firminger, C.R.; Edwards, W.B.; Boyd, S.K. Validation of Bone Density and Microarchitecture Measurements of the Load-Bearing Femur in the Human Knee Obtained Using In Vivo HR-PQCT Protocol. J. Clin. Densitom. Off. J. Int. Soc. Clin. Densitom. 2021, 24, 651–657. [Google Scholar] [CrossRef] [PubMed]
- Mys, K.; Varga, P.; Stockmans, F.; Gueorguiev, B.; Neumann, V.; Vanovermeire, O.; Wyers, C.E.; van den Bergh, J.P.W.; van Lenthe, G.H. High-Resolution Cone-Beam Computed Tomography Is a Fast and Promising Technique to Quantify Bone Microstructure and Mechanics of the Distal Radius. Calcif. Tissue Int. 2021, 108, 314–323. [Google Scholar] [CrossRef] [PubMed]

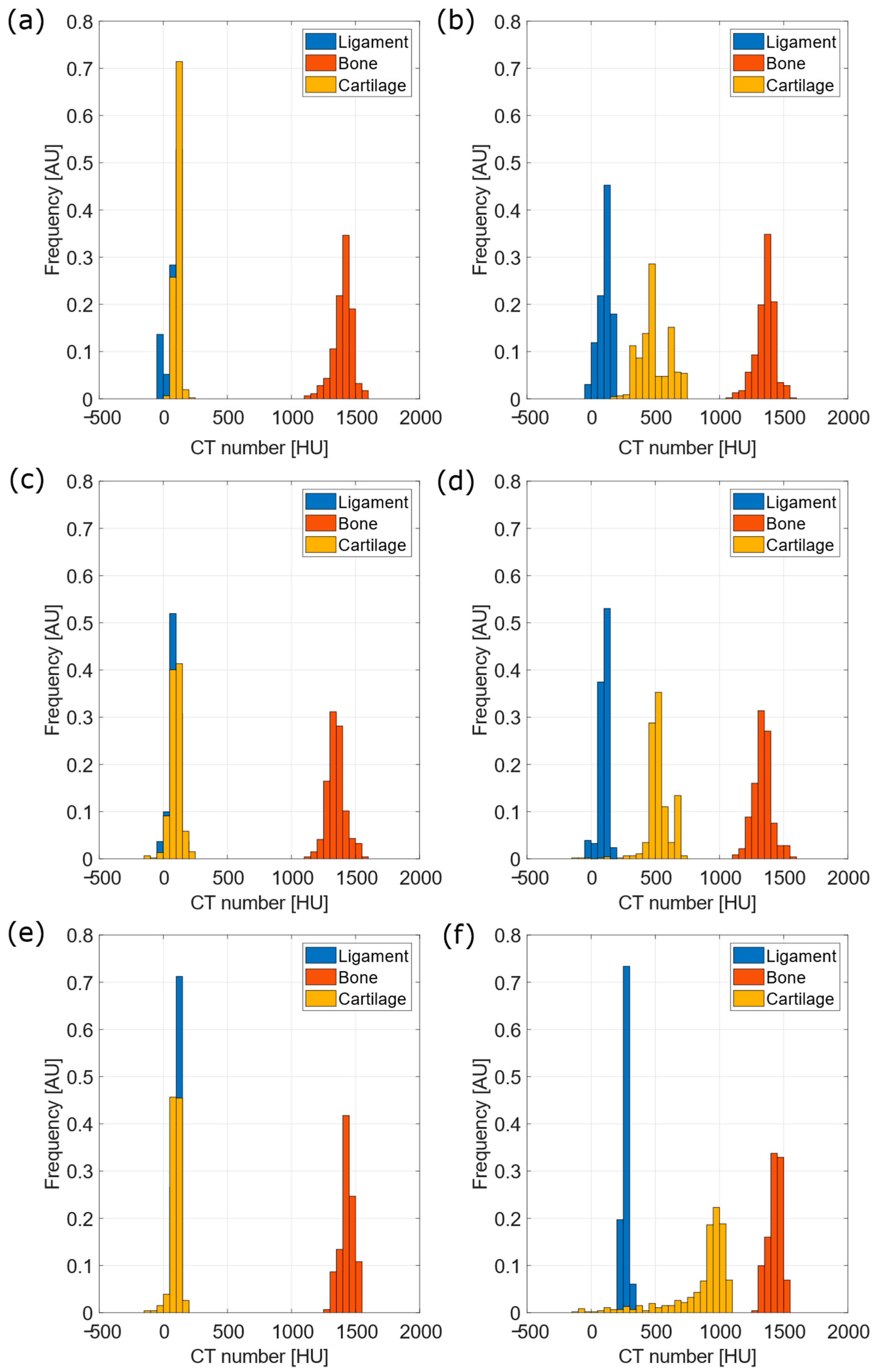
| 5 mgI/mL | 10 mgI/mL | 15 mgI/mL | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Cohen’s d | KS Test | MW Test | Cohen’s d | KS Test | MW Test | Cohen’s d | KS Test | MW Test | |
| L-B | 21.4 | p < 0.001 | p < 0.001 | 22.4 | p < 0.001 | p < 0.001 | 33.1 | p < 0.001 | p < 0.001 |
| B-C | 24.0 | p < 0.001 | p < 0.001 | 21.4 | p < 0.001 | p < 0.001 | 30.1 | p < 0.001 | p < 0.001 |
| C-L | 0.78 | p < 0.001 | p < 0.001 | 0.22 | 0.313 | 0.879 | 0.33 | p < 0.001 | p < 0.001 |
| 5 mgI/mL | 10 mgI/mL | 15 mgI/mL | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Cohen’s d | KS Test | MW Test | Cohen’s d | KS Test | MW Test | Cohen’s d | KS Test | MW Test | |
| L-B | 19.5 | p < 0.001 | p < 0.001 | 21.6 | p < 0.001 | p < 0.001 | 28.6 | p < 0.001 | p < 0.001 |
| B-C | 8.7 | p < 0.001 | p < 0.001 | 9.5 | p < 0.001 | p < 0.001 | 3.3 | p < 0.001 | p < 0.001 |
| C-L | 4.3 | p < 0.001 | p < 0.001 | 5.9 | p < 0.001 | p < 0.001 | 3.5 | p < 0.001 | p < 0.001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fantoni, S.; Gabucci, I.; Cardarelli, P.; Paternò, G.; Taibi, A.; Cristofori, V.; Trapella, C.; Bazzani, A.; Assenza, M.; Zanna Bonacorsi, A.; et al. A Cationic Contrast Agent in X-ray Imaging of Articular Cartilage: Pre-Clinical Evaluation of Diffusion and Attenuation Properties. Diagnostics 2022, 12, 2111. https://doi.org/10.3390/diagnostics12092111
Fantoni S, Gabucci I, Cardarelli P, Paternò G, Taibi A, Cristofori V, Trapella C, Bazzani A, Assenza M, Zanna Bonacorsi A, et al. A Cationic Contrast Agent in X-ray Imaging of Articular Cartilage: Pre-Clinical Evaluation of Diffusion and Attenuation Properties. Diagnostics. 2022; 12(9):2111. https://doi.org/10.3390/diagnostics12092111
Chicago/Turabian StyleFantoni, Simone, Ilenia Gabucci, Paolo Cardarelli, Gianfranco Paternò, Angelo Taibi, Virginia Cristofori, Claudio Trapella, Armando Bazzani, Marta Assenza, Alice Zanna Bonacorsi, and et al. 2022. "A Cationic Contrast Agent in X-ray Imaging of Articular Cartilage: Pre-Clinical Evaluation of Diffusion and Attenuation Properties" Diagnostics 12, no. 9: 2111. https://doi.org/10.3390/diagnostics12092111
APA StyleFantoni, S., Gabucci, I., Cardarelli, P., Paternò, G., Taibi, A., Cristofori, V., Trapella, C., Bazzani, A., Assenza, M., Zanna Bonacorsi, A., Conti, D., & Baruffaldi, F. (2022). A Cationic Contrast Agent in X-ray Imaging of Articular Cartilage: Pre-Clinical Evaluation of Diffusion and Attenuation Properties. Diagnostics, 12(9), 2111. https://doi.org/10.3390/diagnostics12092111







