Usefulness of Contrast-Enhanced Harmonic Endoscopic Ultrasonography for Diagnosis of Malignancy in Intraductal Papillary Mucinous Neoplasm
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Patients
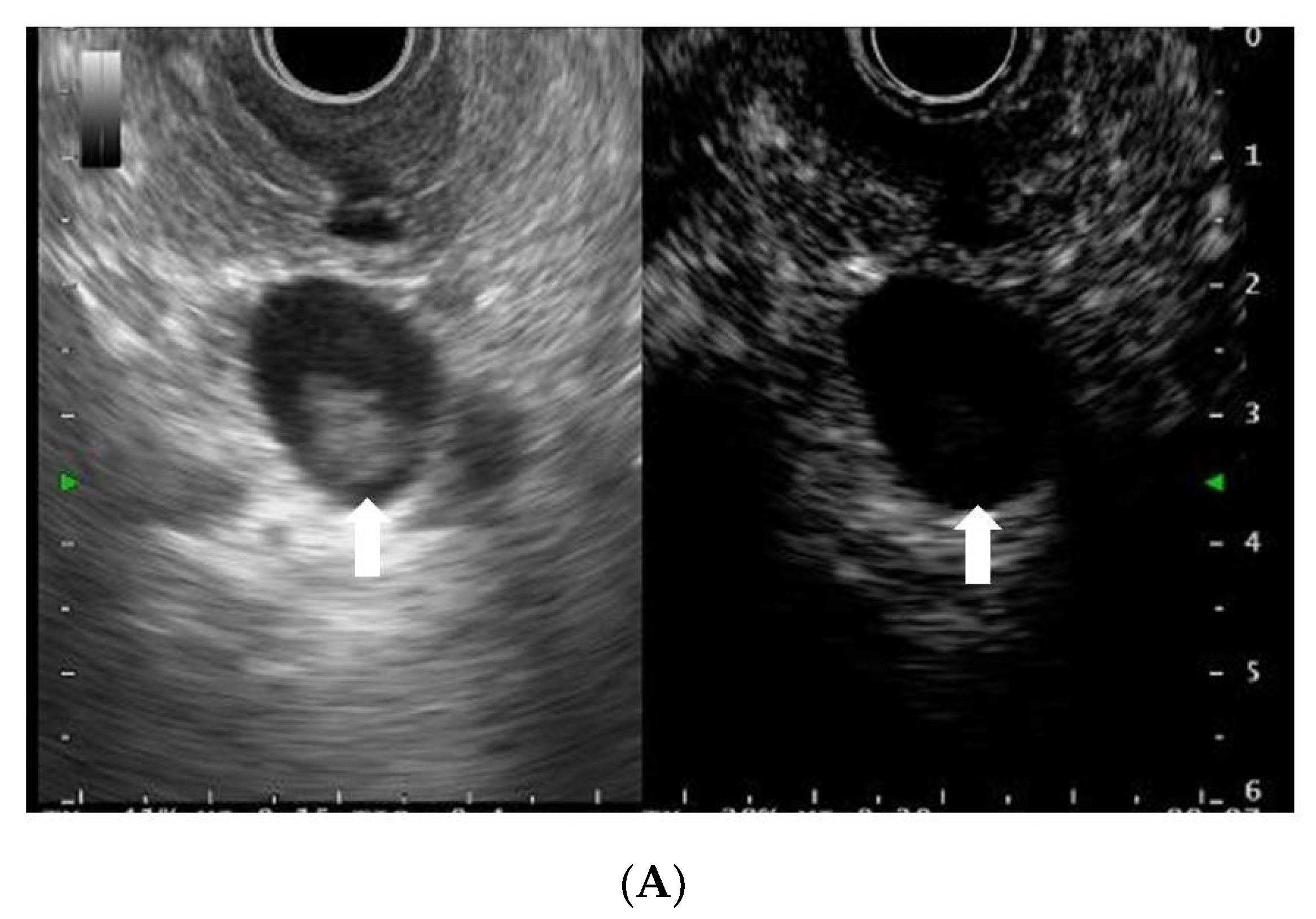
2.3. EUS Procedure
2.4. Definitions
2.5. Statistical Analysis
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ohashi, K.; Murakami, Y.; Takekoshi, T. Four cases of mucin producing cancer of the pancreas on specific findings of the papilla of Vater. Prog. Dig. Endosc. 1982, 20, 348–351. [Google Scholar]
- Khan, S.; Sclabas, G.; Reid-Lombardo, K.M. Population-based epidemiology, risk factors and screening of intraductal papillary mucinous neoplasm patients. World J. Gastrointest. Surg. 2010, 2, 314–318. [Google Scholar] [CrossRef] [PubMed]
- Akita, H.; Takeda, Y.; Hoshino, H.; Wada, H.; Kobayashi, S.; Marubashi, S.; Eguchi, H.; Tanemura, M.; Mori, M.; Doki, Y.; et al. Mural lesion in branch duct-type intraductal papillary mucinous neoplasms of the pancreas is a marker of malignant transformation and indication for surgery. Am. J. Surg. 2011, 202, 214–219. [Google Scholar] [CrossRef] [PubMed]
- Kamata, K.; Kitano, M.; Omoto, S.; Kadosaka, K.; Miyata, T.; Yamao, K.; Imai, H.; Sakamoto, H.; Harwani, Y.; Chikugo, T.; et al. Contrast-enhanced harmonic endoscopic ultrasonography for differential diagnosis of pancreatic cysts. Endoscopy 2016, 48, 35–41. [Google Scholar] [CrossRef]
- Yamashita, Y.; Ueda, K.; Itonaga, M.; Yoshida, T.; Maeda, H.; Maekita, T.; Iguchi, M.; Tamai, H.; Ichinose, M.; Kato, J. Usefulness of contrast-enhanced endoscopic sonography for discriminating mural lesions from mucous clots in intraductal papillary mucinous neoplasms: A single-center prospective study. J. Ultrasound. Med. 2013, 32, 61–68. [Google Scholar] [CrossRef] [PubMed]
- Yamashita, Y.; Shimokawa, T.; Ashida, R.; Napoléon, B.; Lisotti, A.; Fusaroli, P.; Gincul, R.; Dietrich, C.F.; Omoto, S.; Kitano, M. Comparison of endoscopic ultrasonography with and without contrast enhancement for characterization of pancreatic tumors: A meta-analysis. Endosc. Int. Open. 2022, 10, E369–E377. [Google Scholar] [CrossRef]
- Yamashita, Y.; Shimokawa, T.; Napoléon, B.; Fusaroli, P.; Gincul, R.; Kudo, M.; Kitano, M. Value of contrast-enhanced harmonic endoscopic ultrasonography with enhancement pattern for diagnosis of pancreatic cancer: A meta-analysis. Dig. Endosc. 2019, 31, 125–133. [Google Scholar] [CrossRef]
- Omoto, S.; Kitano, M.; Fukasawa, M.; Ashida, R.; Kato, H.; Shiomi, H.; Sugimori, K.; Kanno, A.; Chiba, Y.; Takano, S.; et al. Tissue harmonic versus contrast-enhanced harmonic endoscopic ultrasonography for the diagnosis of pancreatic tumors: Prospective multicenter study. Dig. Endosc. 2022, 34, 198–206. [Google Scholar] [CrossRef]
- Yamashita, Y.; Kato, J.; Ueda, K.; Nakamura, Y.; Kawaji, Y.; Abe, H.; Nuta, J.; Tamura, T.; Itonaga, M.; Yoshida, T.; et al. Contrast-Enhanced Endoscopic Ultrasonography for Pancreatic Tumors. BioMed Res. Int. 2015, 2015, 491782. [Google Scholar] [CrossRef]
- Kitano, M.; Kudo, M.; Yamao, K.; Takagi, T.; Sakamoto, H.; Komaki, T.; Kamata, K.; Imai, H.; Chiba, Y.; Okada, M.; et al. Characterization of small solid tumors in the pancreas: The value of contrast-enhanced harmonic endoscopic ultrasonography. Am. J. Gastroenterol. 2012, 107, 303–310. [Google Scholar] [CrossRef]
- Yamashita, Y.; Tanioka, K.; Kawaji, Y.; Tamura, T.; Nuta, J.; Hatamaru, K.; Itonaga, M.; Yoshida, T.; Ida, Y.; Maekita, T.; et al. Utility of Contrast-Enhanced Harmonic Endoscopic Ultrasonography for Early Diagnosis of Small Pancreatic Cancer. Diagnostics 2020, 10, 23. [Google Scholar] [CrossRef] [PubMed]
- Chantarojanasiri, T.; Hirooka, Y.; Kawashima, H.; Ohno, E.; Kuwahara, T.; Yamamura, T.; Funasaka, K.; Nakamura, M.; Miyahara, R.; Ishigami, M.; et al. Endoscopic ultrasound in diagnosis of solid pancreatic lesions: Elastography or contrast-enhanced harmonic alone versus the combination. Endosc. Int. Open 2017, 5, E1136–E1143. [Google Scholar] [CrossRef][Green Version]
- Uekitani, T.; Kaino, S.; Harima, H.; Suenaga, S.; Sen-Yo, M.; Sakaida, I. Efficacy of contrast-enhanced harmonic endoscopic ultrasonography in the diagnosis of pancreatic ductal carcinoma. Saudi J. Gastroenterol. 2016, 22, 198–202. [Google Scholar] [CrossRef] [PubMed]
- Fusaroli, P.; Spada, A.; Mancino, M.G.; Caletti, G. Contrast harmonic echo-endoscopic ultrasound improves accuracy in diagnosis of solid pancreatic masses. Clin. Gastroenterol. Hepatol. 2010, 8, 629–634. [Google Scholar] [CrossRef] [PubMed]
- Bunganič, B.; Laclav, M.; Dvořáková, T.; Bradáč, O.; Traboulsi, E.; Suchánek, Š.; Frič, P.; Zavoral, M. Accuracy of EUS and CEH EUS for the diagnosis of pancreatic tumours. Scand. J. Gastroenterol. 2018, 53, 1411–1417. [Google Scholar] [CrossRef]
- Harmsen, F.R.; Domagk, D.; Dietrich, C.F.; Hocke, M. Discriminating chronic pancreatitis from pancreatic cancer: Contrast-enhanced EUS and multidetector computed tomography in direct comparison. Endosc. Ultrasound 2018, 7, 395–403. [Google Scholar] [CrossRef] [PubMed]
- Hocke, M.; Ignee, A.; Dietrich, C.F. Advanced endosonographic diagnostic tools for discrimination of focal chronic pancreatitis and pancreatic carcinoma--elastography, contrast enhanced high mechanical index (CEHMI) and low mechanical index (CELMI) endosonography in direct comparison. Z. Gastroenterol. 2012, 50, 199–203. [Google Scholar] [CrossRef] [PubMed]
- Ishikawa, R.; Kamata, K.; Hara, A.; Tanaka, H.; Okamoto, A.; Yamazaki, T.; Nakai, A.; Omoto, S.; Minaga, K.; Yamao, K.; et al. Utility of contrast-enhanced harmonic endoscopic ultrasonography for predicting the prognosis of pancreatic neuroendocrine neoplasms. Dig. Endosc. 2021, 33, 829–839. [Google Scholar] [CrossRef] [PubMed]
- Yamashita, Y.; Kato, J.; Ueda, K.; Nakamura, Y.; Abe, H.; Tamura, T.; Itonaga, M.; Yoshida, T.; Maeda, H.; Moribata, K.; et al. Contrast-enhanced endoscopic ultrasonography can predict a higher malignant potential of gastrointestinal stromal tumors by visualizing large newly formed vessels. J. Clin. Ultrasound. 2015, 43, 89–97. [Google Scholar] [CrossRef]
- Sakamoto, H.; Kitano, M.; Matsui, S.; Kamata, K.; Komaki, T.; Imai, H.; Dote, K.; Kudo, M. Estimation of malignant potential of GI stromal tumors by contrast-enhanced harmonic EUS (with videos). Gastrointest. Endosc. 2011, 73, 227–237. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, M.; Fernández-Del Castillo, C.; Kamisawa, T.; Jang, J.Y.; Levy, P.; Ohtsuka, T.; Salvia, R.; Shimizu, Y.; Tada, M.; Wolfgang, C.L. Revisions of international consensus Fukuoka guidelines for the management of IPMN of the pancreas. Pancreatology 2017, 17, 738–753. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, N.; Kato, H.; Tomoda, T.; Matsumoto, K.; Sakakihara, I.; Noma, Y.; Horiguchi, S.; Harada, R.; Tsutsumi, K.; Hori, K.; et al. Contrast-enhanced harmonic endoscopic ultrasonography with time-intensity curve analysis for intraductal papillary mucinous neoplasms of the pancreas. Endoscopy 2016, 48, 26–34. [Google Scholar] [CrossRef]
- Hosmer, D.W.; Lemeshow, S.L. Applied Logistic Regression, 3rd ed.; Wiley: New York, NY, USA, 2013; pp. 1–33. [Google Scholar]
- Yamaguchi, T.; Shirai, Y.; Ishihara, T.; Sudo, K.; Nakagawa, A.; Ito, H.; Miyazaki, M.; Nomura, F.; Saisho, H. Pancreatic juice cytology in the diagnosis of intraductal papillary mucinous neoplasm of the pancreas. Cancer 2005, 104, 2830–2836. [Google Scholar] [CrossRef] [PubMed]
- Pais, S.A.; Attasaranya, S.; Leblanc, J.K.; Sherman, S.; Schmidt, C.M.; DeWitt, J. Role of endoscopic ultrasound in the diagnosis of intraductal papillary mucinous neoplasms: Correlation with surgical histopathology. Clin. Gastroenterol. Hepatol. 2007, 5, 489–495. [Google Scholar] [CrossRef]
- Frossard, J.L.; Amouyal, P.; Amouyal, G.; Palazzo, L.; Amaris, J.; Soldan, M.; Giostra, E.; Spahr, L.; Hadengue, A.; Fabre, M. Performance of endosonography-guided fine needle aspiration and biopsy in the diagnosis of pancreatic cystic lesions. Am. J. Gastroenterol. 2003, 98, 1516–1524. [Google Scholar] [CrossRef]
- Maire, F.; Voitot, H.; Aubert, A.; Palazzo, L.; O'Toole, D.; Couvelard, A.; Levy, P.; Vidaud, M.; Sauvanet, A.; Ruszniewski, P.; et al. Intraductal papillary mucinous neoplasms of the pancreas: Performance of pancreatic fluid analysis for positive diagnosis and the prediction of malignancy. Am. J. Gastroenterol. 2008, 103, 2871–2877. [Google Scholar] [CrossRef] [PubMed]
- Lim, L.G.; Lakhtakia, S.; Ang, T.L.; Vu, C.K.; Dy, F.; Chong, V.H.; Khor, C.J.; Lim, W.C.; Doshi, B.K.; Varadarajulu, S.; et al. Factors determining diagnostic yield of endoscopic ultrasound guided fine-needle aspiration for pancreatic cystic lesions: A multicenter Asian study. Dig. Dis. Sci. 2013, 58, 1751–1757. [Google Scholar]
- DeWitt, J.; Devereaux, B.; Chriswell, M.; McGreevy, K.; Howard, T.; Imperiale, T.F.; Ciaccia, D.; Lane, K.A.; Maglinte, D.; Kopecky, K.; et al. Comparison ofendoscopic ultrasonography and multidetector computedtomography for detecting and staging pancreatic cancer. Ann. Intern. Med. 2004, 141, 753–763. [Google Scholar] [CrossRef]
- Jemaa, Y.; Houissa, F.; Trabelsi, S.; Moussa, A.; Belhouchet, H.; Mouelhi, L.; Bouraoui, M.; Bouzaidi, S.; Debbeche, R.; Ben Yedder, J.; et al. Endoscopic ultrasonog-raphy versus helical CT in diagnosis and staging of pancreaticcancer. Tunis. Med. 2008, 86, 346–349. [Google Scholar]
- Sakamoto, H.; Kitano, M.; Suetomi, Y.; Maekawa, K.; Takeyama, Y.; Kudo, M. Utility of contrast-enhanced endoscopic ultrasonog-raphy for diagnosis of small pancreatic carcinomas. UltrasoundMed. Biol. 2008, 34, 525–532. [Google Scholar] [CrossRef]
- Ardengh, J.C.; de Paulo, G.A.; Ferrari, A.P. Pancreatic carcinomassmaller than 3.0 cm: Endosonography (EUS) in diagnosis, staging and prediction of resectability. HPB 2003, 5, 226–230. [Google Scholar] [CrossRef] [PubMed]
- Harima, H.; Kaino, S.; Shinoda, S.; Kawano, M.; Suenaga, S.; Sakaida, I. Differential diagnosis of benign and malignant branch duct intraductal papillary mucinous neoplasm using contrast-enhanced endoscopic ultrasonography. World J. Gastroenterol. 2015, 21, 6252–6560. [Google Scholar] [CrossRef] [PubMed]
- Itoi, T.; Fujita, M.; Ikeuchi, N.; Sofuni, A.; Tsuchiya, T.; Ishii, K.; Kamada, K.; Umeda, J.; Tanaka, R.; Tonozuka, R.; et al. Effectiveness of contrast-enhanced endoscopic ultrasound for detecting mural nodules in intraductal papillary mucinous neoplasm of the pancreas and for making therapeutic decisions. Endosc. Ultrasound 2016, 5, 377–383. [Google Scholar] [CrossRef]
- Tanaka, M.; Chari, S.; Adsay, V.; Fernandez-del Castillo, C.; Falconi, M.; Shimizu, M.; Yamaguchi, K.; Yamao, K.; Matsuno, S. International consensus guidelines for management of intraductal papillary mucinous neoplasms and mucinous cystic neoplasms of the pancreas. Pancreatology 2006, 6, 17–32. [Google Scholar] [CrossRef]
- Tanaka, M.; Fernández-del Castillo, C.; Adsay, V.; Chari, S.; Falconi, M.; Jang, J.Y.; Kimura, W.; Levy, P.; Pitman, M.B.; Schmidt, C.M.; et al. International consensus guidelines 2012 for the management of IPMN and MCN of the pancreas. Pancreatology 2012, 12, 183–197. [Google Scholar] [CrossRef]
- Hsiao, C.Y.; Yang, C.Y.; Wu, J.M.; Kuo, T.C.; Tien, Y.W. Utility of the 2006 Sendai and 2012 Fukuoka guidelines for the management of intraductal papillary mucinous neoplasm of the pancreas: A single-center experience with 138 surgically treated patients. Medicine 2016, 95, e4922. [Google Scholar] [CrossRef] [PubMed]
- Wada, K.; Kozarek, R.A.; Traverso, L.W. Outcomes following resection of invasive and noninvasive intraductal papillary mucinous neoplasms of the pancreas. Am. J. Surg. 2005, 189, 632–636. [Google Scholar] [CrossRef]
- Marchegiani, G.; Andrianello, S.; Borin, A.; Dal Borgo, C.; Perri, G.; Pollini, T.; Romanò, G.; D’Onofrio, M.; Gabbrielli, A.; Scarpa, A.; et al. Systematic review, meta-analysis, and a high-volume center experience supporting the new role of mural lesions proposed by the updated 2017 international guidelines on IPMN of the pancreas. Surgery 2018, 163, 1272–1279. [Google Scholar] [CrossRef]







| All (n = 115) | LGD/IGD (n = 31) | HGD (n = 46) | Invasive IPMC (n = 38) | |
|---|---|---|---|---|
| Age in years, mean ± SD | 70.5 ± 8.7 | 69.9 ± 7.4 | 70.6 ± 7.9 | 70.9 ± 10.9 |
| Sex, male/female | 63/52 | 19/12 | 27/19 | 17/21 |
| IPMN size (mm) | 29.1 ± 12.4 | 29.1 ± 11.2 | 29.9 ± 11.7 | 28.6 ± 14.6 |
| MPD size (mm) | 5.2 ± 2.4 | 4.3 ± 2.5 | 5.2 ± 2.4 | 5.9 ± 2.3 |
| Stage (number) | - | - | 0 | IA (26); IIA (2); IIB (7); III (2); IV (1) |
| CE-CT | Conventional EUS | CH-EUS | |
|---|---|---|---|
| Sensitivity | 70% (63/90) | 97% (87/90) | 97% (87/90) |
| Specificity | 76% (19/25) | 36% (9/25) | 76% (19/25) |
| Accuracy | 72% (83/115) | 83% (96/115) | 92% (106/115) |
| CE-CT | Conventional EUS | CH-EUS | |
|---|---|---|---|
| Sensitivity for malignancy | 65% (55/84) | 93% (78/84) | 88% (74/84) |
| Specificity for malignancy | 55% (17/31) | 19% (6/31) | 39% (12/31) |
| Accuracy for malignancy | 63% (72/115) | 73% (84/115) | 75% (86/115) |
| CE-CT | Conventional EUS | CH-EUS | |
| Sensitivity for invasive IPMC | 79% (30/38) | 100% (38/38) | 100% (38/38) |
| Specificity for invasive IPMC | 49% (38/77) | 16% (12/77) | 29% (22/77) |
| Accuracy for invasive IPMC | 59% (68/115) | 43% (50/115) | 52% (60/115) |
| Cut-Off Value (mm) | AUROC | Sensitivity (%) | Specificity (%) | |
|---|---|---|---|---|
| CE-CT | 9 | 0.67 | 52 | 74 |
| Conventional EUS | 9.5 | 0.77 | 55 | 46 |
| CH-EUS | 7.8 | 0.80 | 65 | 84 |
| Cut-Off Value (mm) | AUROC | Sensitivity (%) | Specificity (%) | |
|---|---|---|---|---|
| CE-CT | 7 | 0.74 | 76 | 65 |
| Conventional EUS | 9 | 0.76 | 76 | 68 |
| CH-EUS | 7.8 | 0.82 | 87 | 65 |
| Malignancy | Invasive IPMC | |
|---|---|---|
| Sensitivity | 26% (22/84) | 58% (22/38) |
| Specificity | 100% (31/31) | 100% (77/77) |
| Accuracy | 46% (53/115) | 86% (99/115) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yamashita, Y.; Kawaji, Y.; Shimokawa, T.; Yamazaki, H.; Tamura, T.; Hatamaru, K.; Itonaga, M.; Ashida, R.; Kawai, M.; Kitano, M. Usefulness of Contrast-Enhanced Harmonic Endoscopic Ultrasonography for Diagnosis of Malignancy in Intraductal Papillary Mucinous Neoplasm. Diagnostics 2022, 12, 2141. https://doi.org/10.3390/diagnostics12092141
Yamashita Y, Kawaji Y, Shimokawa T, Yamazaki H, Tamura T, Hatamaru K, Itonaga M, Ashida R, Kawai M, Kitano M. Usefulness of Contrast-Enhanced Harmonic Endoscopic Ultrasonography for Diagnosis of Malignancy in Intraductal Papillary Mucinous Neoplasm. Diagnostics. 2022; 12(9):2141. https://doi.org/10.3390/diagnostics12092141
Chicago/Turabian StyleYamashita, Yasunobu, Yuki Kawaji, Toshio Shimokawa, Hirofumi Yamazaki, Takashi Tamura, Keiichi Hatamaru, Masahiro Itonaga, Reiko Ashida, Manabu Kawai, and Masayuki Kitano. 2022. "Usefulness of Contrast-Enhanced Harmonic Endoscopic Ultrasonography for Diagnosis of Malignancy in Intraductal Papillary Mucinous Neoplasm" Diagnostics 12, no. 9: 2141. https://doi.org/10.3390/diagnostics12092141
APA StyleYamashita, Y., Kawaji, Y., Shimokawa, T., Yamazaki, H., Tamura, T., Hatamaru, K., Itonaga, M., Ashida, R., Kawai, M., & Kitano, M. (2022). Usefulness of Contrast-Enhanced Harmonic Endoscopic Ultrasonography for Diagnosis of Malignancy in Intraductal Papillary Mucinous Neoplasm. Diagnostics, 12(9), 2141. https://doi.org/10.3390/diagnostics12092141








