Improved Single Breath-Hold SSFSE Sequence for Liver MRI Based on Compressed Sensing: Evaluation of Image Quality Compared with Conventional T2-Weighted Sequences
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patients
2.2. MR Imaging Techniques
2.3. Compressed Sensing Accelerated SSFSE Sequence
2.4. Image Analysis
2.5. Statistical Analysis
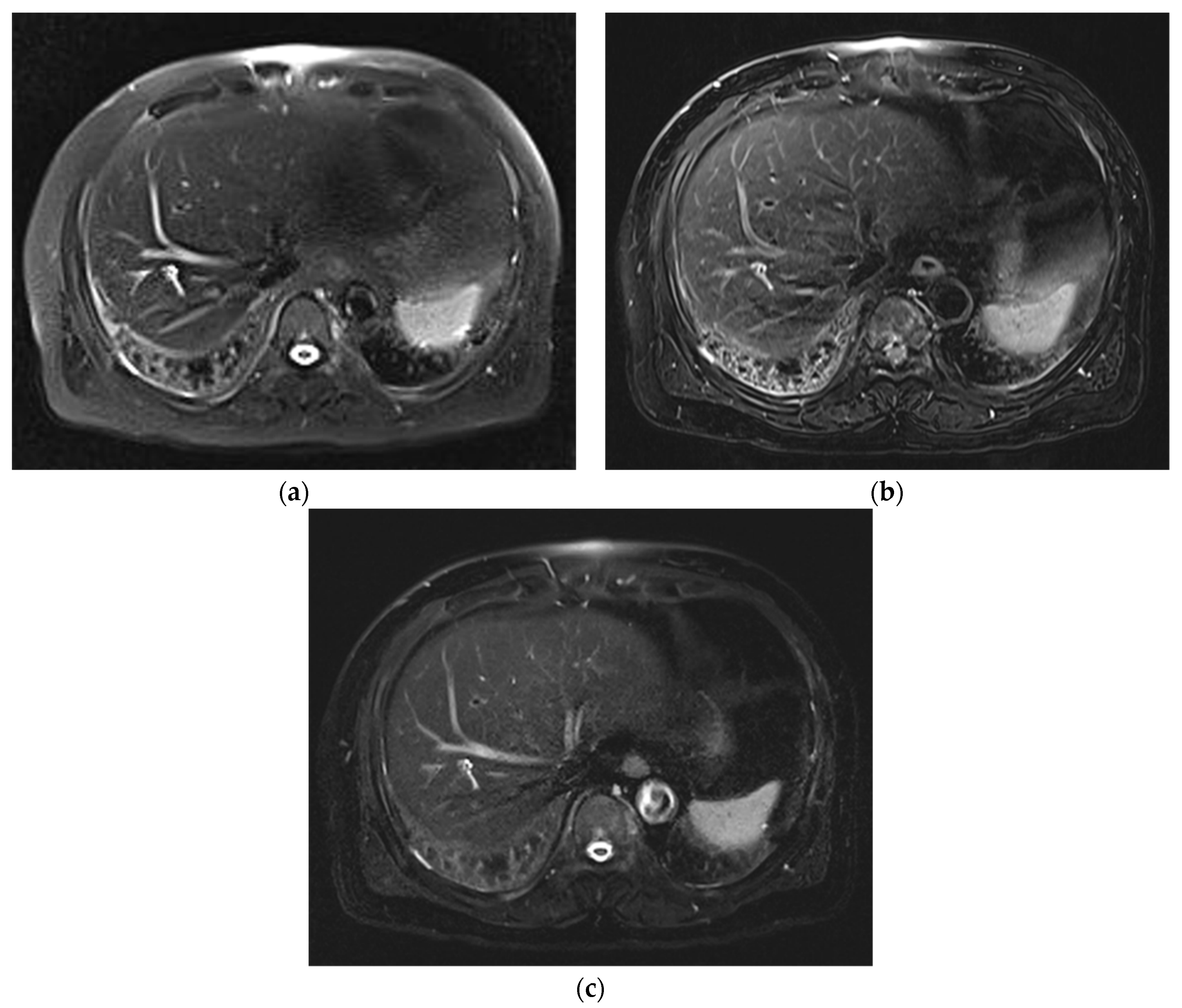
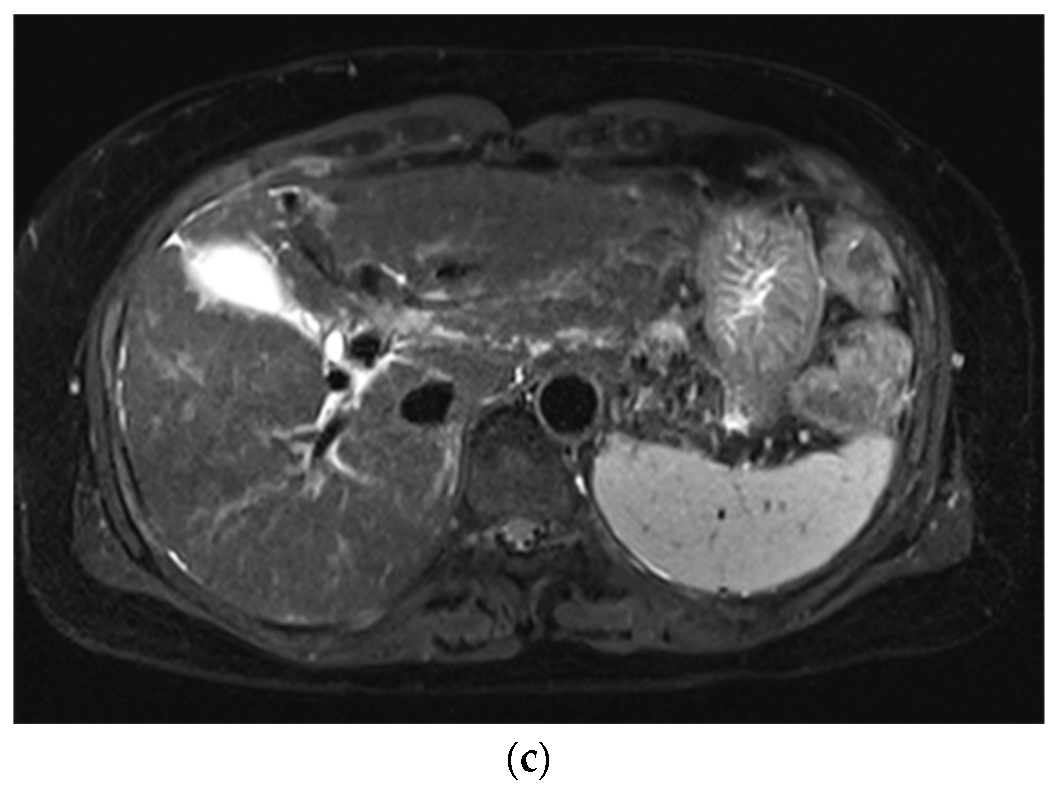
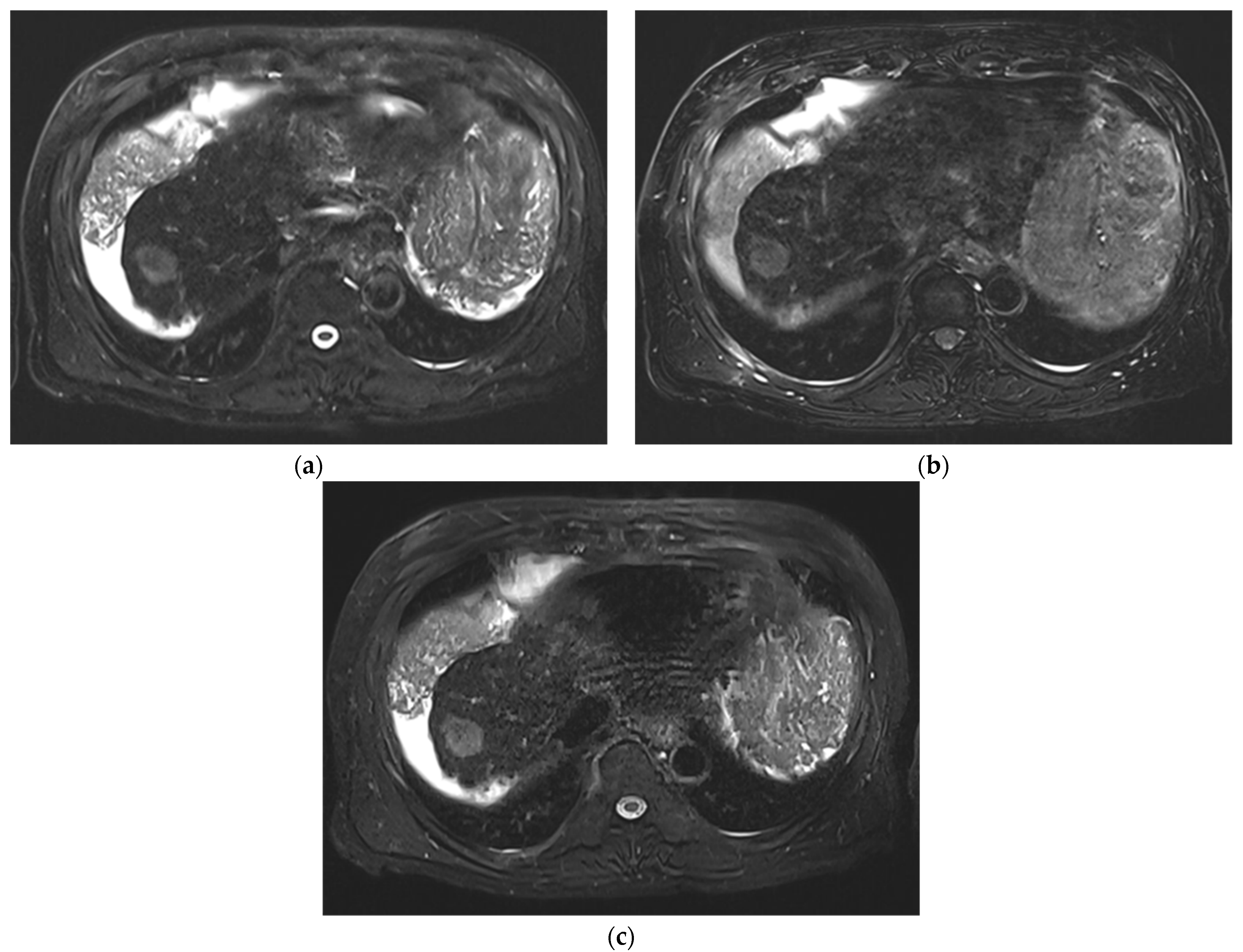
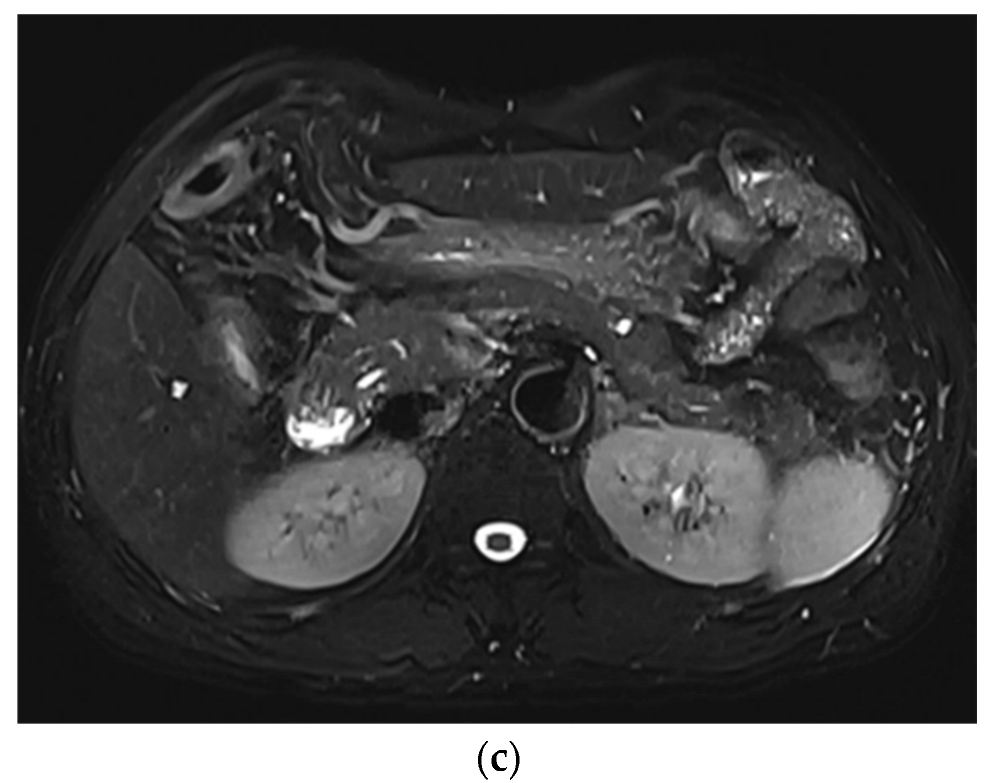
3. Results
3.1. Subjective Image Quality
3.2. Lesion Assessment
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Bashir, M.R.; Castelli, P.; Davenport, M.S.; Larson, D.; Marin, D.; Hussain, H.K.; Jaffe, T.A. Respiratory motion artifact affecting hepatic arterial phase MR imaging with gadoxetate disodium is more common in patients with a prior episode of arterial phase motion associated with gadoxetate disodium. Radiology 2015, 274, 141–148. [Google Scholar] [CrossRef]
- Park, Y.S.; Lee, J.; Kim, J.W.; Park, C.M.; Lee, C.H. Second shot arterial phase to overcome degraded hepatic arterial phase in liver MR imaging. Eur. Radiol. 2019, 29, 2821–2829. [Google Scholar] [CrossRef] [PubMed]
- Hirokawa, Y.; Isoda, H.; Maetani, Y.S.; Arizono, S.; Shimada, K.; Togashi, K. Evaluation of motion correction effect and image quality with the periodically rotated overlapping parallel lines with enhanced reconstruction (PROPELLER) (BLADE) and parallel imaging acquisition technique in the upper abdomen. J. Magn. Reson. Imaging 2008, 28, 957–962. [Google Scholar] [CrossRef]
- Lee, S.S.; Byun, J.H.; Hong, H.S.; Park, S.H.; Won, H.J.; Shin, Y.M.; Lee, M.G. Image quality and focal lesion detection on T2-weighted MR imaging of the liver: Comparison of two high-resolution free-breathing imaging techniques with two breath-hold imaging techniques. J. Magn. Reson. Imaging 2007, 26, 323–330. [Google Scholar] [CrossRef] [PubMed]
- Zech, C.J.; Herrmann, K.A.; Huber, A.; Dietrich, O.; Stemmer, A.; Herzog, P.; Reiser, M.F.; Schoenberg, S.O. High-resolution MR-imaging of the liver with T2-weighted sequences using integrated parallel imaging: Comparison of prospective motion correction and respiratory triggering. J. Magn. Reson. Imaging 2004, 20, 443–450. [Google Scholar] [CrossRef] [PubMed]
- Nakayama, Y.; Yamashita, Y.; Matsuno, Y.; Tang, Y.; Namimoto, T.; Kadota, M.; Mitsuzaki, K.; Abe, Y.; Katahira, K.; Arakawa, A.; et al. Fast breath-hold T2-weighted MRI of the kidney by means of half-Fourier single-shot turbo spin echo: Comparison with high resolution turbo spin echo sequence. J. Comput. Assist. Tomogr. 2001, 25, 55–60. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.G.; Jeong, Y.K.; Kim, J.C.; Kang, E.M.; Kim, P.N.; Auh, Y.H.; Chien, D.; Laub, G. Fast T2-weighted liver MR imaging: Comparison among breath-hold turbo-spin-echo, HASTE, and inversion recovery (IR) HASTE sequences. Abdom. Imaging 2000, 25, 93–99. [Google Scholar] [CrossRef]
- Onishi, N.; Kataoka, M.; Kanao, S.; Sagawa, H.; Iima, M.; Nickel, M.D.; Toi, M.; Togashi, K. Ultrafast dynamic contrast-enhanced mri of the breast using compressed sensing: Breast cancer diagnosis based on separate visualization of breast arteries and veins. J. Magn. Reson. Imaging 2018, 47, 97–104. [Google Scholar] [CrossRef]
- Runge, V.M.; Richter, J.K.; Heverhagen, J.T. Speed in Clinical Magnetic Resonance. Investig. Radiol. 2017, 52, 1–17. [Google Scholar] [CrossRef]
- Song, J.S.; Kim, S.H.; Kuehn, B.; Paek, M.Y. Optimized Breath-Hold Compressed-Sensing 3D MR Cholangiopancreatography at 3T: Image Quality Analysis and Clinical Feasibility Assessment. Diagnostics 2020, 10, 376. [Google Scholar] [CrossRef]
- Zhu, L.; Xue, H.; Sun, Z.; Qian, T.; Weiland, E.; Kuehn, B.; Asbach, P.; Hamm, B.; Jin, Z. Modified breath-hold compressed-sensing 3D MR cholangiopancreatography with a small field-of-view and high resolution acquisition: Clinical feasibility in biliary and pancreatic disorders. J. Magn. Reson. Imaging 2018, 48, 1389–1399. [Google Scholar] [CrossRef]
- Jaspan, O.N.; Fleysher, R.; Lipton, M.L. Compressed sensing MRI: A review of the clinical literature. Br. J. Radiol. 2015, 88, 20150487. [Google Scholar] [CrossRef] [PubMed]
- Ye, J.C. Compressed sensing MRI: A review from signal processing perspective. BMC Biomed. Eng. 2019, 1, 8. [Google Scholar] [CrossRef] [PubMed]
- Lustig, M.; Donoho, D.; Pauly, J.M. Sparse MRI: The application of compressed sensing for rapid MR imaging. Magn. Reson. Med. 2007, 58, 1182–1195. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Chowdhury, S.; Lustig, M.; Barth, R.A.; Alley, M.T.; Grafendorfer, T.; Calderon, P.D.; Robb, F.J.; Pauly, J.M.; Vasanawala, S.S. Clinical performance of contrast enhanced abdominal pediatric MRI with fast combined parallel imaging compressed sensing reconstruction. J. Magn. Reson. Imaging 2014, 40, 13–25. [Google Scholar] [CrossRef]
- Sharma, S.D.; Fong, C.L.; Tzung, B.S.; Law, M.; Nayak, K.S. Clinical image quality assessment of accelerated magnetic resonance neuroimaging using compressed sensing. Investig. Radiol. 2013, 48, 638–645. [Google Scholar] [CrossRef]
- Worters, P.W.; Sung, K.; Stevens, K.J.; Koch, K.M.; Hargreaves, B.A. Compressed-sensing multispectral imaging of the postoperative spine. J. Magn. Reson. Imaging 2013, 37, 243–248. [Google Scholar] [CrossRef]
- Geyer, L.L.; Schoepf, U.J.; Meinel, F.G.; Nance, J.W., Jr.; Bastarrika, G.; Leipsic, J.A.; Paul, N.S.; Rengo, M.; Laghi, A.; De Cecco, C.N. State of the Art: Iterative CT Reconstruction Techniques. Radiology 2015, 276, 339–357. [Google Scholar] [CrossRef]
- Coates, G.G.; Borrello, J.A.; McFarland, E.G.; Mirowitz, S.A.; Brown, J.J. Hepatic T2-weighted MRI: A prospective comparison of sequences, including breath-hold, half-Fourier turbo spin echo (HASTE). J. Magn. Reson. Imaging 1998, 8, 642–649. [Google Scholar] [CrossRef]
- Chen, F.; Taviani, V.; Tamir, J.I.; Cheng, J.Y.; Zhang, T.; Song, Q.; Hargreaves, B.A.; Pauly, J.M.; Vasanawala, S.S. Self-Calibrating Wave-Encoded Variable-Density Single-Shot Fast Spin Echo Imaging. J. Magn. Reson. Imaging 2018, 47, 954–966. [Google Scholar] [CrossRef]
- Chen, F.; Cheng, J.Y.; Taviani, V.; Sheth, V.R.; Brunsing, R.L.; Pauly, J.M.; Vasanawala, S.S. Data-driven self-calibration and reconstruction for non-cartesian wave-encoded single-shot fast spin echo using deep learning. J. Magn. Reson. Imaging 2020, 51, 841–853. [Google Scholar] [CrossRef] [PubMed]
- Herrmann, J.; Nickel, D.; Mugler, J.P., 3rd; Arberet, S.; Gassenmaier, S.; Afat, S.; Nikolaou, K.; Othman, A.E. Development and Evaluation of Deep Learning-Accelerated Single-Breath-Hold Abdominal HASTE at 3 T Using Variable Refocusing Flip Angles. Investig. Radiol. 2021, 56, 645–652. [Google Scholar] [CrossRef] [PubMed]
- Shanbhogue, K.; Tong, A.; Smereka, P.; Nickel, D.; Arberet, S.; Anthopolos, R.; Chandarana, H. Accelerated single-shot T2-weighted fat-suppressed (FS) MRI of the liver with deep learning-based image reconstruction: Qualitative and quantitative comparison of image quality with conventional T2-weighted FS sequence. Eur. Radiol. 2021, 31, 8447–8457. [Google Scholar] [CrossRef]
- Herrmann, J.; Gassenmaier, S.; Nickel, D.; Arberet, S.; Afat, S.; Lingg, A.; Kundel, M.; Othman, A.E. Diagnostic Confidence and Feasibility of a Deep Learning Accelerated HASTE Sequence of the Abdomen in a Single Breath-Hold. Investig. Radiol. 2021, 56, 313–319. [Google Scholar] [CrossRef] [PubMed]


| Parameter | Skyra (n = 52) | Vida (n = 49) | ||||
|---|---|---|---|---|---|---|
| Sequence | SSFSECONV | mTSE | SSFSECS | SSFSECONV | mTSE | SSFSECS |
| TR/TE (ms) | 732/100 | 2040/101 | 500/99 | 1000/99 | 1700/99 | 561/96 |
| FA, degree | 106 | 134 | 118 | 154 | 140 | 135 |
| FOV (mm) | 380 × 380 | 380 × 380 | 380 × 309 | 380 × 309 | 380 × 380 | 380 × 309 |
| Matrix | 256 × 256 | 384 × 307 | 384 × 253 | 384 × 250 | 384 × 307 | 384 × 253 |
| ST (mm) | 5 | 5 | 5 | 5 | 5 | 5 |
| Number of slices | 42 | 42 | 32 | 42 | 42 | 32 |
| Motion management | 3 BH | 4 BH | 1 BH | 3 BH | 4 BH | 1 BH |
| ETL | 128 | 22 | 84 | 125 | 17 | 84 |
| BW (Hz) | 977 | 1085 | 372 | 372 | 303 | 685 |
| Acceleration factor | GRAPPA = 2 | GRAPPA = 2 | CS = 3 | GRAPPA = 2 | GRAPPA = 2 | CS = 3 |
| Scan time (minute) | 0:58 | 1:48 | 0:18 | 1:02 | 1:48 | 0:18 |
| Artifact | Organ Sharpness | Visibility of Small Structures | Overall Image Quality | Conspicuity of Liver Lesion | Conspicuity of Pancreatic Cystic Lesion | |
|---|---|---|---|---|---|---|
| SSFSECONV | 3.75 ± 0.67 | 3.21 ± 0.54 | 3.30 ± 0.59 | 3.35 ± 0.50 | 3.35 ± 0.73 | 4.00 ± 0.96 |
| TSE | 3.43 ± 0.77 | 4.32 ± 0.81 | 3.82 ± 1.03 | 3.68 ± 0.96 | 3.52 ± 1.28 | 2.00 ± 0.96 |
| SSFSECS | 4.15 ± 0.64 | 4.27 ± 0.58 | 4.31 ± 0.58 | 4.16 ± 0.56 | 4.30 ± 0.82 | 4.86 ± 0.36 |
| p value | 0.002 *, <0.001 †‡ | <0.001 *†, 0.554 ‡ | <0.001 *†‡ | 0.003 *, <0.001 †‡ | 0.404 *, <0.001 †‡ | <0.001 *‡, 0.008 † |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, H.K.; Song, J.S.; Jang, W.; Nickel, D.; Paek, M.Y. Improved Single Breath-Hold SSFSE Sequence for Liver MRI Based on Compressed Sensing: Evaluation of Image Quality Compared with Conventional T2-Weighted Sequences. Diagnostics 2022, 12, 2164. https://doi.org/10.3390/diagnostics12092164
Lee HK, Song JS, Jang W, Nickel D, Paek MY. Improved Single Breath-Hold SSFSE Sequence for Liver MRI Based on Compressed Sensing: Evaluation of Image Quality Compared with Conventional T2-Weighted Sequences. Diagnostics. 2022; 12(9):2164. https://doi.org/10.3390/diagnostics12092164
Chicago/Turabian StyleLee, Hyun Kyung, Ji Soo Song, Weon Jang, Dominik Nickel, and Mun Young Paek. 2022. "Improved Single Breath-Hold SSFSE Sequence for Liver MRI Based on Compressed Sensing: Evaluation of Image Quality Compared with Conventional T2-Weighted Sequences" Diagnostics 12, no. 9: 2164. https://doi.org/10.3390/diagnostics12092164
APA StyleLee, H. K., Song, J. S., Jang, W., Nickel, D., & Paek, M. Y. (2022). Improved Single Breath-Hold SSFSE Sequence for Liver MRI Based on Compressed Sensing: Evaluation of Image Quality Compared with Conventional T2-Weighted Sequences. Diagnostics, 12(9), 2164. https://doi.org/10.3390/diagnostics12092164






