Anti-Protein-Arginine Deiminase 4 IgG and IgA Delineate Severe Rheumatoid Arthritis
Abstract
:1. Introduction
2. Materials and Methods
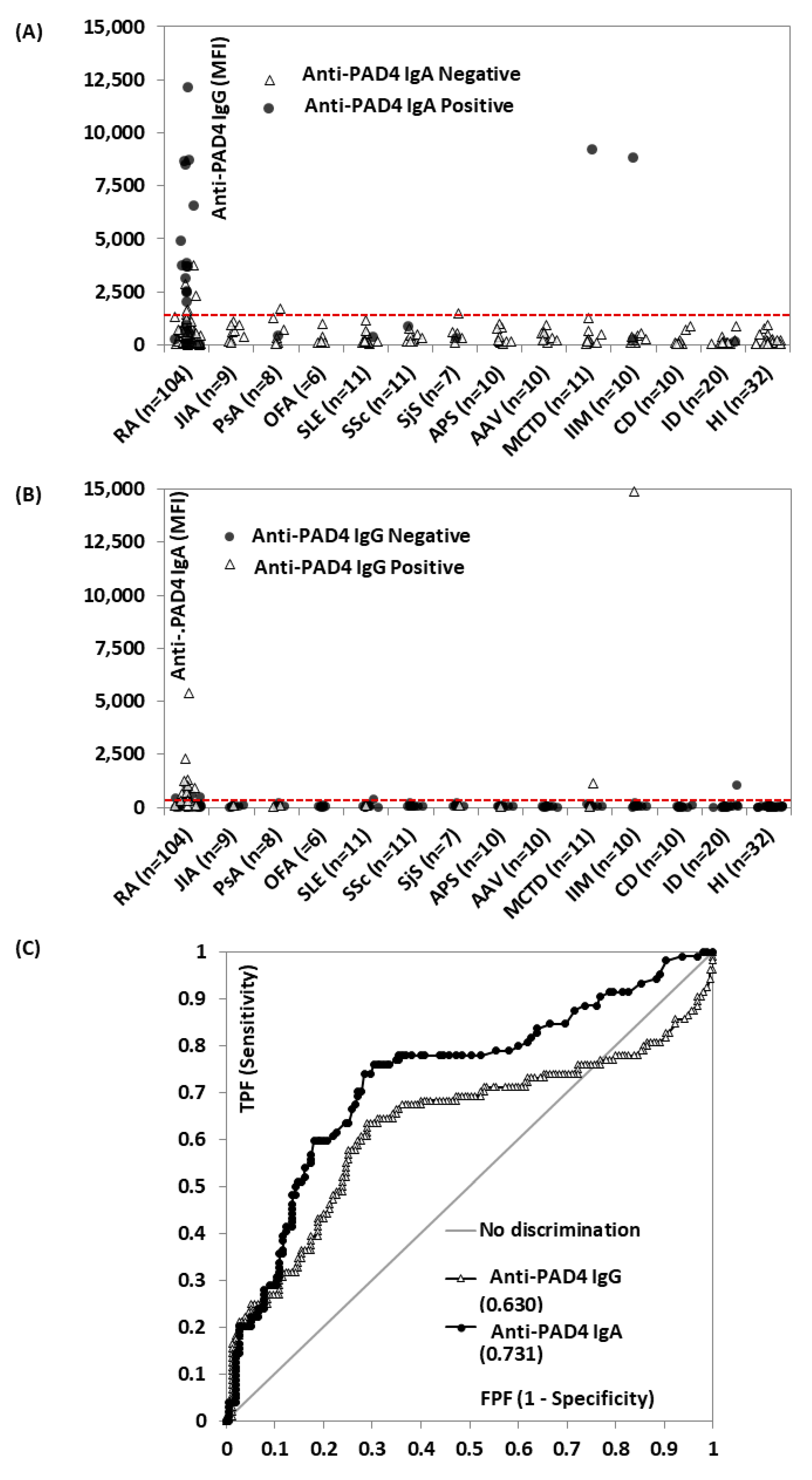
3. Results
4. Discussion
5. Conclusions
6. Patents
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Trouw, L.; Mahler, M. Closing the serological gap: Promising novel biomarkers for the early diagnosis of rheumatoid arthritis. Autoimmun. Rev. 2012, 12, 318–322. [Google Scholar] [CrossRef] [PubMed]
- Demoruelle, M.K.; Deane, K.D. Treatment strategies in early rheumatoid arthritis and prevention of rheumatoid arthritis. Curr. Rheumatol. Rep. 2012, 14, 472–480. [Google Scholar] [CrossRef] [PubMed]
- Smolen, J.S.; Aletaha, D.; Barton, A.; Burmester, G.R.; Emery, P.; Firestein, G.S.; Kavanaugh, A.; McInnes, I.B.; Solomon, D.H.; Strand, V.; et al. Rheumatoid arthritis. Nat. Rev. Dis. Primers 2018, 4, 18001. [Google Scholar] [CrossRef]
- Van Delft, M.A.; Huizinga, T.W. An overview of autoantibodies in rheumatoid arthritis. J. Autoimmun. 2020, 110, 102392. [Google Scholar] [CrossRef] [PubMed]
- Curran, A.M.; Naik, P.; Giles, J.T.; Darrah, E. PAD enzymes in rheumatoid arthritis: Pathogenic effectors and autoimmune targets. Nat. Rev. Rheumatol. 2020, 16, 301–315. [Google Scholar] [CrossRef]
- Darrah, E.; Andrade, F. Rheumatoid arthritis and citrullination. Curr. Opin. Rheumatol. 2018, 30, 72–78. [Google Scholar] [CrossRef]
- Darrah, E.; Giles, J.T.; Ols, M.L.; Bull, H.G.; Andrade, F.; Rosen, A. Erosive Rheumatoid Arthritis Is Associated with Antibodies That Activate PAD4 by Increasing Calcium Sensitivity. Sci. Transl. Med. 2013, 5, 186ra65. [Google Scholar] [CrossRef]
- Darrah, E.; Giles, J.T.; Davis, R.L.; Naik, P.; Wang, H.; Konig, M.F.; Cappelli, L.C.; Bingham, C.O.I.; Danoff, S.K.; Andrade, F. Autoantibodies to peptidylarginine deiminase 2 are associated with less severe disease in rheumatoid arthritis. Front. Immunol. 2018, 9, 2696. [Google Scholar] [CrossRef]
- Halvorsen, E.H.; Pollmann, S.; Gilboe, I.-M.; van der Heijde, D.; Landewe, R.; Odegard, S.; Kvien, T.K.; Molberg, O. Serum IgG antibodies to peptidylarginine deiminase 4 in rheumatoid arthritis and associations with disease severity. Ann. Rheum. Dis. 2008, 67, 414–417. [Google Scholar] [CrossRef]
- Martinez-Prat, L.; Palterer, B.; Vitiello, G.; Parronchi, P.; Robinson, W.H.; Mahler, M. Autoantibodies to protein-arginine deiminase (PAD) 4 in rheumatoid arthritis: Immunological and clinical significance, and potential for precision medicine. Expert Rev. Clin. Immunol. 2019, 15, 1073–1087. [Google Scholar] [CrossRef]
- Ren, J.; Sun, L.; Zhao, J. Meta-analysis: Diagnostic accuracy of antibody against peptidylarginine deiminase 4 by ELISA for rheumatoid arthritis. Clin. Rheumatol. 2017, 36, 2431–2438. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Prat, L.; Lucia, D.; Ibarra, C.; Mahler, M.; Dervieux, T. Antibodies targeting protein-arginine deiminase 4 (PAD4) demonstrate diagnostic value in rheumatoid arthritis. Ann. Rheum. Dis. 2019, 78, 434–436. [Google Scholar] [CrossRef]
- Darrah, E.; Martinez-Prat, L.; Mahler, M. Clinical utility of antipeptidyl arginine deiminase type 4 antibodies. J. Rheumatol. 2019, 46, 329–330. [Google Scholar] [CrossRef] [PubMed]
- Darrah, E.; Yu, F.; Cappelli, L.C.; Rosen, A.; O’Dell, J.R.; Mikuls, T.R. Association of baseline peptidylarginine deiminase 4 autoantibodies with favorable response to treatment escalation in rheumatoid arthritis. Arthritis Rheumatol. 2019, 71, 696–702. [Google Scholar] [CrossRef] [PubMed]
- Cappelli, L.C.; Konig, M.F.; Gelber, A.C.; Iii, C.O.B.; Darrah, E. Smoking is not linked to the development of anti-peptidylarginine deiminase 4 autoantibodies in rheumatoid arthritis. Arthritis Res. Ther. 2018, 20, 1–8. [Google Scholar] [CrossRef]
- Giles, J.T.; Darrah, E.; Danoff, S.; Johnson, C.; Andrade, F.; Rosen, A.; Bathon, J.M. Association of cross-reactive antibodies targeting peptidyl-arginine deiminase 3 and 4 with rheumatoid arthritis-associated interstitial lung disease. PLoS ONE 2014, 9, e98794. [Google Scholar] [CrossRef]
- Holers, V.M.; Demoruelle, M.K.; Kuhn, K.A.; Buckner, J.H.; Robinson, W.H.; Okamoto, Y.; Norris, J.M.; Deane, K.D. Rheumatoid arthritis and the mucosal origins hypothesis: Protection turns to destruction. Nat. Rev. Rheumatol. 2018, 14, 542–557. [Google Scholar] [CrossRef]
- DDemoruelle, M.K.; Wang, H.; Davis, R.L.; Visser, A.; Hoang, J.; Norris, J.M.; Holers, V.M.; Deane, K.D.; Darrah, E. Anti-peptidylarginine deiminase-4 antibodies at mucosal sites can activate peptidylarginine deiminase-4 enzyme activity in rheumatoid arthritis. Arthritis Res. Ther. 2021, 23, 163. [Google Scholar] [CrossRef] [PubMed]
- Lakos, G.; Soós, L.; Fekete, A.; Szabó, Z.; Zeher, M.; Horváth, I.F.; Dankó, K.; Kapitány, A.; Gyetvai, A.; Szegedi, G.; et al. Anti-cyclic citrullinated peptide antibody isotypes in rheumatoid arthritis: Association with disease duration, rheumatoid factor production and the presence of shared epitope. Clin. Exp. Rheumatol. 2008, 26, 253–260. [Google Scholar]
- Kelmenson, L.B.; Wagner, B.D.; McNair, B.K.; Frazer-Abel, A.; Demoruelle, M.K.; Bergstedt, D.T.; Feser, M.L.; Moss, L.K.; Parish, M.C.; Mewshaw, E.A.; et al. Timing of elevations of autoantibody isotypes prior to diagnosis of rheumatoid arthritis. Arthritis Rheumatol. 2020, 72, 251–261. [Google Scholar] [CrossRef]
- Svärd, A.; Kastbom, A.; Reckner-Olsson, Å.; Skogh, T. Presence and utility of IgA-class antibodies to cyclic citrullinated peptides in early rheumatoid arthritis: The Swedish TIRA project. Arthritis Res. Ther. 2008, 10, R75. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sokolova, M.V.; Hagen, M.; Bang, H.; Schett, G.; Rech, J.; Steffen, U.; Haschka, J.; Englbrecht, M.; Hueber, A.J.; Manger, B.; et al. IgA anti-citrullinated protein antibodies are associated with flares during DMARD tapering in rheumatoid arthritis. Rheumatology 2022, 61, 2124–2131. [Google Scholar] [CrossRef] [PubMed]
- Kolfenbach, J.R.; Deane, K.D.; Derber, L.A.; O’Donnell, C.I.; Gilliland, W.R.; Edison, J.D.; Rosen, A.; Darrah, E.; Norris, J.M.; Holers, V.M. Autoimmunity to peptidyl arginine deiminase type 4 precedes clinical onset of rheumatoid arthritis. Arthritis Care Res. 2010, 62, 2633–2639. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Bañuelos, E.; Shi, J.; Wang, H.; Danila, M.; Bridges, S.; Giles, J.; Sims, G.; Andrade, F.; Darrah, E. Heavy chain constant region usage in antibodies to peptidylarginine deiminase 4 distinguishes disease subsets in rheumatoid arthritis. Arthritis Rheumatol. 2022; online ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Arnoux, F.; Mariot, C.; Peen, E.; Lambert, N.C.; Balandraud, N.; Roudier, J.; Auger, I. Peptidyl arginine deiminase immunization induces anticitrullinated protein antibodies in mice with particular MHC types. Proc. Natl. Acad. Sci. USA 2017, 114, E10169–E10177. [Google Scholar] [CrossRef]
- Auger, I.; Balandraud, N.; Massy, E.; Hemon, M.F.; Peen, E.; Arnoux, F.; Mariot, C.; Martin, M.; Lafforgue, P.; Busnel, J.; et al. Peptidylarginine deiminase autoimmunity and the development of anti–citrullinated protein antibody in rheumatoid arthritis: The hapten–carrier model. Arthritis Rheumatol. 2020, 72, 903–911. [Google Scholar] [CrossRef]
- Richards, M.; LA Torre, I.G.-D.; González-Bello, Y.C.; Mercado, M.V.-D.; Andrade-Ortega, L.; Medrano-Ramírez, G.; Navarro-Zarza, J.E.; Maradiaga-Ceceña, M.; Loyo, E.; Rojo-Mejía, A.; et al. Autoantibodies to Mi-2 alpha and Mi-2 beta in patients with idiopathic inflammatory myopathy. Rheumatology 2019, 58, 1655–1661. [Google Scholar] [CrossRef]
- Hecht, C.; Englbrecht, M.; Rech, J.; Schmidt, S.; Araujo, E.; Engelke, K.; Finzel, S.; Schett, G. Additive effect of anti-citrullinated protein antibodies and rheumatoid factor on bone erosions in patients with RA. Ann. Rheum. Dis. 2015, 74, 2151–2156. [Google Scholar] [CrossRef]
- Van Steenbergen, H.W.; Ajeganova, S.; Forslind, K.; Svensson, B.; Van Der Helm-van Mil, A.H.M. The effects of rheumatoid factor and anticitrullinated peptide antibodies on bone erosions in rheumatoid arthritis. Ann. Rheum. Dis. 2015, 74, e3. [Google Scholar] [CrossRef]
- Boer, A.C.; Boonen, A.; van der Helm van Mil, A.H. Is Anti-citrullinated protein antibody-positive rheumatoid arthritis still a more severe disease than anti-citrullinated protein antibody-negative rheumatoid arthritis? A Longitudinal cohort study in rheumatoid arthritis patients diagnosed From 2000 onward. Arthritis Care Res. 2018, 70, 987–996. [Google Scholar] [CrossRef]
- Delacroix, D.; Van Snick, J.; Vaerman, J.; Conley, M.; Mascart-Lemone, F.; Bernier, G. Monoclonal antibodies against isotypic and isoallotypic determinants of human IgA1 and IgA2: Fine specificities and binding properties. Mol. Immunol. 1986, 23, 367–375. [Google Scholar] [CrossRef]
- Boackle, R.J.; Nguyen, Q.L.; Leite, R.S.; Yang, X.; Vesely, J. Complement-coated antibody-transfer (CCAT); serum IgA1 antibodies intercept and transport C4 and C3 fragments and preserve IgG1 deployment (PGD). Mol. Immunol. 2006, 43, 236–245. [Google Scholar] [CrossRef] [PubMed]
- Steffen, U.; Koeleman, C.A.; Sokolova, M.V.; Bang, H.; Kleyer, A.; Rech, J.; Unterweger, H.; Schicht, M.; Garreis, F.; Hahn, J.; et al. IgA subclasses have different effector functions associated with distinct glycosylation profiles. Nat. Commun. 2020, 11, 120. [Google Scholar] [CrossRef] [PubMed]
- Demoruelle, M.K.; Harrall, K.K.; Ho, L.; Purmalek, M.M.; Seto, N.L.; Rothfuss, H.M.; Weisman, M.H.; Solomon, J.J.; Fischer, A.; Okamoto, Y.; et al. Anti–citrullinated protein antibodies are associated with neutrophil extracellular traps in the sputum in relatives of rheumatoid arthritis patients. Arthritis Rheumatol. 2017, 69, 1165–1175. [Google Scholar] [CrossRef] [PubMed] [Green Version]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martinez-Prat, L.; Martinez-Taboada, V.; Santos, C.; Lopez-Hoyos, M.; Mahler, M. Anti-Protein-Arginine Deiminase 4 IgG and IgA Delineate Severe Rheumatoid Arthritis. Diagnostics 2022, 12, 2187. https://doi.org/10.3390/diagnostics12092187
Martinez-Prat L, Martinez-Taboada V, Santos C, Lopez-Hoyos M, Mahler M. Anti-Protein-Arginine Deiminase 4 IgG and IgA Delineate Severe Rheumatoid Arthritis. Diagnostics. 2022; 12(9):2187. https://doi.org/10.3390/diagnostics12092187
Chicago/Turabian StyleMartinez-Prat, Laura, Victor Martinez-Taboada, Cruz Santos, Marcos Lopez-Hoyos, and Michael Mahler. 2022. "Anti-Protein-Arginine Deiminase 4 IgG and IgA Delineate Severe Rheumatoid Arthritis" Diagnostics 12, no. 9: 2187. https://doi.org/10.3390/diagnostics12092187




