The Diagnostic Value of Circulating Biomarkers and Role of Drug-Coated Balloons for In-Stent Restenosis in Patients with Peripheral Arterial Disease
Abstract
:1. Introduction
2. Materials and Methods
3. Results
4. Diagnostic Implications
4.1. Inflammatory Markers
4.2. microRNA
5. Discussion
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| BMS | bare metal stent |
| CLI | critical limb ischemia |
| CSD | covered stent deployment |
| DA | directional atherectomy |
| DCB | drug-coated balloon |
| DCBA | drug-coated balloon angioplasty |
| DES | drug-eluting stent |
| EES | everolimus eluting stent |
| LA | laser atherectomy |
| LD | laser debulking |
| MATH | percutaneous mechanical atherectomy plus thrombectomy |
| MPRT | multicenter prospective randomized controlled trial |
| NRSO | not randomized observational studies |
| PEB | paclitaxel eluting balloon |
| PES | paclitaxel eluting stent |
| PMT | prospective multicenter trial |
| POBA | plain old balloon angioplasty |
| PSMs | propensity score matched study |
| PTA | percutaneous transluminal angioplasty |
| RA | rotational atherectomy |
| RCT | randomized control trial |
| RS | retrospective study |
| TLR | target lesion revascularization |
| VSG | Viabahn Stent-Graft |
References
- Kokkinidis, D.G.; Armstrong, E.J. Current developments in endovascular therapy of peripheral vascular disease. J. Thorac. Dis. 2020, 12, 1681–1694. [Google Scholar] [CrossRef] [PubMed]
- Kayssi, A.; Al-Jundi, W.; Papia, G.; Kucey, D.S.; Forbes, T.; Rajan, D.K.; Neville, R.; Dueck, A.D. Drug-eluting balloon angioplasty versus uncoated balloon angioplasty for the treatment of in-stent restenosis of the femoropopliteal arteries. Cochrane Database Syst. Rev. 2019, 1, CD012510. [Google Scholar] [CrossRef] [PubMed]
- Stilo, F.; Montelione, N.; Calandrelli, R.; Distefano, M.; Spinelli, F.; Di Lazzaro, V.; Pilato, F. The management of carotid restenosis: A comprehensive review. Ann. Transl. Med. 2020, 8, 1272. [Google Scholar] [CrossRef] [PubMed]
- Varela, D.L.; Armstrong, E.J. Endovascular Management of Femoropopliteal In-Stent Restenosis: A Systematic Review. Cardiovasc. Revasc. Med. 2019, 20, 915–925. [Google Scholar] [CrossRef]
- Anantha-Narayanan, M.; Love, K.; Nagpal, S.; Sheikh, A.B.; Regan, C.J.; Mena-Hurtado, C. Safety and efficacy of paclitaxel drug-coated balloon in femoropopliteal in-stent restenosis. Expert Rev. Med Devices 2020, 17, 533–539. [Google Scholar] [CrossRef]
- Spadaccio, C.; Nenna, A.; Rose, D.; Piccirillo, F.; Nusca, A.; Grigioni, F.; Chello, M.; Vlahakes, G.J. The Role of Angiogenesis and Arteriogenesis in Myocardial Infarction and Coronary Revascularization. J. Cardiovasc. Transl. Res. 2022. [Google Scholar] [CrossRef]
- Nusca, A.; Patti, G. Platelet Function and Inhibition in Ischemic Heart Disease. Curr. Cardiol. Rep. 2012, 14, 457–467. [Google Scholar] [CrossRef]
- Nusca, A.; Tuccinardi, D.; Pieralice, S.; Giannone, S.; Carpenito, M.; Monte, L.; Watanabe, M.; Cavallari, I.; Maddaloni, E.; Ussia, G.P.; et al. Platelet Effects of Anti-diabetic Therapies: New Perspectives in the Management of Patients with Diabetes and Cardiovascular Disease. Front. Pharmacol. 2021, 12, 670155. [Google Scholar] [CrossRef]
- Nusca, A.; Viscusi, M.M.; Piccirillo, F.; De Filippis, A.; Nenna, A.; Spadaccio, C.; Nappi, F.; Chello, C.; Mangiacapra, F.; Grigioni, F.; et al. In Stent Neo-Atherosclerosis: Pathophysiology, Clinical Implications, Prevention, and Therapeutic Approaches. Life 2022, 12, 393. [Google Scholar] [CrossRef]
- Sirignano, P.; Citone, M.; Menna, D.; Mansour, W.; Montelione, N.; Capoccia, L.; Speziale, F. Superficial Femoral Artery Stent Disruption Treated by Peripheral Endograft. Ann. Vasc. Surg. 2015, 29, 1661.e5–1661.e8. [Google Scholar] [CrossRef]
- Doshi, R.; Kumar, A.; Adalja, D.; Vaz, I.; Shariff, M. Meta-analysis of Usefulness of Drug Coated Balloon Versus Standard Balloon in the Treatment of Femoropopliteal In-Stent-Restenosis. Am. J. Cardiol. 2020, 133, 170–171. [Google Scholar] [CrossRef] [PubMed]
- Xi, Y.; Chen, J.; Bi, Y.; Xie, S.; Liao, T.; Zhang, Y.; Kislauskis, E.; Wu, T.; Laham, R.; Xiao, J. Long-term clinical safety and efficacy of drug-coated balloon in the treatment of in-stent restenosis: A meta-analysis and systematic review. Catheter. Cardiovasc. Interv. 2020, 96, E129–E141. [Google Scholar] [CrossRef] [PubMed]
- Tong, Z.; Guo, L.; Qi, L.; Cui, S.; Gao, X.; Li, Y.; Guo, J.; Gu, Y. Drug-Coated Balloon Angioplasty and Debulking for the Treatment of Femoropopliteal In-Stent Restenosis: A Systematic Review and Meta-Analysis. BioMed Res. Int. 2020, 2020, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Feng, Z.; Yang, S.; Sang, H.; Xue, G.; Ni, Q.; Zhang, L.; Zhang, W.; Fang, X.; Ye, M. One-Year Clinical Outcome and Risk Factor Analysis of Directional Atherectomy Followed with Drug-Coated Balloon for Femoropopliteal Artery Disease. J. Endovasc. Ther. 2021, 28, 927–937. [Google Scholar] [CrossRef] [PubMed]
- Cao, S.; He, T.; Xie, J.; Feng, H.; Liu, K.; Qu, B.; Wu, X. Drug-coated balloon angioplasty versus balloon angioplasty for treating patients with in-stent restenosis in the femoropopliteal artery: A meta-analysis. Medicine 2021, 100, e25599. [Google Scholar] [CrossRef] [PubMed]
- Nappi, F.; Nenna, A.; Larobina, D.; Martuscelli, G.; Singh, S.S.A.; Chello, M.; Ambrosio, L. The Use of Bioactive Polymers for Intervention and Tissue Engineering: The New Frontier for Cardiovascular Therapy. Polymers 2021, 13, 446. [Google Scholar] [CrossRef] [PubMed]
- Nenna, A.; Nappi, F.; Larobina, D.; Verghi, E.; Chello, M.; Ambrosio, L. Polymers and Nanoparticles for Statin Delivery: Current Use and Future Perspectives in Cardiovascular Disease. Polymers 2021, 13, 711. [Google Scholar] [CrossRef]
- Kokkinidis, D.G.; Behan, S.; Jawaid, O.; Hossain, P.; Giannopoulos, S.; Singh, G.D.; Laird, J.R.; Valle, J.A.; Waldo, S.W.; Armstrong, E.J. Laser atherectomy and drug-coated balloons for the treatment of femoropopliteal in-stent restenosis: 2-Year outcomes. Catheter. Cardiovasc. Interv. 2020, 95, 439–446. [Google Scholar] [CrossRef]
- Kokkinidis, D.G.; Hossain, P.; Jawaid, O.; Alvandi, B.; Foley, T.R.; Singh, G.D.; Waldo, S.W.; Laird, J.R.; Armstrong, E.J. Laser Atherectomy Combined with Drug-Coated Balloon Angioplasty Is Associated with Improved 1-Year Outcomes for Treatment of Femoropopliteal In-Stent Restenosis. J. Endovasc. Ther. 2018, 25, 81–88. [Google Scholar] [CrossRef]
- Schmidt, A.; Zeller, T.; Sievert, H.; Krankenberg, H.; Torsello, G.; Stark, M.A.; Scheinert, D. Photoablation Using theTurbo-Booster andExcimer Laser for In-Stent RestenosisTreatment: Twelve-Month Results from the PATENT Study. J. Endovasc. Ther. 2014, 21, 52–60. [Google Scholar] [CrossRef]
- Brodmann, M.; Keirse, K.; Scheinert, D.; Spak, L.; Jaff, M.R.; Schmahl, R.; Li, P.; Zeller, T. Drug-Coated Balloon Treatment for Femoropopliteal Artery Disease: The IN.PACT Global Study De Novo In-Stent Restenosis Imaging Cohort. JACC: Cardiovasc. Interv. 2017, 10, 2113–2123. [Google Scholar] [CrossRef]
- Horie, K.; Tanaka, A.; Suzuki, K.; Taguri, M.; Inoue, N. Long-term clinical effectiveness of a drug-coated balloon for in-stent restenosis in Femoropopliteal lesions. CVIR Endovasc. 2021, 4, 13. [Google Scholar] [CrossRef] [PubMed]
- Liistro, F.; Angioli, P.; Porto, I.; Ricci, L.; Ducci, K.; Grotti, S.; Falsini, G.; Ventoruzzo, G.; Turini, F.; Bellandi, G.; et al. Paclitaxel-Eluting Balloon vs. Standard Angioplasty to Reduce Recurrent Restenosis in Diabetic Patients with In-Stent Restenosis of the Superficial Femoral and Proximal Popliteal Arteries: The DEBATE-ISR Study. J. Endovasc. Ther. 2014, 21, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Tepe, G.; Schroeder, H.; Albrecht, T.; Reimer, P.; Diehm, N.; Baeriswyl, J.-L.; Brechtel, K.; Speck, U.; Zeller, T. Paclitaxel-Coated Balloon vs Uncoated Balloon Angioplasty for Treatment of In-Stent Restenosis in the Superficial Femoral and Popliteal Arteries: The COPA CABANA Trial. J. Endovasc. Ther. 2020, 27, 276–286. [Google Scholar] [CrossRef]
- Tepe, G.; Zeller, T.; Moscovic, M.; Corpataux, J.-M.; Christensen, J.K.; Keirse, K.; Nano, G.; Schroeder, H.; Binkert, C.A.; Brodmann, M. Paclitaxel-Coated Balloon Angioplasty for the Treatment of Infrainguinal Arteries: 24-Month Outcomes in the Full Cohort of BIOLUX P-III Global Registry. Cardiovasc. Interv. Radiol. 2021, 44, 207–217. [Google Scholar] [CrossRef]
- Liao, C.-J.; Song, S.-H.; Li, T.; Zhang, Y.; Zhang, W.-D. Randomized controlled trial of orchid drug-coated balloon versus standard percutaneous transluminal angioplasty for treatment of femoropopliteal artery in-stent restenosis. Int. Angiol. 2019, 38, 365–371. [Google Scholar] [CrossRef]
- Liao, C.-J.; Song, S.-H.; Li, T.; Zhang, Y.; Zhang, W.-D. Combination of Rotarex Thrombectomy and Drug-Coated Balloon for the Treatment of Femoropopliteal Artery In-Stent Restenosis. Ann. Vasc. Surg. 2019, 60, 301–307. [Google Scholar] [CrossRef]
- Ott, I.; Cassese, S.; Groha, P.; Steppich, B.; Voll, F.; Hadamitzky, M.; Ibrahim, T.; Kufner, S.; Dewitz, K.; Wittmann, T.; et al. ISAR-PEBIS (Paclitaxel-Eluting Balloon Versus Conventional Balloon Angioplasty for In-Stent Restenosis of Superficial Femoral Artery): A Randomized Trial. J. Am. Heart Assoc. 2017, 6, 006321. [Google Scholar] [CrossRef]
- Krankenberg, H.; Tübler, T.; Ingwersen, M.; Schlüter, M.; Scheinert, D.; Blessing, E.; Sixt, S.; Kieback, A.; Beschorner, U.; Zeller, T. Drug-Coated Balloon Versus Standard Balloon for Superficial Femoral Artery In-Stent Restenosis: The Randomized Femoral Artery In-Stent Restenosis (FAIR) Trial. Circulation 2015, 132, 2230–2236. [Google Scholar] [CrossRef]
- Kinstner, C.M.; Lammer, J.; Willfort-Ehringer, A.; Matzek, W.K.; Gschwandtner, M.; Javor, D.; Funovics, M.; Schoder, M.; Koppensteiner, R.; Loewe, C.; et al. Paclitaxel-Eluting Balloon Versus Standard Balloon Angioplasty in In-Stent Restenosis of the Superficial Femoral and Proximal Popliteal Artery: 1-Year Results of the PACUBA Trial. JACC Cardiovasc. Interv. 2016, 9, 1386–1392. [Google Scholar] [CrossRef]
- Armstrong, E.J.; Thiruvoipati, T.; Tanganyika, K.; Singh, G.D.; Laird, J.R. Laser Atherectomy for Treatment of Femoropopliteal In-Stent Restenosis. J. Endovasc. Ther. 2015, 22, 506–513. [Google Scholar] [CrossRef] [PubMed]
- Laird, J.R., Jr.; Yeo, K.K.; Rocha-Singh, K.; Das, T.; Joye, J.; Dippel, E.; Reddy, B.; Botti, C.; Jaff, M.R. Excimer laser with adjunctive balloon angioplasty and heparin-coated self-expanding stent grafts for the treatment of femoropopliteal artery in-stent restenosis: Twelve-month results from the SALVAGE study. Catheter. Cardiovasc. Interv. 2012, 80, 852–859. [Google Scholar] [CrossRef]
- Gandini, R.; Del Giudice, C.; Merolla, S.; Morosetti, D.; Pampana, E.; Simonetti, G. Treatment of Chronic SFA In-Stent Occlusion with Combined Laser Atherectomy and Drug-Eluting Balloon Angioplasty in Patients with Critical Limb Ischemia: A Single-Center, Prospective, Randomized Study. J. Endovasc. Ther. 2013, 20, 805–814. [Google Scholar] [CrossRef] [PubMed]
- Shammas, N.W.; Shammas, G.A.; Banerjee, S.; Popma, J.J.; Mohammad, A.; Jerin, M. JetStream Rotational and Aspiration Atherectomy in Treating In-Stent Restenosis of the Femoropopliteal Arteries: Results of the JETSTREAM-ISR Feasibility Study. J. Endovasc. Ther. 2016, 23, 339–346. [Google Scholar] [CrossRef] [PubMed]
- Sixt, S.; Cancino, O.G.C.; Treszl, A.; Beschorner, U.; Macharzina, R.; Rastan, A.; Krankenberg, H.; Neumann, F.-J.; Zeller, T. Drug-coated balloon angioplasty after directional atherectomy improves outcome in restenotic femoropopliteal arteries. J. Vasc. Surg. 2013, 58, 682–686. [Google Scholar] [CrossRef]
- Bague, N.; Julia, P.; Sauguet, A.; Pernès, J.; Chatelard, P.; Garbé, J.; Penillon, S.; Cardon, J.; Commeau, P.; Planché, O.; et al. Femoropopliteal In-stent Restenosis Repair: Midterm Outcomes After Paclitaxel Eluting Balloon Use (PLAISIR Trial). Eur. J. Vasc. Endovasc. Surg. 2017, 53, 106–113. [Google Scholar] [CrossRef]
- Stabile, E.; Virga, V.; Salemme, L.; Cioppa, A.; Ambrosini, V.; Sorropago, G.; Tesorio, T.; Cota, L.; Popusoi, G.; Pucciarelli, A.; et al. Drug-Eluting Balloon for Treatment of Superficial Femoral Artery In-Stent Restenosis. J. Am. Coll. Cardiol. 2012, 60, 1739–1742. [Google Scholar] [CrossRef]
- Virga, V.; Stabile, E.; Biamino, G.; Salemme, L.; Cioppa, A.; Giugliano, G.; Tesorio, T.; Cota, L.; Popusoi, G.; Pucciarelli, A.; et al. Drug-Eluting Balloons for the Treatment of the Superficial Femoral Artery In-Stent Restenosis: 2-year follow-up. JACC Cardiovasc. Interv. 2014, 7, 411–415. [Google Scholar] [CrossRef]
- Milnerowicz, A.; Milnerowicz, A.; Kuliczkowski, W.; Protasiewicz, M. Rotational Atherectomy Plus Drug-Coated Balloon Angioplasty for the Treatment of Total In-Stent Occlusions in Iliac and Infrainguinal Arteries. J. Endovasc. Ther. 2019, 26, 316–321. [Google Scholar] [CrossRef]
- Giannopoulos, S.; Kokkinidis, D.G.; Jawaid, O.; Behan, S.; Hossain, P.; Alvandi, B.; Foley, T.R.; Singh, G.D.; Waldo, S.W.; Armstrong, E.J. Turbo-Power™ Laser Atherectomy Combined with Drug-coated Balloon Angioplasty is Associated with Improved One-Year Outcomes for the Treatment of Tosaka II and III Femoropopliteal In-stent Restenosis. Cardiovasc. Revasc. Med. 2020, 21, 771–778. [Google Scholar] [CrossRef]
- Thieme, M.; Von Bilderling, P.; Paetzel, C.; Karnabatidis, D.; Delgado, J.P.; Lichtenberg, M. The 24-Month Results of the Lutonix Global SFA Registry: Worldwide Experience with Lutonix Drug-Coated Balloon. JACC Cardiovasc. Interv. 2017, 10, 1682–1690. [Google Scholar] [CrossRef] [PubMed]
- Al Shammeri, O.; Bitar, F.; Ghitelman, J.; Soukas, P.A. Viabahn for femoropopliteal in-stent restenosis. Ann. Saudi Med. 2012, 32, 575–582. [Google Scholar] [CrossRef] [PubMed]
- Bosiers, M.; Deloose, K.; Callaert, J.; Verbist, J.; Hendriks, J.; Lauwers, P.; Schroë, H.; Lansink, W.; Scheinert, D.; Schmidt, A.; et al. Superiority of Stent-Grafts for In-Stent Restenosis in the Superficial Femoral Artery: Twelve-month results from a multicenter randomized trial. J. Endovasc. Ther. 2015, 22, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Zeller, T.; Dake, M.D.; Tepe, G.; Brechtel, K.; Noory, E.; Beschorner, U.; Kultgen, P.L.; Rastan, A. Treatment of Femoropopliteal In-Stent Restenosis with Paclitaxel-Eluting Stents. JACC Cardiovasc. Interv. 2013, 6, 274–281. [Google Scholar] [CrossRef] [PubMed]
- Murata, N.; Takahara, M.; Soga, Y.; Nakano, M.; Yamauchi, Y.; Zen, K.; Kawasaki, D.; Yokoi, H.; Tosaka, A.; Tanaka, N.; et al. Drug-Eluting Stent vs. Percutaneous Transluminal Angioplasty for Treatment of Femoropopliteal In-Stent Restenosis: Results from a Retrospective 1-Year Multicenter Study. J. Endovasc. Ther. 2016, 23, 642–647. [Google Scholar] [CrossRef] [PubMed]
- van den Berg, J.C.; Pedrotti, M.; Canevascini, R.; Chimchila Chevili, S.; Giovannacci, L.; Rosso, R. In-stent restenosis: Mid-term results of debulking using excimer laser and drug-eluting balloons: Sustained benefit? J. Invasive Cardiol. 2014, 26, 333–337. [Google Scholar] [PubMed]
- Liu, M.-Y.; Li, W.; Guo, X.; Zhang, Z.; Liu, B.; Yu, H.; Zhang, Z.; Chen, X.; Feng, H. Percutaneous Mechanical Atherectomy Plus Thrombectomy Using the Rotarex(R)S Device Followed by a Drug-Coated Balloon for the Treatment of Femoropopliteal Artery In-stent Restenosis: A Prospective Single-Center, Single-Arm Efficacy Trial (PERMIT-ISR Trial). Front. Surg. 2021, 8, 671849. [Google Scholar] [CrossRef] [PubMed]
- Tomoi, Y.; Soga, Y.; Okazaki, J.; Iida, O.; Shiraki, T.; Hiramori, S.; Ando, K. Drug-coated stent implantation vs. bypass surgery for in-stent occlusion after femoropopliteal stenting. Heart Vessel. 2021, 36, 646–653. [Google Scholar] [CrossRef]
- Zhang, B.; Niu, G.; Yan, Z.; Zou, Y.; Tong, X.; Yang, M. Drug-Coated Balloon for the Treatment of Femoropopliteal Tosaka Class III In-stent Restenosis Lesions. Front. Surg. 2020, 7, 616414. [Google Scholar] [CrossRef]
- Bosiers, M.; Deloose, K.; Callaert, J.; Verbist, J.; Hendriks, J.; Lauwers, P.; Schroë, H.; Lansink, W.; Scheinert, D.; Schmidt, A.; et al. Stent-grafts are the best way to treat complex in-stent restenosis lesions in the superficial femoral artery: 24-month results from a multicenter randomized trial. J. Cardiovasc. Surg. 2020, 61, 617–625. [Google Scholar] [CrossRef]
- Lee, S.-Y.; Hong, M.-K.; Jang, Y. Formation and Transformation of Neointima after Drug-eluting Stent Implantation: Insights from Optical Coherence Tomographic Studies. Korean Circ. J. 2017, 47, 823–832. [Google Scholar] [CrossRef] [PubMed]
- Walter, D.H.; Fichtlscherer, S.; Sellwig, M.; Auch-Schwelk, W.; Schächinger, V.; Zeiher, A.M. Preprocedural C-reactive protein levels and cardiovascular events after coronary stent implantation. J. Am. Coll. Cardiol. 2001, 37, 839–846. [Google Scholar] [CrossRef]
- Ferrante, G.; Niccoli, G.; Biasucci, L.M.; Liuzzo, G.; Burzotta, F.; Galiuto, L.; Trani, C.; Rebuzzi, A.G.; Crea, F. Association between C-reactive protein and angiographic restenosis after bare metal stents: An updated and comprehensive meta-analysis of 2747 patients. Cardiovasc. Revasc. Med. 2008, 9, 156–165. [Google Scholar] [CrossRef]
- Zhu, X.; Chen, Y.; Xiang, L.; You, T.; Jiao, Y.; Xu, W.; Chen, J. The long-term prognostic significance of high-sensitive C-reactive protein to in-stent restenosis. Medicine 2018, 97, e10679. [Google Scholar] [CrossRef]
- Jakubiak, G.K.; Pawlas, N.; Cies’lar, G.; Stanek, A. Pathogenesis and Clinical Significance of In-Stent Restenosis in Patients with Diabetes. Int. J. Environ. Res. Public Health 2021, 18, 11970. [Google Scholar] [CrossRef]
- Speidl, W.S.; Exner, M.; Amighi, J.; Mlekusch, W.; Sabeti, S.; Kastl, S.P.; Zorn, G.; Maurer, G.; Wagner, O.; Huber, K.; et al. Complement Component C5a Predicts Restenosis After Superficial Femoral Artery Balloon Angioplasty. J. Endovasc. Ther. 2007, 14, 62–69. [Google Scholar] [CrossRef]
- Speidl, W.S.; Exner, M.; Amighi, J.; Kastl, S.P.; Zorn, G.; Maurer, G.; Wagner, O.; Huber, K.; Minar, E.; Wojta, J.; et al. Complement component C5a predicts future cardiovascular events in patients with advanced atherosclerosis. Eur. Heart J. 2005, 26, 2294–2299. [Google Scholar] [CrossRef]
- Yuan, L.; Dong, J.; Zhu, G.; Bao, J.; Lu, Q.; Zhou, J.; Jing, Z. Diagnostic Value of Circulating microRNAs for In-Stent Restenosis in Patients with Lower Extremity Arterial Occlusive Disease. Sci. Rep. 2019, 9, 1–7. [Google Scholar] [CrossRef]
- Stojkovic, S.; Jurisic, M.; Kopp, C.W.; Koppensteiner, R.; Huber, K.; Wojta, J.; Gremmel, T. Circulating microRNAs identify patients at increased risk of in-stent restenosis after peripheral angioplasty with stent implantation. Atherosclerosis 2018, 269, 197–203. [Google Scholar] [CrossRef] [PubMed]
- Li, B.H.; Chan, Y.C.; Cheng, S.W. Evidence for treatment of lower limb in-stent restenosis with drug eluting balloons. J. Cardiovasc. Surg. 2020, 61, 626–631. [Google Scholar] [CrossRef]
- Li, X.; Zhou, M.; Ding, Y.; Wang, Y.; Cai, L.; Shi, Z. A systematic review and meta-analysis of the efficacy of debulking devices for in-stent restenosis of the femoropopliteal artery. J. Vasc. Surg. 2020, 72, 356–366. [Google Scholar] [CrossRef] [PubMed]
- Spadaccio, C.; Antoniades, C.; Nenna, A.; Chung, C.; Will, R.; Chello, M.; Gaudino, M.F.L. Preventing treatment failures in coronary artery disease: What can we learn from the biology of in-stent restenosis, vein graft failure, and internal thoracic arteries? Cardiovasc. Res. 2020, 116, 505–519. [Google Scholar] [CrossRef] [PubMed]
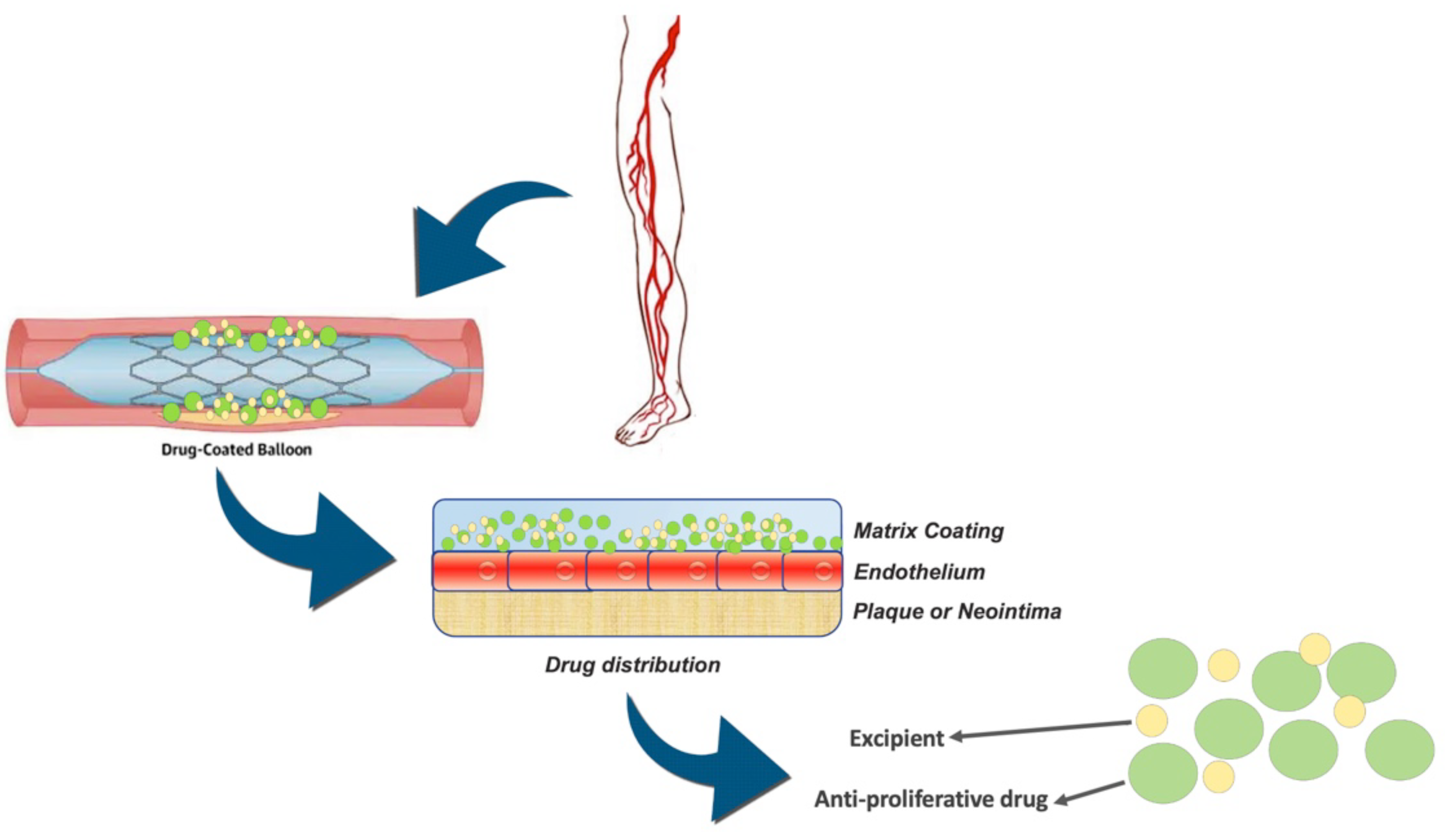

| Study Name | Year | Study Design | Interventions | Inclusion Criteria | Exclusion Criteria | DCB Device | Reference |
|---|---|---|---|---|---|---|---|
| Feng | 2021 | Retrospective | DCB + DA | Claudication (Rutherford classes 2 and 3) or critical limb ischemia (Rutherford classes 4–6); femoropopliteal artery disease | Limited life expectancy (<2 years), acute thrombosis in the target vessel, prior bypass surgery, or thrombolysis within the past 6 weeks | DCB: Orchid; Acotec Scientific, Beijing, China; DA: SilverHawk or TurboHawk devices (Medtronic, formerly Covidien/ev3, Plymouth, MN, USA) | [14] |
| Kokkinidis | 2020 | Retrospective | DCB + LA vs. PTA + LA | Tosaka II or Tosaka III- FP-ISR | Not available | DCB = IN.PACT Admiral paclitaxel-eluting balloon [Medtronic Cardiovascular, Santa Rosa, CA] or Lutonix [Bard Peripheral Vascular, Tempe, AZ]; LA = Turbo Elite (Philips Inc, Colorado Springs, CO), the Turbo-Power excimer laser (Philips Inc), and the Turbo Tandem device (Philips Inc) | [18] |
| Kokkinidis et al. | 2018 | Retrospective | Laser + DCB/Laser + PTA | Patients (mean age 70.3 ± 10.6 years; 86 men) with Tosaka II (n = 29) and Tosaka III (n = 83) FP-ISR retrieved from the vascular databases of 2 academic centers | Not available | Laser Atherectomy + DCB Laser Atherectomy + PTA | [19] |
| PATENT/Schmidt et al. | 2014 | Multicentre RCT | Laser + PTA | Patients between 18 and 85 years of age with peripheral artery disease ranging from intermittent claudication to CLI (Rutherford categories 2 to 5), an existing femoropopliteal stent with suspected ISR, and an ankle-brachial index (ABI) of 0.8 in the target limb. Anatomical inclusion criteria included angiographic evidence of significant ISR 50% stenosis by visual assessment within a previously deployed femoropopliteal stent(s). The target lesion had to be 1 cm and 25 cm, with a reference vessel diameter of 3.5 mm and 7 mm. A minimum of one patent tibial vessel to the ankle without stenosis requiring intervention prior to the 1-month follow-up was required. Additional anatomical inclusion criteria included a popliteal artery free of visually assessed stenosis 0.50% within 1 cm of the knee joint in the anteroposterior projection and angiographic documentation of successful intraluminal guidewire crossing. | Major exclusion criteria included a life expectancy of 12 months, treatment to the target lesion of 3 months prior to study enrollment, serum creatinine of 2.5 mg/dL unless dialysis-dependent, 0.50% diameter stenosis following treatment of inflow lesions in the iliac or common femoral artery, aneurysm within the target lesion, or grade 4/5 stent fracture affecting the target stent or proximal to the target stent | turbo-booster and excimer laser | [20] |
| Brodmann et al. | 2017 | Single arm prospective | DCB | Patients enrolled at sites qualified by the VasCore Duplex Core Lab (Boston, Massachusetts) were screened to meet one or more of the imaging criteria based on an algorithm. The hierarchy for the imaging cohort subgroup assignment was as follows: (1) de novo ISR; (2) long lesions $15 cm; and (3) chronic total occlusions $5 cm. Enrollment in the respective imaging cohorts was only open to subjects that had a de novo ISR, long lesion, or chronic total occlusion at pre-procedure baseline. The only subjects who were included in the respective imaging cohort analyses, however, were those who had the primary target lesions that met the criteria of the respective cohort during the index procedure (i.e., no other types of lesions could have been treated during the index procedure). Subjects with symptoms of intermittent claudication or ischemic rest pain (Rutherford clinical category 2 to 4) and angiographic evidence of occlusion or stenosis (length $2 cm) in the SFA or popliteal artery (including P1 to P3 segments) were eligible for enrollment in the IN.PACT Global study | Subjects with multiple lesions were allowed. Subjects with tissue loss were excluded. | INPACT Admiral | [21] |
| Horie | 2021 | Retrospective | PEB vs. PTA | Age >50 years, symptomatic PAD (Rutherford category 2 to 5), ISR > 70% at the stented site in femoropopliteal segments | Acute limb ischemia and/or short life expectancy | IN.PACT Pacific PCB (Medtronic, Minneapolis, MN) | [22] |
| DEBATE-ISR/Liistro F | 2014 | Multicentre RCT | DCB/PTA | Diabetic patients with femoropopliteal ISR | Paclitaxel allergy; contraindication to combined antiplatelet treatment; life expectancy <1 year | DCB = In Pact Admiral (Medtronic) PTA = Unspecified | [23] |
| COPA CABANA/Tepe G et al. | 2020 | Multicenter RCT | DCB vs. ba | ISR ≥70% or in-stent occlusion 3 to 27 cm long within the stent and adjacent segments of the SFA and/or popliteal artery occurring >3 months after stent implantation; (2) Rutherford category 2 to 5 ischemia; (3) at least 1 patent runoff vessel; and (4) patient willingness and ability to continue study participation after the initial study procedure. | (1) No patent distal runoff vessel; (2) guidewire unable to cross the lesion or a planned sub-intimal approach to the ISR lesion; (3) presence of stent fracture grades 2 to 4; (4) persistent inflow lesion, acute thrombosis of the study lesion or planned major amputation (above the ankle); (5) aneurysm in the target vessel; (6) platelet count <100,000/mm3 or >700,000/mm3, leukocyte count <3000/mm3; (7) contraindication to paclitaxel or any anticoagulation or antiplatelet agent (eg, aspirin, heparin, clopidogrel, ticlopidine, abciximab); and (8) known intolerance or contraindications to contrast agents | Cotavance paclitaxel- coated balloon (MEDRAD Inc, Warrendale, PA, USA) | [24] |
| BIOLUX P-III/Tepe G. et al. | 2021 | Prospective non randomized trial | POBA/CB/SB/AD + DCB (Passeo-18 1x) | Patients were eligible if they had lesions in the infrainguinal arteries suitable for endovascular treatment with the Passeo-18 1x DCB, including below-the-knee lesions and Rutherford 5 or 6, under real-world conditions. | Excluded were patients with a life expectancy of less than one year, or failure to successfully cross the target lesion with a guide wire | Passeo-18 1x (DCB (BIOTRONIK AG, Switzerland) | [25] |
| Liao CJ et al. | 2019 | Retrospective | DCB/PTA | Patients with femoropopliteal occlusive disease treated with DA. | Yntreated ipsilateral iliac artery stenosis, ongoing dialysis treatment, aneurysm within target lesion, known intolerance or allergy to aspirin, heparin, clopidogrel, paclitaxel, or contrast agent, planned amputation of the target limb; and life expectancy <1 year. | SilverHawk | [26,27] |
| ISAR-PEBIS/Ott I et al. | 2017 | Multicentre RCT | DCB/PTA | Symptomatic ISR >70% or occlusion of SFA | Acute ischemia, thrombosis, untreated ipsilateral iliac artery stenosis >70%, severe renal insufficiency, life expectancy <1 year, contraindication to study medications | DCB = In Pact Admiral (Medtronic) PTA = Pacific Xtreme (Medtronic) | [28] |
| FAIR/Krankenberg H et al. | 2015 | Multicentre RCT | DCB/PTA | A SFA ISR up to 20 cm, stenosis >70%; one nfrapopliteal for distal runoff; Rutherford category 2–4. | An untreated ipsilateral iliac artery stenosis; ongoing dialysis treatment; treatment with oral anticoagulants | DCB = In Pact (TM) Admiral (Medtronic) PTA = Admiral Xtreme (Medtronic) | [29] |
| PACUBA/Kinstner CM et al. | 2016 | Multicentre RCT | DCB/PTA | Age >50 years, symptomatic PAD, ISR >50% in the SFA and P1 segment of the popliteal artery, at least 1 patent tibial vessel with distal runoff, Rutherford category 2–3 | Inability to write informed consent; contraindication to study medications; and creatinine >2.5 mg/dL | DCB = Freeway 0.035 DCB (Eurocor) PTA = Unspecified | [30] |
| Armstrong et al. | 2015 | Retrospective | Laser + PTA/PTA | Symptomatic patients (mean age 71 years; 76 men) who underwent endovascular treatment of femoropopliteal ISR between 2006 and 2013. | Not available | Laser Catheter (Turbo Elite) | [31] |
| SALVAGE/Laird et al. | 2012 | Multicentre RCT | Laser + PTA | Patients between 18 and 90 years of age with either moderate-to-severe intermittent claudication or critical limb ischemia (Rutherford categories 2–5). Noninvasive lower extremity arterial studies (resting or exercise) in these subjects had to demonstrate an ankle-brachial index (ABI) equal to or less than 0.8 in the affected leg. Anatomic inclusion criteria included angiographic evidence of significant restenosis (defined as =50% by visual estimate) within a previously deployed femoropopliteal nitinol stent; target lesion length of ISR = 4 cm; femoropopliteal reference luminal diameter of =4.8 mm; and a minimum of one vessel infrapopliteal run-off defined as a one patent tibial vessel to the ankle with less than 50% stenosis. | Patients were excluded if they had a life expectancy <12 months; had undergone previous treatment to the target limb within 3 months of the study procedure; if the target lesion is within or adjacent to an aneurysm; if there were inflow-limiting lesions untreatable in this procedural setting; outflow lesions with >50% stenoses that require treatment in this procedural setting or within the 30-day follow-up; if a trans-lesional gradient >15 mm Hg persisted after optimal laser debulking and PTA; if angiographic evidence of intra-arterial thrombus or atheroembolism from inflow treatment was present; or if there was Grade 4 or 5 stent fracture in the restenotic stent | Excimer Laser with Adjunctive Balloon Angioplasty and Heparin-Coated Self-Expanding Stent Grafts | [32] |
| Gandini et al. | 2013 | RCT | Laser + DCB/DCB | Patients with chronic (0.30 days old) SFA in-stent reste.nosis, with no angiographically detectable antegrade blood flow and recurrent symptoms, were eligible for enrollment if they were 0.18 years old and poor candidates for surgical bypass due to comorbidities or anatomical restraints | Acute leg ischemia with sudden symptom onset and indication for thrombolytic therapy, poor distal SFA outflow (1 vessel), and pregnancy | Laser Atherectomy and Drug-Eluting Balloon Angioplasty | [33] |
| Shammas et al. | 2016 | RCT | JetStream XC + PTA | ≥50% in-stent restenotic lesion in the superficial femoral or popliteal arteries (estimated diameter ≥5 mm), Rutherford category 1–5 ischemia, and at least 1 patent infrapopliteal runoff vessel. | Patients were excluded if they were not able to give informed consent, had a creatinine level >2.5 mg/dL, were unable to take antiplatelet drugs, or had a planned surgical or endovascular procedure within 15 days of the index procedure. | JetStream Rotational and Aspiration Atherectomy | [34] |
| Sixt et al. | 2013 | RS | SilverHawk + DCB/SilverHawk + PTA | Patients with femoropopliteal occlusive disease treated with DA. Rutherford classification (2 to 5) | not available | SilverHawk (LS and LX) | [35] |
| PLAISIR/Bague N et al. | 2017 | Non randomized observational study | DCB | Age 18 years old Symptomatic patient according to Rutherford Class 1, 2, 3, 4 or 5 Clinical degradation by at least 1 Rutherford stage or absence of healing of all skin lesions Symptoms related to SFA ISR defined by PSVR >2.4 within 3 e24 months after SFA stenting of de novo atherosclerotic lesions. Each patient may have either one or both limbs treated in the study The target ISR lesion is fully contained between the origin of the SFA and the distally femoropopliteal crossover (crossing by SFA of medial rim of femur in the PA projection) Adequate SFA inflow and outflow either pre-existing or successfully re-established (outflow defined as patency of at least one infragenicular artery) The target lesion must not extend beyond the stent margin Successful crossing of the target lesion, inflow and outflow lesions with a guidewire; Patient belongs to the French health care system | No atheromatous disease, Asymptomatic lesion. Known allergies to heparin, aspirin, other anti-coagulant/antiplatelet therapies, and/or paclitaxel Acute limb ischaemia; Patient on oral anticoagulation therapy Target lesion requires/has been pretreated with alternative therapy such as: DES, laser, atherectomy, cryoplasty, cutting/scoring balloon, etc; Life expectancy <1 year; Patient involved in another trial; Refusing patient Pregnancy; Patients receiving anticoagulation | INPACT Admiral | [36] |
| Stabile et al. | 2012 | Non randomized observational study | DCB | Superficial Femoral Artery In-Stent Restenosis | Not available | INPACT Admiral | [37] |
| Virga et al. | 2014 | Non randomized observational study | DCB | Superficial Femoral Artery In-Stent Restenosis | Not available | INPACT Admiral | [38] |
| Milnerowicz et al. | 2019 | Non randomized observational study | RA + DCB | Symptomatic patients (mean age 66.7 ± 9.7 years; 49 men) with total occlusion of a previously implanted stent | Not available | Elutax | [39] |
| Giannopoulos et al. | 2020 | Retrospective | DCB + LA vs. PTA + LA | Tosaka II or Tosaka III | Not available | DCB: InPact; LA: Turbo-PowerTM laser (Spectranetics Inc, Colorado Springs, CO, USA), Turbo-EliteTM (Spectranetics Inc) and Turbo-TandemTM (Spectranetics Inc) | [40] |
| Thieme et al. | 2017 | Non randomized observational study | DCB | (1) Patients age 18 years or older; (2) Rutherford Classification category of <4; (3) stenotic or obstructive vascular lesions of the femoropopliteal arteries; (4) lesions treatable with available Lutonix 035 DCB, size per current European Instructions for use version 6 (IFU); (5) at least one patent native outflow artery to the ankle free from significant lesion ($50% stenosis) as confirmed by angiography; and (6) informed consent and willingness to comply with the follow-up schedule. | (1) Enrolled in another clinical trial; (2) unable to take recommended medications as stated in IFU or had a noncontrollable allergy to contrast; (3) pregnant or planning on becoming pregnant; (4) intending to father a child; and (5) Rutherford category >4 | Lutonix | [41] |
| Shammeri et al. | 2012 | Retrospective | VSG | femoropopliteal in-stent restenosis | not available | Viabahn Stent-Graft | [42] |
| RELINE/Bosiers et al. | 2015 | Multicentre RCT | VSG/PTA | Patient presents with lifestyle-limiting claudication, rest pain, or minor tissue loss (Rutherford category 2–5) Patient is willing to comply with specified follow-up evaluations at the specified times Patient is >18 years old Patient understands the nature of the procedure and provides written informed consent prior to enrollment in the study Patient has a projected life expectancy of at least 24 months Noninvasive lower extremity arterial studies (resting or exercise) demonstrate ankle-brachial index ≤0.8 Patient is eligible for treatment with the Viabahn Endoprosthesis Male, infertile female, or female of child bearing potential practicing an acceptable method of birth control with a negative pregnancy test within 7 days prior to study procedure Angiographic Restenotic or reoccluded lesion located in a stent that was previously implanted (>30 days) in the superficial femoral artery (SFA), suitable for endovascular therapy Total target lesion length between 4 and 27 cm (comprising in-stent restenosis and adjacent stenotic disease) Minimum of 1.0 cm of healthy vessel (non-stenotic) both proximal and distal to the treatment Popliteal artery patent at the intercondylar fossa of the femur to P3 Target vessel diameter visually estimated to be >4 and <7.6 mm at the proximal and distal treatment segments within the SFA Guidewire and delivery system successfully traversed lesion Angiographic evidence of at least 1-vessel runoff to the foot that does not require intervention (<50% stenotic) | Untreated flow-limiting aortoiliac stenotic disease Presence of a chronic total occlusion, i.e., a complete occlusion of the failed bare stent that cannot be reopened with thrombolysis or does not allow easy passage of the guidewire Any previous surgery in the target vessel Severe ipsilateral common/deep femoral disease requiring surgical reintervention. Perioperative unsuccessful ipsilateral percutaneous vascular procedure to treat inflow disease just prior to enrollment. Femoral or popliteal aneurysm located at the target vessel. Nonatherosclerotic disease resulting in occlusion (eg, embolism, Buerger’s disease, vasculitis) No patent tibial arteries (>50% stenosis). Prior ipsilateral femoral artery bypass. Severe medical comorbidities (untreated coronary artery disease/congestive heart failure, severe chronic obstructive pulmonary disease, metastatic malignancy, dementia, etc.) or other medical condition that would preclude compliance with the study protocol or result in a 2-year life expectancy. Serum creatinine >2.5 mg/dL within 45 days prior to study procedure unless the subject is currently on dialysis. Major distal amputation (above the transmetatarsal) in the study or nonstudy limb Septicemia or bacteremia. Any previously known coagulation disorder, including hypercoagulability Contraindication to anticoagulation or antiplatelet therapy. Known allergies to stent or stent-graft components (nickel-titanium or polytetrafluoroethylene). Known allergy to contrast media that cannot be adequately pre-medicated prior to the study procedure. Patient with known hypersensitivity to heparin, including those patients who have had a previous incidence of heparin-induced thrombocytopenia type II. Currently participating in another clinical research trial, unless approved by W.L. Gore & Associates in advance of study enrollment. Angiographic evidence of intra-arterial thrombus or atheroembolism from inflow treatment. Any planned surgical intervention/procedure within 30 days of the study procedure. Target lesion access not performed by transfemoral approach. | Viabahn Stent-Graft | [43] |
| ZILVER PTX/Zeller et al. | 2013 | Multicentre RCT | DES | (1) De novo or restenotic lesions of the above-the-knee segment of the femoropopliteal artery with 50% diameter stenosis and baseline clinical symptoms classified as Rutherford category (2) Patients could have multiple lesions requiring treatment, a history of prior stent placement within the lesion, bilateral lesions requiring treatment, and lesions of unlimited length. | Patients treated for multiple lesion types (e.g., both de novo and ISR) were excluded from this analysis | Zilver PTX drug eluting stents | [44] |
| Murato et al. | 2016 | Retrospective | DES/PTA | Femoropopliteal In-Stent Restenosis | not available | Zilver PTX drug eluting stents | [45] |
| Van Den Berg et al. | 2014 | Single arm prospective | Laser + DCB | Clinically relevant (Rutherford 3–6) ISR who were treated with excimer-laser angioplasty and drug-eluting balloons + clinical follow-up of at least 9 months | Not available | INPACT Admiral | [46] |
| PERMIT-ISR Trial | 2021 | Prospective non randomized trial | MATH + DCB | FP artery ISR defined as a peak systolic velocity (PSV) ratio > 2.0 (PSV ≥ 200–250 cm/s) at the target lesion (9); angiographic evidence of significant ISR > 70% by visual assessment within the stent; Rutherford category 2 to 6; reference vessel diameter of 4–8 mm; target ISR lesion >1 and <35 cm; at least 1 non-occluded crural vessel runoff; and ankle–brachial index (ABI) < 0.6. | Serious renal failure (serum creatinine > 2.5 mg/dL), treatment of the target lesion within 3 months before study enrollment, aneurysm in the target lesion, stent fracture, planned amputation of the target limb, and expected follow-up time < 2 years. | MATH: RotareR S System (Straub Medical, Wangs, Switzerland); DCB = OrchidR paclitaxel-coated balloons (Orchid, AcoTec, Beijing, China) | [47] |
| Tomoi Y. et al. | 2021 | Non randomized observational study | POBA and DES vs. BP | Elective treatment of femoropopliteal (FP) in-stent occlusion | Urgent setting, elective treatment with POBA and BMS implantation | not available | [48] |
| Zhang B. et al. | 2020 | Non randomized observational study | RA/RT + POBA + DCB | Patients with femoropopliteal Tosaka class III ISR lesions treated with DCB from September 2016 to September 2018 were enrolled in this single-center study. The inclusion criteria were as follows: (1) patients age 18 years or older; (2) patients diagnosed with Rutherford Classification category 1 or greater; (3) the presence of femoropopliteal Tosaka III ISR lesions; and (4) the lesions were treatable with the available Acotec Orchid DCB. | Pregnant patients or patients who were planning on becoming pregnant were excluded. | Orchid DCB (Acotec, Beijing, China) | [49] |
| Bosiers M. et al. | 2020 | Multicenter RCT | POBA + VSG vs. POBA | General inclusion criteria (1). Patient presenting with lifestyle-limiting claudication, rest pain or minor tissue loss (Rutherford classification from 2 to 5) (2). Patient is willing to comply with specified follow-up evaluations at the specified times (3). Patient is >18 years old (4). Patient understands the nature of the procedure and provides written informed consent, prior to enrolment in the study (5). Patient has a projected life-expectancy of at least 24 months (6). Noninvasive lower extremity arterial studies (resting or exercise) demonstrate ankle-brachial index ≤0.8 (7). Patient is eligible for treatment with the Viabahn® Endoprosthesis (W.L. Gore) (8). Male, infertile female or female of child bearing potential practicing an acceptable method of birth control with a negative pregnancy test within 7 days prior to study procedure Angiographic inclusion criteria (1). Restenotic or reoccluded lesion located in a stent which was previously implanted (>30 days) in the superficial femoral artery, suitable for endovascular therapy (2). Total target lesion length between 4 and 27 cm (comprising in-stent restenosis and adjacent stenotic disease) (3). Minimum of 1.0 cm of healthy vessel (non-stenotic) both proximal and distal to the treatment area (4). Popliteal artery is patent at the intercondylar fossa of the femur to P 3 (5). Target vessel diameter visually estimated to be >4 mm and <7.6 mm at the proximal and distal treatment segments within the SFA (6). Guidewire and delivery system successfully traversed lesion (7). There is angiographic evidence of at least one-vessel-runoff to the foot, that does not require intervention (<50% stenotic) | (1). Untreated flow-limiting aortoiliac stenotic disease (2). Presence of a chronic total occlusion, i.e., a complete occlusion of the failed bare stent that cannot be re-opened with thrombolysis or does not allow easy passage of the guidewire by the physician (3). any previous surgery in the target vessel (4). severe ipsilateral common/deep femoral disease requiring surgical reintervention (5). Perioperative unsuccessful ipsilateral percutaneous vascular procedure to treat inflow disease just prior to enrollment (6). Femoral or popliteal aneurysm located at the target vessel (7). Non-atherosclerotic disease resulting in occlusion (e.g., embolism, Buerger’s disease, vasculitis) (8). No patent tibial arteries (>50% stenosis) (9). Prior ipsilateral femoral artery bypass (10). Severe medical comorbidities (untreated CAD/CHF, severe COPD, metastatic malignancy, dementia, etc.) or other medical condition that would preclude compliance with the study protocol or 2-year life expectancy (11). serum creatinine >2.5 mg/dl within 45 days prior to study procedure unless the subject is currently on dialysis (12). Major distal amputation (above the transmetatarsal) in the study or non-study limb (13). Septicemia or bacteremia (14). Any previously known coagulation disorder, including hypercoagulability (15). Contraindication to anticoagulation or antiplatelet therapy (16). Known allergies to stent or stent graft components (nickel-titanium or ePTFE) (17). Known allergy to contrast media that cannot be adequately premedicated prior to the study procedure (18). Patient with known hypersensitivity to heparin, including those patients who have had a previous incidence of heparin-induced thrombocytopenia (HIT) type II (19). Currently participating in another clinical research trial, unless approved by W.L. Gore & Associates in advance of study enrolmen t (20). Angiographic evidence of intra-arterial thrombus or atheroembolism from inflow treatment (21). Any planned surgical intervention/procedure within 30 days of the study procedure (22). Target lesion access not performed by transfemoral approach | Viabahn Stent-Graft | [50] |
| Study Name | Patients | Mean Age | Diabetes % | Rutherford Class | Duration od Follow-up (m) | Drug Treatment | Reference |
|---|---|---|---|---|---|---|---|
| Feng | 79 | 70.9 | 72.2 | 35 (>4) | 12 | DAPT | [14] |
| Kokkinidis | 117 | 70.0 ± 11.0 | DCB + LA = 33/66; BA + LA = 27/51 | DCB + LA = 27 (>4); BA + LA = 12 (>4) | 24 | DAPT | [18] |
| Kokkinidis et al. | LD + DCB = 62 | 68.5 ± 10 | 50.08 | - | 12 | ASA = 49 | [19] |
| LD + PTA = 50 | 72.5 ± 10.8 | 52 | - | 12 | ASA = 44 | ||
| Schmidt et al. | Laser + PTA = 90 | 69.5 ± 9.3 | 50 | 6 (>4) | 12 | - | [20] |
| Brodmann et al. | DCB = 131 | 67.8 ± 10.1 | 35.1 | 87 (>3) | 12 | - | [21] |
| Horie | 50 | PEB = 71.9 ± 7.5; BA = 71.5 ± 8.9 | PEB = 76.0; BA = 68.0 | 0 (>4); PEB = 24/25 (2/3); BA = 25/25 (2/3) | 60 (PEB = 19/25; BA = 20/25) | DAPT | [22] |
| DEBATE-ISR/Liistro F | 44 | 32 | 100 | 33 (>4) | 36 | DAPT | [23] |
| COPA CABANA/Tepe G et al. | 47 | 68.3 ± 9.6 | 42.5 | 3 (>4) | 22 | DAPT | [24] |
| BIOLUX P-III/Tepe et al. | DCB = 103 | 70.4 ± 9.8 | 42.7 | 75.3% (≥3) | 24 | / | [25] |
| Liao CJ et al. | 74 patients (DCB n = 38)/PTA (n = 36)) | 66.8 ± 7.9 | DCB 50.0/ PTA 47.2 | 2 18.4 vs. 22.2 | 12 | clopidogrel + ASA 3 days before and for 3 months; aspirin was continued as a permanent therapy | [26,27] |
| 3 36.8 vs. 41. 7 | |||||||
| 4 39.5 vs. 30.5 | |||||||
| 5 5.3 vs. 5.6 | |||||||
| ISAR-PEBIS/Ott I et al. | 36 | 70 ± 10 | 4.32 | 1 (>4) | 24 | DAPT | [28] |
| FAIR/Krankenberg H et al. | 62 | 69 ± 8 | 17.36 | 3 (>4) | 12 | DAPT | [29] |
| PACUBA/Kinstner CM et al. | 35 | 68.1 ± 9.2 | 48.5 | 0 (>4) | 12 | DAPT | [30] |
| Armstrong et al. | LD +PTA = 54 | 73 ± 11 | 55.5 | 19 (>4) | 24 | ASA (47) Plavix (33) | [31] |
| PTA = 81 | 69 ± 11 | 55.5 | 37 (>4) | 24 | ASA (77) Plavix (60) | ||
| SALVAGE/Laird et al. | Laser + PTA = 27 | 70.0 ± 10.5 | 59.2 | 2 (>4) | 12 | - | [32] |
| Gandini et al. | Laser + DCB = 24 | 74.1 ± 7.2 | - | - | 12 | - | [33] |
| DCB = 24 | 70.1 ± 11.6 | - | - | 12 | - | ||
| Shammas et al. | JetStream XC + PTA = 29 | 69.9 ± 11.7 | 41 | 10 (>4( | 6 | ASA = 29 | [34] |
| Sixt et al. | SilverHawk + DCB = 29 | 70 ± 13 | 48 | 11 (>4) | 12 | ASA (27) Plavix (28) | [35] |
| SilverHawk + PTA = 60 | 68 ± 10 | 43 | 10 (>4) | 12 | ASA (54) Plavix (56) | ||
| PLAISIR/Bague N et al. | DCB = 53 | 69 ± 12 | 30.18 | 18 | 53 | [36] | |
| Stabile et al. | DCB = 39 | 65.9 ± 9.6 | 48.7 | 2.9 ± 0.7 | 12 | ASA (39) | [37] |
| Virga et al. | DCB = 39 | 65.9 ± 9.6 | 48.7 | 2.9 ± 0.7 | 24 | ASA (39) | [38] |
| Milnerowicz et al. | DCB = 74 | 66.7 ± 9.7 | 25 | 37 (>4) | 12 | ASA (74) | [39] |
| Giannopoulos et al. | Turbo Power + DCB = 27 | 64,7 | 59 | 27 (>3) | 12 | ASA (19) Plavix (7) | [40] |
| Laser + BA = 51 | 72,5 | 52.9 | 46 (>3) | 12 | ASA (45) Plavix (36) | ||
| Thieme et al. | DCB = 89 | 68.2 ± 9.65 | 28.1 | 8 (>4) | 24 | - | [41] |
| Shammeri et al. | DES = 26 | 73 | 55 | 28 (>3) | 36 | ASA (26) Plavix (26) | [42] |
| RELINE/Bosiers et al. | DES = 39 | 67.7 ± 9.8 | 33.3 | 27 (>3) | 12 | - | [43] |
| PTA = 44 | 69.0 ± 9.7 | 36.4 | 38 (>3) | 12 | - | ||
| ZILVER PTX/Zeller et al. | DES = 108 | 68.3 ± 9.4 | 38.9 | - | 12 | Plavix = 108 | [44] |
| Murato et al. | DES = 57 | 74 ± 9 | 60 | - | 12 | DAPT = 57 | [45] |
| PTA = 44 | 69 ± 11 | 57 | - | 12 | DAPT = 44 | ||
| Van Den Berg et al. | Laser + DCB | 78 ± 6.5 | - | - | 9 | - | [46] |
| PERMIT-ISR Trial | 59 | 71.0 ± 11.2 | 45.8 | 48 (class 4–6) | 33 ± 8 | single or dual antiplatelet therapy | [47] |
| Tomoi Y et al. | DES = 28 | 71.2 ± 9.1 | 50 | 3 (3, 4) | 36.6 ± 25.5 | DAPT (2 m) then ASA | [48] |
| Zhang B. et al. | DCB = 28 | 69.3 ± 8.5 | 67.9 | 3 (60.7%) | 21.5 ± 10.3 | DAPT (6 m) then single | [49] |
| Bosiers M. et al. | VSG = 39 | 67.69 ± 9.77 | 33.3 | 3 (56.4%) | 24 | DAPT (6 m) then ASA | [50] |
| Study Name | Patients | Follow-Up Duration | Interventions | Patency | Restenosis | TLR | TVR | Stent Thrombosis | Amputations | Clinical Improvement | Reference |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Feng | 79 | 12 months | DCB + DA | 80.8 1 yy | 7.8 | Superficial femoral and popliteal artery | 0 | 1.2 | [14] | ||
| Kokkinidis et al. | 62 | 12 months | Laser + DCB | 86.7 | 13.3 | 27.5 | Femoropopliteal | 5.2 | [19] | ||
| 50 | 12 months | Laser + PTA | 56.9 | 43.1 | 49.5 | Femoropopliteal | 2.6 | ||||
| Schmidt et al. | 90 | 12 months | Laser + PTA | 37.8 | 62.2 | 27.4 | Femoropopliteal | 2.2 | 0 | Improvement in the Rutherford-Becker categories. ABI, WIQ | [20] |
| Brodmann et al. | 131 | 12 months | DCB | 88.7 | 11,3 | 7.3 | Femoropopliteal | 0.8 | 0 | 75 | [21] |
| Horie | 50 | 18 months | PEB = 25; PTA = 25 | PEB = 65.7; PTA = 18.7 | PEB = 24.0; PTA = 80.0 | PEB = 16.0; PTA = 44.0 | Superficial femoral and proximal popliteal artery | 0 | 0 | [22] | |
| 117 | 24 months | DCB + LA = 66; PTA + LA = 51 | DCB + LA = 45; PTA + LA = 24 | DCB + LA = 55; BA + LA = 76 | DCB + LA = 49; PTA + LA = 55 | Superficial femoral and proximal popliteal artery | 0 | 0 | |||
| DEBATE-ISR/Liistro F | 44 | 12 months | DCB | 80.50 | 19.5 | 13.6 | Superficial femoral proximal popliteal | 0 | 77.3 | [23] | |
| 42 | 12 months | PTA | 28.20 | 71.8 | 31 | Superficial femoral proximal popliteal | 2.4 | 59.5 | |||
| COPA CABANA/Tepe G et al. | 47 | 12 months | DCB | 86.00 | 14, 6 m | 14, 12 m | Superficial femoral proximal popliteal | 4.2 | 24 | [24] | |
| 41 | 12 months | PTA | 41.00 | 59, 6 m | 49, 12 m | Superficial femoral proximal popliteal | 2.4 | 15 | |||
| Tepe G. et al. | 103 | 24 months | POBA/CB/SB/AD + DCB (Passeo-18 1x) | 77.3, 12 m/58.5, 24 m | 10.8, 12 m/21.6, 24 m | Infrainguinal arteries | Major 0; Minor 3.2 at 12 m 4.5 at 24 m | [25] | |||
| Liao CJ et al. | 38 | 12 months | DCB | 89.5 | 10.5 | 6.1 | Femoropopliteal | 0 | 75.8 | [26,27] | |
| 36 | 12 months | PTA | 58.3 | 41.7 | 35.5 | 0 | 51.6 | ||||
| ISAR-PEBIS/Ott I et al. | 36 | 24 monts | DCB | 70, 6 m | 30, 6 m | 19, 24 m | Superficial femoral | 3, 24 m | 0, 24 m | [28] | |
| 34 | 24 months | PTA | 41, 6 m | 59, 6 m | 50, 24 m | Superficial femoral | 0, 24 m | 0, 24 m | |||
| FAIR/Krankenberg H et al. | 62 | 12 months | DCB | 70.50 | 29.5 | 9.2 | Superficial femoral | 2.1 | 0 | 77.8 | [29] |
| 57 | 12 months | PTA | 37.50 | 62.5 | 47.4 | Superficial femoral | 4.5 | 0 | 52.3 | ||
| PACUBA/Kinstner CM et al. | 35 | 12 months | DCB | 40.70 | 59.3 | 51 | Superficial femoral proximal popliteal | 2.8 | 0 | 68.8 | [30] |
| 39 | 12 months | PTA | 13.40 | 86.6 | 77.9 | Superficial femoral proximal popliteal | 0 | 0 | 54.5 | ||
| Armstrong et al. | 54 | 24 months | Laser + PTA | I/II: 6 9 III: 69, 24 m | class I/II FP-ISR:14 III: 43, 24 m | Femoropopliteal | class I/II FP-ISR:26 III: 12, 24 m | 0 | 89, 1 m | [31] | |
| 81 | 24 months | PTA | I/II: 46 III: 100%, 24 m | class I/II FP-ISR: 44 III: 48, 24 m | Femoropopliteal | class I/II FP-ISR:33 III: 71, 24 m | 0 | 81, 1 m | |||
| SALVAGE/Laird et al. | 27 | 12 months | Laser + PTA | 48 | 52 | 17.4 | Femoropopliteal | 0 | 0 | improvements in ABI, walking distance, walking speed, and ability to climb stairs | [32] |
| Gandini et al. | 24 | 12 months | Laser + DCB | 66.7 | 33.3 | 16.7 | Superficial femoral | 8 | Limb salvage 91.7 Healing ulcer 87.5 | [33] | |
| 24 | 12 months | DCB | 37.5 | 62.5 | 50 | Superficial femoral | 46 | Limb salvage 54.2 Healing ulcer 62.5 | |||
| Shammas et al. | 29 | 12 months | JetStream XC + PTA | 72, 6 m | 28 | 14, 6 m/41, 12 m | Femoropopliteal | 0 | 0 | improved significantly | [34] |
| Sixt et al. | 29 | 12 months | SilverHawk + DCB/ | 84.7 | 15.3 | Femoropopliteal | 2/89 all enrolled | no difference between the treatment groups regarding improvement in clinical status | [35] | ||
| 60 | 12 months | SilverHawk + PTA | 43.8 | 56.2 | 2/89 all enrolled | ||||||
| PLAISIR/Bague N et al. | 53 | 18 months | DCB | 78.1 | 21.9 | 23.4 18 m | Femoropopliteal | 9 | 1.88 | 67 18 m | [36] |
| Stabile et al. | 39 | 12 months | DCB | 100 | 0 | Superficial femoral | 0 | 0 | 100 | [37] | |
| Virga et al. | 39 | 24 months | DCB | 70.3 | 29.7 | 21.6 | Superficial femoral | 0 | 100 | [38] | |
| Milnerowicz et al. | 74 | 12 months | RA + DCB | 79.5 | 20.5 | 5.5 | iliac and/or infrainguinal arteries | 1.4 | 89 | [39] | |
| Giannopoulos et al. | 27 | 12 months | Turbo -Power + DCB | 90.8 | 9.2 | 9.1 | Femoropopliteal | MALE: major adverse limb events 5.1 | no significant (p = 0.170) difference between the 2 groups | [40] | |
| 51 | 12 months | LA + PTA | 59.9 | 40.1 | 44.3 | MALE: major adverse limb events 3.7 | |||||
| Thieme et al. | 89 | 24 months | DCB | 66 | 34 | 14,5 | Superficial femoral | Freedom from TVR, major amputation, device- and procedure-related death: 82 | 76 | [41] | |
| Shammeri et al. | 26 | 12 months | VSG | 81.4, 36 m | 18,6 | 25 | Femoropopliteal | 25 | 7.69 | [42] | |
| RELINE/Bosiers et al. | 39 | 12 months | VSG (CSD) | 74.8 | 25.2 | 20.1 | Superficial femoral | 93,6 | [43] | ||
| 44 | 12 months | PTA | 28 | 72 | 57.8 | Superficial femoral | 87.8 | ||||
| ZILVER PTX/Zeller et al. | 108 | 12 months | DES | 78.8 | 21.2 | 19, 12 m/60.8, 24 m | Femoropopliteal | 0 | 60.9, 24 m | [44] | |
| Murato et al. | 57 | 12 months | DES | 49 | 51 | not available | Femoropopliteal | 44.1 | MALE: major adverse limb events: 25.5 | not available | [45] |
| 44 | 12 months | PTA | 14 | 86 | not available | 90.3 | MALE: major adverse limb events 53.6 | not available | |||
| Van Den Berg et al. | 14 | 12 months | Laser + DCB | 91.7 | 8,3 | 7 | Infrainguinal arteries | 7 | improved in all patients | [46] | |
| PERMIT-ISR Trial | 59 | 24 months | MATH + DCB | 82.5 | 17.5 | 15.3 | Superficial femoral and proximal popliteal artery | 0 | 1 | the ABI changed at 12 months were significantly improved from baseline (p < 0.01) | [47] |
| Tomoi Y. et al. | 28 | 36 months | POBA + DES | 32.2% 24 m | 15.3 | Femoropopliteal | 18.4 24 m | MALE: 15.3 at 24 m | 60.7 | [48] | |
| Zhang B. et al. | 28 | 14 months | Debulking (if nedeed) + POBA + DCB | 79.2, 14 m | 8.5, 14 m | Femoropopliteal | 0 | 0 | Clinical symptoms improved by at least 1 Rutherford category in 82.1% of limbs | [49] | |
| Bosiers M. et al. | 39 | 24 months | VSG | 74.8 at 12 m/58.40 at 24 m | 33.7 at 24 m | Femoropopliteal | 0 | 0 | Clinical symptoms improved by at least 1 Rutherford 93.50% at 12 m and 93.10% at 24 m | [50] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Montelione, N.; Catanese, V.; Nenna, A.; Jawabra, M.; Verghi, E.; Loreni, F.; Nappi, F.; Lusini, M.; Mastroianni, C.; Jiritano, F.; et al. The Diagnostic Value of Circulating Biomarkers and Role of Drug-Coated Balloons for In-Stent Restenosis in Patients with Peripheral Arterial Disease. Diagnostics 2022, 12, 2207. https://doi.org/10.3390/diagnostics12092207
Montelione N, Catanese V, Nenna A, Jawabra M, Verghi E, Loreni F, Nappi F, Lusini M, Mastroianni C, Jiritano F, et al. The Diagnostic Value of Circulating Biomarkers and Role of Drug-Coated Balloons for In-Stent Restenosis in Patients with Peripheral Arterial Disease. Diagnostics. 2022; 12(9):2207. https://doi.org/10.3390/diagnostics12092207
Chicago/Turabian StyleMontelione, Nunzio, Vincenzo Catanese, Antonio Nenna, Mohamad Jawabra, Emanuele Verghi, Francesco Loreni, Francesco Nappi, Mario Lusini, Ciro Mastroianni, Federica Jiritano, and et al. 2022. "The Diagnostic Value of Circulating Biomarkers and Role of Drug-Coated Balloons for In-Stent Restenosis in Patients with Peripheral Arterial Disease" Diagnostics 12, no. 9: 2207. https://doi.org/10.3390/diagnostics12092207





