Assessment of Parotid Gland Tumors by Means of Quantitative Multiparametric Ultrasound (mpUS)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patient Selection
2.2. Imaging Protocol
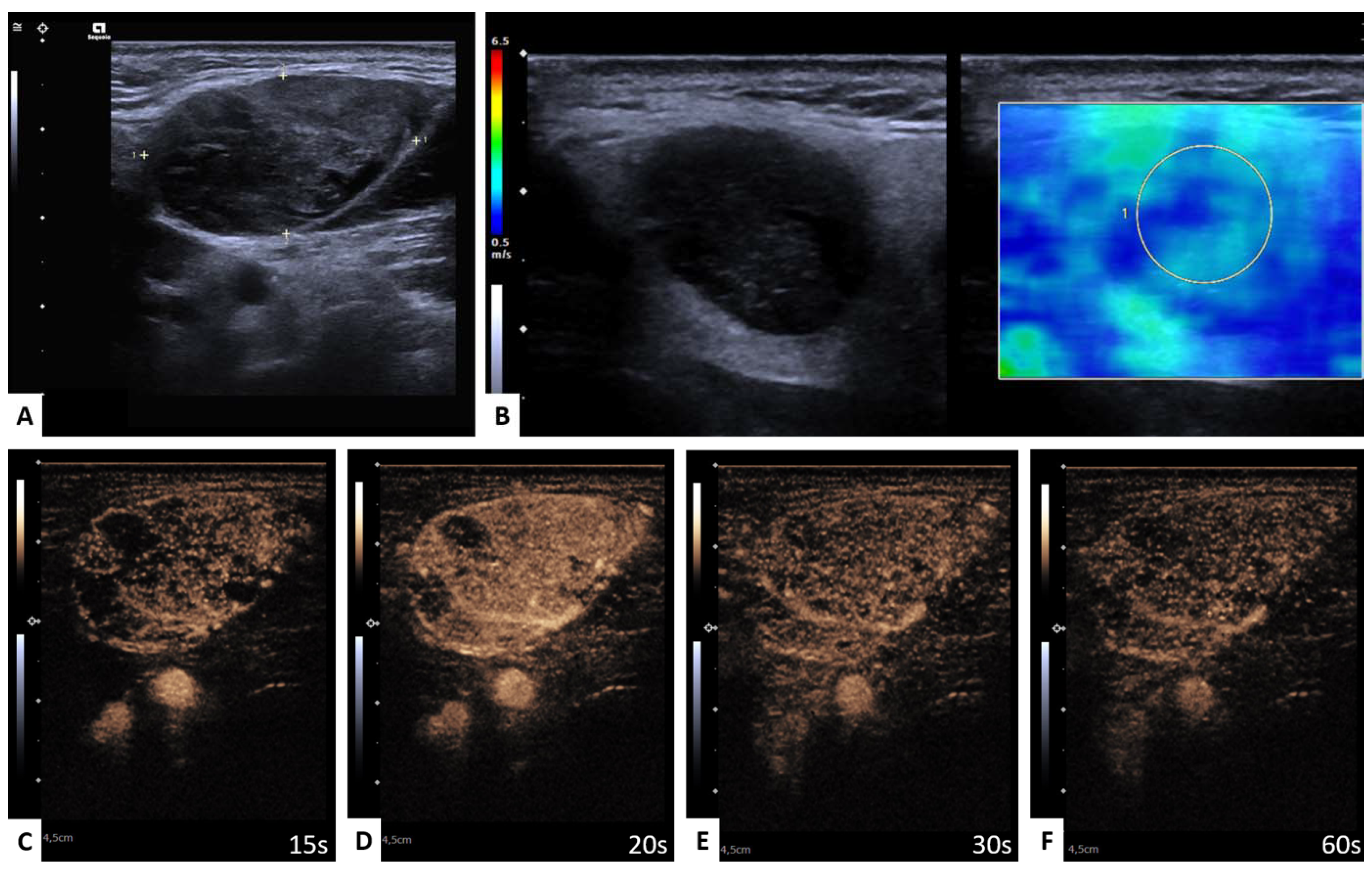
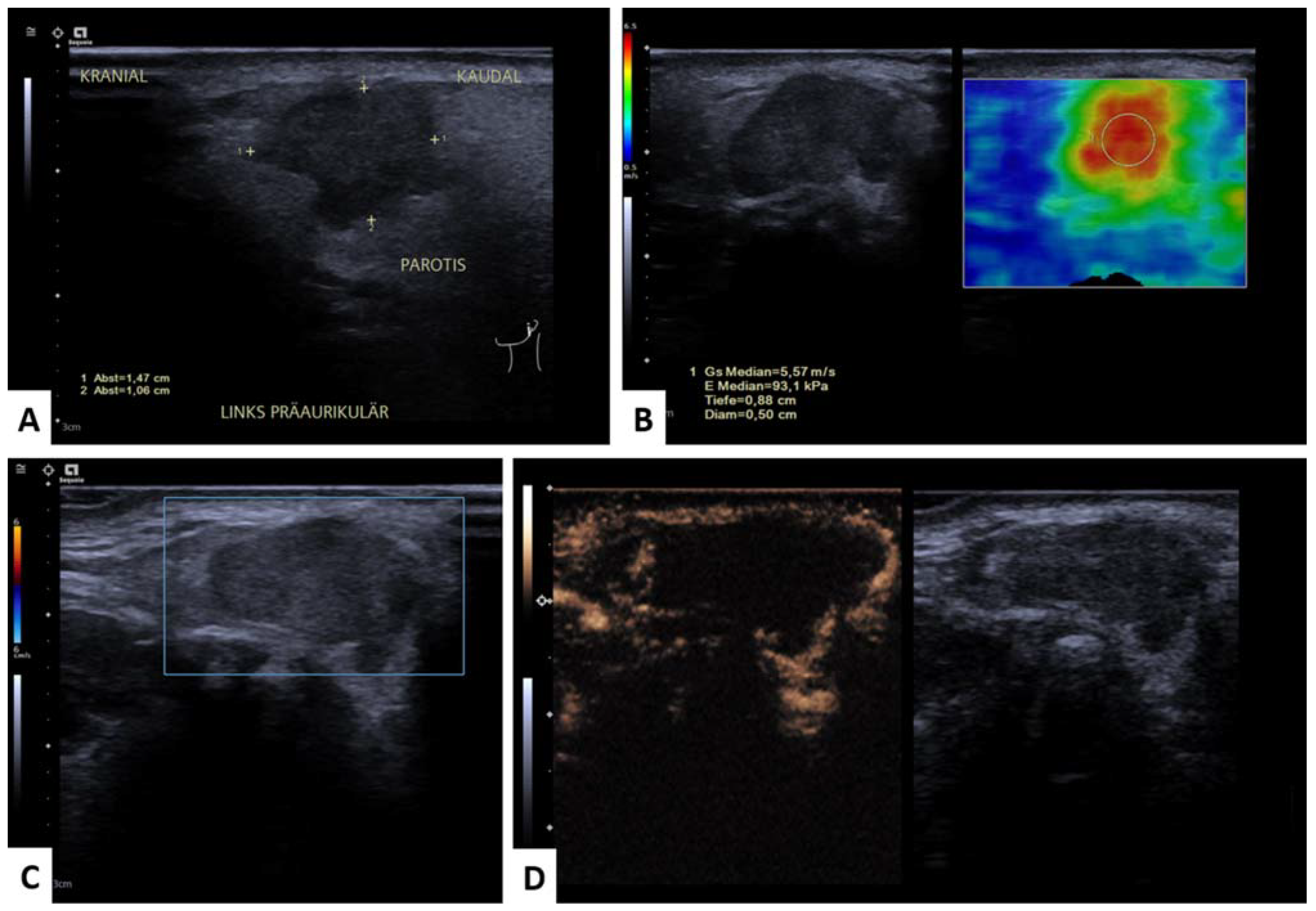
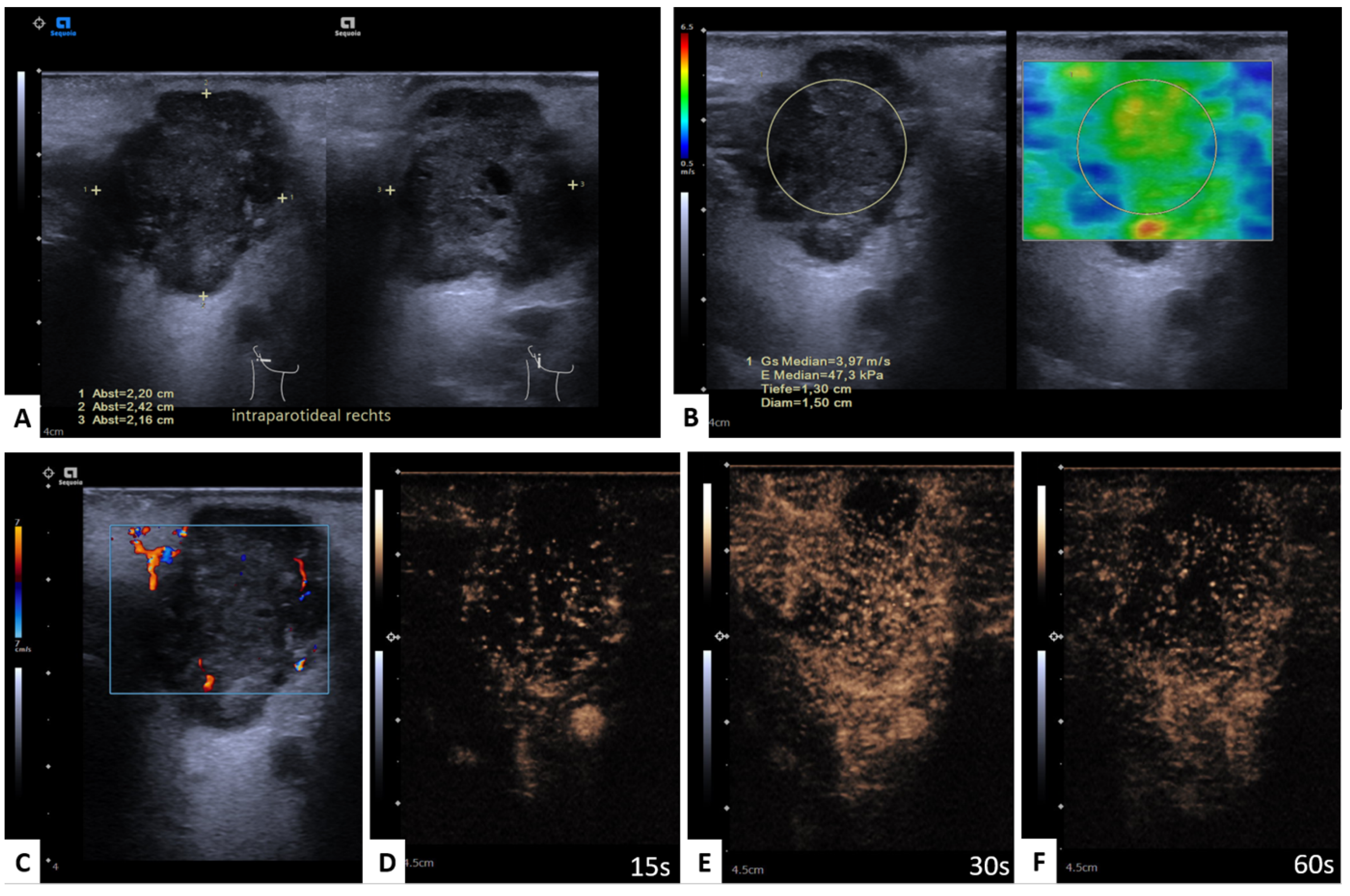
2.3. Shear Wave Elastography
2.4. Perfusion Analysis
2.5. Statistical Analysis
3. Results
3.1. Study Cohort
3.2. Significant mpUS Parameters
3.3. Shear Wave Elastography
3.4. CEUS
3.4.1. WT versus PA
3.4.2. WT versus Malignant Tumors (MT)
3.4.3. WT versus SCC
3.4.4. WT versus PA + SCC
3.4.5. WT + LN versus PA
3.4.6. WT + LN versus Malignant Tumors (MT)
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Psychogios, G.; Rueger, H.; Jering, M.; Tsoures, E.; Kunzel, J.; Zenk, J. Ultrasound can help to indirectly predict contact of parotid tumors to the facial nerve, correct intraglandular localization, and appropriate surgical technique. Head Neck 2019, 41, 3211–3218. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.Y.; Wong, K.T.; King, A.D.; Ahuja, A.T. Imaging of salivary gland tumours. Eur. J. Radiol. 2008, 66, 419–436. [Google Scholar] [CrossRef] [PubMed]
- Ho, K.; Lin, H.; Ann, D.K.; Chu, P.G.; Yen, Y. An overview of the rare parotid gland cancer. Head. Neck. Oncol. 2011, 3, 40. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, C.C.; Tsai, M.H.; Huang, C.C.; Hua, C.H.; Tseng, H.C.; Huang, S.T. Parotid tumors: A 10-year experience. Am. J. Otolaryngol. 2008, 29, 94–100. [Google Scholar] [CrossRef] [PubMed]
- Han, A.Y.; John, M.A.S. Predictors of Nodal Metastasis in Cutaneous Head and Neck Cancers. Curr. Oncol. Rep. 2022, 24, 1145–1152. [Google Scholar] [CrossRef]
- Mourouzis, C.; Boynton, A.; Grant, J.; Umar, T.; Wilson, A.; Macpheson, D.; Pratt, C. Cutaneous head and neck SCCs and risk of nodal metastasis—UK experience. J. Craniomaxillofac. Surg. 2009, 37, 443–447. [Google Scholar] [CrossRef]
- Mayer, M.; Thoelken, R.; Jering, M.; Markl, B.; Zenk, J. Metastases of Cutaneous Squamous Cell Carcinoma Seem to be the Most Frequent Malignancies in the Parotid Gland: A Hospital-Based Study From a Salivary Gland Center. Head Neck Pathol. 2021, 15, 843–851. [Google Scholar] [CrossRef] [PubMed]
- Chooback, N.; Shen, Y.; Jones, M.; Kasaian, K.; Martin, M.; Ng, T.; Thomson, T.; Marra, M.; Laskin, J.; Ho, C. Carcinoma ex pleomorphic adenoma: Case report and options for systemic therapy. Curr. Oncol. 2017, 24, e251–e254. [Google Scholar] [CrossRef] [Green Version]
- Sood, S.; McGurk, M.; Vaz, F. Management of Salivary Gland Tumours: United Kingdom National Multidisciplinary Guidelines. J. Laryngol. Otol. 2016, 130, S142–S149. [Google Scholar] [CrossRef]
- Tholken, R.; Jering, M.; Mayer, M.; Schiele, S.; Muller, G.; Zenk, J. Prospective study on complications using different techniques for parotidectomy for benign tumors. Laryngoscope Investig. Otolaryngol. 2021, 6, 1367–1375. [Google Scholar] [CrossRef]
- Eviston, T.J.; Yabe, T.E.; Gupta, R.; Ebrahimi, A.; Clark, J.R. Parotidectomy: Surgery in evolution. ANZ J. Surg. 2016, 86, 193–199. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Perlman, A.; Kalache, S.; Berman, N.; Seshan, S.; Salvatore, S.; Smith, L.; Wehrli, N.; Waldron, L.; Kodali, H.; et al. Multiparametric Quantitative Ultrasound Imaging in Assessment of Chronic Kidney Disease. J. Ultrasound Med. 2017, 36, 2245–2256. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grgurevic, I.; Tjesic Drinkovic, I.; Pinzani, M. Multiparametric ultrasound in liver diseases: An overview for the practising clinician. Postgrad. Med. J. 2019, 95, 425–432. [Google Scholar] [CrossRef]
- Greis, C. Quantitative evaluation of microvascular blood flow by contrast-enhanced ultrasound (CEUS). Clin. Hemorheol. Microcirc. 2011, 49, 137–149. [Google Scholar] [CrossRef] [PubMed]
- Taljanovic, M.S.; Gimber, L.H.; Becker, G.W.; Latt, L.D.; Klauser, A.S.; Melville, D.M.; Gao, L.; Witte, R.S. Shear-Wave Elastography: Basic Physics and Musculoskeletal Applications. Radiographics 2017, 37, 855–870. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lerchbaumer, M.H.; Wakonig, K.M.; Arens, P.; Dommerich, S.; Fischer, T. Quantitative Multiparametric Ultrasound (mpUS) in the Assessment of Inconclusive Cervical Lymph Nodes. Cancers 2022, 14, 1597. [Google Scholar] [CrossRef]
- O’Brien, C.J. The parotid gland as a metastatic basin for cutaneous cancer. Arch. Otolaryngol. Head Neck Surg. 2005, 131, 551–555. [Google Scholar] [CrossRef] [Green Version]
- Olsen, K.D.; Quer, M.; de Bree, R.; Vander Poorten, V.; Rinaldo, A.; Ferlito, A. Deep lobe parotidectomy-why, when, and how? Eur. Arch. Otorhinolaryngol. 2017, 274, 4073–4078. [Google Scholar] [CrossRef] [Green Version]
- Sonmez Ergun, S.; Gayretli, O.; Buyukpinarbasili, N.; Yildiz, K.; Gurses, I.A.; Avsar, A.; Cavlak, M. Determining the number of intraparotid lymph nodes: Postmortem examination. J. Craniomaxillofac. Surg. 2014, 42, 657–660. [Google Scholar] [CrossRef]
- Sonmez, S.; Orhan, K.S.; Kara, E.; Buyuk, M.; Aydemir, L.; Asliyuksek, H. Determining the number and distribution of intraparotid lymph nodes according to parotidectomy classification of European Salivary Gland Society: Cadaveric study. Head Neck 2020, 42, 3685–3692. [Google Scholar] [CrossRef]
- Colella, G.; Biondi, P.; Itro, A.; Compilato, D.; Campisi, G. Warthin’s tumor distribution within the parotid gland. A feasible etiologic source from lymph nodal tissue. Minerva Stomatol. 2010, 59, 245–252. [Google Scholar] [PubMed]
- Thimsen, V.; Goncalves, M.; Koch, M.; Mantsopoulos, K.; Hornung, J.; Iro, H.; Schapher, M. The current value of quantitative shear wave sonoelastography in parotid gland tumors. Gland Surg. 2021, 10, 1374–1386. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Jiang, L. Application value of shear wave elastography in salivary gland tumors. Oral Radiol. 2021, 37, 653–657. [Google Scholar] [CrossRef] [PubMed]
- Akhtar, K.; Ray, P.S.; Sherwani, R.; Siddiqui, S. Primary squamous cell carcinoma of the parotid gland: A rare entity. BMJ Case Rep. 2013, 2013, bcr2013009467. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xiao, M.; Liu, J.; You, Y.; Yang, X.; Wang, Y. Primary squamous cell carcinoma of the parotid gland: Clinicopathological characteristics, treatment, and prognosis. Int. J. Oral Maxillofac. Surg. 2021, 50, 151–157. [Google Scholar] [CrossRef]
- Gou, J.M.; Chen, Q.; Zhou, Q.; Liu, Y.X. Quantitative diagnosis of salivary gland tumors with contrast-enhanced ultrasound--a preliminary study. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2013, 116, 784–790. [Google Scholar] [CrossRef]
- Bozzato, A.; Zenk, J.; Greess, H.; Hornung, J.; Gottwald, F.; Rabe, C.; Iro, H. Potential of ultrasound diagnosis for parotid tumors: Analysis of qualitative and quantitative parameters. Otolaryngol. Head Neck Surg. 2007, 137, 642–646. [Google Scholar] [CrossRef]
- Klotz, L.V.; Ingrisch, M.; Eichhorn, M.E.; Niemoeller, O.; Siedek, V.; Gurkov, R.; Clevert, D.A. Monitoring parotid gland tumors with a new perfusion software for contrast-enhanced ultrasound. Clin. Hemorheol. Microcirc. 2014, 58, 261–269. [Google Scholar] [CrossRef]
- Wang, Z.; Tang, J.; An, L.; Wang, W.; Luo, Y.; Li, J.; Xu, J. Contrast-enhanced ultrasonography for assessment of tumor vascularity in hepatocellular carcinoma. J. Ultrasound Med. 2007, 26, 757–762. [Google Scholar] [CrossRef]
- David, E.; Cantisani, V.; De Vincentiis, M.; Sidhu, P.S.; Greco, A.; Tombolini, M.; Drudi, F.M.; Messineo, D.; Gigli, S.; Rubini, A.; et al. Contrast-enhanced ultrasound in the evaluation of parotid gland lesions: An update of the literature. Ultrasound 2016, 24, 104–110. [Google Scholar] [CrossRef]
- Welkoborsky, H.J.; Albers, M.; Kustermeyer, J. Perfusion analysis of benign parotid gland tumors by contrast-enhanced ultrasonography (CEUS). Eur. Arch. Otorhinolaryngol. 2022, 279, 4137–4146. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.P. Characteristics of pleomorphic adenomas, adenolymphomas, and malignant tumors of the salivary glands on color doppler ultrasonography and contrast enhanced ultrasonography. Eur. Rev. Med. Pharmacol. Sci. 2020, 24, 11509–11517. [Google Scholar] [CrossRef] [PubMed]
- Yan, M.; Xu, D.; Chen, L.; Zhou, L. Comparative Study of Qualitative and Quantitative Analyses of Contrast-Enhanced Ultrasound and the Diagnostic Value of B-Mode and Color Doppler for Common Benign Tumors in the Parotid Gland. Front. Oncol. 2021, 11, 669542. [Google Scholar] [CrossRef] [PubMed]
- Badea, A.F.; Bran, S.; Tamas-Szora, A.; Floares, A.; Badea, R.; Baciut, G. Solid parotid tumors: An individual and integrative analysis of various ultrasonographic criteria. A prospective and observational study. Med. Ultrason. 2013, 15, 289–298. [Google Scholar] [CrossRef]


| Patients Female–Male Mean age | 96 36/96 (37.5%)–60/96 (62.5%) 62.4 Y (±16.9 Y) |
| Benign PGT | 71/97 (73.2%) |
| Pleomorphic Adenoma | 21/71 |
| Warthin’s Tumor | 38/71 |
| Lymph Node | 7/71 |
| Cyst | 1/71 |
| Oncocytoma | 1/71 |
| Other | 3/71 |
| Malignant PGT | 26/97 (26.8%) |
| Squamous Cell Carcinoma | 12/26 |
| Adenocarcinoma | 4/26 |
| Lymphoma | 4/26 |
| Malignant Melanoma | 3/26 |
| Adenoid Cystic Carcinoma | 1/26 |
| Mucoepidermoid Carcinoma | 1/26 |
| Merkel Cell Carcinoma | 1/26 |
| Comparative PGT | SWE Significance | CEUS Significance |
|---|---|---|
| WT vs. PA | − | MeanLin, PE, WiAUC, WiR, WiPI, WoAUC, WiWoAUC, WoR, mTTI, FT |
| WT vs. MT | − | MeanLin, PE, WiAUC, WiR, WiPi, WoAUC, WiWoAUC, WoR |
| WT vs. SCC | + | WiAUC, WoAUC, WiWoAUC |
| WT vs. PA + SCC | + | MeanLin, PE, WiAUC, WiR, WoAUC, WiWoAUC, WOR |
| WT + LN vs. PA | + | MeanLin, PE, WiAUC, WiR, WiPI, WoAUC, WiWoAUC, WoR, mTTI |
| WT + LN vs. MT | − | MeanLin, PE, WiAUC, WiR, WiPI, WoAUC, WiWoAUC, WoR |
| WT | vs. PA | p | vs. MT | p | vs. SCC | p | vs. PA + SCC | p | |
|---|---|---|---|---|---|---|---|---|---|
| SWE | n = 38 | n = 21 | n = 26 | n = 12 | n = 33 | ||||
| SWE (m/s) | 2.5 ± 0.92 | 2.94 ± 1.03 | 0.069 | 2.63 ± 1 | 0.339 | 3.09 ± 1.04 | 0.046 | 2.73 ± 0.99 | 0.019 |
| CEUS | n = 33 | n = 20 | n = 23 | n = 10 | n = 30 | ||||
| meanLin [a.u] | 2,006,078.77 ± 6,022,658.47 | 59,388.64 ± 175,556.44 | 0.006 | 121,719.86 ± 514,813.21 | 0.008 | 247,028.91 ± 780,708.07 | 0.054 | 1,108,867.64 ± 4,441,027.18 | 0.003 |
| PE [a.u] | 19,072,765.52 ± 61,324,843.66 | 540,672.11 ± 1,592,891.29 | 0.003 | 1,468,390.72 ± 6,490,545.51 | 0.008 | 3,115,717.74 ± 9,851,282.77 | 0.062 | 10,656,696.54 ± 45,119,544.60 | 0.002 |
| WiAUC [a.u] | 51,905,344.84 ± 159,318,110.57 | 1,308,043.56 ± 3,927,811.86 | 0.005 | 3,135,246.66 ± 13,496,189.56 | 0.005 | 6,472,313.56 ± 2,0461,410.99 | 0.047 | 28,631,117.25 ± 117,364,223.12 | 0.002 |
| RT (s) | 6.22 ± 2.59 | 6.51 ± 3.02 | 0.714 | 6.27 ±1.77 | 0.459 | 6.52 ± 1.63 | 0.239 | 6.36 ± 2.58 | 0.401 |
| mTTI (s) | 52.26 ± 63.38 | 76.74 ± 55.22 | 0.015 | 66.08 ± 68.81 | 0.6 | 66.47 ± 59.44 | 0.472 | 62.29 ± 60.36 | 0.03 |
| TTP (s) | 10.10 ± 2.95 | 9.01 ± 3.58 | 0.102 | 9.34 ± 2.08 | 0.566 | 9.83 ± 1.56 | 0.863 | 9.71 ± 3 | 0.253 |
| WiR [a.u] | 582,2087.69 ± 19,288,365.48 | 190,810.89 ± 557,466.92 | 0.003 | 543,573.01 ± 2,446,514.59 | 0.010 | 1,174,780.24 ± 3,714,618.56 | 0.062 | 3,296,712.92 ± 14,189,853.33 | 0.002 |
| WiPI [a.u] | 11,705,571.65 ± 37,461,700.07 | 336,761.33 ± 992,297.43 | 0.003 | 896,490.65 ± 3,958,811.46 | 0.009 | 1,900,363.97 ± 6,008,529.95 | 0.062 | 6,540,043.35 ± 27,567,268.56 | 0.002 |
| WoAUC [a.u] | 74,698,989.56 ± 218,145,521.46 | 2,057,520.60 ± 6,158,753.67 | 0.006 | 4,023,168.72 ± 17,103,206.84 | 0.004 | 8,198,558.42 ± 25,914,563.94 | 0.031 | 40,540,381.65 ± 159,886,884.39 | 0.002 |
| WiWoAUC [a.u] | 128,226,364.53 ± 379,440,971.04 | 3,362,932.11 ± 10,087,063.36 | 0.003 | 7,158,414.81 ± 30,598,900.51 | 0.004 | 14,670,870.98 ± 46,375,971.76 | 0.033 | 69,632,435.75 ± 277,944,250.82 | <0.001 |
| FT (s) | 11.96 ± 5.84 | 16.15 ± 10.39 | 0.037 | 15.10 ± 8.90 | 0.076 | 14.38 ± 5.24 | 0.059 | 13.70 ± 7.66 | 0.014 |
| WoR [a.u] | 3,818,240.26 ± 13,233,643.32 | 95,771.36 ± 278,806.23 | 0.01 | 371,129.02 ± 1,685,672.00 | 0.006 | 809,402.23 ± 2,559,420.97 | 0.063 | 213,2147.39 ± 9,650,318.62 | <0.001 |
| WT + LN | vs. PA | p | vs. MT | p | |
|---|---|---|---|---|---|
| SWE | n = 45 | n = 21 | n = 26 | ||
| SWE (m/s) | 2.33 ± 0.95 | 2.94 ± 1.03 | 0.012 | 2.63 ± 1 | 0.086 |
| CEUS | n = 40 | n = 20 | n = 23 | ||
| MeanLin [a.u] | 1,655,586.27 ± 5,509,624.98 | 59,388.64 ± 175,556.44 | 0.008 | 121,719.86 ± 514,813.21 | 0.012 |
| PE [a.u] | 15,740,147.40 ± 56,030,655.45 | 540,672.11 ± 1,592,891.29 | 0.004 | 1,468,390.72 ± 6,490,545.51 | 0.012 |
| WiAUC [a.u] | 42,835,826.26 ± 145,685,272.30 | 13,08043.56 ± 3,927,811.86 | 0.008 | 3,135,246.66 ± 13,496,189.56 | 0.009 |
| RT (s) | 6.13 ± 2.39 | 6.51 ± 3.02 | 0.649 | 6.27 ±1.77 | 0.392 |
| mTTI (s) | 53.13 ± 59.39 | 76.74 ± 55.22 | 0.019 | 66.08 ± 68.81 | 0.732 |
| TTP (s) | 9.80 ± 2.79 | 9.01 ± 3.58 | 0.158 | 9.34 ± 2.08 | 0.92 |
| WiR [a.u] | 4,804,810.78 ± 17,614,431.92 | 190,810.89 ± 557,466.92 | 0.004 | 543,573.01 ± 2,446,514.59 | 0.013 |
| WiPI [a.u] | 9,660,280.10 ± 34,230,356.76 | 336,761.33 ± 992,297.43 | 0.004 | 896,490.65 ± 3,958,811.46 | 0.012 |
| WoAUC [a.u] | 61,313,661.69 ± 199,153,143.51 | 2,057,520.60 ± 6,158,753.67 | 0.010 | 4,023,168.72 ± 17,103,206.84 | 0.007 |
| WiWoAUC [a.u] | 105,247,832.72 ± 346,310,544.32 | 3,362,932.11 ± 10,087,063.36 | 0.006 | 7,158,414.81 ± 30,598,900.51 | 0.007 |
| FT (s) | 12.08 ± 5.48 | 16.15 ± 10.39 | 0.053 | 15.10 ± 8.90 | 0.107 |
| WoR [a.u] | 313,3745.45 ± 1,204,4372.37 | 95,771.36 ± 278,806.23 | <0.001 | 371,129.02 ± 1,685,672.00 | 0.01 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wakonig, K.M.; Lerchbaumer, M.H.; Dommerich, S.; Olze, H.; Hamm, B.; Fischer, T.; Arens, P. Assessment of Parotid Gland Tumors by Means of Quantitative Multiparametric Ultrasound (mpUS). Diagnostics 2023, 13, 12. https://doi.org/10.3390/diagnostics13010012
Wakonig KM, Lerchbaumer MH, Dommerich S, Olze H, Hamm B, Fischer T, Arens P. Assessment of Parotid Gland Tumors by Means of Quantitative Multiparametric Ultrasound (mpUS). Diagnostics. 2023; 13(1):12. https://doi.org/10.3390/diagnostics13010012
Chicago/Turabian StyleWakonig, Katharina Margherita, Markus Herbert Lerchbaumer, Steffen Dommerich, Heidi Olze, Bernd Hamm, Thomas Fischer, and Philipp Arens. 2023. "Assessment of Parotid Gland Tumors by Means of Quantitative Multiparametric Ultrasound (mpUS)" Diagnostics 13, no. 1: 12. https://doi.org/10.3390/diagnostics13010012






