The Feasibility of Interventional Pulmonology Methods for Detecting the T790M Mutation after the First or Second-Generation EGFR-TKI Resistance of Non-Small Cell Lung Cancer
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Patient Enrollment
2.2. Re-Biopsy Methods and EGFR T790M Mutation Testing
2.3. Statistical Analysis
3. Results
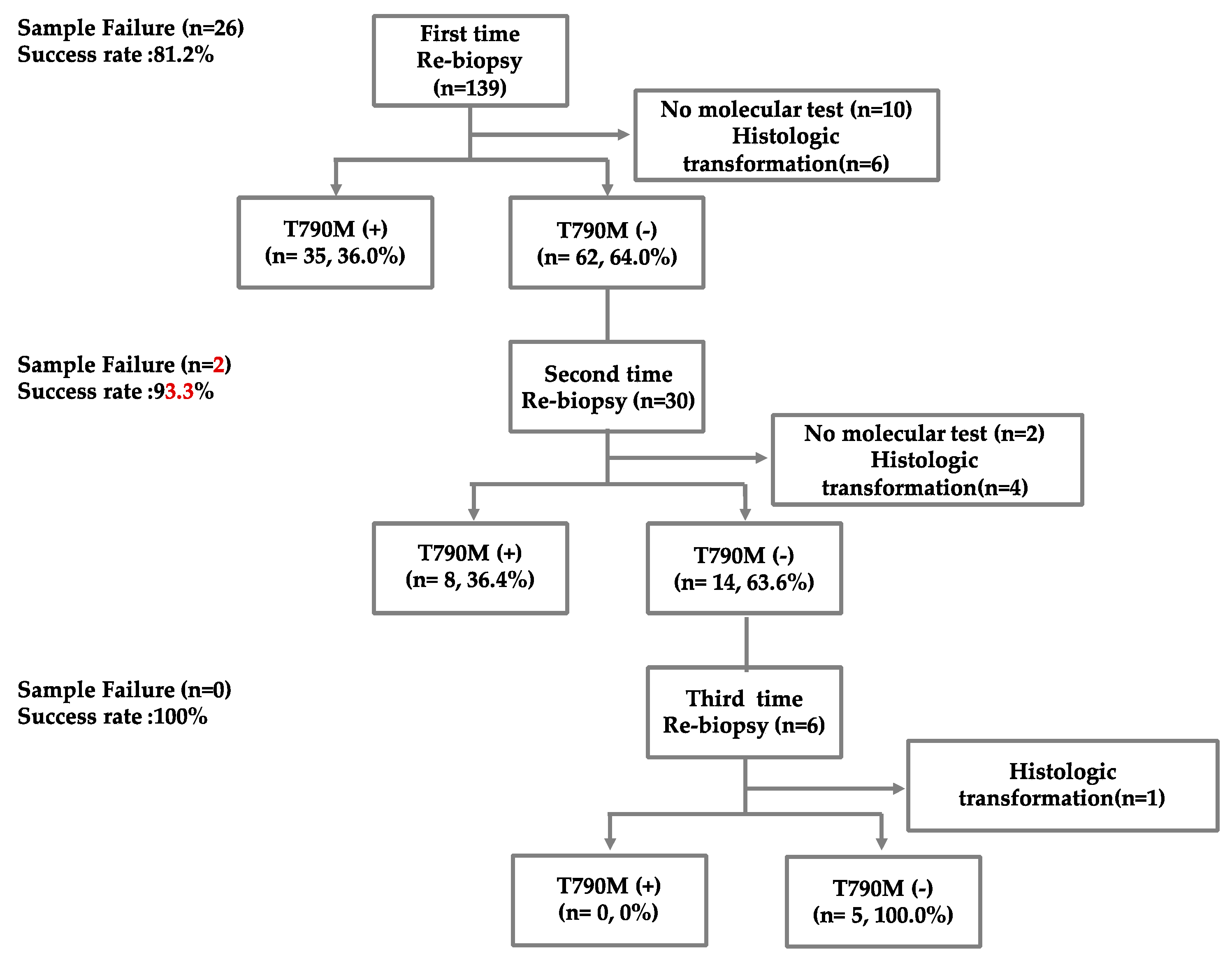
3.1. Patient’s Enrollment and Baseline Characteristics
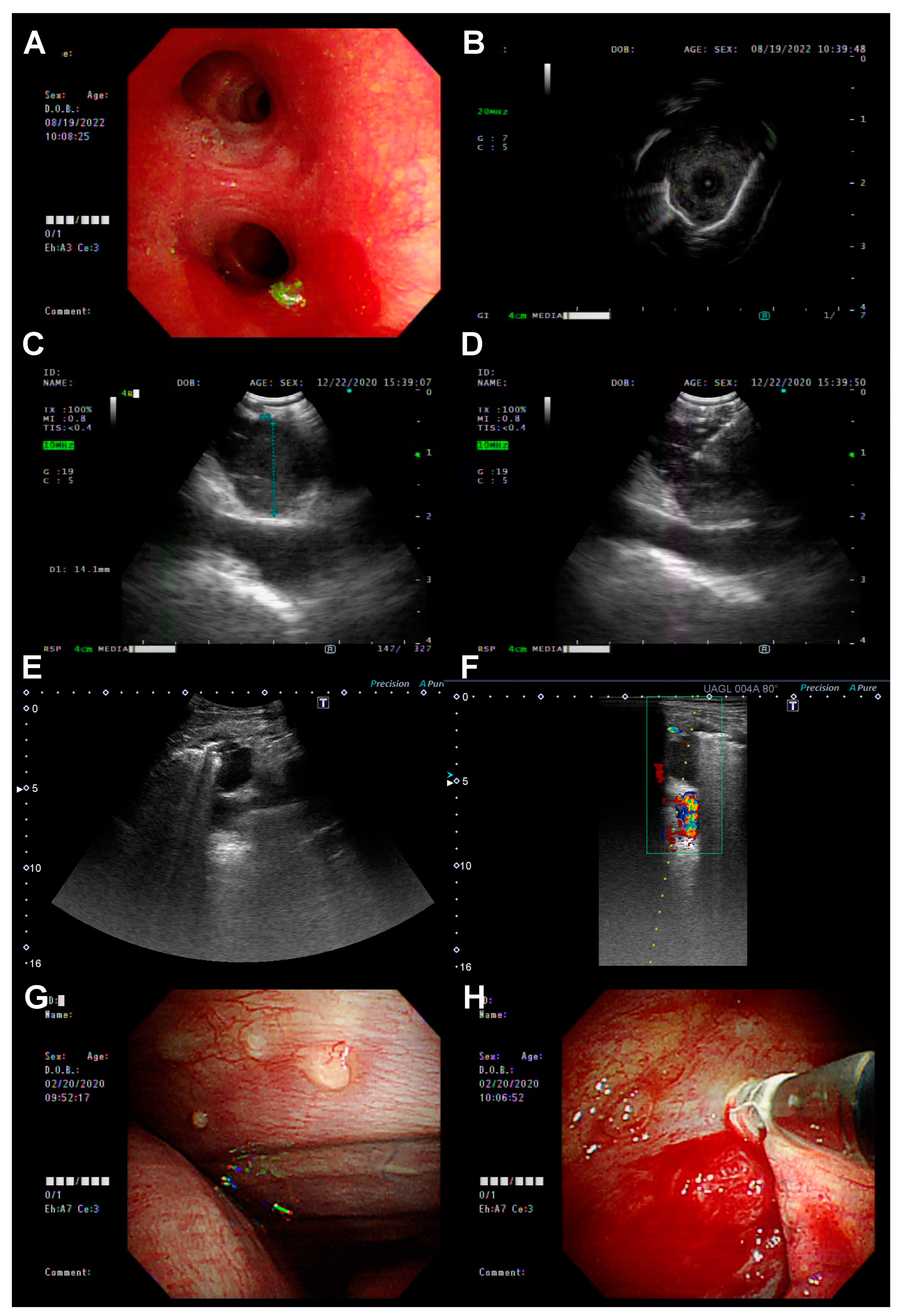
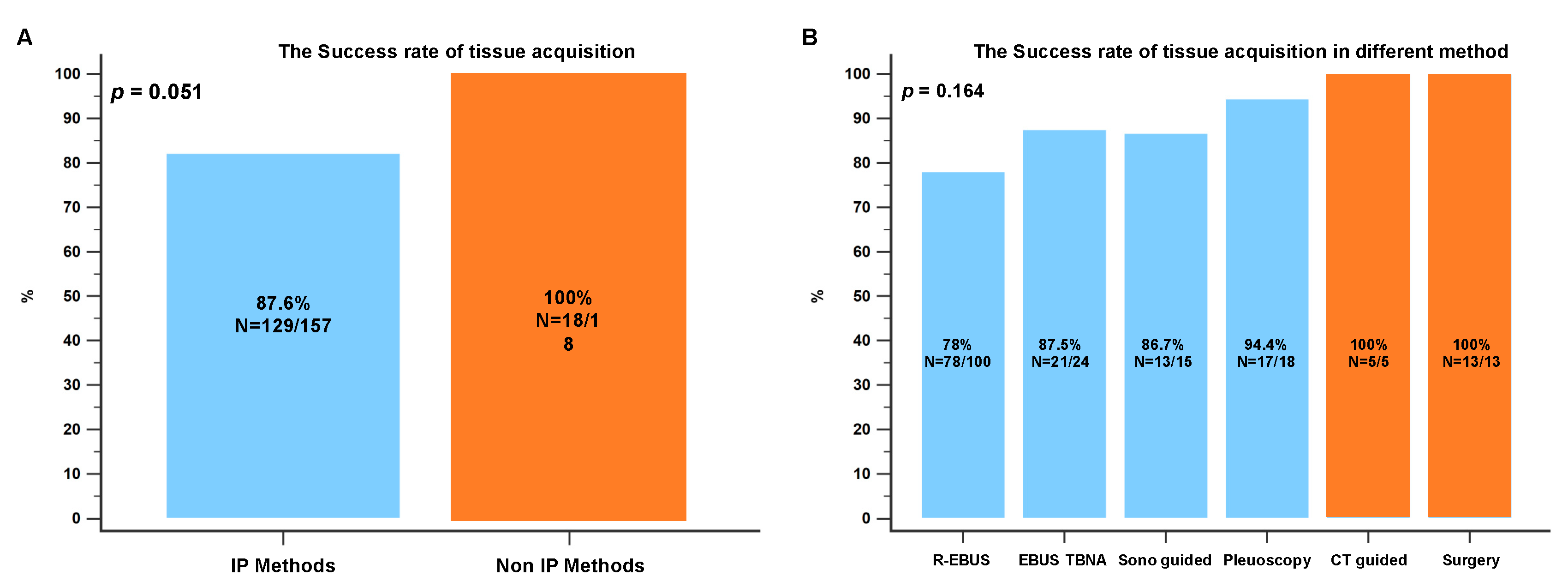
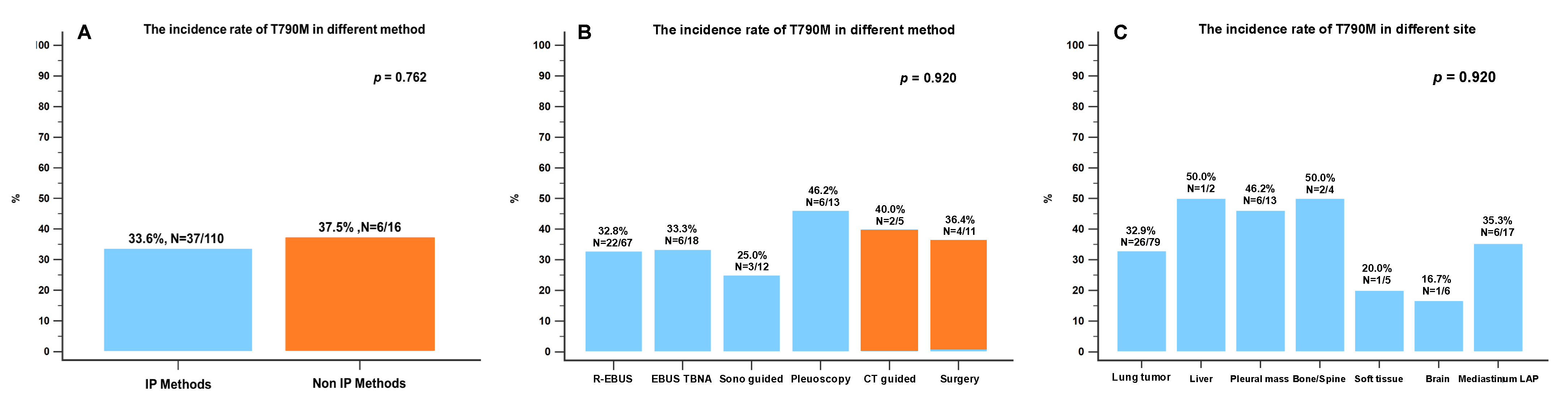
3.2. The Biopsy Tissue Assessment and Diagnosis by Different Biopsy Methods
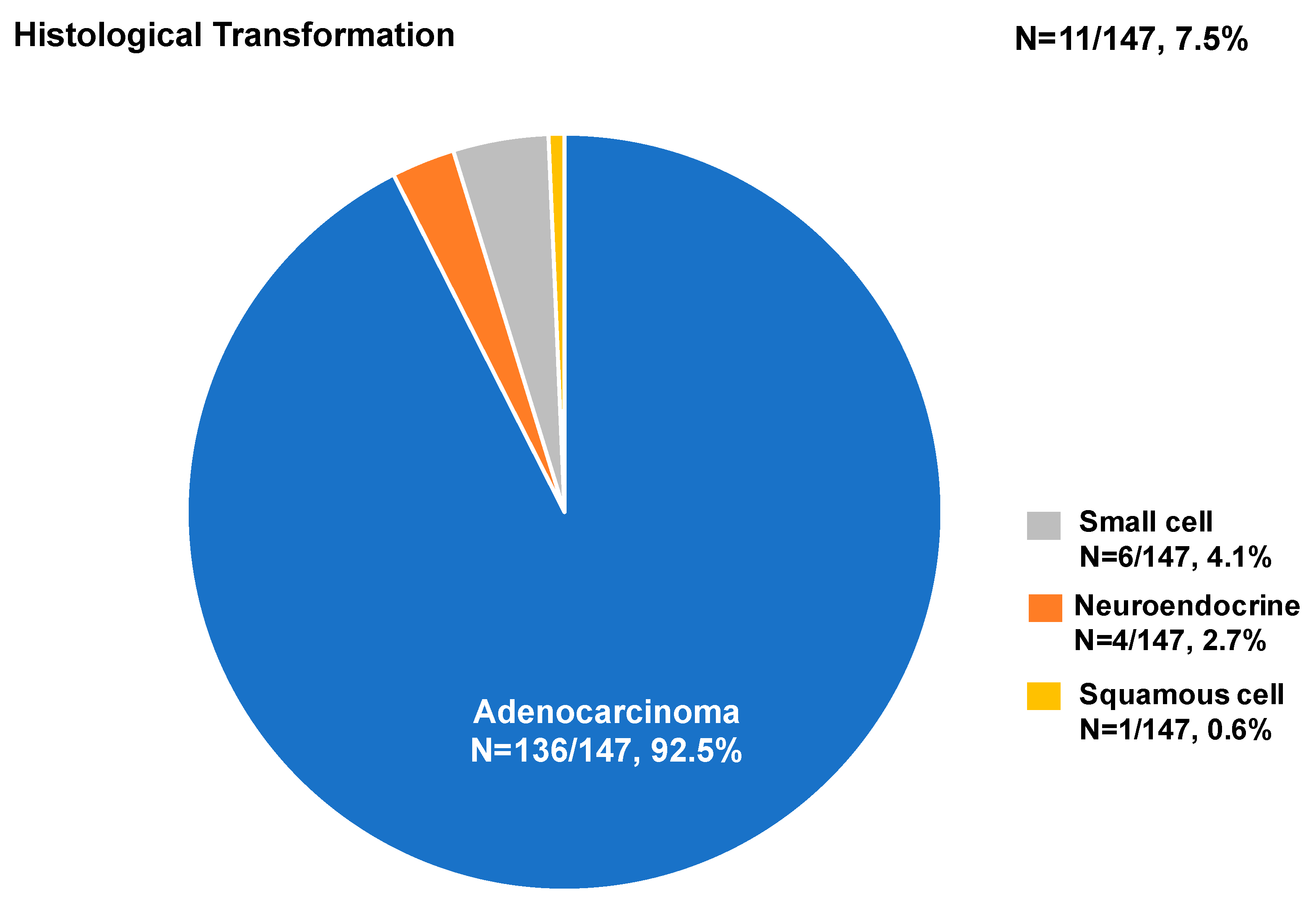
3.3. The Biopsy Tissue Molecular Analysis by Different Biopsy Methods
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fukuoka, M.; Wu, Y.L.; Thongprasert, S.; Sunpaweravong, P.; Leong, S.S.; Sriuranpong, V.; Chao, T.Y.; Nakagawa, K.; Chu, D.T.; Saijo, N.; et al. Biomarker analyses and final overall survival results from a phase III, randomized, open-label, first-line study of gefitinib versus carboplatin/paclitaxel in clinically selected patients with advanced non-small-cell lung cancer in Asia (IPASS). J. Clin. Oncol. 2011, 29, 2866–2874. [Google Scholar] [CrossRef]
- Rosell, R.; Carcereny, E.; Gervais, R.; Vergnenegre, A.; Massuti, B.; Felip, E.; Palmero, R.; Garcia-Gomez, R.; Pallares, C.; Sanchez, J.M.; et al. Erlotinib versus standard chemotherapy as first-line treatment for European patients with advanced EGFR mutation-positive non-small-cell lung cancer (EURTAC): A multicentre, open-label, randomised phase 3 trial. Lancet Oncol. 2012, 13, 239–246. [Google Scholar] [CrossRef]
- Schuler, M.; Paz-Ares, L.; Sequist, L.V.; Hirsh, V.; Lee, K.H.; Wu, Y.L.; Lu, S.; Zhou, C.; Feng, J.; Ellis, S.H.; et al. First-line afatinib for advanced EGFRm+ NSCLC: Analysis of long-term responders in the LUX-Lung 3, 6, and 7 trials. Lung Cancer 2019, 133, 10–19. [Google Scholar] [CrossRef]
- Wu, Y.L.; Cheng, Y.; Zhou, X.; Lee, K.H.; Nakagawa, K.; Niho, S.; Tsuji, F.; Linke, R.; Rosell, R.; Corral, J.; et al. Dacomitinib versus gefitinib as first-line treatment for patients with EGFR-mutation-positive non-small-cell lung cancer (ARCHER 1050): A randomised, open-label, phase 3 trial. Lancet Oncol. 2017, 18, 1454–1466. [Google Scholar] [CrossRef]
- Cho, B.C.; Chewaskulyong, B.; Lee, K.H.; Dechaphunkul, A.; Sriuranpong, V.; Imamura, F.; Nogami, N.; Kurata, T.; Okamoto, I.; Zhou, C.; et al. Osimertinib versus Standard of Care EGFR TKI as First-Line Treatment in Patients with EGFRm Advanced NSCLC: FLAURA Asian Subset. J. Thorac. Oncol. 2019, 14, 99–106. [Google Scholar] [CrossRef]
- Cross, D.A.; Ashton, S.E.; Ghiorghiu, S.; Eberlein, C.; Nebhan, C.A.; Spitzler, P.J.; Orme, J.P.; Finlay, M.R.; Ward, R.A.; Mellor, M.J.; et al. AZD9291, an irreversible EGFR TKI, overcomes T790M-mediated resistance to EGFR inhibitors in lung cancer. Cancer Discov. 2014, 4, 1046–1061. [Google Scholar] [CrossRef]
- Janne, P.A.; Yang, J.C.; Kim, D.W.; Planchard, D.; Ohe, Y.; Ramalingam, S.S.; Ahn, M.J.; Kim, S.W.; Su, W.C.; Horn, L.; et al. AZD9291 in EGFR inhibitor-resistant non-small-cell lung cancer. N. Engl. J. Med. 2015, 372, 1689–1699. [Google Scholar] [CrossRef]
- Hochmair, M.J.; Morabito, A.; Hao, D.; Yang, C.T.; Soo, R.A.; Yang, J.C.; Gucalp, R.; Halmos, B.; Wang, L.; Marten, A.; et al. Sequential afatinib and osimertinib in patients with EGFR mutation-positive non-small-cell lung cancer: Updated analysis of the observational GioTag study. Futur. Oncol. 2019, 15, 2905–2914. [Google Scholar] [CrossRef]
- Popat, S.; Jung, H.A.; Lee, S.Y.; Hochmair, M.J.; Lee, S.H.; Escriu, C.; Lee, M.K.; Migliorino, M.R.; Lee, Y.C.; Girard, N.; et al. Sequential afatinib and osimertinib in patients with EGFR mutation-positive NSCLC and acquired T790M: A global non-interventional study (UpSwinG). Lung Cancer 2021, 162, 9–15. [Google Scholar] [CrossRef]
- Zhou, J.; Zhao, C.; Zhao, J.; Wang, Q.; Chu, X.; Li, J.; Zhou, F.; Ren, S.; Li, X.; Su, C.; et al. Re-biopsy and liquid biopsy for patients with non-small cell lung cancer after EGFR-tyrosine kinase inhibitor failure. Thorac. Cancer 2019, 10, 957–965. [Google Scholar] [CrossRef]
- Cescon, D.W.; Bratman, S.V.; Chan, S.M.; Siu, L.L. Circulating tumor DNA and liquid biopsy in oncology. Nat. Cancer 2020, 1, 276–290. [Google Scholar] [CrossRef]
- Bencze, E.; Bogos, K.; Kohánka, A.; Báthory-Fülöp, L.; Sárosi, V.; Csernák, E.; Bittner, N.; Melegh, Z.; Tóth, E. EGFR T790M Mutation Detection in Patients With Non-Small Cell Lung Cancer After First Line EGFR TKI Therapy: Summary of Results in a Three-Year Period and a Comparison of Commercially Available Detection Kits. Pathol. Oncol. Res. 2022, 28, 1610607. [Google Scholar] [CrossRef]
- Pathak, R.; Villaflor, V.M. Histologic Transformation in EGFR-Mutant Lung Adenocarcinomas: Mechanisms and Therapeutic Implications. Cancers 2021, 13, 4641. [Google Scholar] [CrossRef]
- French, K.D.; Desai, N.R.; Diamond, E.; Kovitz, K.L. Developing an Interventional Pulmonary Service in a Community-Based Private Practice: A Case Study. Chest 2016, 149, 1094–1101. [Google Scholar] [CrossRef]
- Kirita, K.; Izumo, T.; Matsumoto, Y.; Hiraishi, Y.; Tsuchida, T. Bronchoscopic Re-biopsy for Mutational Analysis of Non-small Cell Lung Cancer. Lung 2016, 194, 371–378. [Google Scholar] [CrossRef]
- Goag, E.K.; Lee, J.M.; Chung, K.S.; Kim, S.Y.; Leem, A.Y.; Song, J.H.; Jung, J.Y.; Park, M.S.; Chang, Y.S.; Kim, Y.S.; et al. Usefulness of Bronchoscopic Rebiopsy of Non-Small Cell Lung Cancer with Acquired Resistance to Epidermal Growth Factor Receptor-Tyrosine Kinase Inhibitor. J. Cancer 2018, 9, 1113–1120. [Google Scholar] [CrossRef]
- Izumo, T.; Matsumoto, Y.; Chavez, C.; Tsuchida, T. Re-biopsy by endobronchial ultrasound procedures for mutation analysis of non-small cell lung cancer after EGFR tyrosine kinase inhibitor treatment. BMC Pulm. Med. 2016, 16, 106. [Google Scholar] [CrossRef]
- Li, Q.; Zhang, L.; Liao, X.; Tang, S.; Li, Z. Ultrasound-guided percutaneous needle biopsies of peripheral pulmonary lesions: Diagnostic efficacy and risk factors for diagnostic failure. Ann. Palliat. Med. 2021, 10, 9772–9783. [Google Scholar] [CrossRef]
- Hamaji, M.; Motoyama, H.; Menju, T.; Chen-Yoshikawa, T.F.; Sonobe, M.; Kim, Y.H.; Date, H. Thoracoscopic rebiopsy to detect the T790M mutation after postoperative recurrence. Interact. Cardiovasc. Thorac. Surg. 2018, 27, 606–608. [Google Scholar] [CrossRef]
- Matsuda, A.; Fuchimoto, Y.; Wada, S.; Tanaka, T.; Takeguchi, T.; Mitsumune, S.; Takigawa, Y.; Miyamoto, Y.; Ozaki, S.; Iga, N.; et al. Rebiopsy with Thoracoscopy under Local Anesthesia for the Detection of EGFR T790M Mutation. Case Rep. Oncol. 2019, 12, 918–921. [Google Scholar] [CrossRef]
- Chen, C.H.; Liao, W.C.; Wu, B.R.; Chen, C.Y.; Chen, W.C.; Hsia, T.C.; Cheng, W.C.; Tu, C.Y.; Hsu, W.H. Endobronchial Ultrasound Changed the World of Lung Cancer Patients: A 11-Year Institutional Experience. PLoS ONE 2015, 10, e0142336. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Hata, A.; Katakami, N.; Yoshioka, H.; Takeshita, J.; Tanaka, K.; Nanjo, S.; Fujita, S.; Kaji, R.; Imai, Y.; Monden, K.; et al. Rebiopsy of non-small cell lung cancer patients with acquired resistance to epidermal growth factor receptor-tyrosine kinase inhibitor: Comparison between T790M mutation-positive and mutation-negative populations. Cancer 2013, 119, 4325–4332. [Google Scholar] [CrossRef] [PubMed]
- Bosc, C.; Ferretti, G.R.; Cadranel, J.; Audigier-Valette, C.; Besse, B.; Barlesi, F.; Decroisette, C.; Lantuejoul, S.; Arbib, F.; Moro-Sibilot, D. Rebiopsy during disease progression in patients treated by TKI for oncogene-addicted NSCLC. Target. Oncol. 2015, 10, 247–253. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Kang, H.J.; Moon, S.H.; Lee, J.M.; Kim, H.Y.; Lee, G.K.; Lee, J.S.; Hwangbo, B. Endobronchial Ultrasound-Guided Transbronchial Needle Aspiration for Re-biopsy in Previously Treated Lung Cancer. Cancer Res. Treat. 2019, 51, 1488–1499. [Google Scholar] [CrossRef]
- Yoon, H.J.; Lee, H.Y.; Lee, K.S.; Choi, Y.L.; Ahn, M.J.; Park, K.; Ahn, J.S.; Sun, J.M.; Kim, J.; Kim, T.S.; et al. Repeat biopsy for mutational analysis of non-small cell lung cancers resistant to previous chemotherapy: Adequacy and complications. Radiology 2012, 265, 939–948. [Google Scholar] [CrossRef]
- Kim, H.; Chae, K.J.; Yoon, S.H.; Kim, M.; Keam, B.; Kim, T.M.; Kim, D.W.; Goo, J.M.; Park, C.M. Repeat biopsy of patients with acquired resistance to EGFR TKIs: Implications of biopsy-related factors on T790M mutation detection. Eur. Radiol. 2018, 28, 861–868. [Google Scholar] [CrossRef]
- Poudel, B.; Desman, J.; Aihara, G.; Weidman, D.I.; Tsang, A.; Kovrizhkin, K.; Pereira, T.; Arun, S.; Pradeep, T.; Matin, S.; et al. Adequacy of samples obtained via percutaneous core-needle rebiopsy for EGFR T790M molecular analysis in patients with non-small cell lung cancer following acquired resistance to first-line therapy: A systematic review and meta-analysis. Cancer Treat. Res. Commun. 2021, 29, 100470. [Google Scholar] [CrossRef]
- Wu, S.G.; Chiang, C.L.; Liu, C.Y.; Wang, C.C.; Su, P.L.; Hsia, T.C.; Shih, J.Y.; Chang, G.C. An Observational Study of Acquired EGFR T790M-Dependent Resistance to EGFR-TKI Treatment in Lung Adenocarcinoma Patients in Taiwan. Front. Oncol. 2020, 10, 1481. [Google Scholar] [CrossRef]
- Hong, M.H.; Kim, H.R.; Ahn, B.C.; Heo, S.J.; Kim, J.H.; Cho, B.C. Real-World Analysis of the Efficacy of Rebiopsy and EGFR Mutation Test of Tissue and Plasma Samples in Drug-Resistant Non-Small Cell Lung Cancer. Yonsei Med. J. 2019, 60, 525–534. [Google Scholar] [CrossRef]
- Kudo, K.; Nishii, K.; Makimoto, G.; Ishikawa, N.; Tsubata, Y.; Kodani, M.; Fujimoto, N.; Yamasaki, M.; Kubota, T.; Takigawa, N.; et al. First and repeat rebiopsy for detecting EGFR T790M mutation in non-small-cell lung cancer: CS-Lung-003 prospective observational registry study. J. Cancer Res. Clin. Oncol. 2022, 148, 1869–1877. [Google Scholar] [CrossRef]
- Wu, S.G.; Shih, J.Y. Management of acquired resistance to EGFR TKI-targeted therapy in advanced non-small cell lung cancer. Mol. Cancer 2018, 17, 38. [Google Scholar] [CrossRef] [PubMed]
- Fujimoto, D.; Akamatsu, H.; Morimoto, T.; Wakuda, K.; Sato, Y.; Kawa, Y.; Yokoyama, T.; Tamiya, M.; Hiraoka, R.; Shingu, N.; et al. Histologic transformation of epidermal growth factor receptor-mutated lung cancer. Eur. J. Cancer 2022, 166, 41–50. [Google Scholar] [CrossRef] [PubMed]
- Pereira, I.; Gaspar, C.; Pina, M.; Azevedo, I.; Rodrigues, A. Real-World T790M Mutation Frequency and Impact of Rebiopsy in Patients With EGFR-Mutated Advanced Non-Small Cell Lung Cancer. Cureus 2020, 12, e12128. [Google Scholar] [CrossRef] [PubMed]
- Ninomaru, T.; Hata, A.; Kokan, C.; Okada, H.; Tomimatsu, H.; Ishida, J. Higher osimertinib introduction rate achieved by multiple repeated rebiopsy after acquired resistance to first/second generation EGFR-TKIs. Thorac. Cancer 2021, 12, 746–751. [Google Scholar] [CrossRef]
- Lee, K.; Kim, Y.; Jung, H.A.; Lee, S.H.; Ahn, J.S.; Ahn, M.J.; Park, K.; Choi, Y.L.; Sun, J.M. Repeat biopsy procedures and T790M rates after afatinib, gefitinib, or erlotinib therapy in patients with lung cancer. Lung Cancer 2019, 130, 87–92. [Google Scholar] [CrossRef]
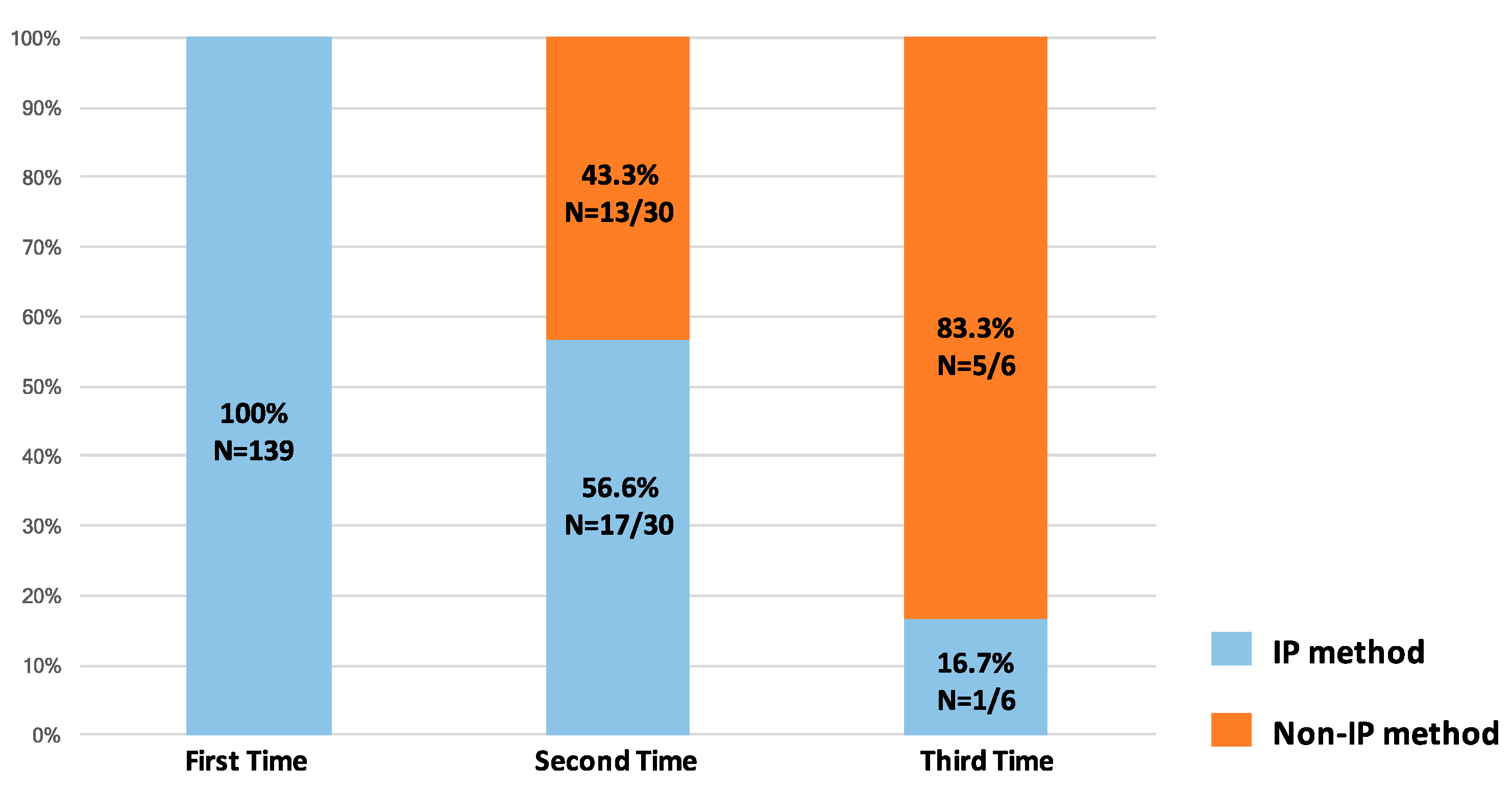
| All (n = 139) | |
|---|---|
| Age, year (SD) | 64.2 (10.4) |
| Male (%) | 43 (30.9) |
| Smoking (%) | 29 (20.9) |
| ECOG PS ≥ 2 (%) | 7 (5.0) |
| EGFR mutation (%) | |
| Del 19 | 67 (48.2) |
| L858R | 65 (46.8) |
| Other | 7 (5.1) |
| Stage | |
| IIIA | 3(2.2) |
| IIIB | 8 (5.8) |
| IIIC | 2 (1.4) |
| IVA | 55 (39.6) |
| IVB | 61 (43.9) |
| Post-OP recurrence | 10 (7.2) |
| EGFR-TKI | |
| Gefitinib | 34 (24.5) |
| Erlotinib | 48 (34.5) |
| Afatinib | 54 (38.8) |
| Other | 3 (2.2) |
| Time to re-biopsy, month (95% CI) | 18.6 (16.5–20.9) |
| IP Methods (n = 157) | Non-IP Methods (n = 18) | p-Value | |
|---|---|---|---|
| Biopsy method | <0.001 | ||
| Bronchoscopy | 100 (57.1) | 0 (0) | |
| EBUS-TBNA | 24 (13.7) | 0 (0) | |
| Ultrasound-guided | 15 (8.6) | 0 (0) | |
| Medical pleuroscopy | 18 (10.3) | 0 (0) | |
| CT-guided biopsy | 0 (0) | 5 (2.9) | |
| Surgery | 0 (0) | 13 (7.4) | |
| Time to re-biopsy, months | 18.1 (11.8–30.1) | 21.6 (16.0–20.4) | 0.601 |
| Biopsy site | 0.009 | ||
| Primary site | 109 (69.4) | 7 (38.9) | |
| Metastatic site | 48 (30.6) | 11 (61.1) | |
| Biopsy organ | <0.001 | ||
| Lung mass | 108 (93.9) | 7 (6.1) | |
| Liver | 4 (100) | 0 (0) | |
| Pleural mass | 18 (100) | 0 (0) | |
| Spine/bone | 0 (0) | 4 (100) | |
| Soft tissue | 6 (100) | 0 (0) | |
| Brain tumor | 0 (0) | 7 (100) | |
| Mediastinum lymph node | 21 (100) | 0 (0) | |
| Histology result | 0.099 | ||
| Adenocarcinoma | 121 (77.1) | 15 (83) | |
| Neuroendocrine | 3 (1.9) | 1 (5.6) | |
| Small-cell carcinoma | 4 (2.5) | 2 (11.1) | |
| Squamous cell carcinoma | 1 (0.6) | 0 (0) | |
| No malignancy | 28 (17.8) | 0 (0) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cheng, W.-C.; Shen, Y.-C.; Chen, C.-L.; Liao, W.-C.; Chen, H.-J.; Hsia, T.-C.; Chen, C.-H.; Tu, C.-Y. The Feasibility of Interventional Pulmonology Methods for Detecting the T790M Mutation after the First or Second-Generation EGFR-TKI Resistance of Non-Small Cell Lung Cancer. Diagnostics 2023, 13, 129. https://doi.org/10.3390/diagnostics13010129
Cheng W-C, Shen Y-C, Chen C-L, Liao W-C, Chen H-J, Hsia T-C, Chen C-H, Tu C-Y. The Feasibility of Interventional Pulmonology Methods for Detecting the T790M Mutation after the First or Second-Generation EGFR-TKI Resistance of Non-Small Cell Lung Cancer. Diagnostics. 2023; 13(1):129. https://doi.org/10.3390/diagnostics13010129
Chicago/Turabian StyleCheng, Wen-Chien, Yi-Cheng Shen, Chieh-Lung Chen, Wei-Chih Liao, Hung-Jen Chen, Te-Chun Hsia, Chia-Hung Chen, and Chih-Yen Tu. 2023. "The Feasibility of Interventional Pulmonology Methods for Detecting the T790M Mutation after the First or Second-Generation EGFR-TKI Resistance of Non-Small Cell Lung Cancer" Diagnostics 13, no. 1: 129. https://doi.org/10.3390/diagnostics13010129
APA StyleCheng, W.-C., Shen, Y.-C., Chen, C.-L., Liao, W.-C., Chen, H.-J., Hsia, T.-C., Chen, C.-H., & Tu, C.-Y. (2023). The Feasibility of Interventional Pulmonology Methods for Detecting the T790M Mutation after the First or Second-Generation EGFR-TKI Resistance of Non-Small Cell Lung Cancer. Diagnostics, 13(1), 129. https://doi.org/10.3390/diagnostics13010129







