Abstract
Colon capsule endoscopy (CCE) is a promising modality for colonic investigations, but completion rates (CR) and adequate cleansing rates (ACR) must be improved to meet established standards for optical colonoscopy. Improvements should be made with patient acceptability in mind. We aimed to compare a very low-volume polyethylene glycol (PEG) laxative to a conventional high-volume laxative. We carried out a single-center retrospective comparative cohort study including patients referred for CCE. One hundred and sixty-six patients were included in the final analysis, with eighty-three patients in each group. We found a CR and ACR of 77% and 67% in the high-volume group and 72% and 75% in the very low-volume group, respectively. In the high-volume group, 54% had complete transit and adequate cleansing, whereas this was the case for 63% in the very low-volume group. No statistically significant difference in CR, ACR, or a combination of the two was found. A very low-volume bowel preparation regimen was non-inferior to a high-volume regimen before CCE in terms of CR and ACR.
1. Introduction
Colon capsule endoscopy (CCE) is a promising modality for lower gastrointestinal (GI) investigations in both clinical routine and screening [1,2,3,4,5,6,7,8,9,10]. Furthermore, the double-headed camera capsules are being applied for panenteric investigations, with promising results [11,12,13,14,15,16]. To achieve viable wider CCE adoption, challenges regarding completion rates (CR) and adequate cleanliness rates (ACR) must be handled [17,18]. CR and ACR should be improved to meet the standards for optical colonoscopy (OC) from the European Society of GI Endoscopy (ESGE). ESGE recommends both CR and ACR ≥ 90% [19]. Recently, a meta-analysis of preparation regimens for CCE confirmed that CR and ACR were suboptimal [20]. Although polyethylene glycol (PEG)-based laxatives and sodium phosphate (NaP)-based boosters were the most commonly used, the combination did not lead to higher CR or ACR. A meta-analysis exploring patient preferences for CCE and OC found that procedural adverse events with CCE were rare, the tolerability was high for both CCE and OC, and patient preferences for the two procedures did not differ significantly even though the tolerability for CCE was rated higher than for OC [21]. The results of the meta-analysis are based on preference for the entire pathway and patients undergoing only one of the examinations were excluded. The lack of polypectomy and biopsy capabilities in CCE and the risk of a second round of bowel preparation may sway the vote in favor of OC for patients with pathology where therapeutic procedures are needed. Bowel cleansing is known to be an obstacle to patient compliance with endoscopic procedures. A very low-volume laxative was developed to pursue a more tolerable cleansing regimen. So far, this regimen was only studied for bowel preparation before colonoscopy [22,23,24,25,26]. Therefore, this study aimed to investigate the CR, ACR, and diagnostic yield (DY) of a very low-volume PEG-based laxative compared to a conventional high-volume laxative.
2. Materials and Methods
We conducted a retrospective comparative cohort study. All consecutive patients referred to clinical CCE at Skåne University Hospital, Malmö, in the 3 years from July 2019 to June 2022, were included. According to the clinical protocol, patients under the age of 18 and patients with swallowing difficulties or previous intestinal resection were not eligible for CCE. We used the PillCam® Crohn’s capsule (PCC) (Medtronic, Minneapolis, MN, USA) for all investigations. The PCC is used in clinical practice at Skåne University Hospital to enable panintestinal investigation, as a large proportion of the population referred to endoscopy at the department are patients with known or suspected inflammatory bowel disease (IBD). All patients were treated as day cases. The patient ingested only the first dose of laxative at home without supervision. A nurse with experience in CCE supervised the remaining steps in the procedure (Table 1). Patients stayed at the clinic until capsule excretion or until 4 pm.

Table 1.
Bowel preparation regimens.
2.1. Bowel Preparation
The same protocol was followed for all patients except for the laxatives used (Table 1). The high-volume group received 4 L of PEG solution (Laxabon®, Karo Pharma AB, Stockholm, Sweden). In contrast, the very low-volume group received 1 L PEG solution with ascorbic acid (PEG-ASC) (Plenvu®, Norgine, Amsterdam, The Netherlands) and 2 L of additional clear liquids. Both are taken in split doses. Sodium phosphate (Phosphoral, Recordati AB, Kista, Sweden) was used as a booster for all. If the capsule was not passed from the stomach to the small bowel (SB) within two hours from ingestion, it was endoscopically delivered to the duodenum.
2.2. CCE Evaluation
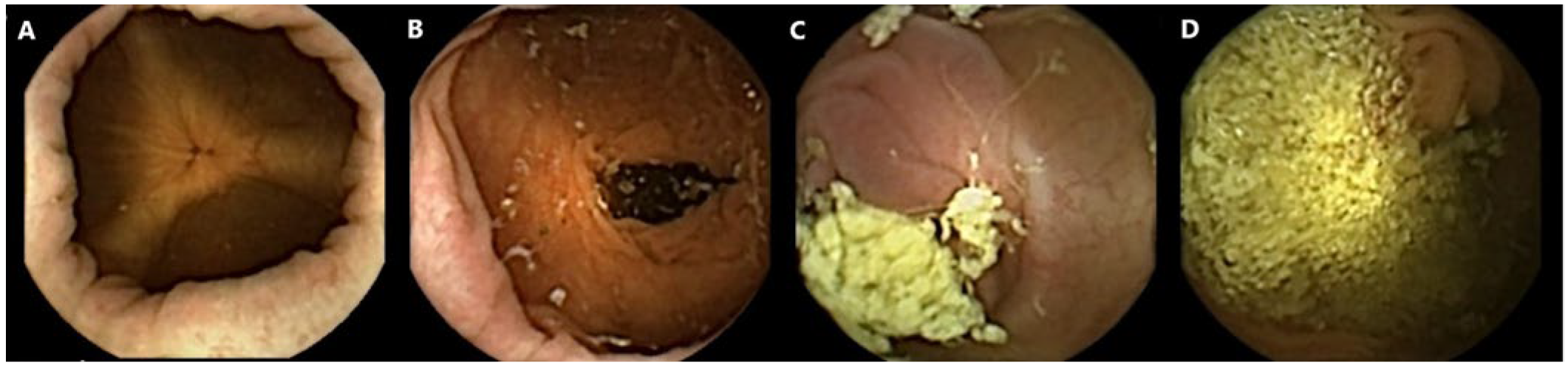
All capsule videos were analyzed using PillCam™ reader software v9 (Given Imaging Inc., Duluth, GA, USA). A gastroenterologist with extensive experience in SB capsule endoscopy (CE) and CCE evaluated cleanliness on the 4-point Leighton–Rex scale [27]. The grades excellent and good were considered adequate, while the grades fair and poor were considered inadequate (Figure 1). The reader was blinded to the bowel preparation regimen used. Complete CCE was defined as visualization of the hemorrhoidal plexus or an excreted capsule. The transit times were calculated for the stomach, SB, and colon separately by PillCam™ reader software.
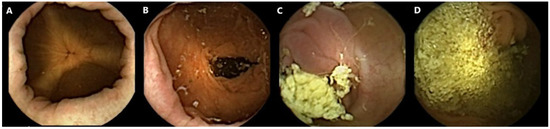
Figure 1.
Examples of colon capsule video frames graded according to the Leighton–Rex scale. (A) Excellent. (B) Good. (C) Fair. (D) Poor.
2.3. Statistical Analysis
Baseline characteristics were compared between the high-volume and very low-volume groups, including age, sex, BMI, prior colonoscopy, and CCE indication. CE performance (completion, cleansing grade, retention, and transit times) and DY were additionally compared between the groups. DY and performance measures (except CR) were stratified by colon and SB. Transit time was additionally reported for the stomach. As the continuous variables were not normally distributed, the non-parametric Wilcoxon rank sum test was used for comparison. Categorical variables were compared using the chi2 test. Ordinal logistic regression models were conducted testing predictors of prolonged transit times (divided by 33rd and 67th percentiles), including age, sex, BMI, laxative, and indication for CE in the models. Data management and statistical analysis were conducted in SAS 9.4 (SAS, Gary, NC, USA).
3. Results
Study Population
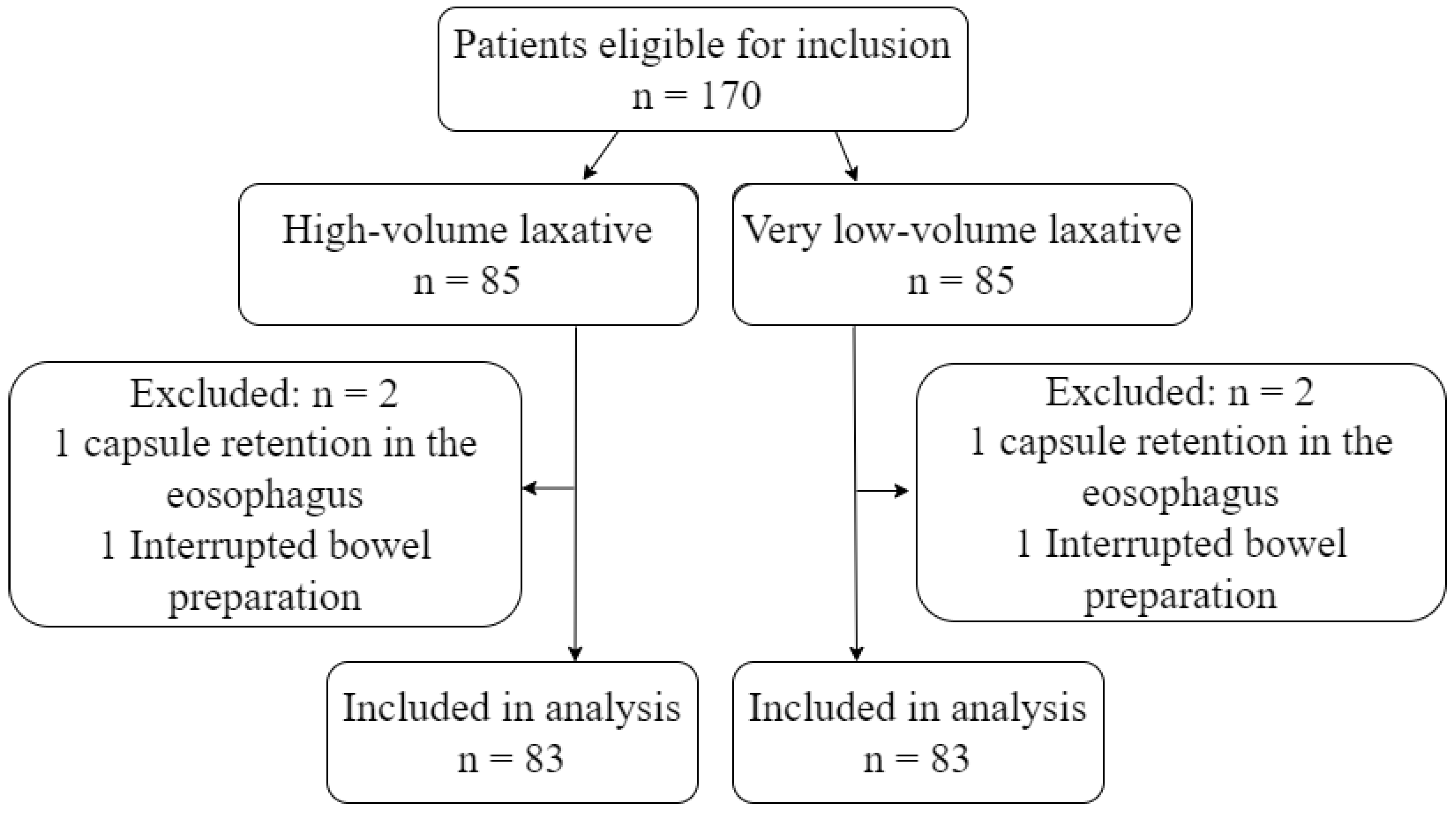
During the study period, 170 eligible patients underwent CCE. Four patients were excluded due to capsule retention in the esophagus or interruption in the bowel preparation phase (Figure 2). The number of excluded patients and the reason for exclusion was identical in the two groups. Patients were between 19 and 87 years old and were referred based on varying indications. The most common referral indication was known or suspected IBD, followed by overt and occult GI bleeding. The remaining 20% of patients were referred due to abdominal pain/diverticulitis, colon cancer screening, and weight loss (Table 2).
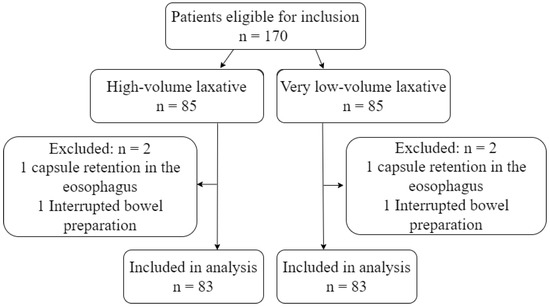
Figure 2.
Flowchart.

Table 2.
Baseline information.
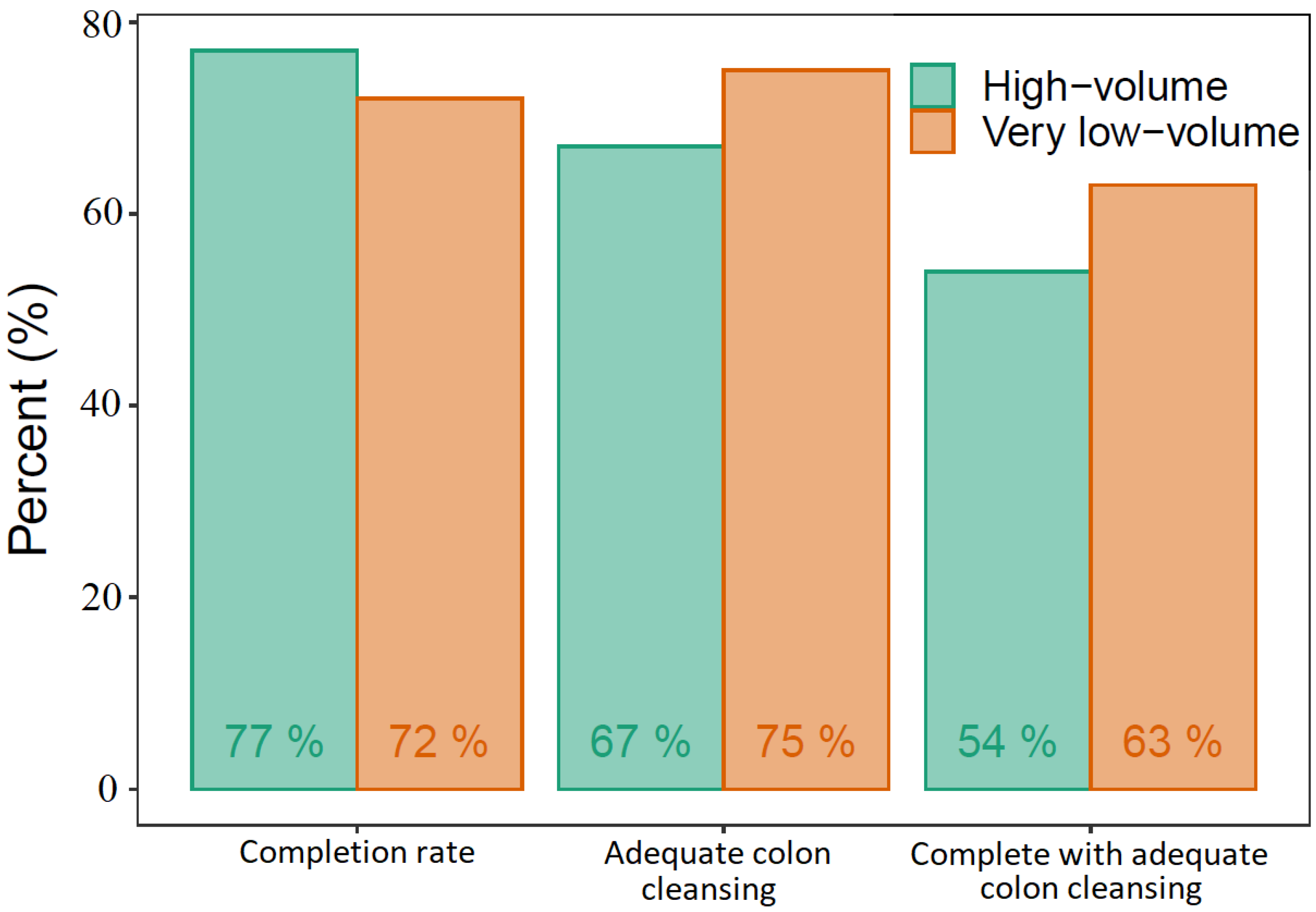
We found a CR and colonic ACR of 77% and 67%, respectively, in the high-volume group and 72% and 75% in the very low-volume group. In the high-volume group, 54% had complete transit with adequate colon cleansing, whereas this was the case for 63% in the very low-volume group (Figure 3). The differences between the two groups regarding CR, colonic ACR, or a combination of the two were not statistically significant. The only statistically significant difference was the stomach transit time, longer in the very low-volume group (Table 3). The DY was similar in the two groups, 59% in the high-volume group and 66% in the very low-volume group (Table 4). Ordinal logistic regression models found that increased age increased the total transit time whereas very low volume PEG increased the stomach transit time, adjusted for BMI, sex, and indication for CE. No significant adverse events requiring medical intervention or hospitalization occurred in either of the study groups.
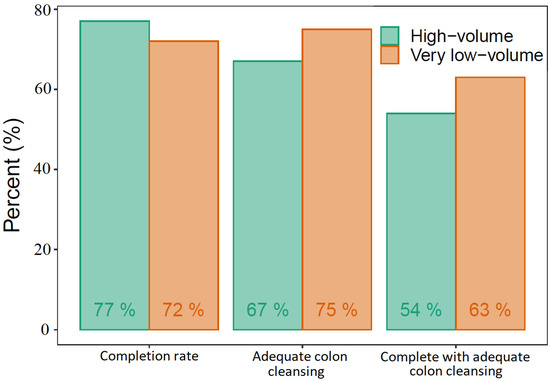
Figure 3.
Capsule endoscopy performance.

Table 3.
Capsule endoscopy performance.

Table 4.
Capsule endoscopy outcome.
4. Discussion
Our results show that a very low-volume bowel cleansing regimen before CCE performs equal to a high-volume regimen. We found no difference between the two groups except for a longer stomach transit time in the very low-volume group. As per the clinical routine at Skåne University Hospital, a gastroscopy was performed to deliver the capsule to the small bowel if it did not progress from the stomach within two hours. Considering this, stomach transit times could potentially be longer, with possible segmental delay or even stomach retention as a result.
The recent European introduction of a very low-volume PEG bowel preparation for OC prompted comparisons to established low- and high-volume regimens. So far, the very low-volume preparation shows to be at least non-inferior to both 2 L PEG-ASC and 4 L PEG solutions, with favorable patient experience, tolerability, and high adherence [22,23,24,25,26].
A recently published Swedish study on bowel preparation before OC showed that vomiting was more frequent in patients who received the very low-volume regimen [22]. Clinically, this could be a symptom of gastric transit delay, which is in line with our findings. The high dose of ascorbic acid in the very low-volume laxative (7.54 g) [28] is likely a large contributor to the GI symptoms. High oral doses of ascorbic acid is associated with GI symptoms such as abdominal pain, bloating and transient osmotic diarrhea [29]. The hyper-osmolality created by the ascorbic acid delivered in the low volume of the laxative solution facilitates bowel cleansing [30].
The results from the Swedish study showed that despite the vomiting associated with the very low-volume regimen, the overall experience reported by the patients was better than in the high-volume group [22]. Similar results for tolerability were found in an Italian study comparing high and very low-volume PEG regimens [23].
Our study showed a tendency towards higher small bowel and colon ACRs in the very low-volume group despite arithmetically lower CR. Considering this, choosing a different booster than the NaP-based booster could be a part of the solution. The results from a recent meta-analysis on bowel preparation for CCE suggest that adding the booster gastrografin could improve the CR [20]. The prokinetic prucalopride is also a promising option, as shown in a recent Danish study, where both CR and ACR were significantly improved with the addition of prucalopride to the booster regimen [31].
The 1 L PEG-ASC laxative is classified as a very low-volume laxative with only 1 L of active substance compared to 4 L in the high-volume regimens. Even though the laxative volume is 1 L, the total liquid volume is only 1 L less than in the high-volume regimens when adding the additional liquid recommended. However, it might be easier for the patients to accept the intake of additional fluids instead of the laxative solution, leading to the higher tolerability reported for 1 L-PEG-ASC compared to high-volume laxatives [22,23]. The additional liquid intake seems essential in improving CR. A study found that a water intake of ≥12 mL per minute during the CCE was an independent predictor for a complete CCE [32].
In some countries, CCE is already a part of the clinical practice. In March 2021, NHS England announced that CCE would be offered instead of OC to certain patient cohorts with low-risk colonic symptoms. Across NHS health boards in Scotland, CCE was implemented as a supplement to OC [33]. It was introduced as part of the ScotCap study, which found that CCE is both safe and well tolerated and that the proportion of patients in need of OC can be reduced. However, the Scottish team did find a need for improvement as the CR and ACR in their cohort were below 80%. Real-world data from Denmark and France confirm that outside the scope of academic trials, improvement is needed to increase the reliability of CCE [34,35]. Less than half of the French sample CCEs were considered optimal, i.e., complete with adequate bowel cleansing and in the Danish introduction of CCE in patients with incomplete colonoscopy, a CR of 60% and ACR of 54% was achieved, while only 40% had complete CCE with adequate bowel cleansing.
The present study is, to our knowledge, the first one examining the very low-volume bowel preparation in CCE specifically. Often it is difficult to report on patient adherence to the bowel preparation, as the data are often self-reported, introducing the risk of recall bias. In our study, dedicated endoscopy nurses supervised the bowel preparation (except for the first dose of laxatives), thereby controlling patients’ adherence to the protocol. CCE faces the challenge of delivering results on ACR comparable to OC, which is currently the gold standard for investigating colonic symptoms. However, the interest in CCE and the use of the modality is growing.
We acknowledge some limitations to our study. Because of the retrospective design, information on patient tolerability is not available. As the patients’ experience is very important when it comes to bowel cleansing, such data would heighten the conclusions that we can draw from this study. The design where study groups are not included in parallel introduces the risk of external factors affecting the results. No significant changes were made during the 3-year study period, and staff as well as protocols were consistent, minimizing the risk of biased results.
To investigate the possibilities for very low-volume bowel preparation in CCE further, studies in a prospective multicenter setting are needed.
5. Conclusions
A very low-volume bowel preparation regimen performed equal to a high-volume regimen before CCE in terms of CR and ACR. The markedly lower volume of active substance could be an advantage in regard to patient acceptability. To improve CR and possibly ACR further, studies on different boosters in addition to the laxative are warranted.
Author Contributions
Conceptualization, E.T. and A.K.; methodology, E.T. and A.K.; formal analysis, U.D.; investigation, E.T., G.W.J. and A.N.; data curation, E.T., G.W.J. and A.N.; writing—original draft preparation, B.S-O.; writing—review and editing, A.K., U.D., T.B.-M., G.B., E.T. and H.T.; visualization, B.S.-O. and U.D.; supervision, A.K., G.B., E.T. and H.T. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Swedish Cancer Society, grant number 19 0386.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by the Swedish Ethical Review Authority (2021-04252, 20 October 2021).
Informed Consent Statement
Informed consent was obtained from the patients for the CCE procedure according to the guidelines at Skåne University Hospital.
Data Availability Statement
The data sets are not publicly available due to the clinical nature of the data but are available from the corresponding author on reasonable request.
Conflicts of Interest
Ervin Toth has received research support and consultancy fees from Medtronic, Olympus, AnX Robotica and Norgine. Anastasios Koulaouzidis is co-director and shareholder of iCERV Ltd. He has received consultancy fees and travel support from Jinshan Ltd., Diagmed Healthcare Ltd. and research support (grant) from ESGE/Given Imaging Ltd. and IntroMedic/SynMed, honoraria from Jinshan and Medtronic and has participated in advisory board meetings hosted by ANKON.
References
- Bjorsum-Meyer, T.; Baatrup, G.; Koulaouzidis, A. Colon capsule endoscopy as a diagnostic adjunct in patients with symptoms from the lower gastrointestinal tract. Diagnostics 2021, 11, 1671. [Google Scholar] [CrossRef] [PubMed]
- Cash, B.D.; Fleisher, M.R.; Fern, S.; Rajan, E.; Haithcock, R.; Kastenberg, D.M.; Pound, D.; Papageorgiou, N.P.; Fernández-Urién, I.; Schmelkin, I.J.; et al. Multicentre, prospective, randomised study comparing the diagnostic yield of colon capsule endoscopy versus CT colonography in a screening population (the TOPAZ study). Gut 2021, 70, 2115–2122. [Google Scholar] [CrossRef] [PubMed]
- Ismail, M.S.; Semenov, S.; Sihag, S.; Manoharan, T.; Douglas, A.R.; Reill, P.; Kelly, M.; Boran, G.; O’Connor, A.; Breslin, N.; et al. Colon capsule endoscopy is a viable alternative to colonoscopy for the investigation of intermediate- and low-risk patients with gastrointestinal symptoms: Results of a pilot study. Endosc. Int. Open 2021, 9, E965–E970. [Google Scholar] [CrossRef] [PubMed]
- Halder, W.; Laskaratos, F.M.; El-Mileik, H.; Coda, S.; Fox, S.; Banerjee, S.; Epstein, O. Review: Colon capsule endoscopy in inflammatory bowel disease. Diagnostics 2022, 12, 149. [Google Scholar] [CrossRef] [PubMed]
- Mollers, T.; Schwab, M.; Gildein, L.; Hoffmeister, M.; Albert, J.; Brenner, H.; Jäger, S. Second-generation colon capsule endoscopy for detection of colorectal polyps: Systematic review and meta-analysis of clinical trials. Endosc. Int. Open 2021, 9, E562–E571. [Google Scholar] [CrossRef]
- Kjolhede, T.; Olholm, A.M.; Kaalby, L.; Kidholm, K.; Qvist, N.; Baatrup, G. Diagnostic accuracy of capsule endoscopy compared with colonoscopy for polyp detection: Systematic review and meta-analyses. Endoscopy 2021, 53, 713–721. [Google Scholar] [CrossRef]
- Hausmann, J.; Tal, A.; Gomer, A.; Philipper, M.; Moog, G.; Hohn, H.; Hesselbarth, N.; Plass, H.; Albert, J.; Finkelmeier, F. Colon capsule endoscopy: Indications, findings, and complications—Data from a Prospective German Colon Capsule Registry Trial (DEKOR). Clin. Endosc. 2021, 54, 92–99. [Google Scholar] [CrossRef]
- Pecere, S.; Senore, C.; Hassan, C.; Riggi, E.; Segnan, N.; Pennazio, M.; Sprujievnik, T.; Rondonotti, E.; Baccarin, A.; Quintero, E.; et al. Accuracy of colon capsule endoscopy for advanced neoplasia. Gastrointest. Endosc. 2020, 91, 406–414.e1. [Google Scholar] [CrossRef]
- Hussey, M.; Holleran, G.; Stack, R.; Moran, N.; Tersaruolo, C.; McNamara, D. Same-day colon capsule endoscopy is a viable means to assess unexplored colonic segments after incomplete colonoscopy in selected patients. United Eur. Gastroenterol. J. 2018, 6, 1556–1562. [Google Scholar] [CrossRef]
- Toth, E.; Yung, D.E.; Nemeth, A.; Wurm Johansson, G.; Thorlacius, H.; Koulaouzidis, A. Video capsule colonoscopy in routine clinical practice. Ann. Transl. Med. 2017, 5, 195. [Google Scholar] [CrossRef]
- Vuik, F.E.R.; Moen, S.; Nieuwenburg, S.A.V.; Schreuders, E.H.; Kuipers, E.J.; Spaander, M.C.W. Applicability of colon capsule endoscopy as pan-endoscopy: From bowel preparation, transit, and rating times to completion rate and patient acceptance. Endosc. Int. Open 2021, 9, E1852–E1859. [Google Scholar] [CrossRef] [PubMed]
- Carretero, C.; Prieto de Frias, C.; Angos, R.; Betes, M.; Herraiz, M.; de la Riva, S.; Zozaya, F.; Fernández-Calderón, M.; Rodríguez-Lago, I.; Navas, M.M. Pan-enteric capsule for bleeding high-risk patients. Can we limit endoscopies? Rev. Esp. Enferm. Dig. 2021, 113, 580–584. [Google Scholar] [CrossRef] [PubMed]
- Cortegoso Valdivia, P.; Elosua, A.; Houdeville, C.; Pennazio, M.; Fernandez-Urien, I.; Dray, X.; Toth, E.; Eliakim, R.; Koulaouzidis, A. Clinical feasibility of panintestinal (or panenteric) capsule endoscopy: A systematic review. Eur. J. Gastroenterol. Hepatol. 2021, 33, 949–955. [Google Scholar] [CrossRef] [PubMed]
- Mussetto, A.; Arena, R.; Fuccio, L.; Trebbi, M.; Garribba, A.T.; Gasperoni, S.; Manzi, I.; Triossi, O.; Rondonotti, E. A new panenteric capsule endoscopy-based strategy in patients with melena and a negative upper gastrointestinal endoscopy: A prospective feasibility study. Eur. J. Gastroenterol. Hepatol. 2021, 33, 686–690. [Google Scholar] [CrossRef] [PubMed]
- Tai, F.W.D.; Ellul, P.; Elosua, A.; Fernandez-Urien, I.; Tontini, G.E.; Elli, L.; Eliakim, R.; Kopylov, U.; Koo, S.; Parker, C.; et al. Panenteric capsule endoscopy identifies proximal small bowel disease guiding upstaging and treatment intensification in Crohn’s disease: A European multicentre observational cohort study. United Eur. Gastroenterol. J. 2021, 9, 248–255. [Google Scholar] [CrossRef]
- Eliakim, R.; Spada, C.; Lapidus, A.; Eyal, I.; Pecere, S.; Fernández-Urién, I.; Lahat, A.; Costamagna, G.; Schwartz, A.; Ron, Y.; et al. Evaluation of a new pan-enteric video capsule endoscopy system in patients with suspected or established inflammatory bowel disease—feasibility study. Endosc. Int. Open 2018, 6, E1235–E1246. [Google Scholar] [CrossRef]
- Spada, C.; Hassan, C.; Bellini, D.; Burling, D.; Cappello, G.; Carretero, C.; Dekker, E.; Eliakim, R.; de Haan, M.; Kaminski, M.F.; et al. Imaging alternatives to colonoscopy: CT colonography and colon capsule. European Society of Gastrointestinal Endoscopy (ESGE) and European Society of Gastrointestinal and Abdominal Radiology (ESGAR) Guideline—Update 2020. Eur. Radiol. 2021, 31, 2967–2982. [Google Scholar] [CrossRef]
- Bjoersum-Meyer, T.; Spada, C.; Watson, A.; Eliakim, R.; Baatrup, G.; Toth, E.; Koulaouzidis, A. What holds back colon capsule endoscopy from being the main diagnostic test for the large bowel in cancer screening? Gastrointest. Endosc. 2022, 95, 168–170. [Google Scholar] [CrossRef]
- Kaminski, M.F.; Thomas-Gibson, S.; Bugajski, M.; Bretthauer, M.; Rees, C.J.; Dekker, E.; Hoff, G.; Jover, R.; Suchanek, S.; Ferlitsch, M.; et al. Performance measures for lower gastrointestinal endoscopy: A European Society of Gastrointestinal Endoscopy (ESGE) quality improvement initiative. United Eur. Gastroenterol. J. 2017, 5, 309–334. [Google Scholar] [CrossRef]
- Bjoersum-Meyer, T.; Skonieczna-Zydecka, K.; Cortegoso Valdivia, P.; Stenfors, I.; Lyutakov, I.; Rondonotti, E.; Pennazio, M.; Marlicz, W.; Baatrup, G.; Koulaouzidis, A.; et al. Efficacy of bowel preparation regimens for colon capsule endoscopy: A systematic review and meta-analysis. Endosc. Int. Open 2021, 9, E1658–E1673. [Google Scholar] [CrossRef]
- Deding, U.; Cortegoso Valdivia, P.; Koulaouzidis, A.; Baatrup, G.; Toth, E.; Spada, C.; Fernández-Urién, I.; Pennazio, M.; Bjørsum-Meyer, T. Patient-reported outcomes and preferences for colon capsule endoscopy and colonoscopy: A systematic review with meta-analysis. Diagnostics 2021, 11, 1730. [Google Scholar] [CrossRef] [PubMed]
- Bednarska, O.; Nyhlin, N.; Schmidt, P.T.; Johansson, G.W.; Toth, E.; Lindfors, P. The effectiveness and tolerability of a very low-volume bowel preparation for colonoscopy compared to low and high-volume polyethylene glycol-solutions in the real-life setting. Diagnostics 2022, 12, 1155. [Google Scholar] [CrossRef] [PubMed]
- Maida, M.; Sinagra, E.; Morreale, G.C.; Sferrazza, S.; Scalisi, G.; Schillaci, D.; Ventimiglia, M.; Macaluso, F.S.; Vettori, G.; Conoscenti, G.; et al. Effectiveness of very low-volume preparation for colonoscopy: A prospective, multicenter observational study. World J. Gastroenterol. 2020, 26, 1950–1961. [Google Scholar] [CrossRef] [PubMed]
- Baile-Maxia, S.; Amlani, B.; Martinez, R.J. Bowel-cleansing efficacy of the 1L polyethylene glycol-based bowel preparation NER1006 (PLENVU) in patient subgroups in two phase III trials. Therap. Adv. Gastroenterol. 2021, 14, 17562848211020286. [Google Scholar] [CrossRef]
- Clayton, L.B.; Tayo, B.; Halphen, M.; Kornberger, R. Novel 1 L polyethylene glycol-based bowel preparation (NER1006): Proof of concept assessment versus standard 2 L polyethylene glycol with ascorbate—A randomized, parallel group, phase 2, colonoscopist-blinded trial. BMC Gastroenterol. 2019, 19, 79. [Google Scholar] [CrossRef]
- Epstein, M.; Halonen, J.; Sharma, P. Bowel preparation with 1L polyethylene glycol and ascorbate NER1006 doubles the chance to detect three or more adenomas in overweight or obese males. Endosc. Int. Open 2021, 9, E1324–E1334. [Google Scholar] [CrossRef]
- Leighton, J.A.; Rex, D.K. A grading scale to evaluate colon cleansing for the PillCam COLON capsule: A reliability study. Endoscopy 2011, 43, 123–127. [Google Scholar] [CrossRef]
- Plenvu Prescribing information. Available online: https://www.plenvu.com/hcp/prescribing-information (accessed on 3 November 2022).
- Dosedel, M.; Jirkovsky, E.; Macakova, K.; Krcmova, L.K.; Javorska, L.; Pourova, J.; Mercolini, L.; Remião, F.; Nováková, L.; Mladěnka, P.; et al. Vitamin C-sources, physiological role, kinetics, deficiency, use, toxicity, and determination. Nutrients 2021, 13, 615. [Google Scholar] [CrossRef]
- Radaelli, F. The paradox of the novel 1 L polyethylene glycol bowel preparation: Efficacy, not tolerability, is the great new! Endoscopy 2019, 51, 7–9. [Google Scholar] [CrossRef]
- Deding, U.; Kaalby, L.; Baatrup, G.; Kobaek-Larsen, M.; Thygesen, M.K.; Epstein, O.; Bjørsum-Meyer, T. The effect of Prucalopride on the completion rate and polyp detection rate of colon capsule endoscopies. Clin. Epidemiol. 2022, 14, 437–444. [Google Scholar] [CrossRef]
- Sato, J.; Nakamura, M.; Watanabe, O.; Yamamura, T.; Funasaka, K.; Ohno, E.; Miyahara, R.; Kawashima, H.; Goto, H.; Hirooka, Y.; et al. Prospective study of factors important to achieve observation of the entire colon on colon capsule endoscopy. Therap. Adv. Gastroenterol. 2017, 10, 20–31. [Google Scholar] [CrossRef] [PubMed]
- MacLeod, C.; Hudson, J.; Brogan, M.; Cotton, S.; Treweek, S.; MacLennan, G.; Watson, A.J.M. ScotCap—A large observational cohort study. Colorectal. Dis. 2022, 24, 411–421. [Google Scholar] [CrossRef] [PubMed]
- Havshoi, A.V.; Deding, U.; Jensen, S.S.; Andersen, P.V.; Kaalby, L.; Al-Najami, I. Colon capsule endoscopy following incomplete colonoscopy in routine clinical settings. Surg. Endosc. 2022. [Google Scholar] [CrossRef]
- Benech, N.; Vinet, O.; Gaudin, J.L.; Benamouzig, R.; Dray, X.; Ponchon, T.; Galmiche, J.-P.; Sacher-Huvelin, S.; Samaha, E.; Saurin, J.-C.; et al. Colon capsule endoscopy in clinical practice: Lessons from a national 5-year observational prospective cohort. Endosc. Int. Open 2021, 9, E1542–E1548. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).