Suboptimal Performance of Hepatocellular Carcinoma Prediction Models in Patients with Hepatitis B Virus-Related Cirrhosis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design and Participants
2.2. HCC Surveillance
2.3. Calculation of HCC Risk Scores from Prediction Models
2.4. Statistical Analysis
3. Results
3.1. Baseline Characteristics and HCC Development
3.2. Independent Predictive Factors of HCC Development in Cirrhotic Patients with CHB
3.3. Predictive Performance and HCC
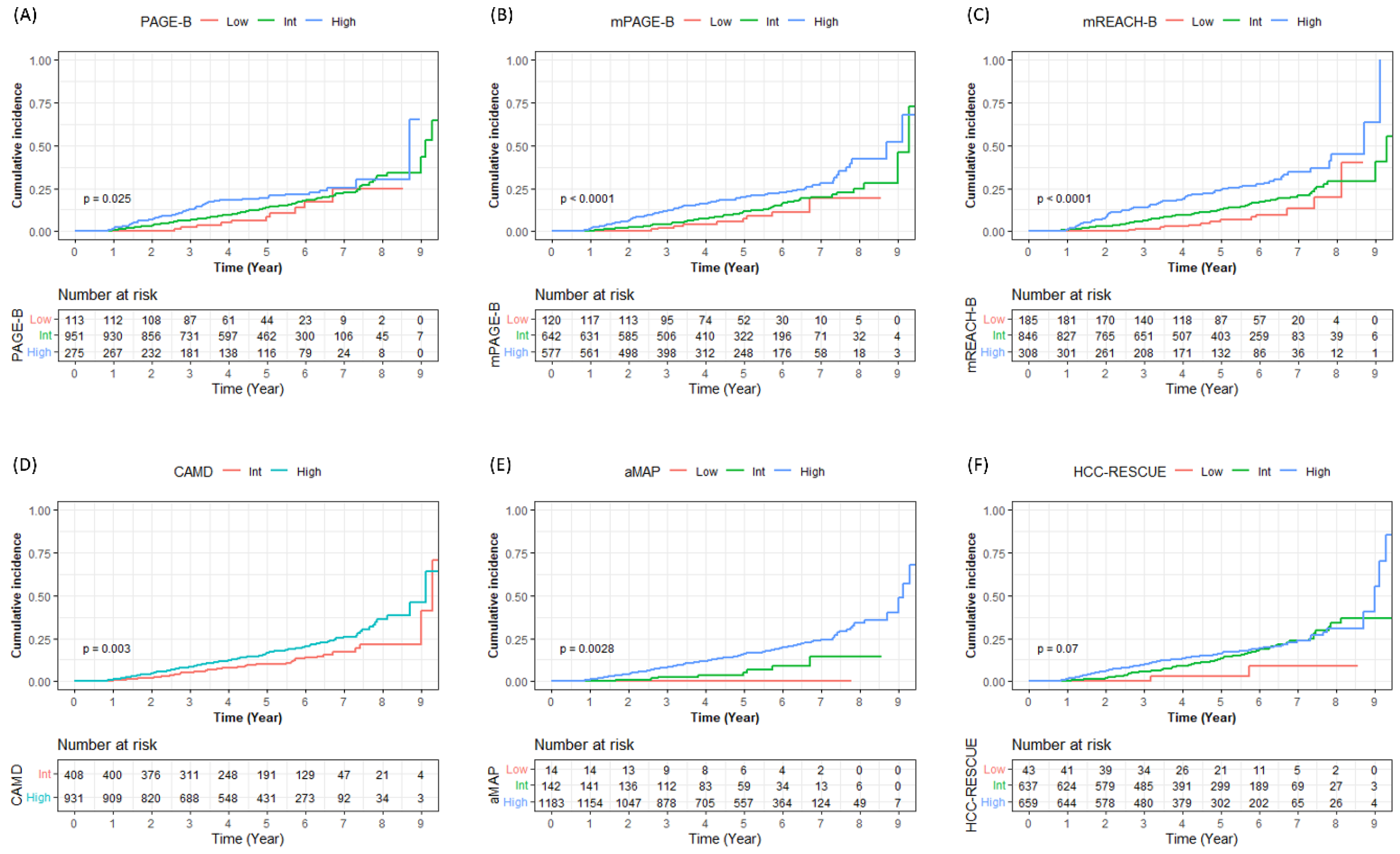
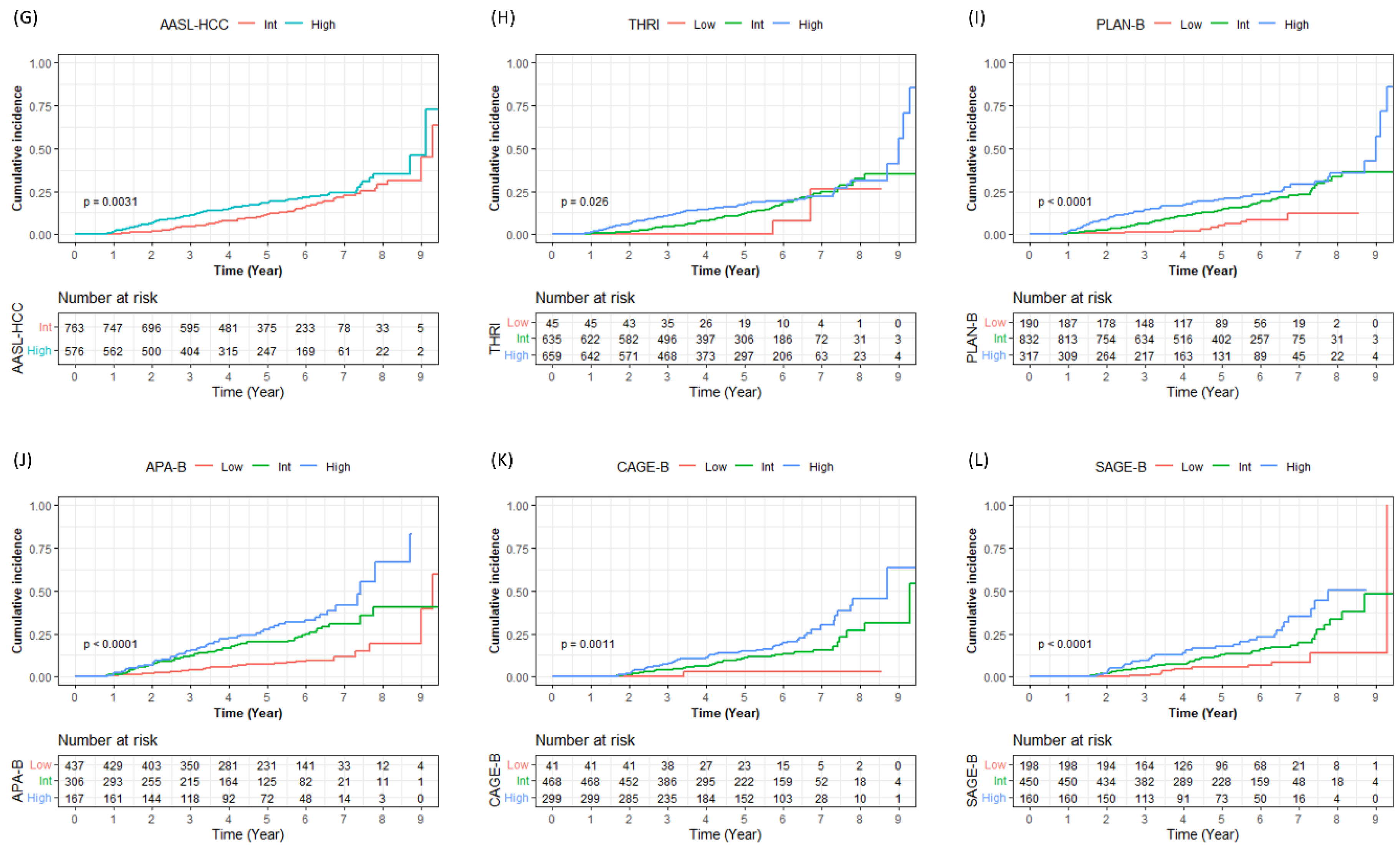
3.4. Risk Stratification in Cirrhotic Patients with CHB
3.5. On-Treatment LS Value in Cirrhotic Patients with CHB
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Terrault, N.A.; Bzowej, N.H.; Chang, K.M.; Hwang, J.P.; Jonas, M.M.; Murad, M.H. Aasld guidelines for treatment of chronic hepatitis B. Hepatology 2016, 63, 261–283. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.J.; Yang, H.I.; Su, J.; Jen, C.L.; You, S.L.; Lu, S.N.; Huang, G.T.; Iloeje, U.H. Risk of hepatocellular carcinoma across a biological gradient of serum hepatitis B virus DNA level. JAMA 2006, 295, 65–73. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sarin, S.K.; Kumar, M.; Lau, G.K.; Abbas, Z.; Chan, H.L.; Chen, C.J.; Chen, D.S.; Chen, H.L.; Chen, P.J.; Chien, R.N.; et al. Asian-pacific clinical practice guidelines on the management of hepatitis B: A 2015 update. Hepatol. Int. 2016, 10, 1–98. [Google Scholar] [CrossRef] [PubMed]
- Torimura, T.; Iwamoto, H. Optimizing the management of intermediate-stage hepatocellular carcinoma: Current trends and prospects. Clin. Mol. Hepatol. 2021, 27, 236–245. [Google Scholar] [CrossRef]
- Sohn, W.; Kang, D.; Kang, M.; Guallar, E.; Cho, J.; Paik, Y.H. Impact of nationwide hepatocellular carcinoma surveillance on the prognosis in patients with chronic liver disease. Clin. Mol. Hepatol. 2022, 28, 851–863. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Jin, Y.J.; Shin, S.K.; Kwon, J.H.; Kim, S.G.; Suh, Y.J.; Jeong, Y.; Yu, J.H.; Lee, J.W.; Kwon, O.S.; et al. Surgery versus radiofrequency ablation in patients with child- pugh class-a/single small (≤3 cm) hepatocellular carcinoma. Clin. Mol. Hepatol. 2022, 28, 207–218. [Google Scholar] [CrossRef]
- Korean Liver Cancer Association (KLCA) and National Cancer Center (NCC) Korea. 2022 KLCA-NCC Korea practice guidelines for the management of hepatocellular carcinoma. Clin. Mol. Hepatol. 2022, 28, 583–705. [Google Scholar] [CrossRef]
- Kim, J.M.; Kim, D.G.; Kim, J.; Lee, K.; Lee, K.W.; Ryu, J.H.; Kim, B.W.; Choi, D.L.; You, Y.K.; Kim, D.S.; et al. Outcomes after liver transplantation in Korea: Incidence and risk factors from Korean transplantation registry. Clin. Mol. Hepatol. 2021, 27, 451–462. [Google Scholar] [CrossRef]
- Lee, S.W.; Choi, J.; Kim, S.U.; Lim, Y.S. Entecavir versus tenofovir in patients with chronic hepatitis B: Enemies or partners in the prevention of hepatocellular carcinoma. Clin. Mol. Hepatol. 2021, 27, 402–412. [Google Scholar] [CrossRef]
- Sohn, W.; Lee, H.W.; Lee, S.; Lim, J.H.; Lee, M.W.; Park, C.H.; Yoon, S.K. Obesity and the risk of primary liver cancer: A systematic review and meta-analysis. Clin. Mol. Hepatol. 2021, 27, 157–174. [Google Scholar] [CrossRef]
- Kim, M.N.; Han, K.; Yoo, J.; Hwang, S.G.; Ahn, S.H. Increased risk of hepatocellular carcinoma and mortality in chronic viral hepatitis with concurrent fatty liver. Aliment. Pharmacol. Ther. 2022, 55, 97–107. [Google Scholar] [CrossRef] [PubMed]
- Yun, B.; Ahn, S.H.; Oh, J.; Yoon, J.H.; Kim, B.K. Effect of metabolic dysfunction-associated fatty liver disease on liver cancer risk in a population with chronic hepatitis B virus infection: A nationwide study. Hepatol. Res. 2022, 52, 975–984. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.H.; Cho, S.G.; Jin, Y.J.; Lee, J.W. The best predictive model for hepatocellular carcinoma in patients with chronic hepatitis B infection. Clin. Mol. Hepatol. 2022, 28, 351–361. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.H.; Suh, Y.J.; Jin, Y.J.; Heo, N.Y.; Jang, J.W.; You, C.R.; An, H.Y.; Lee, J.W. Prediction model for hepatocellular carcinoma risk in treatment-naive chronic hepatitis B patients receiving entecavir/tenofovir. Eur. J. Gastroenterol. Hepatol. 2019, 31, 865–872. [Google Scholar] [CrossRef]
- Cheng, R.; Xu, X. Validation of hepatocellular carcinoma risk prediction models in patients with hepatitis B-related cirrhosis. J. Hepatocell. Carcinoma 2022, 9, 987–997. [Google Scholar] [CrossRef]
- Papatheodoridis, G.; Dalekos, G.; Sypsa, V.; Yurdaydin, C.; Buti, M.; Goulis, J.; Calleja, J.L.; Chi, H.; Manolakopoulos, S.; Mangia, G.; et al. Page-b predicts the risk of developing hepatocellular carcinoma in Caucasians with chronic hepatitis B on 5-year antiviral therapy. J. Hepatol. 2016, 64, 800–806. [Google Scholar] [CrossRef]
- Kim, J.H.; Kim, Y.D.; Lee, M.; Jun, B.G.; Kim, T.S.; Suk, K.T.; Kang, S.H.; Kim, M.Y.; Cheon, G.J.; Kim, D.J.; et al. Modified page-b score predicts the risk of hepatocellular carcinoma in Asians with chronic hepatitis B on antiviral therapy. J. Hepatol. 2018, 69, 1066–1073. [Google Scholar] [CrossRef]
- Lee, H.W.; Yoo, E.J.; Kim, B.K.; Kim, S.U.; Park, J.Y.; Kim, D.Y.; Ahn, S.H.; Han, K.H. Prediction of development of liver-related events by transient elastography in hepatitis B patients with complete virological response on antiviral therapy. Am. J. Gastroenterol. 2014, 109, 1241–1249. [Google Scholar] [CrossRef]
- Hsu, Y.C.; Yip, T.C.; Ho, H.J.; Wong, V.W.; Huang, Y.T.; El-Serag, H.B.; Lee, T.Y.; Wu, M.S.; Lin, J.T.; Wong, G.L.; et al. Development of a scoring system to predict hepatocellular carcinoma in Asians on antivirals for chronic hepatitis B. J. Hepatol. 2018, 69, 278–285. [Google Scholar] [CrossRef]
- Papatheodoridis, G.V.; Sypsa, V.; Dalekos, G.N.; Yurdaydin, C.; Van Boemmel, F.; Buti, M.; Calleja, J.L.; Chi, H.; Goulis, J.; Manolakopoulos, S.; et al. Hepatocellular carcinoma prediction beyond year 5 of oral therapy in a large cohort of Caucasian patients with chronic hepatitis B. J. Hepatol. 2020, 72, 1088–1096. [Google Scholar] [CrossRef]
- Chon, H.Y.; Lee, J.S.; Lee, H.W.; Chun, H.S.; Kim, B.K.; Tak, W.Y.; Park, J.Y.; Kweon, Y.O.; Kim, D.Y.; Ahn, S.H.; et al. Predictive performance of cage-b and sage-b models in Asian treatment-naive patients who started entecavir for chronic hepatitis B. Clin. Gastroenterol. Hepatol. 2022, 20, e794–e807. [Google Scholar] [CrossRef]
- Lee, J.S.; Lee, H.W.; Lim, T.S.; Shin, H.J.; Lee, H.W.; Kim, S.U.; Park, J.Y.; Kim, D.Y.; Ahn, S.H.; Kim, B.K. Novel liver stiffness-based nomogram for predicting hepatocellular carcinoma risk in patients with chronic hepatitis B virus infection initiating antiviral therapy. Cancers 2021, 13, 5892. [Google Scholar] [CrossRef] [PubMed]
- Liang, L.Y.; Lee, H.W.; Wong, V.W.; Yip, T.C.; Tse, Y.K.; Hui, V.W.; Lui, G.C.; Chan, H.L.; Wong, G.L. Serum fibrosis index-based risk score predicts hepatocellular carcinoma in untreated patients with chronic hepatitis B. Clin. Mol. Hepatol. 2021, 27, 499–509. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.H.; Yang, H.I.; Liu, J.; Batrla-Utermann, R.; Jen, C.L.; Iloeje, U.H.; Lu, S.N.; You, S.L.; Wang, L.Y.; Chen, C.J. Prediction models of long-term cirrhosis and hepatocellular carcinoma risk in chronic hepatitis B patients: Risk scores integrating host and virus profiles. Hepatology 2013, 58, 546–554. [Google Scholar] [CrossRef]
- Wong, V.W.; Chan, S.L.; Mo, F.; Chan, T.C.; Loong, H.H.; Wong, G.L.; Lui, Y.Y.; Chan, A.T.; Sung, J.J.; Yeo, W.; et al. Clinical scoring system to predict hepatocellular carcinoma in chronic hepatitis B carriers. J. Clin. Oncol. 2010, 28, 1660–1665. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yuen, M.F.; Tanaka, Y.; Fong, D.Y.; Fung, J.; Wong, D.K.; Yuen, J.C.; But, D.Y.; Chan, A.O.; Wong, B.C.; Mizokami, M.; et al. Independent risk factors and predictive score for the development of hepatocellular carcinoma in chronic hepatitis B. J. Hepatol. 2009, 50, 80–88. [Google Scholar] [CrossRef]
- Wong, G.L.; Chan, H.L.; Wong, C.K.; Leung, C.; Chan, C.Y.; Ho, P.P.; Chung, V.C.; Chan, Z.C.; Tse, Y.K.; Chim, A.M.; et al. Liver stiffness-based optimization of hepatocellular carcinoma risk score in patients with chronic hepatitis B. J. Hepatol. 2014, 60, 339–345. [Google Scholar] [CrossRef]
- Yang, H.I.; Yuen, M.F.; Chan, H.L.; Han, K.H.; Chen, P.J.; Kim, D.Y.; Ahn, S.H.; Chen, C.J.; Wong, V.W.; Seto, W.K. Risk estimation for hepatocellular carcinoma in chronic hepatitis B (reach-b): Development and validation of a predictive score. Lancet Oncol. 2011, 12, 568–574. [Google Scholar] [CrossRef]
- The Korean Association for the Study of the Liver (KASL). KASL clinical practice guidelines for liver cirrhosis: Varices, hepatic encephalopathy, and related complications. Clin. Mol. Hepatol. 2020, 26, 83–127. [Google Scholar] [CrossRef]
- Yoo, S.H.; Lim, T.S.; Lee, H.W.; Kim, J.K.; Lee, J.S.; Lee, H.W.; Kim, B.K.; Park, J.Y.; Kim, D.Y.; Ahn, S.H.; et al. Risk assessment of hepatocellular carcinoma and liver-related events using ultrasonography and transient elastography in patients with chronic hepatitis B. J. Viral Hepat. 2021, 28, 1362–1372. [Google Scholar] [CrossRef]
- Heimbach, J.K.; Kulik, L.M.; Finn, R.S.; Sirlin, C.B.; Abecassis, M.M.; Roberts, L.R.; Zhu, A.X.; Murad, M.H.; Marrero, J.A. Aasld guidelines for the treatment of hepatocellular carcinoma. Hepatology 2018, 67, 358–380. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fan, R.; Papatheodoridis, G.; Sun, J.; Innes, H.; Toyoda, H.; Xie, Q.; Mo, S.; Sypsa, V.; Guha, I.N.; Kumada, T.; et al. Amap risk score predicts hepatocellular carcinoma development in patients with chronic hepatitis. J. Hepatol. 2020, 73, 1368–1378. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.A.; Kowgier, M.; Hansen, B.E.; Brouwer, W.P.; Maan, R.; Wong, D.; Shah, H.; Khalili, K.; Yim, C.; Heathcote, E.J.; et al. Toronto hcc risk index: A validated scoring system to predict 10-year risk of hcc in patients with cirrhosis. J. Hepatol. 2017. [Google Scholar] [CrossRef] [PubMed]
- Sohn, W.; Cho, J.Y.; Kim, J.H.; Lee, J.I.; Kim, H.J.; Woo, M.A.; Jung, S.H.; Paik, Y.H. Risk score model for the development of hepatocellular carcinoma in treatment-naïve patients receiving oral antiviral treatment for chronic hepatitis B. Clin. Mol. Hepatol. 2017, 23, 170–178. [Google Scholar] [CrossRef]
- Kim, H.Y.; Lampertico, P.; Nam, J.Y.; Lee, H.C.; Kim, S.U.; Sinn, D.H.; Seo, Y.S.; Lee, H.A.; Park, S.Y.; Lim, Y.S.; et al. An artificial intelligence model to predict hepatocellular carcinoma risk in Korean and Caucasian patients with chronic hepatitis B. J. Hepatol. 2022, 76, 311–318. [Google Scholar] [CrossRef]
- Chen, C.H.; Lee, C.M.; Lai, H.C.; Hu, T.H.; Su, W.P.; Lu, S.N.; Lin, C.H.; Hung, C.H.; Wang, J.H.; Lee, M.H.; et al. Prediction model of hepatocellular carcinoma risk in Asian patients with chronic hepatitis B treated with entecavir. Oncotarget 2017, 8, 92431–92441. [Google Scholar] [CrossRef] [Green Version]
- Chon, Y.E.; Park, J.Y.; Myoung, S.M.; Jung, K.S.; Kim, B.K.; Kim, S.U.; Kim, D.Y.; Ahn, S.H.; Han, K.H. Improvement of liver fibrosis after long-term antiviral therapy assessed by fibroscan in chronic hepatitis B patients with advanced fibrosis. Am. J. Gastroenterol. 2017, 112, 882–891. [Google Scholar] [CrossRef]
- Lee, H.W.; Kim, S.U.; Park, J.Y.; Kim, D.Y.; Ahn, S.H.; Han, K.H.; Kim, B.K. External validation of the modified page-b score in asian chronic hepatitis B patients receiving antiviral therapy. Liver Int. 2019, 39, 1624–1630. [Google Scholar] [CrossRef]
- Kim, M.N.; Hwang, S.G.; Kim, B.K.; Park, J.Y.; Kim, D.Y.; Han, K.H.; Kim, S.U.; Ahn, S.H. Liver cirrhosis, not antiviral therapy, predicts clinical outcome in cohorts with heterogeneous hepatitis b viral status. Gut Liver 2019, 13, 197–205. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.S.; Lee, H.W.; Kim, B.K.; Park, J.Y.; Kim, D.Y.; Ahn, S.H.; Jang, J.Y.; Park, S.Y.; Lee, H.W.; Lee, C.K.; et al. Comparison of fibroscan-aspartate aminotransferase (fast) score and other non-invasive surrogates in predicting high-risk non-alcoholic steatohepatitis criteria. Front. Med. 2022, 9, 869190. [Google Scholar] [CrossRef]
- Cheng, R.; Xu, J.; Tan, N.; Luo, H.; Pan, J.; Xu, X. Predictive nomograms for clinical outcomes in hepatitis B-related cirrhosis patients receiving antiviral therapy. Infect. Drug Resist. 2021, 14, 2707–2719. [Google Scholar] [CrossRef] [PubMed]
- Nam, J.Y.; Sinn, D.H.; Bae, J.; Jang, E.S.; Kim, J.W.; Jeong, S.H. Deep learning model for prediction of hepatocellular carcinoma in patients with hbv-related cirrhosis on antiviral therapy. JHEP Rep. 2020, 2, 100175. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Wang, H.; Zhang, W.; Gu, E. Verification of hepatitis b-related hepatocellular carcinoma predictive models to evaluate the risk of hcc in patients with liver cirrhosis under antiviral treatment. Eur. J. Gastroenterol. Hepatol. 2022, 34, 546–552. [Google Scholar] [CrossRef] [PubMed]
- Tchelepi, H.; Ralls, P.W.; Radin, R.; Grant, E. Sonography of diffuse liver disease. J. Ultrasound Med. 2002, 21, 1023–1032; quiz 1033–1024. [Google Scholar] [CrossRef] [PubMed]
- Bruix, J.; Sherman, M. Management of hepatocellular carcinoma: An update. Hepatology 2011, 53, 1020–1022. [Google Scholar] [CrossRef] [Green Version]
- Wu, J.W.; Kao, J.H.; Tseng, T.C. Three heads are better than two: Hepatitis B core-related antigen as a new predictor of hepatitis B virus-related hepatocellular carcinoma. Clin. Mol. Hepatol. 2021, 27, 524–534. [Google Scholar] [CrossRef]
- Kim, S.W.; Yoon, J.S.; Lee, M.; Cho, Y. Toward a complete cure for chronic hepatitis B: Novel therapeutic targets for hepatitis B virus. Clin. Mol. Hepatol. 2022, 28, 17–30. [Google Scholar] [CrossRef]
- Jang, J.W.; Kim, J.S.; Kim, H.S.; Tak, K.Y.; Nam, H.; Sung, P.S.; Bae, S.H.; Choi, J.Y.; Yoon, S.K.; Roberts, L.R. Persistence of intrahepatic hepatitis B virus DNA integration in patients developing hepatocellular carcinoma after hepatitis B surface antigen seroclearance. Clin. Mol. Hepatol. 2021, 27, 207–218. [Google Scholar] [CrossRef]
- Kang, S.H.; Lee, H.W.; Yoo, J.J.; Cho, Y.; Kim, S.U.; Lee, T.H.; Jang, B.K.; Kim, S.G.; Ahn, S.B.; Kim, H.; et al. Kasl clinical practice guidelines: Management of nonalcoholic fatty liver disease. Clin. Mol. Hepatol. 2021, 27, 363–401. [Google Scholar] [CrossRef]
- Rasha, F.; Paul, S.; Simon, T.G.; Hoshida, Y. Hepatocellular carcinoma chemoprevention with generic agents. Semin. Liver Dis. 2022, 42, 501–513. [Google Scholar] [CrossRef]
- Jung, K.S.; Kim, S.U.; Song, K.; Park, J.Y.; Kim, D.Y.; Ahn, S.H.; Kim, B.K.; Han, K.H. Validation of hepatitis b virus-related hepatocellular carcinoma prediction models in the era of antiviral therapy. Hepatology 2015, 62, 1757–1766. [Google Scholar] [CrossRef] [PubMed]
| Variables | Total (n = 1339) |
|---|---|
| Age (year) | 54 (47, 59) |
| <40 | 87 (6.5) |
| 40–50 | 343 (25.6) |
| 50–60 | 578 (43.2) |
| 60–70 | 265 (19.8) |
| ≥70 | 51 (3.8) |
| Male sex | 717 (53.5) |
| Diabetes mellitus | 190 (14.2) |
| HBeAg positivity | 514 (38.4) |
| TDF use (vs. ETV) | 684 (51.1) |
| Platelet count (×103/μL) | 134 (99, 172) |
| Total bilirubin (mg/dL) | 0.9 (0.7, 1.3) |
| Serum albumin (g/dL) | 4.2 (3.8, 4.4) |
| Prothrombin time (INR) | 1.04 (0.98, 1.13) |
| Aspartate aminotransferase (IU/L) | 39 (28, 59) |
| Alanine aminotransferase (IU/L) | 37 (25, 59) |
| Alpha-fetoprotein (ng/mL) (n = 910) | 4.48 (2.74, 8.57) |
| Liver stiffness value † (kPa) | 11.2 (7.4, 17.3) |
| 1 year after AVT (kPa) (n = 808) | 8.9 (2.7, 13.4) |
| Follow-up and treatment duration (month) | 56.8 (35.6, 75.3) |
| PAGE-B | 15 (12, 18) |
| Modified PAGE-B | 12 (10, 14) |
| Modified REACH-B | 9 (8, 11) |
| CAMD | 15 (13, 16) |
| aMAP | 67.3 (63.0, 71.3) |
| HCC-RESCUE | 84 (77, 92) |
| AASL-HCC | 19 (17, 20) |
| Toronto HCC Risk Index | 236 (197, 297) |
| PLAN-B | 0.395 (0.306, 0.493) |
| APA-B (n = 910) | 6 (3, 8) |
| CAGE-B (n = 808) | 9 (8, 11) |
| SAGE-B (n = 808) | 6 (6, 9) |
| Variables | Without HCC (n = 1128) | HCC (n = 211) | p Value |
|---|---|---|---|
| Age (year) | 53 (47, 59) | 55 (50, 60) | 0.003 |
| <40 | 83 (7.4) | 4 (1.9) | 0.004 |
| 40–50 | 295 (26.2) | 48 (22.7) | |
| 50–60 | 473 (41.9) | 105 (49.8) | |
| 60–70 | 220 (19.5) | 45 (21.3) | |
| ≥70 | 42 (3.7) | 9 (4.3) | |
| Male sex | 605 (53.6) | 112 (53.1) | 0.942 |
| Diabetes mellitus | 151 (13.4) | 39 (18.5) | 0.066 |
| HBeAg positivity | 414 (36.7) | 100 (47.4) | 0.004 |
| TDF use (vs. ETV) | 576 (51.1) | 108 (51.2) | >0.999 |
| Platelet count (×103/μL) | 138 (102, 175) | 115 (87, 156) | <0.001 |
| Total bilirubin (mg/dL) | 0.9 (0.7, 1.2) | 1.0 (0.7, 1.5) | 0.065 |
| Serum albumin (g/dL) | 4.2 (3.9, 4.4) | 4.0 (3.4, 4.3) | <0.001 |
| Prothrombin time (INR) | 1.04 (0.98, 1.11) | 1.05 (1.0, 1.16) | 0.007 |
| Aspartate aminotransferase (IU/L) | 38 (27, 56) | 49 (37, 76) | <0.001 |
| Alanine aminotransferase (IU/L) | 36 (24, 56) | 44 (30, 78) | 0.218 |
| Alpha-fetoprotein (ng/mL) | 3.96 (2.59, 7.46) | 7.21 (4.66, 14.69) | <0.001 |
| Liver stiffness value † (kPa) | 10.3 (6.9, 16.6) | 14.2 (10.0, 22.3) | <0.001 |
| 1 year after AVT (kPa) (n = 808) | 8.7 (6.1, 12.5) | 11.8 (8.65, 16.6) | <0.001 |
| Follow-up and treatment duration (month) | 60.1 (38.1, 76.1) | 40.7 (24.6, 60.9) | <0.001 |
| PAGE-B | 15 (12, 18) | 16 (13, 18) | <0.001 |
| Modified PAGE-B | 12 (10, 14) | 13 (11, 15) | <0.001 |
| Modified REACH-B | 9 (7, 11) | 11 (9, 12) | <0.001 |
| CAMD | 14 (13, 16) | 15 (14, 16) | 0.022 |
| aMAP | 66.8 (62.6, 71.1) | 69.4 (66.1, 72.6) | <0.001 |
| HCC-RESCUE | 84 (76, 92) | 86 (79, 93) | 0.013 |
| AASL-HCC | 19 (17, 20) | 20 (17, 22) | <0.001 |
| Toronto HCC Risk Index | 236 (197, 297) | 247 (217, 297) | 0.001 |
| PLAN-B | 0.382 (0.294, 0.479) | 0.434 (0.370, 0.527) | <0.001 |
| APA-B (n = 910) | 5 (3, 8) | 7 (6, 10) | <0.001 |
| CAGE-B (n = 808) | 9 (7, 11) | 11 (9, 12) | <0.001 |
| SAGE-B (n = 808) | 6 (4, 9) | 8 (6, 11) | <0.001 |
| Variable | Univariate | Multivariate Analysis | |
|---|---|---|---|
| p Value | p Value | Hazard Ratio (95% CI) | |
| Age (year) | <0.001 | 0.003 | 1.023 (1.008, 1.038) |
| Diabetes mellitus | 0.032 | 0.207 | 1.259 (0.881, 1.800) |
| HBeAg positivity | 0.012 | 0.066 | 1.302 (0.982, 1.725) |
| Platelet count (×103/μL) | <0.001 | 0.015 | 0.997 (0.994, 0.999) |
| Total bilirubin (mg/dL) | 0.048 | 0.439 | 0.971 (0.901, 1.046) |
| Serum albumin (g/dL) | <0.001 | <0.001 | 0.578 (0.446, 0.751) |
| Prothrombin time (INR) | 0.014 | 0.589 | 0.829 (0.420, 1.636) |
| Liver stiffness value † (kPa) | <0.001 | 0.026 | 1.012 (1.002, 1.024) |
| Scoring Systems | Harrell’s c-Index (95% CI) | Integrated AUC * (95% CI) | TDAUC at 1 Year (95% CI) | TDAUC at 2 Year (95% CI) | TDAUC at 3 Year (95% CI) | TDAUC at 5 Year (95% CI) | TDAUC at 8 Year (95% CI) |
|---|---|---|---|---|---|---|---|
| PAGE-B | 0.605 (0.568, 0.644) | 0.573 (0.536, 0.609) | 0.674 (0.513, 0.835) | 0.689 (0.624, 0.754) | 0.658 (0.601, 0.714) | 0.596 (0.547, 0.644) | 0.568 (0.475, 0.661) |
| Modified PAGE-B | 0.640 (0.601, 0.676) | 0.611 (0.577, 0.644) | 0.747 (0.592, 0.902) | 0.714 (0.650, 0.777) | 0.701 (0.648, 0.754) | 0.630 (0.581, 0.678) | 0.641 (0.550, 0.732) |
| Modified REACH-B | 0.667 (0.630, 0.702) | 0.643 (0.606, 0.674) | 0.662 (0.516, 0.808) | 0.732 (0.670, 0.795) | 0.704 (0.654, 0.753) | 0.663 (0.616, 0.709) | 0.608 (0.515, 0.700) |
| CAMD | 0.565 (0.528, 0.604) | 0.553 (0.517, 0.588) | 0.674 (0.545, 0.804) | 0.626 (0.556, 0.697) | 0.603 (0.545, 0.661) | 0.553 (0.506, 0.600) | 0.576 (0.482, 0.671) |
| aMAP | 0.603 (0.564, 0.641) | 0.610 (0.573, 0.645) | 0.725 (0.571, 0.879) | 0.713 (0.653, 0.774) | 0.706 (0.655, 0.758) | 0.630 (0.582, 0..678) | 0.629 (0.536, 0.722) |
| HCC-RESCUE | 0.588 (0.547, 0.623) | 0.560 (0.524, 0.593) | 0.761 (0.646, 0.876) | 0.692 (0.630, 0.754) | 0.645 (0.591, 0.700) | 0.571 (0.523, 0.619) | 0.513 (0.416, 0.610) |
| AASL-HCC | 0.616 (0.578, 0.655) | 0.590 (0.557, 0.623) | 0.798 (0.679, 0.916) | 0.724 (0.662, 0.787) | 0.680 (0.625, 0.735) | 0.603 (0.553, 0.653) | 0.578 (0.486, 0.670) |
| Toronto HCC Risk Index | 0.603 (0.564, 0.641) | 0.572 (0.537, 0.608) | 0.693 (0.591, 0.794) | 0.709 (0.651, 0.768) | 0.667 (0.613, 0.721) | 0.589 (0.541, 0.637) | 0.536 (0.437, 0.634) |
| PLAN-B | 0.638 (0.600, 0.675) | 0.613 (0.578, 0.650) | 0.634 (0.489, 0.779) | 0.727 (0.658, 0.797) | 0.691 (0.638, 0.743) | 0.625 (0.576, 0.673) | 0.462 (0.365, 0.560) |
| APA-B (n = 910) † | 0.661 (0.615, 0.703) | 0.655 (0.618, 0.691) | 0.608 (0.447, 0.769) | 0.651 (0.573, 0.729) | 0.664 (0.603, 0.725) | 0.679 (0.626, 0.732) | 0.718 (0.604, 0.832) |
| CAGE-B (n = 808) ‡ | 0.621 (0.571, 0.675) | 0.622 (0.579, 0.661) | - | 0.645 (0.533, 0.757) | 0.679 (0.606, 0.753) | 0.679 (0.606, 0.753) | 0.675 (0.556, 0.794) |
| SAGE-B (n = 808) ‡ | 0.639 (0.587, 0.691) | 0.637 (0.593, 0.678) | - | 0.659 (0.540, 0.777) | 0.705 (0.633, 0.777) | 0.610 (0.547, 0.674) | 0.667 (0.546, 0.786) |
| Risk Stratification | Patient No. (%) | Cumulative Incidence of HCC | Log Rank p Value | Log Rank p Value vs. | |||||
|---|---|---|---|---|---|---|---|---|---|
| 1 Year | 2 Year | 3 Year | 5 Year | 8 Year | |||||
| All Patients | 1339 (100) | 12 (0.9) | 49 (3.8) | 91 (7.4) | 155 (14.6) | 203 (31.7) | |||
| PAGE-B | |||||||||
| Low (0–9) | 113 (8.4) | 0 (0.0) | 0 (0.0) | 2 (2.2) | 6 (8.5) | 10 (24.7) | 0.025 | Int | 0.200 |
| Int (10–17) | 951 (71.0) | 7 (0.7) | 31 (3.4) | 57 (6.5) | 105 (13.9) | 142 (32.3) | High | 0.030 | |
| High (18–25) | 275 (20.5) | 5 (1.8) | 18 (6.8) | 32 (12.9) | 44 (19.3) | 51 (30.4) | Low | 0.020 | |
| Modified PAGE-B | |||||||||
| Low (0–8) | 120 (9.0) | 0 (0.0) | 0 (0.0) | 2 (2.0) | 6 (7.3) | 9 (19.4) | <0.001 | Int | 0.200 |
| Int (9–12) | 642 (47.9) | 2 (0.3) | 13 (2.1) | 25 (4.2) | 56 (11.4) | 79 (24.9) | High | <0.001 | |
| High (13–21) | 577 (43.1) | 10 (1.8) | 36 (6.4) | 64 (12.1) | 93 (19.7) | 115 (42.4) | Low | 0.002 | |
| Modified REACH-B | |||||||||
| Low (0–6) | 185 (13.8) | 0 (0.0) | 0 (0.0) | 2 (1.3) | 8 (6.7) | 12 (19.8) | <0.001 | Int | 0.020 |
| Int (7–11) | 846 (63.2) | 8 (1.0) | 24 (2.9) | 49 (6.3) | 86 (12.8) | 116 (28.9) | High | <0.001 | |
| High (12–16) | 308 (23.0) | 4 (1.3) | 25 (8.4) | 40 (14.0) | 61 (24.0) | 75 (45.1) | Low | <0.001 | |
| CAMD | |||||||||
| Low (0–7) | - | - | - | - | - | 0.003 | - | ||
| Int (8–13) | 408 (30.5) | 2 (0.5) | 8 (2.0) | 19 (5.1) | 33 (10.2) | 44 (21.4) | |||
| High (14–23) | 931 (69.5) | 10 (1.1) | 41 (4.6) | 72 (8.4) | 122 (16.5) | 159 (36.3) | |||
| aMAP | |||||||||
| Low (1–50) | 14 (1.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | - | 0.003 | Int | 0.400 |
| Int (50–60) | 142 (10.6) | 0 (0.0) | 1 (0.7) | 3 (2.4) | 5 (5.1) | 8 (14.2) | High | 0.002 | |
| High (60–100) | 1183 (88.3) | 12 (1.0) | 48 (4.2) | 88 (8.1) | 150 (15.8) | 195 (33.8) | Low | 0.100 | |
| HCC-RESCUE | |||||||||
| Low ( ≤ 64) | 43 (3.2) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (3.1) | 2 (9.2) | 0.070 | Int | 0.080 |
| Int (65–84) | 637 (47.6) | 2 (0.3) | 11 (1.8) | 31 (5.4) | 65 (13.5) | 93 (33.9) | High | 0.200 | |
| High (≥85) | 659 (49.2) | 10 (1.5) | 38 (5.9) | 60 (9.8) | 89 (16.3) | 108 (30.8) | Low | 0.050 | |
| AASL-HCC | |||||||||
| Low (0–5) | - | - | - | - | - | - | 0.003 | - | |
| Int (6–19) | 763 (57.0) | 2 (0.3) | 10 (1.6) | 29 (4.5) | 66 (11.7) | 96 (29.0) | |||
| High (20–29) | 576 (43.0) | 10 (1.8) | 27 (6.6) | 50 (11.2) | 77 (18.4) | 95 (35.3) | |||
| Toronto HCC Risk Index | |||||||||
| Low (0–120) | 45 (3.4) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 2 (26.2) | 0.030 | Int | 0.100 |
| Int (120–240) | 635 (47.4) | 3 (0.5) | 9 (1.5) | 25 (4.4) | 59 (12.4) | 90 (32.4) | High | 0.050 | |
| High (240–366) | 659 (49.2) | 9 (1.4) | 40 (6.3) | 66 (10.9) | 96 (17.6) | 111 (31.5) | Low | 0.040 | |
| PLAN-B | |||||||||
| Low (0.075–0.250) | 190 (20.9) | 1 (0.5) | 1 (0.5) | 2 (1.1) | 6 (4.9) | 10 (12.1) | <0.001 | Int | <0.001 |
| Int (0.250–0.500) | 832 (91.4) | 6 (0.7) | 20 (2.5) | 47 (6.2) | 95 (14.6) | 127 (33.4) | High | 0.020 | |
| High (0.500–1.000) | 317 (34.8) | 5 (1.6) | 28 (9.2) | 42 (14.3) | 54 (20.2) | 66 (35.8) | Low | <0.001 | |
| APA-B (n = 910) † | |||||||||
| Low (0–5) | 437 (48.0) | 2 (0.5) | 8 (1.9) | 16 (4.0) | 27 (7.5) | 34 (19.5) | <0.001 | Int | <0.001 |
| Int (6–9) | 306 (33.6) | 4 (1.3) | 21 (7.3) | 33 (11.9) | 50 (20.1) | 63 (40.6) | High | 0.050 | |
| High (10–15) | 167 (18.4) | 4 (2.5) | 11 (6.8) | 24 (15.6) | 38 (27.3) | 50 (66.7) | Low | <0.001 | |
| CAGE-B (n = 808) ‡ | |||||||||
| Low | 41 (5.1) | - | 0 (0.0) | 0 (0.0) | 1 (3.1) | 1 (3.1) | 0.001 | Int | 0.080 |
| Int | 468 (57.9) | - | 6 (1.3) | 17 (3.8) | 40 (11.2) | 51 (26.8) | High | 0.003 | |
| High | 299 (37.0) | - | 6 (2.0) | 22 (7.9) | 37 (14.9) | 56 (45.7) | Low | 0.010 | |
| SAGE-B (n = 808) ‡ | |||||||||
| Low | 198 (24.5) | - | 0 (0.0) | 1 (0.6) | 8 (5.4) | 11 (14.0) | <0.001 | Int | 0.003 |
| Int | 450 (55.7) | - | 8 (1.8) | 24 (5.5) | 47 (13.0) | 62 (33.7) | High | 0.002 | |
| High | 160 (19.8) | - | 4 (2.5) | 14 (9.5) | 23 (17.8) | 35 (50.5) | Low | <0.001 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, J.S.; Lim, T.S.; Lee, H.W.; Kim, S.U.; Park, J.Y.; Kim, D.Y.; Ahn, S.H.; Lee, H.W.; Lee, J.I.; Kim, J.K.; et al. Suboptimal Performance of Hepatocellular Carcinoma Prediction Models in Patients with Hepatitis B Virus-Related Cirrhosis. Diagnostics 2023, 13, 3. https://doi.org/10.3390/diagnostics13010003
Lee JS, Lim TS, Lee HW, Kim SU, Park JY, Kim DY, Ahn SH, Lee HW, Lee JI, Kim JK, et al. Suboptimal Performance of Hepatocellular Carcinoma Prediction Models in Patients with Hepatitis B Virus-Related Cirrhosis. Diagnostics. 2023; 13(1):3. https://doi.org/10.3390/diagnostics13010003
Chicago/Turabian StyleLee, Jae Seung, Tae Seop Lim, Hye Won Lee, Seung Up Kim, Jun Yong Park, Do Young Kim, Sang Hoon Ahn, Hyun Woong Lee, Jung Il Lee, Ja Kyung Kim, and et al. 2023. "Suboptimal Performance of Hepatocellular Carcinoma Prediction Models in Patients with Hepatitis B Virus-Related Cirrhosis" Diagnostics 13, no. 1: 3. https://doi.org/10.3390/diagnostics13010003
APA StyleLee, J. S., Lim, T. S., Lee, H. W., Kim, S. U., Park, J. Y., Kim, D. Y., Ahn, S. H., Lee, H. W., Lee, J. I., Kim, J. K., Min, I. K., & Kim, B. K. (2023). Suboptimal Performance of Hepatocellular Carcinoma Prediction Models in Patients with Hepatitis B Virus-Related Cirrhosis. Diagnostics, 13(1), 3. https://doi.org/10.3390/diagnostics13010003








