Decrease Retinal Thickness in Patients with Chronic Migraine Evaluated by Optical Coherence Tomography
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Sample
2.2. Procedures
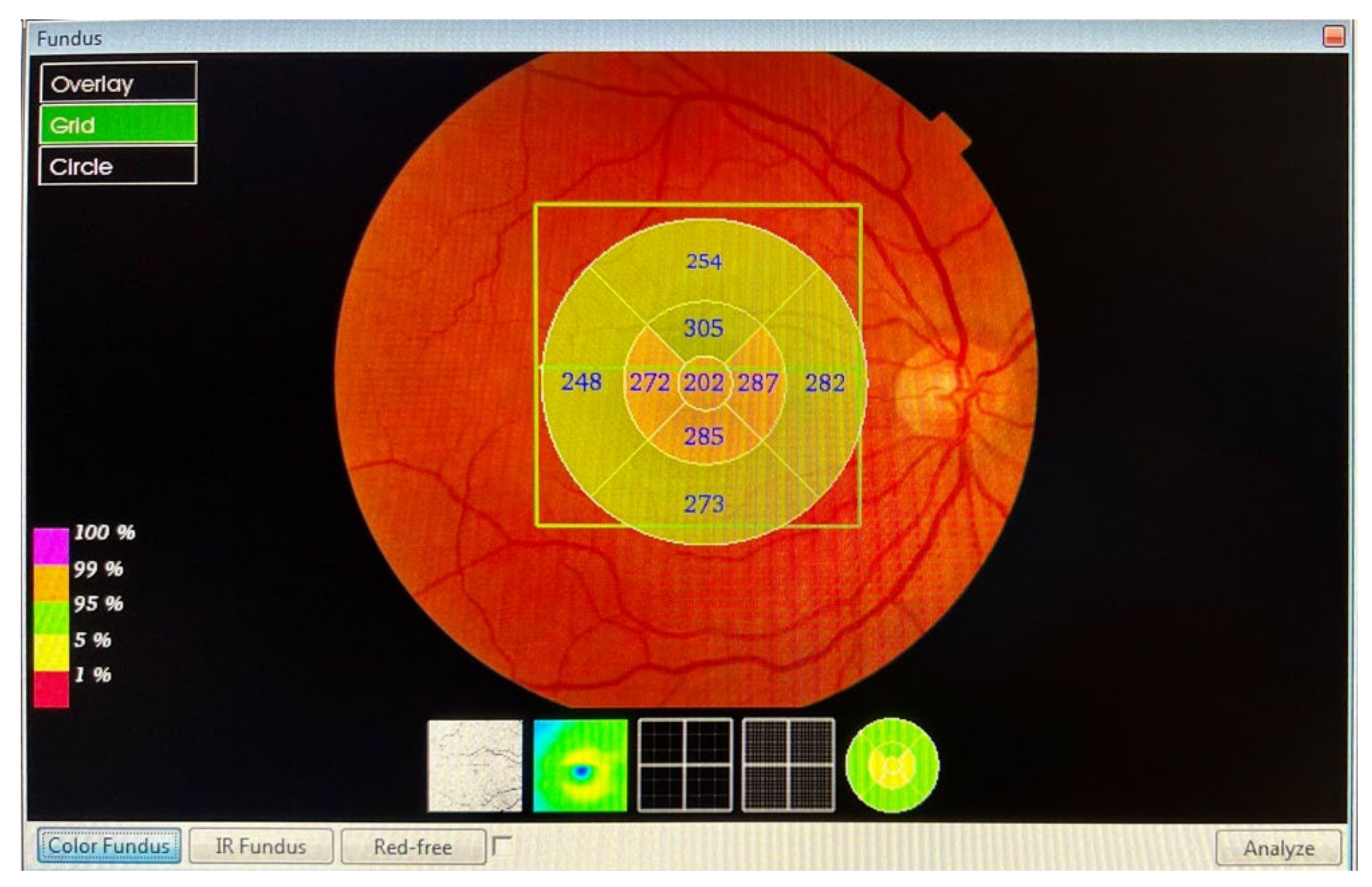
- Macula: from the inner limiting membrane (ILM) to the retinal pigment epithelium (RPE), the profile included, in nine ETDRS quadrants, the superior peripheral, superior, central, inferior, inferior peripheral, temporal peripheral, temporal, and nasal peripheral. The profile centred on the fovea.
- RNFL macular: from the ILM to RNFL, the profile included, in nine ETDRS quadrants, the superior peripheral, superior, central, inferior, inferior peripheral, temporal peripheral, temporal, and nasal peripheral. The profile focused on the fovea.
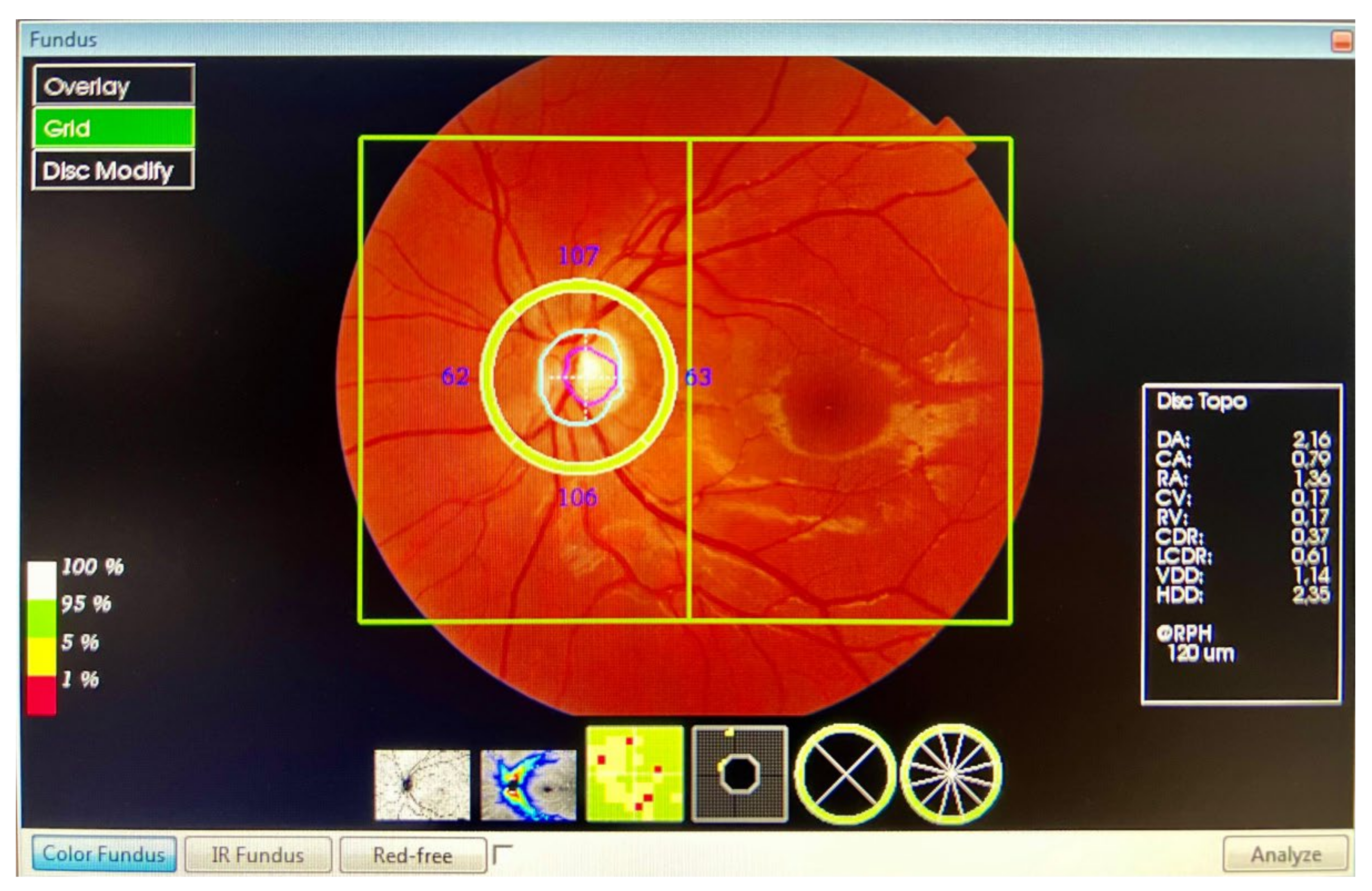
- Peripapillary RNFL: from ILM to the GCL, in four quadrants: superior, inferior, nasal, and temporal. The profile centred on the optic nerve.
- GCL++: from ILM to the IPL, in six ETDRS quadrants: superior, superior temporal, superior nasal, inferior, inferior temporal, and inferior nasal. The profile centred on the fovea.
- GCL+: from GCL to the IPL, in six ETDRS quadrants: superior, superior temporal, superior nasal, inferior, inferior temporal, and inferior nasal. The profile centred on the fovea.
2.3. Sample Size Calculation
2.4. Statistical Analysis
3. Results
3.1. Correlation between the Years with CM Diagnosis and Retinal Thickness
3.2. Relationship between Retinal Thickness and Pain Laterality regarding Chronic Migraines
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ferrari, M.D. The economic burden of migraine to society. PharmacoEconomics 1998, 13, 667–676. [Google Scholar] [CrossRef] [PubMed]
- Edmeads, J.; Mackell, J.A. The Economic Impact of Migraine: An Analysis of Direct and Indirect Costs. Headache: J. Head Face Pain 2002, 42, 501–509. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Doane, M.J.; Gupta, S.; Fang, J.; Laflamme, A.K.; Vo, P. The Humanistic and Economic Burden of Migraine in Europe: A Cross-Sectional Survey in Five Countries. Neurol. Ther. 2020, 9, 535–549. [Google Scholar] [CrossRef] [PubMed]
- Belvis, R.; Mas, N.; Roig, C. Changes introduced into the recent International Classification of Headache Disorders: ICHD-III beta classification. Rev. Neurol. 2015, 60, 81–89. [Google Scholar] [PubMed]
- Guldiken, B.; Guldiken, S.; Taskiran, B.; Koc, G.; Turgut, N.; Kabayel, L.; Tugrul, A. Migraine in Metabolic Syndrome. Neurol. 2009, 15, 55–58. [Google Scholar] [CrossRef]
- Granella, F.; Farina, S.; Malferrari, G.; Manzoni, G.C. Drug Abuse in Chronic Headache: A Clinico-Epidemiologic Study. Cephalalgia 1987, 7, 15–19. [Google Scholar] [CrossRef]
- Bigal, M.E.; Sheftell, F.D.; Rapoport, A.M.; Tepper, S.J.; Lipton, R.B. Chronic Daily Headache: Identification of Factors Associated With Induction and Transformation. Headache J. Head Face Pain 2002, 42, 575–581. [Google Scholar] [CrossRef]
- Barbanti, P.; Aurilia, C.; Egeo, G.; Fofi, L. Hypertension as a risk factor for migraine chronification. Neurol. Sci. 2010, 31, 41–43. [Google Scholar] [CrossRef]
- Ducros, A. Genetics of migraine. Pathol. Biol. 2000, 48, 658–662. [Google Scholar]
- Goadsby, P.; Charbit, A.; Andreou, A.; Akerman, S.; Holland, P. Neurobiology of migraine. Neuroscience 2009, 161, 327–341. [Google Scholar] [CrossRef]
- Ayata, C. Cortical Spreading Depression Triggers Migraine Attack: Pro. Headache: J. Head Face Pain 2010, 50, 725–730. [Google Scholar] [CrossRef]
- Karalezli, A.; Simsek, C.; Celik, G.; Eroglu, F.C. Evaluation of choroidal thickness using spectral-domain optical coherence tomog-raphy in migraine patients during acute migraine attacks: A comparative study. Eye 2014, 28, 1477–1481. [Google Scholar] [CrossRef] [Green Version]
- Demircan, S.; Atas, M.; Yüksel, S.A.; Ulusoy, M.D.; Yuvacı, I.; Arifoğlu, H.B.; Baskan, B.; Zararsız, G. The Impact of Migraine on Posterior Ocular Structures. J. Ophthalmol. 2015, 2015, 868967. [Google Scholar] [CrossRef]
- Abdul-Rahman, A.M.; Gilhotra, J.S.; Selva, D. Dynamic focal retinal arteriolar vasospasm in migraine. Indian J. Ophthalmol. 2011, 59, 51–53. [Google Scholar] [CrossRef]
- Fercher, A.F.; Drexler, W.; Hitzenberger, C.K.; Lasser, T. Optical coherence tomography—Principles and applications. Rep. Prog. Phys. 2003, 66, 239–303. [Google Scholar] [CrossRef]
- Gabriele, M.L.; Wollstein, G.; Ishikawa, H.; Kagemann, L.; Xu, J.; Folio, L.S.; Schuman, J.S. Optical coherence tomography: History, current status, and laboratory work. Investig. Ophthalmol. Vis. Sci. 2011, 52, 2425–2436. [Google Scholar] [CrossRef]
- Huang, D.; Swanson, E.A.; Lin, C.P.; Schuman, J.S.; Stinson, W.G.; Chang, W.; Hee, M.R.; Flotte, T.; Gregory, K.; Puliafito, C.A.; et al. Optical coherence tomography. Science 1991, 254, 1178–1181. [Google Scholar] [CrossRef] [Green Version]
- Blumenthal, E.Z.; Williams, J.M.; Weinreb, R.N.; Girkin, C.A.; Berry, C.C.; Zangwill, L.M. Reproducibility of nerve fiber layer thickness measurements by use of optical coherence tomography. Ophthalmology 2000, 107, 2278–2282. [Google Scholar] [CrossRef]
- Grulkowski, I.; Liu, J.J.; Potsaid, B.; Jayaraman, V.; Lu, C.D.; Jiang, J.; Cable, A.E.; Duker, J.S.; Fujimoto, J.G. Retinal, anterior segment and full eye imaging using ultrahigh speed swept source OCT with vertical-cavity surface emitting lasers. Biomed. Opt. Express 2012, 3, 2733–2751. [Google Scholar] [CrossRef] [Green Version]
- Konstantopoulos, A.; Hossain, P.; Anderson, D.F. Recent advances in ophthalmic anterior segment imaging: A new era for oph-thalmic diagnosis? Br. J. Ophthalmol. 2007, 91, 551–557. [Google Scholar] [CrossRef] [Green Version]
- Ran, A.R.; Cheung, C.Y.; Wang, X.; Chen, H.; Luo, L.Y.; Chan, P.P.; Wong, M.O.; Chang, R.T.; Mannil, S.S.; Young, A.L.; et al. Detection of glaucomatous optic neuropathy with spec-tral-domain optical coherence tomography: A retrospective training and validation deep-learning analysis. Lancet Digit. Health 2019, 1, e172–e182. [Google Scholar] [CrossRef] [PubMed]
- Daneshvar, R.; Yarmohammadi, A.; Alizadeh, R.; Henry, S.; Law, S.K.; Caprioli, J.; Nouri-Mahdavi, K. Prediction of Glaucoma Progression with Structural Parameters: Comparison of Optical Coherence Tomography and Clinical Disc Parameters. Am. J. Ophthalmol. 2019, 208, 19–29. [Google Scholar] [CrossRef] [PubMed]
- Medeiros, F.A.; Zangwill, L.M.; Bowd, C.; Vessani, R.M.; Susanna, R., Jr.; Weinreb, R.N. Evaluation of retinal nerve fiber layer, optic nerve head, and macular thickness measurements for glaucoma detection using optical coherence tomography. Am. J. Ophthalmol. 2005, 139, 44–55. [Google Scholar] [CrossRef] [PubMed]
- Avery, R.L.; Pieramici, D.J.; Rabena, M.D.; Castellarin, A.A.; Nasir, M.A.; Giust, M.J. Intravitreal Bevacizumab (Avastin) for Neovascular Age-Related Macular Degeneration. Ophthalmology 2006, 113, 363–372.e5. [Google Scholar] [CrossRef] [PubMed]
- Wojtkowski, M.; Srinivasan, V.; Fujimoto, J.G.; Ko, T.; Schuman, J.S.; Kowalczyk, A.; Duker, J.S. Three-dimensional Retinal Imaging with High-Speed Ultrahigh-Resolution Optical Coherence Tomography. Ophthalmology 2005, 112, 1734–1746. [Google Scholar] [CrossRef] [Green Version]
- Hee, M.R.; Puliafito, C.A.; Duker, J.S.; Reichel, E.; Coker, J.G.; Wilkins, J.R.; Schuman, J.S.; Swanson, E.A.; Fujimoto, J.G. Topography of diabetic macular edema with optical coherence tomography. Ophthalmology 1998, 105, 360–370. [Google Scholar] [CrossRef] [Green Version]
- Thomas, D.; Duguid, G. Optical coherence tomography—A review of the principles and contemporary uses in retinal investi-gation. Eye 2004, 18, 561–570. [Google Scholar] [CrossRef] [Green Version]
- Gupta, S.; Zivadinov, R.; Ramanathan, M.; Weinstock-Guttman, B. Optical coherence tomography and neurodegeneration: Are eyes the windows to the brain? Expert Rev. Neurother. 2016, 16, 765–775. [Google Scholar] [CrossRef]
- London, A.; Benhar, I.; Schwartz, M. The retina as a window to the brain—From eye research to CNS disorders. Nat. Rev. Neurol. 2013, 9, 44–53. [Google Scholar] [CrossRef]
- Ratchford, J.N.; Saidha, S.; Sotirchos, E.S.; Oh, J.A.; Seigo, M.A.; Eckstein, C.; Durbin, M.K.; Oakley, J.D.; Meyer, S.A.; Conger, A.; et al. Active MS is associated with accelerated retinal ganglion cell/inner plexiform layer thinning. Neurology 2013, 80, 47–54. [Google Scholar] [CrossRef] [Green Version]
- Maldonado, R.S.; Mettu, P.; El-Dairi, M.; Bhatti, M.T. The application of optical coherence tomography in neurologic diseases. Neurol. Clin. Pract. 2015, 5, 460–469. [Google Scholar] [CrossRef]
- Saidha, S.; Al-Louzi, O.; Ratchford, J.N.; Bhargava, P.; Oh, J.; Newsome, S.D.; Prince, J.L.; Pham, D.; Roy, S.; van Zijl, P.; et al. Optical coherence tomography reflects brain at-rophy in multiple sclerosis: A four-year study. Ann. Neurol. 2015, 78, 801–813. [Google Scholar] [CrossRef] [Green Version]
- Ascaso, F.J.; Cruz, N.; Modrego, P.J.; Lopez-Anton, R.; Santabárbara, J.; Pascual, L.F.; Lobo, A.; Cristóbal, J.A. Retinal alterations in mild cognitive im-pairment and Alzheimer’s disease: An optical coherence tomography study. J. Neurol. 2014, 261, 1522–1530. [Google Scholar] [CrossRef]
- González de la Aleja, J.; Guerrero-Molina, M.; Saíz-Díaz, R.A.; López-Muñoz, F.; Raga-Martínez, I.; Hernández-Gallego, J.; Navarrete-Chamorro, P.; Povedano-Montero, F.J. Peripapillary retinal nerve fibre layer thinning in genetic generalized epilepsy. Seizure 2019, 71, 201–206. [Google Scholar] [CrossRef]
- Álvarez-Sesmero, S.; Povedano-Montero, F.J.; Arias-Horcajadas, F.; Marín-Mayor, M.; Navarrete-Chamorro, P.; Raga-Martínez, I.; Rubio, G.; López-Muñoz, F. Retinal Nerve Fiber Layer in Patients with Alcohol Use Disorder. Appl. Sci. 2019, 9, 5331. [Google Scholar] [CrossRef] [Green Version]
- Martinez, A.; Proupim, N.; Sanchez, M. Retinal nerve fibre layer thickness measurements using optical coherence tomography in migraine patients. Br. J. Ophthalmol. 2008, 92, 1069–1075. [Google Scholar] [CrossRef]
- Stewart, W.F.; Lipton, R.B.; Kolodner, K.B.; Sawyer, J.; Lee, C.; Liberman, J.N. Validity of the Migraine Disability Assessment (MIDAS) score in comparison to a diary-based measure in a population sample of migraine sufferers. Pain 2000, 88, 41–52. [Google Scholar] [CrossRef]
- Stewart, W.F.; Lipton, R.B.; Whyte, J.; Dowson, A.; Kolodner, K.; Liberman, J.N.; Sawyer, J. An international study to assess reliability of the Migraine Disability Assessment (MIDAS) score. Neurology 1999, 53, 988. [Google Scholar] [CrossRef]
- Hospital, E.O.; Palomar, E.V.; Cipres, M.; Obis, J.; Rodrigo, M.; Satue, M.; Garcia-Martin, E. Reproducibility of the measurements taken with swept source optical coherence tomography. Acta Ophthalmol. 2017, 95, s259. [Google Scholar] [CrossRef]
- Hong, E.H.; Ryu, S.J.; Kang, M.H.; Seong, M.; Cho, H.; Yeom, J.H.; Shin, Y.U. Comparison of repeatability of swept-source and spectral-domain optical coherence tomography for measuring inner retinal thickness in retinal disease. PLoS ONE 2019, 14, e0210729. [Google Scholar] [CrossRef] [Green Version]
- Feng, Y.-F.; Guo, H.; Huang, J.-H.; Yu, J.-G.; Yuan, F. Retinal Nerve Fiber Layer Thickness Changes in Migraine: A Meta-Analysis of Case–Control Studies. Curr. Eye Res. 2016, 41, 814–822. [Google Scholar] [CrossRef] [PubMed]
- Kowalska, M.; Prendecki, M.; Kozubski, W.; Lianeri, M.; Dorszewska, J. Molecular factors in migraine. Oncotarget 2016, 7, 50708–50718. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gipponi, S.; Scaroni, N.; Venturelli, E.; Forbice, E.; Rao, R.; Liberini, P.; Padovani, A.; Semeraro, F. Reduction in retinal nerve fiber layer thickness in migraine patients. Neurol. Sci. 2013, 34, 841–845. [Google Scholar] [CrossRef] [PubMed]
- Colak, H.N.; Kantarci, F.A.; Tatar, M.G.; Eryilmaz, M.; Uslu, H.; Goker, H.; Yildirim, A.; Gürler, B. Retinal nerve fiber layer, ganglion cell complex, and choroidal thicknesses in migraine. Arq. Bras. de Oftalmol. 2015, 79, 78–81. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kirbas, S.; Tufekci, A.; Turkyilmaz, K.; Kirbas, A.; Oner, V.; Durmus, M. Evaluation of the retinal changes in patients with chronic migraine. Acta Neurol. Belg. 2013, 113, 167–172. [Google Scholar] [CrossRef]
- Ekinci, M.; Ceylan, E.; Çağatay, H.H.; Keles, S.; Huseyinoglu, N.; Tanyıldız, B.; Çakıcı, Ö.; Kartal, B. Retinal nerve fibre layer, ganglion cell layer and choroid thinning in migraine with aura. BMC Ophthalmol. 2014, 14, 75. [Google Scholar] [CrossRef] [Green Version]
- Yülek, F.; Dirik, E.B.; Eren, Y.; Simavli, H.; Uğurlu, N.; Çağıl, N.; Şimşek, Ş. Macula and retinal nerve fiber layer in migraine patients: Analysis by spectral domain optic coherence tomography. Semin. Ophthalmol. 2015, 30, 124–128. [Google Scholar] [CrossRef]
- Reggio, E.; Chisari, C.G.; Ferrigno, G.; Patti, F.; Donzuso, G.; Sciacca, G.; Avitabile, T.; Faro, S.; Zappia, M. Migraine causes retinal and choroidal structural changes: Evaluation with ocular coherence tomography. J. Neurol. 2017, 264, 494–502. [Google Scholar] [CrossRef]
- Kara, S.A.; Erdemoǧlu, A.K.; Karadeniz, M.Y.; Altmok, D. Color Doppler sonography of orbital and vertebral arteries in migraineurs without aura. J. Clin. Ultrasound 2003, 31, 308–314. [Google Scholar] [CrossRef]
- McKendrick, A.M.; Vingrys, A.; Badcock, D.; Heywood, J.T. Visual field losses in subjects with migraine headaches. Investig. Ophthalmol. Vis. Sci. 2000, 41, 1239–1247. [Google Scholar]
- Lewis, R.A.; Vijayan, N.; Watson, C.; Keltner, J.; Johnson, C.A. Visual Field Loss in Migraine. Ophthalmology 1989, 96, 321–326. [Google Scholar] [CrossRef]
- McKendrick, A.M.; Badcock, D.R. Decreased visual field sensitivity measured 1 day, then 1 week, after migraine. Investig. Opthalmology Vis. Sci. 2004, 45, 1061–1070. [Google Scholar] [CrossRef]
- Omoǧlu, S.; Yarangümeli, A.; Köz, Ö.G.; Elhan, A.H.; Kural, G. Glaucomatous visual field defects in patients with migraine. J. Neurol. 2003, 250, 201–206. [Google Scholar]
- McKendrick, A.M.; Cioffi, G.A.; Johnson, C.A. Short-wavelength sensitivity deficits in patients with migraine. Arch. Ophthalmol. 2002, 120, 154–161. [Google Scholar] [CrossRef]
- Tietjen, G.E. Migraine as a Systemic Vasculopathy. Cephalalgia 2009, 29, 989–996. [Google Scholar] [CrossRef]
- Bigal, M.E.; Kurth, T.; Hu, H.; Santanello, N.; Lipton, R.B. Migraine and cardiovascular disease: Possible mechanisms of interaction. Neurology 2009, 72, 1864–1871. [Google Scholar] [CrossRef] [Green Version]
- Yanagi, M.; Kawasaki, R.; Wang, J.J.; Wong, T.Y.; Crowston, J.; Kiuchi, Y. Vascular risk factors in glaucoma: A review. Clin. Exp. Ophthalmol. 2011, 39, 252–258. [Google Scholar] [CrossRef]
- Kim, C.; Kim, T.-W. Comparison of Risk Factors for Bilateral and Unilateral Eye Involvement in Normal-Tension Glaucoma. Investig. Opthalmology Vis. Sci. 2009, 50, 1215–1220. [Google Scholar] [CrossRef]
- Drance, S.; Anderson, D.R.; Schulzer, M.; the Collaborative Normal-Tension Glaucoma Study Group. Risk factors for progression of visual field abnormalities in normal-tension glaucoma. Am. J. Ophthalmol. 2001, 131, 699–708. [Google Scholar] [CrossRef]
- Jie Jin, W.; Mitchell, P.; Smith, W. Is there an association between migraine headache and open-angle glaucoma? Findings from the Blue Mountains Eye Study. Ophthalmology 1997, 104, 1714–1719. [Google Scholar]
- Gunes, A.; Demirci, S.; Tok, L.; Tok, O.; Kutluhan, S. Is Retinal Nerve Fiber Layer Thickness Change Related to Headache Lateral-ization in Migraine? Korean J. Ophthalmol. 2016, 30, 134–139. [Google Scholar] [CrossRef] [PubMed]



| CM Patients (n = 90) | Control Subjects (n = 90) | |
|---|---|---|
| Age (years) | 42.3 (9.2) | 41.6 (10.6) |
| Males/Females | 12 (14.3%)/78 (85.7%) | 12 (14.3%)/78 (85.7%) |
| Intraocular pressure (mm Hg) | 15.9 (2.1) | 15.7 (1.2) |
| Spherical equivalent (dp) | 0.19 (1.98) | 0.15 (1.82) |
| Visual acuity (decimal) | 0.96 (0.18) | 1.06 (0.05) |
| Evolution years EM | 20.4 (10.3) | |
| Evolution years CM | 8.8 (3.8) | |
| Laterality CM | 54 patients (60%) right 36 patients (40%) left | |
| Visual aura | 14 women (15.6%) 2 men (2.2%) | |
| Attacks per month | 18.4 (4.7) | |
| MIDAS scale | 38.5 (13.7) grade IV |
| Control Subjects n = 90 | SD Control (±) | Migraine Patients n = 90 | SD Migraine (±) | p Value |
|---|---|---|---|---|
| 286.09 | 11.74 | 281.52 | 12.41 | 0.012 |
| Quadrants (µm) | Control Subjects n = 90 | SD Control (±) | Migraine Patients n = 90 | SD Migraine (±) | p Value |
|---|---|---|---|---|---|
| Peripheral superior | 275.49 | 14.61 | 272.02 | 13.70 | 0.102 |
| Superior | 315.33 | 12.50 | 308.74 | 14.90 | 0.002 * |
| Central | 239.52 | 18.45 | 234.12 | 21.13 | 0.070 |
| Inferior | 313.14 | 12.42 | 307.42 | 15.15 | 0.006 |
| Peripheral inferior | 267.80 | 16.47 | 261.87 | 13.55 | 0.009 |
| Peripheral temporal | 257.81 | 14.28 | 254.93 | 12.83 | 0.157 |
| Temporal | 301.07 | 11.95 | 296.26 | 15.32 | 0.020 |
| Nasal temporal | 289.13 | 15.65 | 285.72 | 14.86 | 0.131 |
| Nasal | 315.84 | 12.89 | 310.08 | 15.68 | 0.008 |
| Control Subjects n = 90 | SD Control (±) | Migraine Patients n = 90 | SD Migraine (±) | p Value |
|---|---|---|---|---|
| 106.53 | 9.36 | 104.96 | 9.23 | 0.261 |
| Quadrants (µm) | Control Subjects n = 90 | SD Control (±) | Migraine Patients n = 90 | SD Migraine (±) | p Value |
|---|---|---|---|---|---|
| Superior | 129.90 | 15.43 | 125.87 | 14.19 | 0.036 |
| Inferior | 136.81 | 15.23 | 133.31 | 14.65 | 0.118 |
| Temporal | 74.24 | 11.81 | 72.57 | 11.20 | 0.329 |
| Nasal | 74.80 | 14.61 | 75.69 | 12.57 | 0.662 |
| Quadrants (µm) | Control Subjects n = 90 | SD Control (±) | Migraine Patients n = 90 | SD Migraine (±) | p Value |
|---|---|---|---|---|---|
| RNFL macular | 28.90 | 2.01 | 28.13 | 2.58 | 0.026 |
| GCL++ 1 | 108.76 | 6.55 | 105.85 | 8.61 | 0.012 |
| GCL+ 2 | 73.76 | 5.20 | 71.68 | 5.53 | 0.010 |
| Quadrants (µm) | Control Subjects n = 90 | SD Control (±) | Migraine Patients n = 90 | SD Migraine (±) | p Value |
|---|---|---|---|---|---|
| Peripheral superior | 40.08 | 3.78 | 38.79 | 3.95 | 0.026 |
| Superior | 27.32 | 2.25 | 26.80 | 2.25 | 0.121 |
| Central | 3.94 | 2.19 | 3.60 | 2.67 | 0.345 |
| Inferior | 28.41 | 4.07 | 27.27 | 2.41 | 0.023 |
| Peripheral inferior | 41.77 | 3.67 | 40.38 | 5.54 | 0.050 |
| Peripheral temporal | 22.89 | 2.43 | 22.91 | 2.50 | 0.952 |
| Temporal | 20.68 | 1.87 | 20.22 | 1.92 | 0.109 |
| Nasal temporal | 51.52 | 5.05 | 48.89 | 2.63 | 0.014 |
| Nasal | 23.82 | 1.91 | 23.11 | 2.31 | 0.026 |
| Quadrants (µm) | Control Subjects n = 90 | SD Control (±) | Migraine Patients n = 90 | SD Migraine (±) | p Value |
|---|---|---|---|---|---|
| Superior | 109.83 | 6.97 | 106.61 | 7.67 | 0.004 * |
| Superior temporal | 96.46 | 6.00 | 94.11 | 7.26 | 0.085 |
| Superior nasal | 118.17 | 13.55 | 113.84 | 16.09 | 0.050 |
| Inferior | 107.81 | 6.96 | 105.00 | 9.01 | 0.021 |
| Inferior temporal | 99.56 | 8.45 | 97.46 | 11.70 | 0.169 |
| Inferior nasal | 121.93 | 7.19 | 118.46 | 11.16 | 0.014 |
| Quadrants (µm) | Control Subjects n = 90 | SD Control (±) | Migraine Patients n = 90 | SD Migraine (±) | p Value |
|---|---|---|---|---|---|
| Superior | 73.26 | 5.17 | 71.04 | 5.40 | 0.006 * |
| Superior temporal | 72.50 | 5.42 | 70.17 | 6.13 | 0.007 * |
| Superior nasal | 76.38 | 5.94 | 74.52 | 5.84 | 0.036 |
| Inferior | 70.42 | 5.25 | 68.37 | 5.44 | 0.011 |
| Inferior temporal | 74.63 | 5.54 | 72.32 | 6.69 | 0.012 |
| Inferior nasal | 75.94 | 5.75 | 73.92 | 5.63 | 0.018 |
| Average Macula | Years | ||
|---|---|---|---|
| Average macula | Pearson correlation | 1 | −0.873 * |
| Sig. (bilateral) | <0.01 | ||
| n | 90 | 90 | |
| Years | Pearson correlation | −0.873 * | 1 |
| Sig. (bilateral) | <0.01 | ||
| n | 90 | 90 | |
| Average GCL++ | Years | ||
|---|---|---|---|
| Average GCL++ | Pearson correlation | 1 | −0.545 * |
| Sig. (bilateral) | <0.01 | ||
| n | 90 | 90 | |
| Years | Pearson correlation | −0.545 * | 1 |
| Sig. (bilateral) | <0.01 | ||
| n | 90 | 90 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Raga-Martínez, I.; Povedano-Montero, F.J.; Hernández-Gallego, J.; López-Muñoz, F. Decrease Retinal Thickness in Patients with Chronic Migraine Evaluated by Optical Coherence Tomography. Diagnostics 2023, 13, 5. https://doi.org/10.3390/diagnostics13010005
Raga-Martínez I, Povedano-Montero FJ, Hernández-Gallego J, López-Muñoz F. Decrease Retinal Thickness in Patients with Chronic Migraine Evaluated by Optical Coherence Tomography. Diagnostics. 2023; 13(1):5. https://doi.org/10.3390/diagnostics13010005
Chicago/Turabian StyleRaga-Martínez, Isidoro, Francisco J. Povedano-Montero, Jesús Hernández-Gallego, and Francisco López-Muñoz. 2023. "Decrease Retinal Thickness in Patients with Chronic Migraine Evaluated by Optical Coherence Tomography" Diagnostics 13, no. 1: 5. https://doi.org/10.3390/diagnostics13010005
APA StyleRaga-Martínez, I., Povedano-Montero, F. J., Hernández-Gallego, J., & López-Muñoz, F. (2023). Decrease Retinal Thickness in Patients with Chronic Migraine Evaluated by Optical Coherence Tomography. Diagnostics, 13(1), 5. https://doi.org/10.3390/diagnostics13010005