Advanced MR Imaging for Knee Osteoarthritis: A Review on Local and Brain Effects
Abstract
:1. Introduction
2. Hyaline Articular Cartilage of the Knee: Anatomy and Biomechanics
3. Role of Standard Magnetic Resonance Imaging Sequences in Knee Osteoarthritis Diagnosis
4. Role of Novel Compositional MR Imaging Techniques for Hyaline Knee Cartilage Evaluation
4.1. T1ρ Mapping
4.2. T2 Mapping
4.3. T2 Star (T2*) Mapping
4.4. Ultrashort TE
4.5. Diffusion-Weighted Imaging (DWI) and Diffusion Tensor Imaging (DTI)
4.6. Sodium Magnetic Resonance Imaging (23Na+-MRI)
4.7. Glycosaminoglycan Chemical Exchange Saturation Transfer (GagCEST)
4.8. Delayed Gadolinium-Enhanced MRI of Cartilage (dGEMRIC)
5. Diagnostic Perspective: Artificial Intelligence
6. Treatment of Osteoarthritis and MRI as a Tool to Follow-Up Therapies
7. MRI Effects of Knee Osteoarthritis on the Brain
7.1. Structural Changes
7.2. Functional Connectivity
7.3. Cerebral Blood Flow
8. Summary
9. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sharma, L. Osteoarthritis of the Knee. N. Engl. J. Med. 2021, 384, 51–59. [Google Scholar] [CrossRef] [PubMed]
- Cross, M.; Smith, E.; Hoy, D.; Nolte, S.; Ackerman, I.; Fransen, M.; Bridgett, L.; Williams, S.; Guillemin, F.; Hill, C.L.; et al. The global burden of hip and knee osteoarthritis: Estimates from the Global Burden of Disease 2010 study. Ann. Rheum. Dis. 2014, 73, 1323–1330. [Google Scholar] [CrossRef]
- Hunter, D.J.; Bierma-Zeinstra, S. Osteoarthritis. Lancet 2019, 393, 1745–1759. [Google Scholar] [CrossRef] [PubMed]
- Krakowski, P.; Karpiński, R.; Maciejewski, R.; Jonak, J.; Jurkiewicz, A. Short-Term Effects of Arthroscopic Microfracturation of Knee Chondral Defects in Osteoarthritis. Appl. Sci. 2020, 10, 8312. [Google Scholar] [CrossRef]
- Oei, E.; Hirvasniemi, J.; van Zadelhoff, T.; van der Heijden, R. Osteoarthritis year in review 2021: Imaging. Osteoarthr. Cartil. 2021, 30, 226–236. [Google Scholar] [CrossRef] [PubMed]
- Hsu, H.; Siwiec, R.M. Knee Osteoarthritis; StatPearls: Tampa, FL, USA, 2022. [Google Scholar]
- Krakowski, P.; Nogalski, A.; Jurkiewicz, A.; Karpiński, R.; Maciejewski, R.; Jonak, J. Comparison of Diagnostic Accuracy of Physical Examination and MRI in the Most Common Knee Injuries. Appl. Sci. 2019, 9, 4102. [Google Scholar] [CrossRef] [Green Version]
- Jain, S.; Gupta, S.; Naugraiya, T. Changes in Hematobiochemical, Radiological, and Synovial Fluid Parameter in Patients of Osteoarthritis Knee with Effusion: A Prospective Observational Study. J. Orthop. CASE Rep. 2021, 11, 87–91. [Google Scholar] [CrossRef]
- Quattrocchi, C.C.; Giona, A.; Di Martino, A.; Gaudino, F.; Mallio, C.A.; Errante, Y.; Occhicone, F.; Vitali, M.A.; Zobel, B.B.; Denaro, V. Lumbar subcutaneous edema and degenerative spinal disease in patients with low back pain: A retrospective MRI study. Musculoskelet. Surg. 2015, 99, 159–163. [Google Scholar] [CrossRef]
- Hayashi, D.; Roemer, F.W.; Guermazi, A. Imaging of Osteoarthritis by Conventional Radiography, MR Imaging, PET–Computed Tomography, and PET–MR Imaging. PET Clin. 2018, 14, 17–29. [Google Scholar] [CrossRef]
- Heidari, B. Knee osteoarthritis prevalence, risk factors, pathogenesis and features: Part I. Caspian J. Intern. Med. 2011, 2, 205–212. [Google Scholar]
- Mathiessen, A.; Cimmino, M.A.; Hammer, H.B.; Haugen, I.K.; Iagnocco, A.; Conaghan, P.G. Imaging of osteoarthritis (OA): What is new? Best Pr. Res. Clin. Rheumatol. 2016, 30, 653–669. [Google Scholar] [CrossRef] [PubMed]
- Karpiński, R. Knee joint osteoarthritis diagnosis based on selected acoustic signal discriminants using machine learning. Appl. Comput. Sci. 2022, 18, 71–85. [Google Scholar] [CrossRef]
- Ahn, J.M.; El-Khoury, G.Y. Computed Tomography of Knee Injuries. Imaging Decis. MRI 2006, 10, 14–23. [Google Scholar] [CrossRef]
- Karpiński, R.; Krakowski, P.; Jonak, J.; Machrowska, A.; Maciejewski, M.; Nogalski, A. Diagnostics of Articular Cartilage Damage Based on Generated Acoustic Signals Using ANN—Part I: Femoral-Tibial Joint. Sensors 2022, 22, 2176. [Google Scholar] [CrossRef] [PubMed]
- Yusuf, E.; Kortekaas, M.C.; Watt, I.; Huizinga, T.W.J.; Kloppenburg, M. Do knee abnormalities visualised on MRI explain knee pain in knee osteoarthritis? A systematic review. Ann. Rheum. Dis. 2011, 70, 60–67. [Google Scholar] [CrossRef]
- Chaudhari, A.S.; Kogan, F.; Pedoia, V.; Majumdar, S.; Gold, G.E.; Hargreaves, B.A. Rapid Knee MRI Acquisition and Analysis Techniques for Imaging Osteoarthritis. J. Magn. Reson. Imaging 2020, 52, 1321–1339. [Google Scholar] [CrossRef]
- Koff, M.F.; Amrami, K.K.; Kaufman, K.R. Clinical evaluation of T2 values of patellar cartilage in patients with osteoarthritis. Osteoarthr. Cartil. 2007, 15, 198–204. [Google Scholar] [CrossRef] [Green Version]
- Stehling, C.; Liebl, H.; Krug, R.; Lane, N.E.; Nevitt, M.C.; Lynch, J.; McCulloch, C.E.; Link, T.M. Patellar Cartilage: T2 Values and Morphologic Abnormalities at 3.0-T MR Imaging in Relation to Physical Activity in Asymptomatic Subjects from the Osteoarthritis Initiative. Radiology 2010, 254, 509–520. [Google Scholar] [CrossRef] [Green Version]
- Mittal, S.; Pradhan, G.; Singh, S.; Batra, R. T1 and T2 mapping of articular cartilage and menisci in early osteoarthritis of the knee using 3-Tesla magnetic resonance imaging. Pol. J. Radiol. 2019, 84, 549–564. [Google Scholar] [CrossRef]
- Dunn, T.C.; Lu, Y.; Jin, H.; Ries, M.D.; Majumdar, S. T2 Relaxation Time of Cartilage at MR Imaging: Comparison with Severity of Knee Osteoarthritis. Radiology 2004, 232, 592–598. [Google Scholar] [CrossRef]
- Emanuel, K.S.; Kellner, L.J.; Peters, M.J.M.; Haartmans, M.J.J.; Hooijmans, M.T.; Emans, P.J. The relation between the biochemical composition of knee articular cartilage and quantitative MRI: A systematic review and meta-analysis. Osteoarthr. Cartil. 2021, 30, 650–662. [Google Scholar] [CrossRef] [PubMed]
- Arendt, E.A.; Miller, L.; Block, J.E. Early knee osteoarthritis management should first address mechanical joint overload. Orthop. Rev. 2014, 6, 5188. [Google Scholar] [CrossRef] [PubMed]
- Wheaton, A.J.; Dodge, G.R.; Borthakur, A.; Kneeland, J.B.; Schumacher, H.R.; Reddy, R. Detection of changes in articular cartilage proteoglycan byT1ρ magnetic resonance imaging. J. Orthop. Res. 2005, 23, 102–108. [Google Scholar] [CrossRef] [Green Version]
- Sonin, A.H.; Pensy, R.A.; Mulligan, M.E.; Hatem, S. Grading Articular Cartilage of the Knee Using Fast Spin-Echo Proton Density-Weighted MR Imaging Without Fat Suppression. Am. J. Roentgenol. 2002, 179, 1159–1166. [Google Scholar] [CrossRef] [PubMed]
- Crema, M.D.; Roemer, F.W.; Marra, M.D.; Burstein, D.; Gold, G.E.; Eckstein, F.; Baum, T.; Mosher, T.; Carrino, J.A.; Guermazi, A. Articular Cartilage in the Knee: Current MR Imaging Techniques and Applications in Clinical Practice and Research. Radiographics 2011, 31, 37–61. [Google Scholar] [CrossRef] [Green Version]
- Rodrigues, M.B.; Camanho, G.L. MRI evaluation of knee cartilage. Rev. Bras. Ortop. 2010, 45, 340–346. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gold, G.E.; Hargreaves, B.A.; Stevens, K.J.; Beaulieu, C.F. Advanced Magnetic Resonance Imaging of Articular Cartilage. Orthop. Clin. N. Am. 2006, 37, 331–347. [Google Scholar] [CrossRef]
- Stahl, R.; Luke, A.; Li, X.; Carballido-Gamio, J.; Ma, C.B.; Majumdar, S.; Link, T.M. T1rho, T2 and focal knee cartilage abnormalities in physically active and sedentary healthy subjects versus early OA patients—A 3.0-Tesla MRI study. Eur. Radiol. 2008, 19, 132–143. [Google Scholar] [CrossRef]
- Banjar, M.; Horiuchi, S.; Gedeon, D.N.; Yoshioka, H. Review of Quantitative Knee Articular Cartilage MR Imaging. Magn. Reson. Med. Sci. 2022, 21, 29–40. [Google Scholar] [CrossRef]
- Marchiori, G.; Cassiolas, G.; Berni, M.; Grassi, A.; Fabbro, G.D.; Fini, M.; Filardo, G.; Zaffagnini, S.; Lopomo, N.F. A Comprehensive Framework to Evaluate the Effects of Anterior Cruciate Ligament Injury and Reconstruction on Graft and Cartilage Status through the Analysis of MRI T2 Relaxation Time and Knee Laxity: A Pilot Study. Life 2021, 11, 1383. [Google Scholar] [CrossRef]
- Mallio, C.A.; Vadalà, G.; Russo, F.; Bernetti, C.; Ambrosio, L.; Zobel, B.B.; Quattrocchi, C.C.; Papalia, R.; Denaro, V. Novel Magnetic Resonance Imaging Tools for the Diagnosis of Degenerative Disc Disease: A Narrative Review. Diagnostics 2022, 12, 420. [Google Scholar] [CrossRef] [PubMed]
- Krakowski, P.; Karpiński, R.; Jojczuk, M.; Nogalska, A.; Jonak, J. Knee MRI Underestimates the Grade of Cartilage Lesions. Appl. Sci. 2021, 11, 1552. [Google Scholar] [CrossRef]
- Link, T.M.; Neumann, J.; Li, X. Prestructural cartilage assessment using MRI. J. Magn. Reson. Imaging 2017, 45, 949–965. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guermazi, A.; Alizai, H.; Crema, M.D.; Trattnig, S.; Regatte, R.; Roemer, F. Compositional MRI techniques for evaluation of cartilage degeneration in osteoarthritis. Osteoarthr. Cartil. 2015, 23, 1639–1653. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nishioka, H.; Nakamura, E.; Hirose, J.; Okamoto, N.; Yamabe, S.; Mizuta, H. MRI T1ρ and T2 mapping for the assessment of articular cartilage changes in patients with medial knee osteoarthritis after hemicallotasis osteotomy. Bone Jt. Res. 2016, 5, 294–300. [Google Scholar] [CrossRef]
- Lin, G.; Lan, F.; Wu, D.; Cao, G.; Li, Z.; Qi, Z.; Liu, Y.; Yang, S.; Lu, J.; Wang, T. Resting-state functional connectivity alteration in elderly patients with knee osteoarthritis and declined cognition: An observational study. Front. Aging Neurosci. 2022, 14, 1002642. [Google Scholar] [CrossRef]
- Goto, H.; Iwama, Y.; Fujii, M.; Aoyama, N.; Kubo, S.; Kuroda, R.; Ohno, Y.; Sugimura, K. A preliminary study of the T1rho values of normal knee cartilage using 3T-MRI. Eur. J. Radiol. 2012, 81, e796–e803. [Google Scholar] [CrossRef]
- Carballido-Gamio, J.; Joseph, G.B.; Lynch, J.A.; Link, T.M.; Majumdar, S. Longitudinal analysis of MRI T 2 knee cartilage laminar organization in a subset of patients from the osteoarthritis initiative: A texture approach. Magn. Reson. Med. 2010, 65, 1184–1194. [Google Scholar] [CrossRef]
- Hofmann, F.C.; Neumann, J.; Heilmeier, U.; Joseph, G.B.; Nevitt, M.C.; McCulloch, C.E.; Link, T.M. Conservatively treated knee injury is associated with knee cartilage matrix degeneration measured with MRI-based T2 relaxation times: Data from the osteoarthritis initiative. Skelet. Radiol. 2018, 47, 93–106. [Google Scholar] [CrossRef] [Green Version]
- Zhang, P.; Yu, B.; Zhang, R.; Chen, X.; Shao, S.; Zeng, Y.; Cui, J.; Zhao, J. Longitudinal study of the morphological and T2* changes of knee cartilages of marathon runners using prototype software for automatic cartilage segmentation. Br. J. Radiol. 2021, 94, 20200833. [Google Scholar] [CrossRef]
- Williams, A.A.; Erhart-Hledik, J.C.; Asay, J.L.; Mahtani, G.B.; Titchenal, M.R.; Lutz, A.M.; Andriacchi, T.P.; Chu, C.R. Patient-Reported Outcomes and Knee Mechanics Correlate with Patellofemoral Deep Cartilage UTE-T2* 2 Years After Anterior Cruciate Ligament Reconstruction. Am. J. Sports Med. 2021, 49, 675–683. [Google Scholar] [CrossRef] [PubMed]
- Ukai, T.; Sato, M.; Yamashita, T.; Imai, Y.; Mitani, G.; Takagaki, T.; Serigano, K.; Mochida, J. Diffusion tensor imaging can detect the early stages of cartilage damage: A comparison study. BMC Musculoskelet. Disord. 2015, 16, 35. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zaric, O.; Juras, V.; Szomolanyi, P.; Schreiner, M.; Raudner, M.; Giraudo, C.; Trattnig, S. Frontiers of Sodium MRI Revisited: From Cartilage to Brain Imaging. J. Magn. Reson. Imaging 2020, 54, 58–75. [Google Scholar] [CrossRef] [PubMed]
- Brinkhof, S.; Haghnejad, A.A.; Ito, K.; Bloch, K.M.; Klomp, D. Uncompromised MRI of knee cartilage while incorporating sensitive sodium MRI. NMR Biomed. 2019, 32, e4173. [Google Scholar] [CrossRef] [Green Version]
- Madelin, G.; Xia, D.; Brown, R.; Babb, J.; Chang, G.; Krasnokutsky, S.; Regatte, R.R. Longitudinal study of sodium MRI of articular cartilage in patients with knee osteoarthritis: Initial experience with 16-month follow-up. Eur. Radiol. 2017, 28, 133–142. [Google Scholar] [CrossRef]
- Joseph, G.B.; McCulloch, C.E.; Nevitt, M.C.; Link, T.M.; Sohn, J.H. Machine learning to predict incident radiographic knee osteoarthritis over 8 Years using combined MR imaging features, demographics, and clinical factors: Data from the Osteoarthritis Initiative. Osteoarthr. Cartil. 2021, 30, 270–279. [Google Scholar] [CrossRef]
- Soellner, S.T.; Welsch, G.H.; Gelse, K.; Goldmann, A.; Kleyer, A.; Schett, G.; Pachowsky, M.L. gagCEST imaging at 3 T MRI in patients with articular cartilage lesions of the knee and intraoperative validation. Osteoarthr. Cartil. 2021, 29, 1163–1172. [Google Scholar] [CrossRef]
- Hangaard, S.; Gudbergsen, H.; Skougaard, M.; Bliddal, H.; Nybing, J.D.; Tiderius, C.J.; Boesen, M. Point of no return for improvement of cartilage quality indicated by dGEMRIC before and after weight loss in patients with knee osteoarthritis: A cohort study. Acta Radiol. 2018, 59, 336–340. [Google Scholar] [CrossRef]
- Mallio, C.A.; Quattrocchi, C.C.; Rovira, À.; Parizel, P.M. Gadolinium Deposition Safety: Seeking the Patient’s Perspective. Am. J. Neuroradiol. 2020, 41, 944–946. [Google Scholar] [CrossRef]
- Greco, F.; Mallio, C.A. Artificial intelligence and abdominal adipose tissue analysis: A literature review. Quant. Imaging Med. Surg. 2021, 11, 4461–4474. [Google Scholar] [CrossRef]
- Mora, J.C.; Przkora, R.; Cruz-Almeida, Y. Knee osteoarthritis: Pathophysiology and current treatment modalities. J. Pain Res. 2018, 11, 2189–2196. [Google Scholar] [CrossRef] [PubMed]
- Afzali, T.; Fangel, M.V.; Vestergaard, A.S.; Rathleff, M.S.; Ehlers, L.H.; Jensen, M.B. Cost-effectiveness of treatments for non-osteoarthritic knee pain conditions: A systematic review. PLoS ONE 2018, 13, e0209240. [Google Scholar] [CrossRef]
- Cottam, W.; Iwabuchi, S.; Drabek, M.M.; Reckziegel, D.; Auer, D.P. Altered connectivity of the right anterior insula drives the pain connectome changes in chronic knee osteoarthritis. Pain 2018, 159, 929–938. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Soni, A.; Wanigasekera, V.; Mezue, M.; Cooper, C.; Javaid, M.; Price, A.J.; Tracey, I. Central Sensitization in Knee Osteoarthritis: Relating Presurgical Brainstem Neuroimaging and Pain DETECT -Based Patient Stratification to Arthroplasty Outcome. Arthritis Rheumatol. 2019, 71, 550–560. [Google Scholar] [CrossRef] [PubMed]
- Kang, B.; Ma, J.; Shen, J.; Xu, H.; Wang, H.; Zhao, C.; Xie, J.; Zhong, S.; Gao, C.; Xu, X.; et al. Altered brain activity in end-stage knee osteoarthritis revealed by resting-state functional magnetic resonance imaging. Brain Behav. 2022, 12, e2479. [Google Scholar] [CrossRef]
- Liao, X.; Mao, C.; Wang, Y.; Zhang, Q.; Cao, D.; Seminowicz, D.A.; Zhang, M.; Yang, X. Brain gray matter alterations in Chinese patients with chronic knee osteoarthritis pain based on voxel-based morphometry. Medicine 2018, 97, e0145. [Google Scholar] [CrossRef]
- Guo, H.; Wang, Y.; Qiu, L.; Huang, X.; He, C.; Zhang, J.; Gong, Q. Structural and Functional Abnormalities in Knee Osteoarthritis Pain Revealed with Multimodal Magnetic Resonance Imaging. Front. Hum. Neurosci. 2021, 15, 783355. [Google Scholar] [CrossRef]
- Alshuft, H.M.; Condon, L.A.; Dineen, R.A.; Auer, D.P. Cerebral Cortical Thickness in Chronic Pain Due to Knee Osteoarthritis: The Effect of Pain Duration and Pain Sensitization. PLoS ONE 2016, 11, e0161687. [Google Scholar] [CrossRef] [Green Version]
- Mallio, C.A.; Zobel, B.B.; Quattrocchi, C.C. Evaluating rehabilitation interventions in Parkinson′s disease with functional MRI: A promising neuroprotective strategy. Neural Regen. Res. 2015, 10, 702–703. [Google Scholar] [CrossRef]
- Zhang, X.; Chen, Q.; Su, Y.; Meng, J.; Qiu, J.; Zheng, W. Pain in the default mode network: A voxel-based morphometry study on thermal pain sensitivity. NeuroReport 2020, 31, 1030–1035. [Google Scholar] [CrossRef]
- Lan, F.; Lin, G.; Cao, G.; Li, Z.; Ma, D.; Liu, F.; Duan, M.; Fu, H.; Xiao, W.; Qi, Z.; et al. Altered Intrinsic Brain Activity and Functional Connectivity Before and After Knee Arthroplasty in the Elderly: A Resting-State fMRI Study. Front. Neurol. 2020, 11, 556028. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Chen, L.; Tu, Y.; Chen, X.; Hu, K.; Tu, Y.; Lin, M.; Xie, G.; Chen, S.; Huang, J.; et al. Different exercise modalities relieve pain syndrome in patients with knee osteoarthritis and modulate the dorsolateral prefrontal cortex: A multiple mode MRI study. Brain Behav. Immun. 2019, 82, 253–263. [Google Scholar] [CrossRef] [PubMed]
- Goulden, N.; Khusnulina, A.; Davis, N.J.; Bracewell, R.M.; Bokde, A.L.; McNulty, J.P.; Mullins, P.G. The salience network is responsible for switching between the default mode network and the central executive network: Replication from DCM. Neuroimage 2014, 99, 180–190. [Google Scholar] [CrossRef] [PubMed]
- Reckziegel, D.; Raschke, F.; Cottam, W.J.; Auer, D.P. Cingulate GABA levels inversely correlate with the intensity of ongoing chronic knee osteoarthritis pain. Mol. Pain 2016, 12, 1744806916650690. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, H.; Tanner, J.; Parvataneni, H.; Rice, M.; Horgas, A.; Ding, M.; Price, C. Impact of Total Knee Arthroplasty with General Anesthesia on Brain Networks: Cognitive Efficiency and Ventricular Volume Predict Functional Connectivity Decline in Older Adults. J. Alzheimer’s Dis. 2018, 62, 319–333. [Google Scholar] [CrossRef] [Green Version]
- Iwabuchi, S.J.; Xing, Y.; Cottam, W.J.; Drabek, M.M.; Tadjibaev, A.; Fernandes, G.S.; Petersen, K.K.; Arendt-Nielsen, L.; Graven-Nielsen, T.; Valdes, A.M.; et al. Brain perfusion patterns are altered in chronic knee pain: A spatial covariance analysis of arterial spin labelling MRI. Pain 2020, 161, 1255–1263. [Google Scholar] [CrossRef]
- Zhang, W.; Nuki, G.; Moskowitz, R.; Abramson, S.; Altman, R.; Arden, N.; Bierma-Zeinstra, S.; Brandt, K.; Croft, P.; Doherty, M.; et al. OARSI recommendations for the management of hip and knee osteoarthritis: Part III: Changes in evidence following systematic cumulative update of research published through January 2009. Osteoarthr. Cartil. 2010, 18, 476–499. [Google Scholar] [CrossRef] [Green Version]
- Zhang, W.; Moskowitz, R.; Nuki, G.; Abramson, S.; Altman, R.; Arden, N.; Bierma-Zeinstra, S.; Brandt, K.; Croft, P.; Doherty, M.; et al. OARSI recommendations for the management of hip and knee osteoarthritis, Part I: Critical appraisal of existing treatment guidelines and systematic review of current research evidence. Osteoarthr. Cartil. 2007, 15, 981–1000. [Google Scholar] [CrossRef] [Green Version]
- Zhang, W.; Moskowitz, R.W.; Nuki, G.; Abramson, S.; Altman, R.D.; Arden, N.; Bierma-Zeinstra, S.; Brandt, K.D.; Croft, P.; Doherty, M.; et al. OARSI recommendations for the management of hip and knee osteoarthritis, Part II: OARSI evidence-based, expert consensus guidelines. Osteoarthr. Cartil. 2008, 16, 137–162. [Google Scholar] [CrossRef] [Green Version]
- Befrui, N.; Elsner, J.; Flesser, A.; Huvanandana, J.; Jarrousse, O.; Le, T.N.; Müller, M.; Schulze, W.H.W.; Taing, S.; Weidert, S. Vibroarthrography for early detection of knee osteoarthritis using normalized frequency features. Med. Biol. Eng. Comput. 2018, 56, 1499–1514. [Google Scholar] [CrossRef]
- Hunter, D.; Zhang, W.; Conaghan, P.; Hirko, K.; Menashe, L.; Li, L.; Reichmann, W.; Losina, E. Systematic review of the concurrent and predictive validity of MRI biomarkers in OA. Osteoarthr. Cartil. 2011, 19, 557–588. [Google Scholar] [CrossRef] [PubMed]
- Conaghan, P.; Felson, D.; Gold, G.; Lohmander, S.; Totterman, S.; Altman, R. MRI and non-cartilaginous structures in knee osteoarthritis. Osteoarthr. Cartil. 2006, 14, 87–94. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Desai, A.D.; Caliva, F.; Iriondo, C.; Mortazi, A.; Jambawalikar, S.; Bagci, U.; Perslev, M.; Igel, C.; Dam, E.B.; Gaj, S.; et al. The International Workshop on Osteoarthritis Imaging Knee MRI Segmentation Challenge: A Multi-Institute Evaluation and Analysis Framework on a Standardized Dataset. Radiol. Artif. Intell. 2021, 3, e200078. [Google Scholar] [CrossRef] [PubMed]
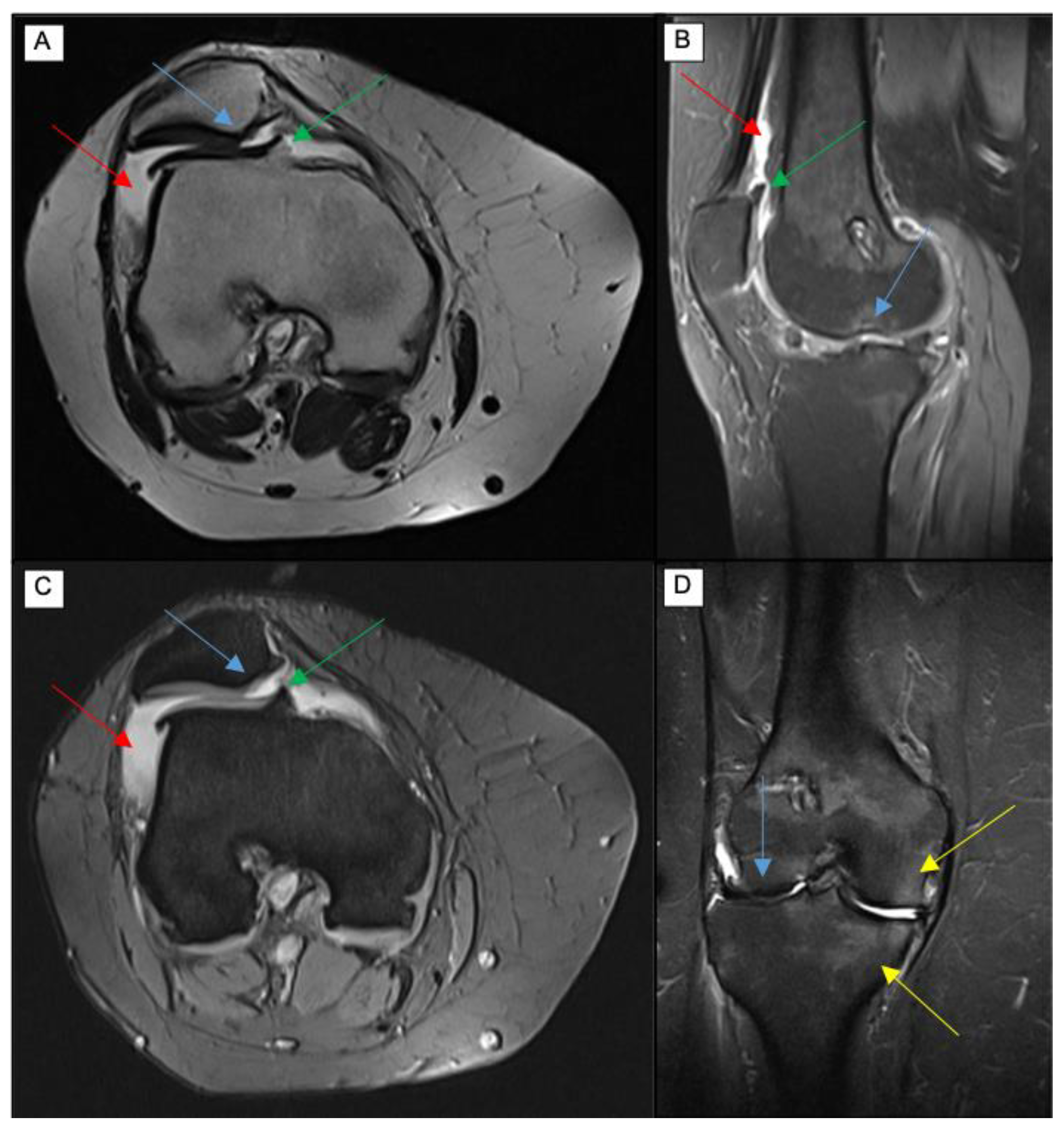
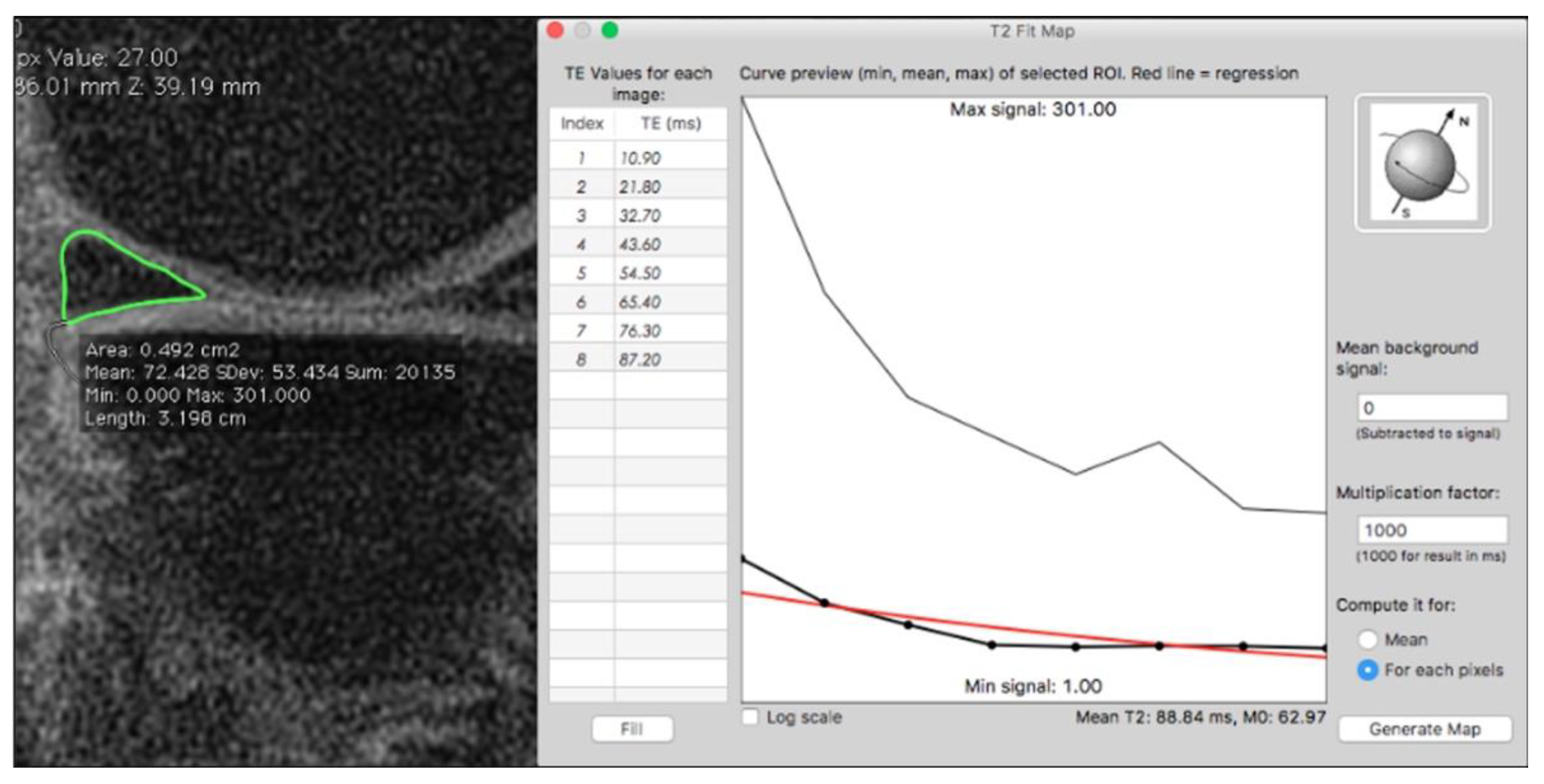
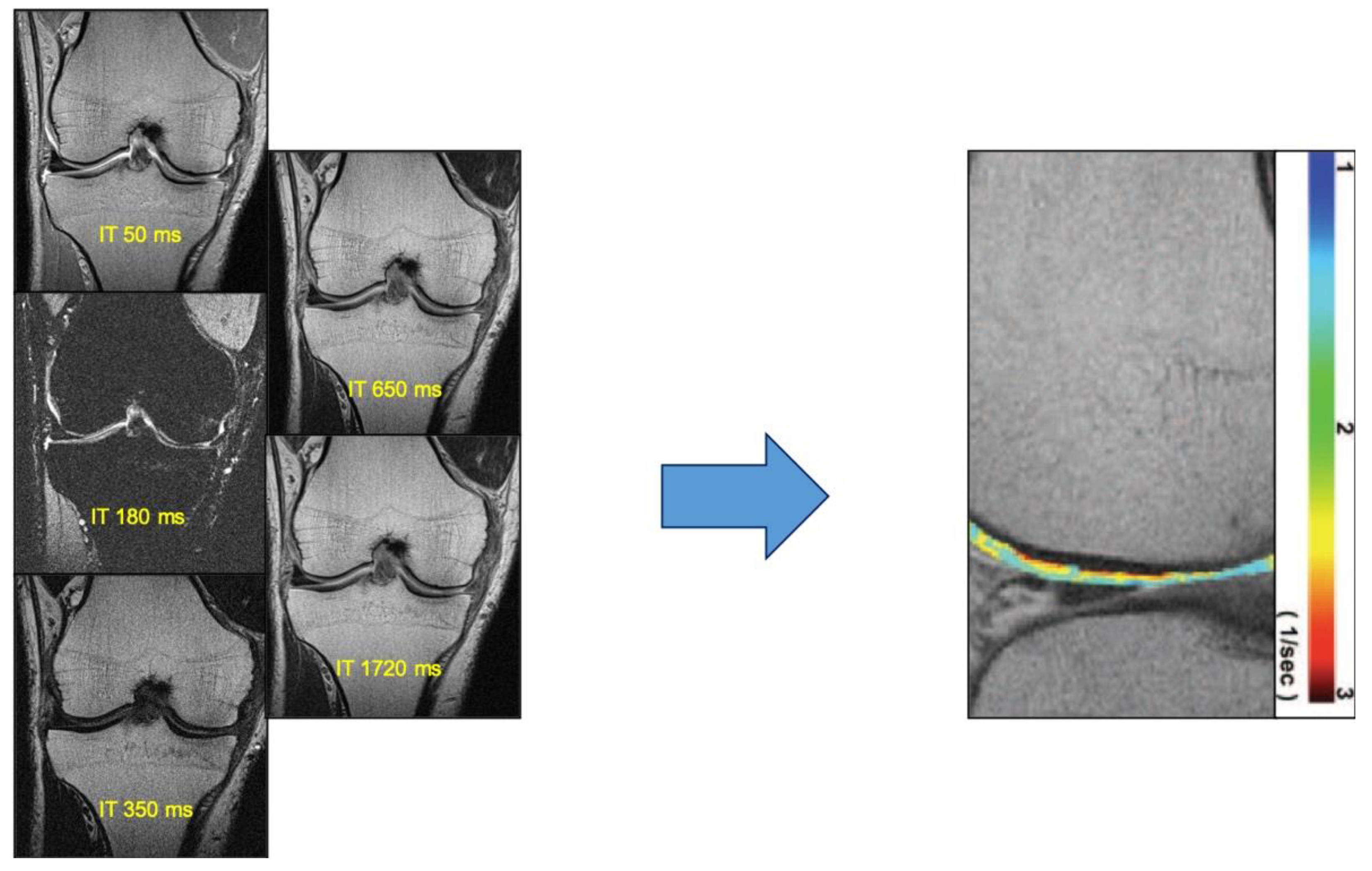
| Technique | Biochemical Changes Evaluated | Clinical Feasibility |
|---|---|---|
| T1ρ | PG and water content, collagen anisotropy | +++ |
| T2 | PG and water content | +++ |
| T2 * | Macromolecule architecture and water mobility | +++ |
| Ultrashort TE | Tissue composition and organization | +++ |
| DWI with ADC DTI with FA | Water diffusion, tissue composition and organization | + |
| 23Na-MRI | Na+ concentration, indirectly GAG/PG content | + |
| GagCEST | Exchange of hydroxyl-protons between GAG and bulk water, GAG content | + |
| dGEMRIC | Diffusion rate, GAG content indirectly | + |
| Authors | Country | Aim/Rationale | Patients | MRI | Sequence | Main conclusion |
|---|---|---|---|---|---|---|
| H. Nishioka et al. [36] | Kumamoto, Japan | To perform qualitative evaluations of reparative tissue on articular surface of medial compartment after HCO with MRI T1ρ and T2 mapping | 20 | 3 T scanner (Philips Healthcare, Best, The Netherlands) | T1ρ-WI T2-WI | T1ρ and T2 mapping revealed that the repaired tissue was fibrocartilage |
| Robert Stahl et al. [29] | San Francisco, USA | To evaluate the diagnostic value of T2 and T1ρ in identifying focal cartlage lesions in asymptomatic physically active subjects | 37 | 3 T scanner (Signa, GE Medical Systems, Waukesha, WI) | T1ρ-WI T2-WI | T1ρ and T2 imaging demonstrated a different cartilage composition in active subjects with and without focal cartilage abnormalities |
| Hajimu Goto et al. [38] | Kobe, Japan | To investigate effect of aging and weight-bearing on T1ρ values in cartilage | 32 | 3 T scanner (Philips Healthcare, Best, The Netherlands) | T1ρ-WI | The degree of weight-bearing and, in particular aging, correlate with changes in cartilage T1rho values |
| Timothy C. Dunn et al. [21] | San Francisco, USA | To determine differences in T2 values in femoral and tibial cartilage in patients with varying degrees of OA | 55 | 1.5 T scanner (GE Medical Systems, Milwaukee, Wis) | T2-WI | T2 values of femoral and medial tibial cartilage increase with the severity of OA |
| M. F. Koff et al. [18] | Rochester, USA | To study T2 values of patellar cartilage grouped by radiographic stage of patello-femoral OA and by BMI | 113 | 1.5 T scanner (Signa, GE Medical Systems, Waukesha, WI) | T2-WI | T2 values are not sensitive to changes in radiographic stages of OA and BMI could be considered a factor for a potential increase of T2 values |
| Ping Zhang et al. [41] | Shijiazhuang, China | To study effects of long-distance running on knee cartilage with T2*-WI | 12 | 3 T scanner (Magnetom; Siemens Healthcare, Erlangen, Germany) | T2*-WI | An increase in T2* values of knee cartilage happened right after long distance running with a following reduction in the 2 months later |
| Ashley A. Williams et al. [42] | California, USA | To evaluate with UTE-T2* relationship between cartilage chenges, knee function, pain and gait metrics, 2 years after ACLR | 60 | 3 T scanner (Signa, GE Medical Systems, Waukesha, WI) | UTE-T2* | Patellofemoral deep cartilage matrix disruption, as assessed by MRI UTE-T2*, was associated with reduced sports and recreational function and with gait metrics reflective of altered patellofemoral loading |
| Taku Ukai et al. [43] | Kanagawa, Japan | To measure damaged areas of cartilage with ADC, T2 values and FA | 41 | 3 T scanner (Achieva 3 Tesla, Philips Healthcare, Best, The Netherlands) | ADC FA T2-WI | T2 mapping is useful for detecting moderate or severe cartilage damage. ADC can be used to detect early stage cartilage damage, FA can also distinguish normal from damaged cartilage |
| S. T. Soellner et al. [48] | Erlangen, Germany | To compare gagCEST of knee cartilage with intraoperative results for the assessment of early OA and to define gagCEST values for the differentiation between healthy and degenerated cartilage | 21 | 3 T scanner (Signa, GE Medical Systems, Waukesha, WI) | gagCEST | gagCEST might provide a diagnostic tool for the detection of early knee-joint cartilage damage and grading |
| Stine Hangaard et al. [49] | Copenhagen, Denmark | To evaluate changes in quality of cartilage after weight loss | 19 | 1.5 T scanner (Philips Healthcare, Best, The Netherlands) | dGEMRIC | Improvement of cartilage quality, assessed with dGEMRIC, after weight loss might be possible only in early stage of KOA |
| Guillaume Madelin et al. [46] | New York, USA | To evaluate the potential of sodium MRI to detect changes over time of apparent sodium concentration (ASC) in articular cartilage in patients with KOA | 12 | 7 T scanner (Siemens Healthcare, Erlangen, Germany) | 23Na+-MRI | Quantitative sodium MRI has the potential to detect a decrease of ASC over time in articular cartilage of patients with KOA |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mallio, C.A.; Bernetti, C.; Agostini, F.; Mangone, M.; Paoloni, M.; Santilli, G.; Martina, F.M.; Quattrocchi, C.C.; Zobel, B.B.; Bernetti, A. Advanced MR Imaging for Knee Osteoarthritis: A Review on Local and Brain Effects. Diagnostics 2023, 13, 54. https://doi.org/10.3390/diagnostics13010054
Mallio CA, Bernetti C, Agostini F, Mangone M, Paoloni M, Santilli G, Martina FM, Quattrocchi CC, Zobel BB, Bernetti A. Advanced MR Imaging for Knee Osteoarthritis: A Review on Local and Brain Effects. Diagnostics. 2023; 13(1):54. https://doi.org/10.3390/diagnostics13010054
Chicago/Turabian StyleMallio, Carlo A., Caterina Bernetti, Francesco Agostini, Massimiliano Mangone, Marco Paoloni, Gabriele Santilli, Francesca Maria Martina, Carlo C. Quattrocchi, Bruno Beomonte Zobel, and Andrea Bernetti. 2023. "Advanced MR Imaging for Knee Osteoarthritis: A Review on Local and Brain Effects" Diagnostics 13, no. 1: 54. https://doi.org/10.3390/diagnostics13010054
APA StyleMallio, C. A., Bernetti, C., Agostini, F., Mangone, M., Paoloni, M., Santilli, G., Martina, F. M., Quattrocchi, C. C., Zobel, B. B., & Bernetti, A. (2023). Advanced MR Imaging for Knee Osteoarthritis: A Review on Local and Brain Effects. Diagnostics, 13(1), 54. https://doi.org/10.3390/diagnostics13010054









