Pancreatic Cystic Lesions: A Focused Review on Cyst Clinicopathological Features and Advanced Diagnostics
Abstract
:1. Introduction
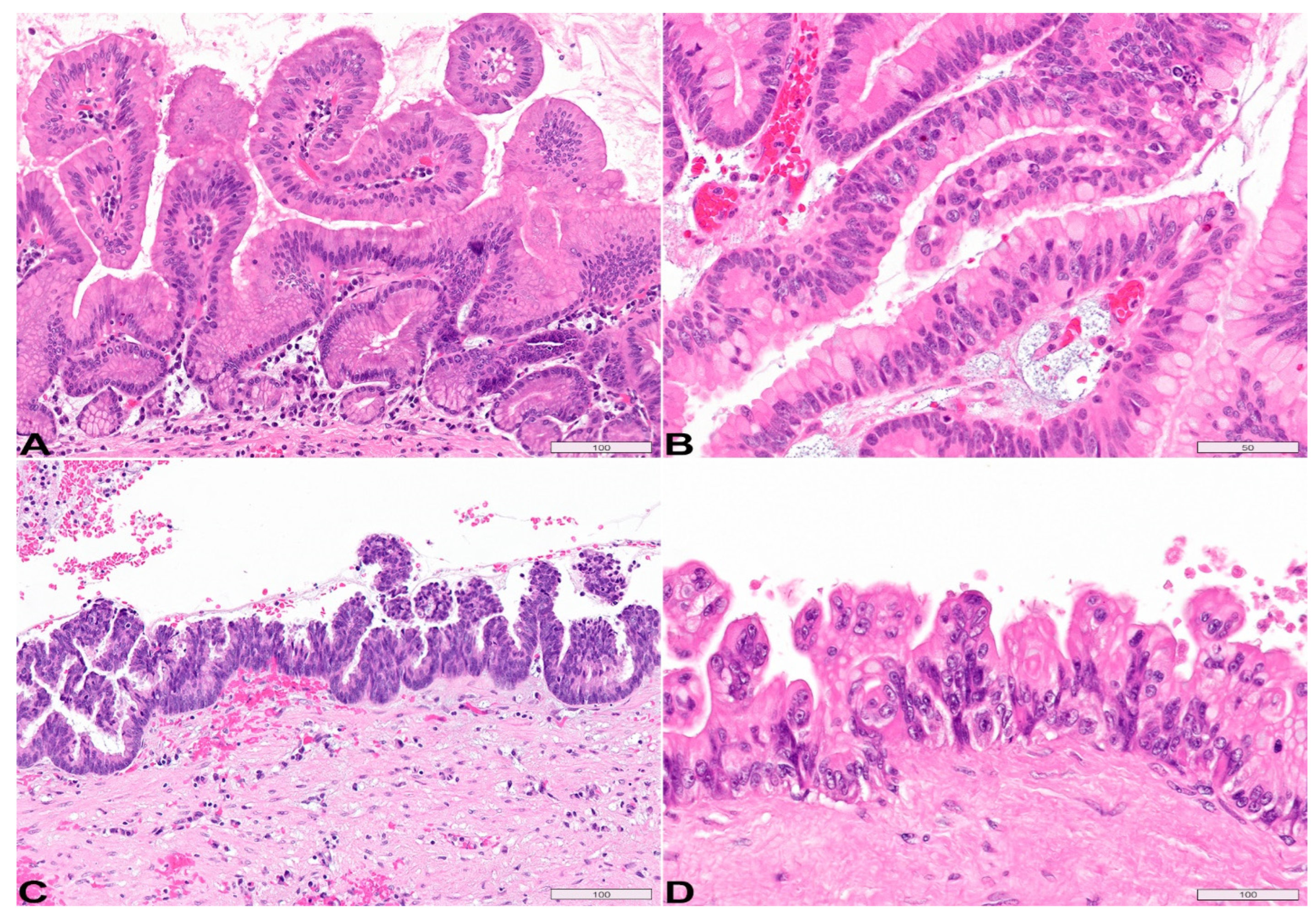
2. Intraductal Papillary Mucinous Neoplasm
Histopathology
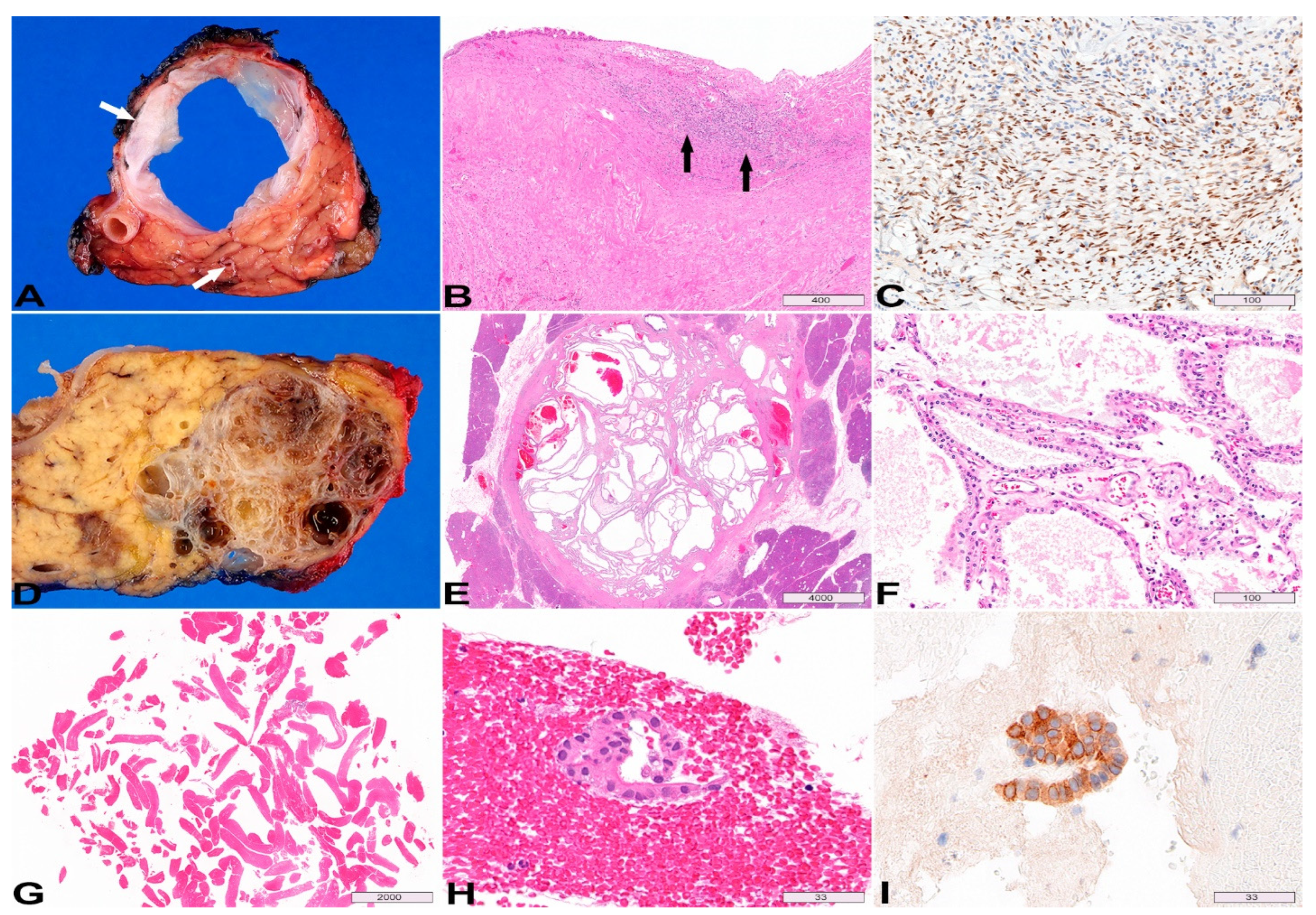
3. Mucinous Cystic Neoplasm
4. Serous Cystadenoma
5. Cystic Neuroendocrine Tumor
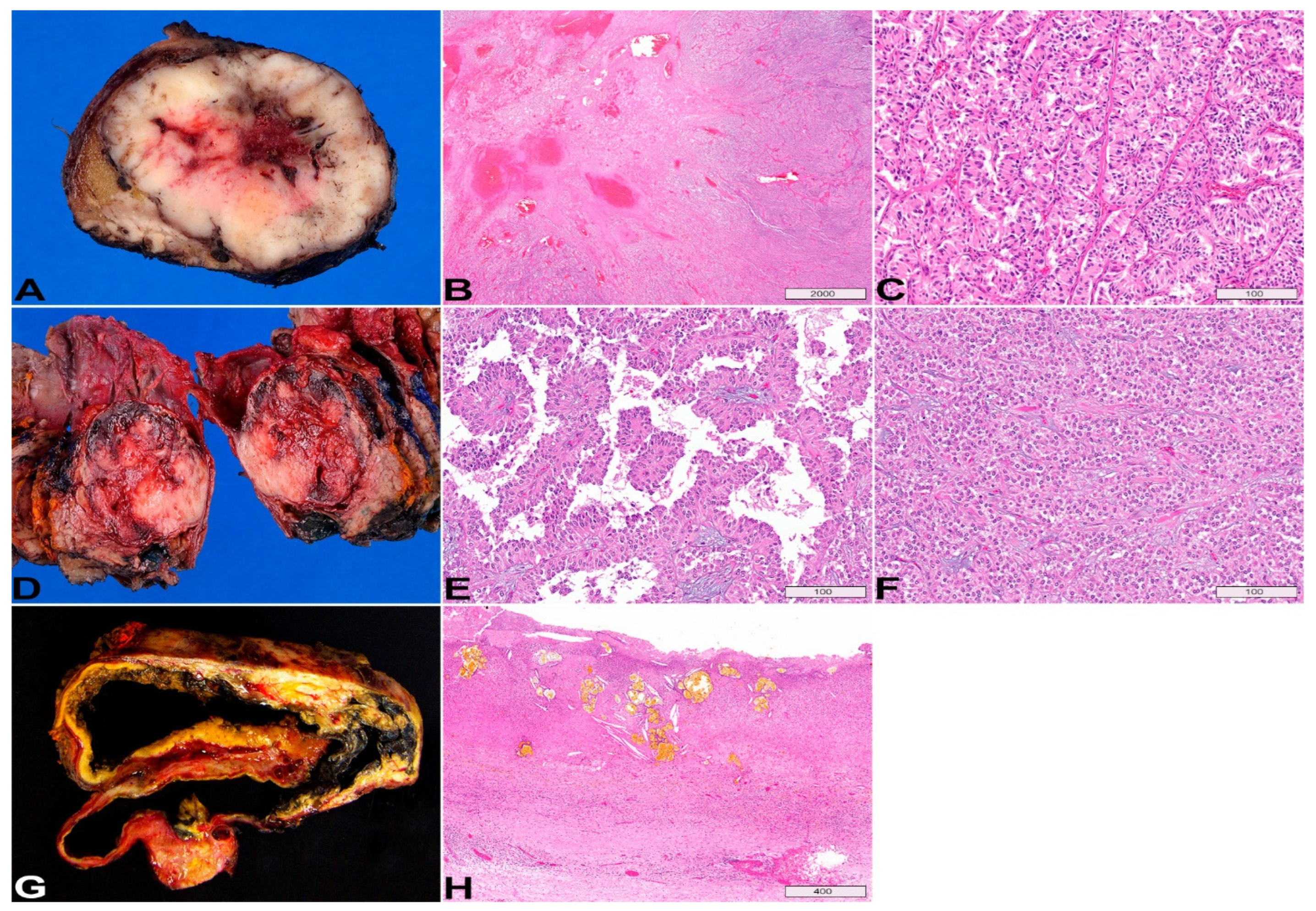
6. Solid Pseudopapillary Neoplasm
7. Pseudocyst
8. Squamous Lined Epithelial Cysts
9. Simple Mucinous Cyst
10. Advanced and Emerging Diagnostic Tools for Pancreatic Cystic Lesions
11. Summary
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tanaka, M.; Fernández-del Castillo, C.; Kamisawa, T.; Jang, J.Y.; Levy, P.; Ohtsuka, T.; Salvia, R.; Shimizu, Y.; Tada, M.; Wolfgang, C.L. Revisions of international consensus Fukuoka guidelines for the management of IPMN of the pancreas. Pancreatology 2017, 17, 738–753. [Google Scholar] [CrossRef] [PubMed]
- Ardeshna, D.R.; Cao, T.; Rodgers, B.; Onongaya, C.; Jones, D.; Chen, W.; Koay, E.J.; Krishna, S.G. Recent advances in the diagnostic evaluation of pancreatic cystic lesions. World J. Gastroenterol. 2022, 28, 624–634. [Google Scholar] [CrossRef] [PubMed]
- Buerlein, R.C.D.; Shami, V.M. Management of pancreatic cysts and guidelines: What the gastroenterologist needs to know. Ther. Adv. Gastrointest. Endosc. 2021, 14, 26317745211045769. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.S.; Jang, J.-Y. Management Algorithms for Pancreatic Cystic Neoplasms. Arch. Pathol. Lab. Med. 2022, 146, 322–329. [Google Scholar] [CrossRef] [PubMed]
- Turner, R.C.; Melnychuk, J.T.; Chen, W.; Jones, D.; Krishna, S.G. Molecular Analysis of Pancreatic Cyst Fluid for the Management of Intraductal Papillary Mucinous Neoplasms. Diagnostics 2022, 12, 2573. [Google Scholar] [CrossRef] [PubMed]
- De Pretis, N.; Mukewar, S.; Aryal-Khanal, A.; Bi, Y.; Takahashi, N.; Chari, S. Pancreatic cysts: Diagnostic accuracy and risk of inappropriate resections. Pancreatology 2017, 17, 267–272. [Google Scholar] [CrossRef]
- Sharib, J.M.; Fonseca, A.L.; Swords, D.S.; Jaradeh, K.; Bracci, P.M.; Firpo, M.A.; Hatcher, S.; Scaife, C.L.; Wang, H.; Kim, G.E. Surgical overtreatment of pancreatic intraductal papillary mucinous neoplasms: Do the 2017 International Consensus Guidelines improve clinical decision making? Surgery 2018, 164, 1178–1184. [Google Scholar] [CrossRef]
- Aziz, H.; Acher, A.W.; Krishna, S.G.; Cloyd, J.M.; Pawlik, T.M. Comparison of Society Guidelines for the Management and Surveillance of Pancreatic Cysts: A Review. JAMA Surg. 2022, 157, 723–730. [Google Scholar] [CrossRef]
- Paniccia, A.; Polanco, P.M.; Boone, B.A.; Wald, A.I.; McGrath, K.; Brand, R.E.; Khalid, A.; Kubiliun, N.; O’Broin-Lennon, A.M.; Park, W.G.; et al. Prospective, Multi-Institutional, Real-Time Next-Generation Sequencing of Pancreatic Cyst Fluid Reveals Diverse Genomic Alterations That Improve the Clinical Management of Pancreatic Cysts. Gastroenterology 2022, 164, 117–133.e7. [Google Scholar] [CrossRef]
- Gaujoux, S.; Brennan, M.F.; Gonen, M.; D’Angelica, M.I.; DeMatteo, R.; Fong, Y.; Schattner, M.; DiMaio, C.; Janakos, M.; Jarnagin, W.R.; et al. Cystic Lesions of the Pancreas: Changes in the Presentation and Management of 1,424 Patients at a Single Institution over a 15-Year Time Period. J. Am. Coll. Surg. 2011, 212, 590–600. [Google Scholar] [CrossRef]
- Kleeff, J.; Michalski, C.; Kong, B.; Erkan, M.; Roth, S.; Siveke, J.; Friess, H.; Esposito, I. Surgery for cystic pancreatic lesions in the post-sendai era: A single institution experience. HPB Surg. 2015, 2015, 847837. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Valsangkar, N.P.; Morales-Oyarvide, V.; Thayer, S.P.; Ferrone, C.R.; Wargo, J.A.; Warshaw, A.L.; Fernandez-del Castillo, C. 851 resected cystic tumors of the pancreas: A 33-year experience at the Massachusetts General Hospital. Surg. 2012, 152, S4–S12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Esposito, I.; Haeberle, L. Nonmucinous Cystic Lesions of the Pancreas. Arch. Pathol. Lab. Med. 2022, 146, 312–321. [Google Scholar] [CrossRef] [PubMed]
- WHO Classification of Tumours-IARC. In Tumours of the pancreas.Digestive System Tumours, 5th ed.; International Agency for Research on Cancer: Lyon, France, 2019; Volume 1.
- Adsay, N.V.; Longnecker, D.S.; Klimstra, D.S. Pancreatic tumors with cystic dilatation of the ducts: Intraductal papillary mucinous neoplasms and intraductal oncocytic papillary neoplasms. Semin. Diagn. Pathol. 2000, 17, 16–30. [Google Scholar] [PubMed]
- Jang, J.Y.; Kim, S.W.; Ahn, Y.J.; Yoon, Y.S.; Choi, M.G.; Lee, K.U.; Han, J.K.; Kim, W.H.; Lee, Y.J.; Kim, S.C.; et al. Multicenter analysis of clinicopathologic features of intraductal papillary mucinous tumor of the pancreas: Is it possible to predict the malignancy before surgery? Ann. Surg. Oncol. 2005, 12, 124–132. [Google Scholar] [CrossRef]
- Loftus, E.V., Jr.; Olivares-Pakzad, B.A.; Batts, K.P.; Adkins, M.C.; Stephens, D.H.; Sarr, M.G.; DiMagno, E.P. Intraductal papillary-mucinous tumors of the pancreas: Clinicopathologic features, outcome, and nomenclature. Members of the Pancreas Clinic, and Pancreatic Surgeons of Mayo Clinic. Gastroenterology 1996, 110, 1909–1918. [Google Scholar] [CrossRef]
- Pelaez-Luna, M.; Chari, S.T.; Smyrk, T.C.; Takahashi, N.; Clain, J.E.; Levy, M.J.; Pearson, R.K.; Petersen, B.T.; Topazian, M.D.; Vege, S.S.; et al. Do Consensus indications for resection in branch duct intraductal papillary mucinous neoplasm predict malignancy? A study of 147 patients. Am. J. Gastroenterol. 2007, 102, 1759–1764. [Google Scholar] [CrossRef]
- Sohn, T.A.; Yeo, C.J.; Cameron, J.L.; Hruban, R.H.; Fukushima, N.; Campbell, K.A.; Lillemoe, K.D. Intraductal papillary mucinous neoplasms of the pancreas: An updated experience. Ann. Surg. 2004, 239, 788–799. [Google Scholar] [CrossRef]
- Tanaka, M.; Sawai, H.; Okada, Y.; Yamamoto, M.; Funahashi, H.; Takeyama, H.; Manabe, T. Clinicopathologic study of intraductal papillary-mucinous tumors and mucinous cystic tumors of the pancreas. Hepatogastroenterology 2006, 53, 783–787. [Google Scholar]
- Klöppel, G.; Basturk, O.; Schlitter, A.M.; Konukiewitz, B.; Esposito, I. Intraductal neoplasms of the pancreas. Semin. Diagn. Pathol. 2014, 31, 452–466. [Google Scholar] [CrossRef]
- Yamaguchi, K.; Tanaka, M. Mucin-hypersecreting tumor of the pancreas with mucin extrusion through an enlarged papilla. Am. J. Gastroenterol. 1991, 86, 835–839. [Google Scholar] [PubMed]
- Krishna, S.G.; Hart, P.A.; Malli, A.; Kruger, A.J.; McCarthy, S.T.; El-Dika, S.; Walker, J.P.; Dillhoff, M.E.; Manilchuk, A.; Schmidt, C.R.; et al. Endoscopic Ultrasound-Guided Confocal Laser Endomicroscopy Increases Accuracy of Differentiation of Pancreatic Cystic Lesions. Clin. Gastroenterol. Hepatol. Off. Clin. Pract. J. Am. Gastroenterol. Assoc. 2019, 18, 432–440.e6. [Google Scholar] [CrossRef] [PubMed]
- Krishna, S.G.; Hart, P.A.; DeWitt, J.M.; DiMaio, C.J.; Kongkam, P.; Napoleon, B.; Othman, M.O.; Yew Tan, D.M.; Strobel, S.G.; Stanich, P.P.; et al. EUS-guided confocal laser endomicroscopy: Prediction of dysplasia in intraductal papillary mucinous neoplasms (with video). Gastrointest. Endosc. 2020, 91, 551–563.e5. [Google Scholar] [CrossRef] [PubMed]
- Machicado, J.D.; Chao, W.L.; Carlyn, D.E.; Pan, T.Y.; Poland, S.; Alexander, V.L.; Maloof, T.G.; Dubay, K.; Ueltschi, O.; Middendorf, D.M.; et al. High performance in risk stratification of intraductal papillary mucinous neoplasms by confocal laser endomicroscopy image analysis with convolutional neural networks (with video). Gastrointest. Endosc. 2021, 94, 78–87.e2. [Google Scholar] [CrossRef] [PubMed]
- Adsay, N.V.; Adair, C.F.; Heffess, C.S.; Klimstra, D.S. Intraductal oncocytic papillary neoplasms of the pancreas. Am. J. Surg. Pathol. 1996, 20, 980–994. [Google Scholar] [CrossRef] [PubMed]
- Basturk, O.; Chung, S.M.; Hruban, R.H.; Adsay, N.V.; Askan, G.; Iacobuzio-Donahue, C.; Balci, S.; Zee, S.Y.; Memis, B.; Shia, J.; et al. Distinct pathways of pathogenesis of intraductal oncocytic papillary neoplasms and intraductal papillary mucinous neoplasms of the pancreas. Virchows Arch. 2016, 469, 523–532. [Google Scholar] [CrossRef] [Green Version]
- Basturk, O.; Tan, M.; Bhanot, U.; Allen, P.; Adsay, V.; Scott, S.N.; Shah, R.; Berger, M.F.; Askan, G.; Dikoglu, E.; et al. The oncocytic subtype is genetically distinct from other pancreatic intraductal papillary mucinous neoplasm subtypes. Mod. Pathol. 2016, 29, 1058–1069. [Google Scholar] [CrossRef] [Green Version]
- Marchegiani, G.; Mino-Kenudson, M.; Ferrone, C.R.; Warshaw, A.L.; Lillemoe, K.D.; Fernández-del Castillo, C. Oncocytic-type intraductal papillary mucinous neoplasms: A unique malignant pancreatic tumor with good long-term prognosis. J. Am. Coll. Surg. 2015, 220, 839–844. [Google Scholar] [CrossRef]
- Ban, S.; Naitoh, Y.; Mino-Kenudson, M.; Sakurai, T.; Kuroda, M.; Koyama, I.; Lauwers, G.Y.; Shimizu, M. Intraductal papillary mucinous neoplasm (IPMN) of the pancreas: Its histopathologic difference between 2 major types. Am. J. Surg. Pathol. 2006, 30, 1561–1569. [Google Scholar] [CrossRef]
- Adsay, N.V.; Conlon, K.C.; Zee, S.Y.; Brennan, M.F.; Klimstra, D.S. Intraductal papillary-mucinous neoplasms of the pancreas: An analysis of in situ and invasive carcinomas in 28 patients. Cancer 2002, 94, 62–77. [Google Scholar] [CrossRef]
- Adsay, N.V.; Merati, K.; Basturk, O.; Iacobuzio-Donahue, C.; Levi, E.; Cheng, J.D.; Sarkar, F.H.; Hruban, R.H.; Klimstra, D.S. Pathologically and biologically distinct types of epithelium in intraductal papillary mucinous neoplasms: Delineation of an “intestinal” pathway of carcinogenesis in the pancreas. Am. J. Surg. Pathol. 2004, 28, 839–848. [Google Scholar] [CrossRef] [PubMed]
- Furukawa, T.; Klöppel, G.; Volkan Adsay, N.; Albores-Saavedra, J.; Fukushima, N.; Horii, A.; Hruban, R.H.; Kato, Y.; Klimstra, D.S.; Longnecker, D.S.; et al. Classification of types of intraductal papillary-mucinous neoplasm of the pancreas: A consensus study. Virchows Arch. 2005, 447, 794–799. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, A.; Horinouchi, M.; Goto, M.; Nagata, K.; Sakoda, K.; Takao, S.; Imai, K.; Kim, Y.S.; Sato, E.; Yonezawa, S. New classification of pancreatic intraductal papillary-mucinous tumour by mucin expression: Its relationship with potential for malignancy. J. Pathol. 2002, 197, 201–210. [Google Scholar] [CrossRef] [PubMed]
- Jabłońska, B.; Szmigiel, P.; Mrowiec, S. Pancreatic intraductal papillary mucinous neoplasms: Current diagnosis and management. World J. Gastrointest. Oncol. 2021, 13, 1880–1895. [Google Scholar] [CrossRef] [PubMed]
- Basturk, O.; Hong, S.M.; Wood, L.D.; Adsay, N.V.; Albores-Saavedra, J.; Biankin, A.V.; Brosens, L.A.; Fukushima, N.; Goggins, M.; Hruban, R.H.; et al. A Revised Classification System and Recommendations From the Baltimore Consensus Meeting for Neoplastic Precursor Lesions in the Pancreas. Am. J. Surg. Pathol. 2015, 39, 1730–1741. [Google Scholar] [CrossRef] [PubMed]
- Amato, E.; Molin, M.D.; Mafficini, A.; Yu, J.; Malleo, G.; Rusev, B.; Fassan, M.; Antonello, D.; Sadakari, Y.; Castelli, P.; et al. Targeted next-generation sequencing of cancer genes dissects the molecular profiles of intraductal papillary neoplasms of the pancreas. J. Pathol. 2014, 233, 217–227. [Google Scholar] [CrossRef] [PubMed]
- Ren, R.; Krishna, S.G.; Chen, W.; Frankel, W.L.; Shen, R.; Zhao, W.; Avenarius, M.R.; Garee, J.; Caruthers, S.; Jones, D. Activation of the RAS pathway through uncommon BRAF mutations in mucinous pancreatic cysts without KRAS mutation. Mod. Pathol. 2021, 34, 438–444. [Google Scholar] [CrossRef]
- Molin, M.D.; Matthaei, H.; Wu, J.; Blackford, A.; Debeljak, M.; Rezaee, N.; Wolfgang, C.L.; Butturini, G.; Salvia, R.; Bassi, C.; et al. Clinicopathological correlates of activating GNAS mutations in intraductal papillary mucinous neoplasm (IPMN) of the pancreas. Ann. Surg. Oncol. 2013, 20, 3802–3808. [Google Scholar] [CrossRef]
- Tan, M.C.; Basturk, O.; Brannon, A.R.; Bhanot, U.; Scott, S.N.; Bouvier, N.; LaFemina, J.; Jarnagin, W.R.; Berger, M.F.; Klimstra, D.; et al. GNAS and KRAS Mutations Define Separate Progression Pathways in Intraductal Papillary Mucinous Neoplasm-Associated Carcinoma. J. Am. Coll. Surg. 2015, 220, 845–854.e1. [Google Scholar] [CrossRef] [Green Version]
- Wu, J.; Matthaei, H.; Maitra, A.; Dal Molin, M.; Wood, L.D.; Eshleman, J.R.; Goggins, M.; Canto, M.I.; Schulick, R.D.; Edil, B.H.; et al. Recurrent GNAS mutations define an unexpected pathway for pancreatic cyst development. Sci. Transl. Med. 2011, 3, 92ra66. [Google Scholar] [CrossRef] [Green Version]
- Sakamoto, H.; Kuboki, Y.; Hatori, T.; Yamamoto, M.; Sugiyama, M.; Shibata, N.; Shimizu, K.; Shiratori, K.; Furukawa, T. Clinicopathological significance of somatic RNF43 mutation and aberrant expression of ring finger protein 43 in intraductal papillary mucinous neoplasms of the pancreas. Mod. Pathol. 2015, 28, 261–267. [Google Scholar] [CrossRef] [PubMed]
- Bell, P.D.; Singhi, A.D. Integrating Molecular Analysis into the Pathologic Evaluation of Pancreatic Cysts. Surg. Pathol. Clin. 2022, 15, 455–468. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Carracedo, D.; Chen, Z.-M.; Qiu, W.; Huang, A.S.; Tang, S.M.; Hruban, R.H.; Su, G.H. PIK3CA Mutations in mucinous cystic neoplasms of the pancreas. Pancreas 2014, 43, 245–249. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kanda, M.; Sadakari, Y.; Borges, M.; Topazian, M.; Farrell, J.; Syngal, S.; Lee, J.; Kamel, I.; Lennon, A.M.; Knight, S.; et al. Mutant TP53 in duodenal samples of pancreatic juice from patients with pancreatic cancer or high-grade dysplasia. Clin. Gastroenterol. Hepatol. Off. Clin. Pract. J. Am. Gastroenterol. Assoc. 2013, 11, 719–730.e5. [Google Scholar] [CrossRef] [Green Version]
- Theisen, B.K.; Wald, A.I.; Singhi, A.D. Molecular Diagnostics in the Evaluation of Pancreatic Cysts. Surgical Pathology Clinics 2016, 9, 441–456. [Google Scholar] [CrossRef]
- Pea, A.; Yu, J.; Rezaee, N.; Luchini, C.; He, J.; Dal Molin, M.; Griffin, J.F.; Fedor, H.; Fesharakizadeh, S.; Salvia, R.; et al. Targeted DNA Sequencing Reveals Patterns of Local Progression in the Pancreatic Remnant Following Resection of Intraductal Papillary Mucinous Neoplasm (IPMN) of the Pancreas. Ann. Surg. 2017, 266, 133–141. [Google Scholar] [CrossRef] [Green Version]
- Rosenbaum, M.W.; Jones, M.; Dudley, J.C.; Le, L.P.; Iafrate, A.J.; Pitman, M.B. Next-generation sequencing adds value to the preoperative diagnosis of pancreatic cysts. Cancer Cytopathol. 2017, 125, 41–47. [Google Scholar] [CrossRef]
- Schönleben, F.; Qiu, W.; Ciau, N.T.; Ho, D.J.; Li, X.; Allendorf, J.D.; Remotti, H.E.; Su, G.H. PIK3CA mutations in intraductal papillary mucinous neoplasm/carcinoma of the pancreas. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2006, 12, 3851–3855. [Google Scholar] [CrossRef] [Green Version]
- Furukawa, T.; Hatori, T.; Fujita, I.; Yamamoto, M.; Kobayashi, M.; Ohike, N.; Morohoshi, T.; Egawa, S.; Unno, M.; Takao, S.; et al. Prognostic relevance of morphological types of intraductal papillary mucinous neoplasms of the pancreas. Gut 2011, 60, 509–516. [Google Scholar] [CrossRef]
- Hara, T.; Ikebe, D.; Odaka, A.; Sudo, K.; Nakamura, K.; Yamamoto, H.; Itami, M.; Hirata, T.; Kashimura, J.; Yamaguchi, T. Preoperative histological subtype classification of intraductal papillary mucinous neoplasms (IPMN) by pancreatic juice cytology with MUC stain. Ann. Surg. 2013, 257, 1103–1111. [Google Scholar] [CrossRef]
- Ozcan, K.; Klimstra, D.S. A Review of Mucinous Cystic and Intraductal Neoplasms of the Pancreatobiliary Tract. Arch. Pathol. Lab. Med. 2022, 146, 298–311. [Google Scholar] [CrossRef] [PubMed]
- Schnelldorfer, T.; Sarr, M.G.; Nagorney, D.M.; Zhang, L.; Smyrk, T.C.; Qin, R.; Chari, S.T.; Farnell, M.B. Experience with 208 resections for intraductal papillary mucinous neoplasm of the pancreas. Arch. Surg. 2008, 143, 639–646. [Google Scholar] [CrossRef] [Green Version]
- Shimizu, Y.; Yamaue, H.; Maguchi, H.; Yamao, K.; Hirono, S.; Osanai, M.; Hijioka, S.; Hosoda, W.; Nakamura, Y.; Shinohara, T.; et al. Predictors of malignancy in intraductal papillary mucinous neoplasm of the pancreas: Analysis of 310 pancreatic resection patients at multiple high-volume centers. Pancreas 2013, 42, 883–888. [Google Scholar] [CrossRef] [PubMed]
- Hosoda, W.; Sasaki, E.; Murakami, Y.; Yamao, K.; Shimizu, Y.; Yatabe, Y. GNAS mutation is a frequent event in pancreatic intraductal papillary mucinous neoplasms and associated adenocarcinomas. Virchows Arch. 2015, 466, 665–674. [Google Scholar] [CrossRef]
- Matthaei, H.; Dal Molin, M.; Maitra, A. Identification and analysis of precursors to invasive pancreatic cancer. Methods Mol. Biol. 2013, 980, 1–12. [Google Scholar] [CrossRef]
- Jang, K.T.; Park, S.M.; Basturk, O.; Bagci, P.; Bandyopadhyay, S.; Stelow, E.B.; Walters, D.M.; Choi, D.W.; Choi, S.H.; Heo, J.S.; et al. Clinicopathologic characteristics of 29 invasive carcinomas arising in 178 pancreatic mucinous cystic neoplasms with ovarian-type stroma: Implications for management and prognosis. Am. J. Surg. Pathol. 2015, 39, 179–187. [Google Scholar] [CrossRef]
- Thompson, L.D.; Becker, R.C.; Przygodzki, R.M.; Adair, C.F.; Heffess, C.S. Mucinous cystic neoplasm (mucinous cystadenocarcinoma of low-grade malignant potential) of the pancreas: A clinicopathologic study of 130 cases. Am. J. Surg. Pathol. 1999, 23, 1–16. [Google Scholar] [CrossRef] [Green Version]
- Yamao, K.; Yanagisawa, A.; Takahashi, K.; Kimura, W.; Doi, R.; Fukushima, N.; Ohike, N.; Shimizu, M.; Hatori, T.; Nobukawa, B.; et al. Clinicopathological features and prognosis of mucinous cystic neoplasm with ovarian-type stroma: A multi-institutional study of the Japan pancreas society. Pancreas 2011, 40, 67–71. [Google Scholar] [CrossRef]
- Zamboni, G.; Scarpa, A.; Bogina, G.; Iacono, C.; Bassi, C.; Talamini, G.; Sessa, F.; Capella, C.; Solcia, E.; Rickaert, F.; et al. Mucinous Cystic Tumors of the Pancreas: Clinicopathological features, prognosis, and relationship to other mucinous cystic tumors. Am. J. Surg. Pathol. 1999, 23, 410–422. [Google Scholar] [CrossRef]
- Sun, Y.; Zhou, F.; Liu, F.; Hu, Y.; Tan, S.; Liang, P.; Linghu, E.; Yu, X. Discrimination of serous cystadenoma from mucinous cystadenoma in the pancreas with contrast-enhanced ultrasonography: A prospective study in 61 patients. OncoTargets Ther. 2017, 10, 1285–1294. [Google Scholar] [CrossRef] [Green Version]
- Fukushima, N.; Zamboni, G. Mucinous cystic neoplasms of the pancreas: Update on the surgical pathology and molecular genetics. Semin. Diagn. Pathol. 2014, 31, 467–474. [Google Scholar] [CrossRef]
- Buetow, P.C.; Rao, P.; Thompson, L.D. From the Archives of the AFIP. Mucinous cystic neoplasms of the pancreas: Radiologic-pathologic correlation. Radiographics 1998, 18, 433–449. [Google Scholar] [CrossRef] [Green Version]
- Testini, M.; Gurrado, A.; Lissidini, G.; Venezia, P.; Greco, L.; Piccinni, G. Management of mucinous cystic neoplasms of the pancreas. World J. Gastroenterol. 2010, 16, 5682–5692. [Google Scholar] [CrossRef]
- Sarr, M.G.; Carpenter, H.A.; Prabhakar, L.P.; Orchard, T.F.; Hughes, S.; van Heerden, J.A.; DiMagno, E.P. Clinical and Pathologic Correlation of 84 Mucinous Cystic Neoplasms of the Pancreas: Can one reliably differentiate benign from malignant (or premalignant) neoplasms? Ann. Surg. 2000, 231, 205–212. [Google Scholar] [CrossRef]
- Pittman, M.E.; Rao, R.; Hruban, R.H. Classification, Morphology, Molecular Pathogenesis, and Outcome of Premalignant Lesions of the Pancreas. Arch. Pathol. Lab. Med. 2017, 141, 1606–1614. [Google Scholar] [CrossRef] [Green Version]
- Springer, S.; Masica, D.L.; Dal Molin, M.; Douville, C.; Thoburn, C.J.; Afsari, B.; Li, L.; Cohen, J.D.; Thompson, E.; Allen, P.J.; et al. A multimodality test to guide the management of patients with a pancreatic cyst. Sci. Transl. Med. 2019, 11, eaav4772. [Google Scholar] [CrossRef]
- Compagno, J.; Oertel, J.E. Microcystic adenomas of the pancreas (glycogen-rich cystadenomas): A clinicopathologic study of 34 cases. Am. J. Clin. Pathol. 1978, 69, 289–298. [Google Scholar] [CrossRef]
- Galanis, C.; Zamani, A.; Cameron, J.L.; Campbell, K.A.; Lillemoe, K.D.; Caparrelli, D.; Chang, D.; Hruban, R.H.; Yeo, C.J. Resected serous cystic neoplasms of the pancreas: A review of 158 patients with recommendations for treatment. J. Gastrointest. Surg. 2007, 11, 820–826. [Google Scholar] [CrossRef]
- Kosmahl, M.; Pauser, U.; Peters, K.; Sipos, B.; Lüttges, J.; Kremer, B.; Klöppel, G. Cystic neoplasms of the pancreas and tumor-like lesions with cystic features: A review of 418 cases and a classification proposal. Virchows Arch. 2004, 445, 168–178. [Google Scholar] [CrossRef]
- Reid, M.D.; Choi, H.-J.; Memis, B.; Krasinskas, A.M.; Jang, K.T.; Akkas, G.; Maithel, S.K.; Sarmiento, J.M.; Kooby, D.A.; Basturk, O.; et al. Serous Neoplasms of the Pancreas: A Clinicopathologic Analysis of 193 Cases and Literature Review With New Insights on Macrocystic and Solid Variants and Critical Reappraisal of So-called “Serous Cystadenocarcinoma”. Am. J. Surg. Pathol. 2015, 39, 1597–1610. [Google Scholar] [CrossRef]
- Kim, M.; Karadsheh, Z.; Levy, A.; Al-Haddad, M.A. Management of Incidental Pancreatic Cystic Lesions: Integrating Novel Diagnostic and Prognostic Factors With CurrentClinical Guidelines. J. Clin. Gastroenterol. 2020, 54, 415–427. [Google Scholar] [CrossRef] [PubMed]
- Vortmeyer, A.O.; Lubensky, I.A.; Fogt, F.; Linehan, W.M.; Khettry, U.; Zhuang, Z. Allelic deletion and mutation of the von Hippel-Lindau (VHL) tumor suppressor gene in pancreatic microcystic adenomas. Am. J. Pathol. 1997, 151, 951–956. [Google Scholar] [PubMed]
- Kosmahl, M.; Seada, L.S.; Jänig, U.; Harms, D.; Klöppel, G. Solid-pseudopapillary tumor of the pancreas: Its origin revisited. Virchows Arch. 2000, 436, 473–480. [Google Scholar] [CrossRef] [PubMed]
- Klöppel, G.; Morohoshi, T.; John, H.D.; Oehmichen, W.; Opitz, K.; Angelkort, A.; Lietz, H.; Rückert, K. Solid and cystic acinar cell tumour of the pancreas. A tumour in young women with favourable prognosis. Virchows Arch A Pathol Anat Histol. 1981, 392, 171–183. [Google Scholar] [CrossRef] [PubMed]
- Terris, B.; Cavard, C. Diagnosis and molecular aspects of solid-pseudopapillary neoplasms of the pancreas. Semin. Diagn. Pathol. 2014, 31, 484–490. [Google Scholar] [CrossRef] [PubMed]
- Din, N.U.; Rahim, S.; Abdul-Ghafar, J.; Ahmed, A.; Ahmad, Z. Clinicopathological and immunohistochemical study of 29 cases of solid-pseudopapillary neoplasms of the pancreas in patients under 20 years of age along with detailed review of literature. Diagn. Pathol. 2020, 15, 139. [Google Scholar] [CrossRef]
- Foo, W.-C.; Harrison, G.; Zhang, X. Immunocytochemistry for SOX-11 and TFE3 as diagnostic markers for solid pseudopapillary neoplasms of the pancreas in FNA biopsies. Cancer Cytopathol. 2017, 125, 831–837. [Google Scholar] [CrossRef] [Green Version]
- Harrison, G.; Hemmerich, A.; Guy, C.; Perkinson, K.; Fleming, D.; McCall, S.; Cardona, D.; Zhang, X. Overexpression of SOX11 and TFE3 in Solid-Pseudopapillary Neoplasms of the Pancreas. Am. J. Clin. Pathol. 2017, 149, 67–75. [Google Scholar] [CrossRef]
- Jiang, Y.; Xie, J.; Wang, B.; Mu, Y.; Liu, P. TFE3 is a diagnostic marker for solid pseudopapillary neoplasms of the pancreas. Hum. Pathol. 2018, 81, 166–175. [Google Scholar] [CrossRef]
- Abraham, S.C.; Klimstra, D.S.; Wilentz, R.E.; Yeo, C.J.; Conlon, K.; Brennan, M.; Cameron, J.L.; Wu, T.-T.; Hruban, R.H. Solid-pseudopapillary tumors of the pancreas are genetically distinct from pancreatic ductal adenocarcinomas and almost always harbor β-catenin mutations. Am. J. Pathol. 2002, 160, 1361–1369. [Google Scholar] [CrossRef]
- Tanaka, Y.; Kato, K.; Notohara, K.; Hojo, H.; Ijiri, R.; Miyake, T.; Nagahara, N.; Sasaki, F.; Kitagawa, N.; Nakatani, Y.; et al. Frequent beta-catenin mutation and cytoplasmic/nuclear accumulation in pancreatic solid-pseudopapillary neoplasm. Cancer Res. 2001, 61, 8401–8404. [Google Scholar] [PubMed]
- Adsay, N.V.; Hasteh, F.; Cheng, J.D.; Klimstra, D.S. Squamous-lined cysts of the pancreas: Lymphoepithelial cysts, dermoid cysts (teratomas), and accessory-splenic epidermoid cysts. Semin. Diagn. Pathol. 2000, 17, 56–65. [Google Scholar]
- Adsay, N.V.; Hasteh, F.; Cheng, J.D.; Bejarano, P.A.; Lauwers, G.Y.; Batts, K.P.; Klöppel, G.; Klimstra, D.S. Lymphoepithelial cysts of the pancreas: A report of 12 cases and a review of the literature. Mod. Pathol. 2002, 15, 492–501. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Truong, L.D.; Rangdaeng, S.; Jordan, P.H., Jr. Lymphoepithelial cyst of the pancreas. Am. J. Surg. Pathol. 1987, 11, 899–903. [Google Scholar] [CrossRef]
- Lüchtrath, H.; Schriefers, K.H. [A pancreatic cyst with features of a so-called branchiogenic cyst]. Der. Pathol. 1985, 6, 217–219. [Google Scholar]
- Hisaoka, M.; Haratake, J.; Horie, A.; Yasunami, Y.; Kimura, T. Lymphoepithelial cyst of the pancreas in a 65-year-old man. Hum. Pathol. 1991, 22, 924–926. [Google Scholar] [CrossRef] [PubMed]
- Lane, J.; Vance, A.; Finelli, D.; Williams, G.; Ravichandran, P. Dermoid cyst of the pancreas: A case report with literature review. J. Radiol. Case Rep. 2012, 6, 17–25. [Google Scholar] [CrossRef]
- Yildiz, A.E.; Ariyurek, M.O.; Karcaaltincaba, M. Splenic anomalies of shape, size, and location: Pictorial essay. Sci. World J. 2013, 2013, 321810. [Google Scholar] [CrossRef] [Green Version]
- Li, B.Q.; Lu, J.; Seery, S.; Guo, J.C. Epidermoid cyst in intrapancreatic accessory spleen: A systematic review. Pancreatology 2019, 19, 10–16. [Google Scholar] [CrossRef]
- Mege, D.; Grégoire, E.; Barbier, L.; Del Grande, J.; Le Treut, Y.P. Lymphoepithelial cyst of the pancreas: An analysis of 117 patients. Pancreas 2014, 43, 987–995. [Google Scholar] [CrossRef]
- Osiro, S.; Rodriguez, J.R.; Tiwari, K.J.; Rodriguez, I.I.; Mathenge, N.; Tubbs, R.S.; Loukas, M. Is preoperative diagnosis possible? A clinical and radiological review of lymphoepithelial cysts of the pancreas. JOP. J. Pancreas 2013, 14, 15–20. [Google Scholar] [CrossRef]
- Tucci, G.; Muzi, M.G.; Nigro, C.; Cadeddu, F.; Amabile, D.; Servadei, F.; Farinon, A.M. Dermoid cyst of the pancreas: Presentation and management. World J. Surg. Oncol. 2007, 5, 85. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Seki, M.; Ninomiya, E.; Aruga, A.; Yamada, K.; Koga, R.; Saiura, A.; Yamamoto, J.; Yamaguchi, T.; Takano, K.; Fujita, R.; et al. Image-diagnostic features of mature cystic teratomas of the pancreas: Report on two cases difficult to diagnose preoperatively. J. Hepato-Biliary-Pancreat. Surg. 2005, 12, 336–340. [Google Scholar] [CrossRef] [PubMed]
- Zavras, N.; Machairas, N.; Foukas, P.; Lazaris, A.; Patapis, P.; Machairas, A. Epidermoid cyst of an intrapancreatic accessory spleen: A case report and literature review. World J. Surg. Oncol. 2014, 12, 92. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kato, S.; Mori, H.; Zakimi, M.; Yamada, K.; Chinen, K.; Arashiro, M.; Shinoura, S.; Kikuchi, K.; Murakami, T.; Kunishima, F. Epidermoid Cyst in an Intrapancreatic Accessory Spleen: Case Report and Literature Review of the Preoperative Imaging Findings. Intern. Med. 2016, 55, 3445–3452. [Google Scholar] [CrossRef] [Green Version]
- Krishna, S.G.; Modi, R.M.; Kamboj, A.K.; Swanson, B.J.; Hart, P.A.; Dillhoff, M.E.; Manilchuk, A.; Schmidt, C.R.; Conwell, D.L. In vivo and ex vivo confocal endomicroscopy of pancreatic cystic lesions: A prospective study. World J. Gastroenterol. 2017, 23, 3338–3348. [Google Scholar] [CrossRef]
- Capitanich, P.; Iovaldi, M.L.; Medrano, M.; Malizia, P.; Herrera, J.; Celeste, F.; Boerr, L.A.; Obiol, C.M.; Mezzadri, N.A. Lymphoepithelial cysts of the pancreas: Case report and review of the literature. J. Gastrointest. Surg. 2004, 8, 342–345. [Google Scholar] [CrossRef]
- Centeno, B.A.; Stockwell, J.W.; Lewandrowski, K.B. Cyst fluid cytology and chemical features in a case of lymphoepithelial cyst of the pancreas: A rare and difficult preoperative diagnosis. Diagn. Cytopathol. 1999, 21, 328–330. [Google Scholar] [CrossRef]
- Ciers, P.; Vanderhaeghe, D.; Vansteenkiste, F.; Moubax, K.; Vanooteghem, S.; Vanneste, A.; Van Moerkercke, W. Lymphoepithelial cysts of the pancreas: Case report and review of the literature. Acta Chir. Belg. 2022, 1–5. [Google Scholar] [CrossRef]
- Raval, J.S.; Zeh, H.J.; Moser, A.J.; Lee, K.K.; Sanders, M.K.; Navina, S.; Kuan, S.F.; Krasinskas, A.M. Pancreatic lymphoepithelial cysts express CEA and can contain mucous cells: Potential pitfalls in the preoperative diagnosis. Mod. Pathol. 2010, 23, 1467–1476. [Google Scholar] [CrossRef] [Green Version]
- Adsay, V.; Mino-Kenudson, M.; Furukawa, T.; Basturk, O.; Zamboni, G.; Marchegiani, G.; Bassi, C.; Salvia, R.; Malleo, G.; Paiella, S.; et al. Pathologic Evaluation and Reporting of Intraductal Papillary Mucinous Neoplasms of the Pancreas and Other Tumoral Intraepithelial Neoplasms of Pancreatobiliary Tract: Recommendations of Verona Consensus Meeting. Ann. Surg. 2016, 263, 162–177. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Krasinskas, A.M.; Oakley, G.J.; Bagci, P.; Jang, K.T.; Kuan, S.F.; Reid, M.D.; Erbarut, I.; Adsay, V. “Simple Mucinous Cyst” of the Pancreas: A Clinicopathologic Analysis of 39 Examples of a Diagnostically Challenging Entity Distinct From Intraductal Papillary Mucinous Neoplasms and Mucinous Cystic Neoplasms. Am. J. Surg. Pathol. 2017, 41, 121–127. [Google Scholar] [CrossRef] [PubMed]
- Kosmahl, M.; Egawa, N.; Schröder, S.; Carneiro, F.; Lüttges, J.; Klöppel, G. Mucinous nonneoplastic cyst of the pancreas: A novel nonneoplastic cystic change? Mod. Pathol. 2002, 15, 154–158. [Google Scholar] [CrossRef]
- Schechter, S.; Shi, J. Simple Mucinous Cyst of the Pancreas: Review and Update. Arch. Pathol. Lab. Med. 2017, 141, 1330–1335. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Attiyeh, M.; Zhang, L.; Iacobuzio-Donahue, C.; Allen, P.; Imam, R.; Basturk, O.; Klimstra, D.S.; Sigel, C.S. Simple mucinous cysts of the pancreas have heterogeneous somatic mutations. Hum. Pathol. 2020, 101, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Reid, M.D. Cytologic Assessment of Cystic/Intraductal Lesions of the Pancreatobiliary Tract. Arch. Pathol. Lab. Med. 2022, 146, 280–297. [Google Scholar] [CrossRef] [PubMed]
- Durkin, C.; Krishna, S.G. Advanced diagnostics for pancreatic cysts: Confocal endomicroscopy and molecular analysis. World J. Gastroenterol. 2019, 25, 2734–2742. [Google Scholar] [CrossRef]
- Chin, Y.K.; Wu, C.C.H.; Tan, D.M.Y. The Role of Needle-Based Confocal Laser Endomicroscopy in the Evaluation of Pancreatic Cystic Lesions: A Systematic Review. Clin. Endosc. 2021, 54, 38–47. [Google Scholar] [CrossRef] [Green Version]
- Singh, R.R.; Perisetti, A.; Pallav, K.; Chandan, S.; De Leon, M.R.; Sharma, N.R. Risk Stratification of Pancreatic Cysts With Confocal Laser Endomicroscopy. Gastro Hep Adv. 2021, 1, 160–170. [Google Scholar] [CrossRef]
- Tacelli, M.; Celsa, C.; Magro, B.; Barchiesi, M.; Barresi, L.; Capurso, G.; Arcidiacono, P.G.; Cammà, C.; Crinò, S.F. Diagnostic performance of endoscopic ultrasound through-the-needle microforceps biopsy of pancreatic cystic lesions: Systematic review with meta-analysis. Dig. Endosc. Off. J. Jpn. Gastroenterol. Endosc. Soc. 2020, 32, 1018–1030. [Google Scholar] [CrossRef] [Green Version]
- Rift, C.V.; Scheie, D.; Toxværd, A.; Kovacevic, B.; Klausen, P.; Vilmann, P.; Hansen, C.P.; Lund, E.L.; Hasselby, J.P. Diagnostic accuracy of EUS-guided through-the-needle-biopsies and simultaneously obtained fine needle aspiration for cytology from pancreatic cysts: A systematic review and meta-analysis. Pathol.-Res. Pract. 2021, 220, 153368. [Google Scholar] [CrossRef] [PubMed]
- Kovacevic, B.; Klausen, P.; Rift, C.V.; Toxværd, A.; Grossjohann, H.; Karstensen, J.G.; Brink, L.; Hassan, H.; Kalaitzakis, E.; Storkholm, J.; et al. Clinical impact of endoscopic ultrasound-guided through-the-needle microbiopsy in patients with pancreatic cysts. Endoscopy 2021, 53, 44–52. [Google Scholar] [CrossRef] [PubMed]
- Facciorusso, A.; Ramai, D.; Gkolfakis, P.; Shapiro, A.; Arvanitakis, M.; Lisotti, A.; Triantafyllou, K.; Fusaroli, P.; Papanikolaou, I.S.; Crinò, S.F. Through-the-needle biopsy of pancreatic cystic lesions: Current evidence and implications for clinical practice. Expert Rev. Med. Devices 2021, 18, 1165–1174. [Google Scholar] [CrossRef] [PubMed]
- Chong, Y.K.; Ho, C.C.; Leung, S.Y.; Lau, S.K.P.; Woo, P.C.Y. Clinical Mass Spectrometry in the Bioinformatics Era: A Hitchhiker’s Guide. Comput. Struct. Biotechnol. J. 2018, 16, 316–334. [Google Scholar] [CrossRef]
- Ge, P.; Luo, Y.; Chen, H.; Liu, J.; Guo, H.; Xu, C.; Qu, J.; Zhang, G.; Chen, H. Application of Mass Spectrometry in Pancreatic Cancer Translational Research. Front. Oncol. 2021, 11, 667427. [Google Scholar] [CrossRef] [PubMed]
- Bannaga, A.S.; Tyagi, H.; Daulton, E.; Covington, J.S.; Arasaradnam, R.P. Exploratory Study Using Urinary Volatile Organic Compounds for the Detection of Hepatocellular Carcinoma. Molecules 2021, 26, 2447. [Google Scholar] [CrossRef]
- Woollam, M.; Wang, L.; Grocki, P.; Liu, S.; Siegel, A.P.; Kalra, M.; Goodpaster, J.V.; Yokota, H.; Agarwal, M. Tracking the Progression of Triple Negative Mammary Tumors over Time by Chemometric Analysis of Urinary Volatile Organic Compounds. Cancers 2021, 13, 1462. [Google Scholar] [CrossRef]
- Paziewska, A.; Polkowski, M.; Rubel, T.; Karczmarski, J.; Wiechowska-Kozlowska, A.; Dabrowska, M.; Mikula, M.; Dadlez, M.; Ostrowski, J. Mass Spectrometry-Based Comprehensive Analysis of Pancreatic Cyst Fluids. BioMed Res. Int. 2018, 2018, 7169595. [Google Scholar] [CrossRef] [Green Version]
- Scarlett, C.J.; Samra, J.S.; Xue, A.; Baxter, R.C.; Smith, R.C. Classification of pancreatic cystic lesions using SELDI-TOF mass spectrometry. ANZ J. Surg. 2007, 77, 648–653. [Google Scholar] [CrossRef]
- Park, J.; Han, D.; Do, M.; Woo, J.; Wang, J.I.; Han, Y.; Kwon, W.; Kim, S.-W.; Jang, J.-Y.; Kim, Y. Proteome characterization of human pancreatic cyst fluid from intraductal papillary mucinous neoplasm by liquid chromatography/tandem mass spectrometry. Rapid Commun. Mass Spectrom. 2017, 31, 1761–1772. [Google Scholar] [CrossRef]
- Liu, X.; Zheng, W.; Wang, W.; Shen, H.; Liu, L.; Lou, W.; Wang, X.; Yang, P. A new panel of pancreatic cancer biomarkers discovered using a mass spectrometry-based pipeline. Br. J. Cancer 2017, 117, 1846–1854. [Google Scholar] [CrossRef] [PubMed]
- Jabbar, K.S.; Arike, L.; Verbeke, C.S.; Sadik, R.; Hansson, G.C. Highly Accurate Identification of Cystic Precursor Lesions of Pancreatic Cancer Through Targeted Mass Spectrometry: A Phase IIc Diagnostic Study. J. Clin. Oncol. 2018, 36, 367–375. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wojtkowski, M.; Bajraszewski, T.; Gorczynska, I.; Targowski, P.; Kowalczyk, A.; Wasilewski, W.; Radzewicz, C. Ophthalmic imaging by spectral optical coherence tomography. Am. J. Ophthalmol. 2004, 138, 412–419. [Google Scholar] [CrossRef] [PubMed]
- Welzel, J. Optical coherence tomography in dermatology: A review. Ski. Res. Technol. 2001, 7, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Bergmark, B.; Dallan, L.A.P.; Pereira, G.T.R.; Kuder, J.F.; Murphy, S.A.; Buccola, J.; Wollmuth, J.; Lopez, J.; Spinelli, J.; Meinen, J.; et al. Decision-Making During Percutaneous Coronary Intervention Guided by Optical Coherence Tomography: Insights From the LightLab Initiative. Circ. Cardiovasc. Interv. 2022, 15, 872–881. [Google Scholar] [CrossRef] [PubMed]
- Lu, L.; Hu, Z.; Frankel, W.; Shen, R.; Chen, W.; Pan, X.; Grecula, J.C.; Bloomston, M.P.; Dillhoff, M.E. Using Endoscopic Optical Coherence Tomography to Detect and Treat Early-Stage Pancreatic Cancers. Front. Oncol. 2021, 11, 591484. [Google Scholar] [CrossRef] [PubMed]
- Ardeshna, D.R.; Rangwani, S.; Cao, T.; Pawlik, T.M.; Stanich, P.P.; Krishna, S.G. Intraductal Papillary Mucinous Neoplasms in Hereditary Cancer Syndromes. Biomedicines 2022, 10, 1475. [Google Scholar] [CrossRef]




| IPMN | MCN | SCA | cNET | SPN | Pseudocyst | |
|---|---|---|---|---|---|---|
| Incidence * | 45% | 16% | 16% | 5% | 3% | - |
| Mean Age (year) | 65 | 45 | 62 | - | 25–30 | - |
| Sex | M > F | F >> M | F > M | - | F >> M | - |
| Mucinous | Yes | Yes | No | No | No | No |
| Ductal communication | Yes | No | No | No | No | No |
| Mean size | Variable | 6 cm | 4 cm | Variable | 8 cm | Variable |
| Most common location | Head | Tail/ body | Body/tail | Variable | Tail | Usually outside pancreas; exophytic |
| Cyst fluid | High viscosity, high amylase, CEA > 192 ng/mL | High viscosity, CEA > 192 ng/mL | Low viscosity, low CEA < 192 ng/mL | Low viscosity, low CEA < 192 ng/mL | - | Low viscosity, high amylase |
| Macroscopic features | Variable wall thickness, smooth to papillary lining epithelium | Thick wall ≥ 3 mm, unilocular or multilocular with few septa | Thin wall < 3 mm, smooth cyst lining, microcystic (honey comb) > macro/oligocystic > solid | Unilocular, hemorrhage, serous fluid | Solid with cystic spaces, hemorrhage, necrosis | Unilocular, no lining epithelium, dark cloudy fluid, ultimately thick fibrous wall |
| EUS-nCLE | Papillary projections with outer epithelium and inner vascular core | Horizon-type epithelial bands without papillae conformation | Distinct vascular pattern–an intricate fern pattern of capillary networks | Dark clusters (trabeculae) of cells in cords or nests separated by stroma | Dark clusters (trabeculae) of cells in cords or nests separated by stroma | Clumps of inflammatory cells. Dark background due to the absence of epithelium and associated vascular interstitium |
| Histology | Papillary mucinous epithelium | Mucinous epithelium, ovarian-type stroma | Flat serous epithelium, clear cytoplasm, subepithelial capillary network | Nests/trabeculae of cells separated by fibrous bands | Solid nested areas and pseudopapillary structures | No cyst epithelium, inflammatory/fibrotic wall |
| IHC | Gastric-type: MUC5AC+; Intestinal-type: MUC2+/CDX2+; Pancreatobiliary type: MUC1/MUC6+ | PR > ER+, inhibin+ | Inhibin+ | synaptophysin+, chromogranin+ | Beta-catenin (nuclear), SOX11+, TFE3+ | - |
| Molecular alteration | MAPK/GNAS, RNF43. Advanced neoplasia: TP53, SMAD, CDKN2A, mTOR | MAPK, RNF43. Advanced neoplasia: TP53, SMAD, CDKN2A, mTOR | VHL | MEN1, LOH | CTNNB1 | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, W.; Ahmed, N.; Krishna, S.G. Pancreatic Cystic Lesions: A Focused Review on Cyst Clinicopathological Features and Advanced Diagnostics. Diagnostics 2023, 13, 65. https://doi.org/10.3390/diagnostics13010065
Chen W, Ahmed N, Krishna SG. Pancreatic Cystic Lesions: A Focused Review on Cyst Clinicopathological Features and Advanced Diagnostics. Diagnostics. 2023; 13(1):65. https://doi.org/10.3390/diagnostics13010065
Chicago/Turabian StyleChen, Wei, Nehaal Ahmed, and Somashekar G. Krishna. 2023. "Pancreatic Cystic Lesions: A Focused Review on Cyst Clinicopathological Features and Advanced Diagnostics" Diagnostics 13, no. 1: 65. https://doi.org/10.3390/diagnostics13010065
APA StyleChen, W., Ahmed, N., & Krishna, S. G. (2023). Pancreatic Cystic Lesions: A Focused Review on Cyst Clinicopathological Features and Advanced Diagnostics. Diagnostics, 13(1), 65. https://doi.org/10.3390/diagnostics13010065







