Spectral CT: Current Liver Applications
Abstract
:1. Introduction
2. Materials and Methods
3. Results
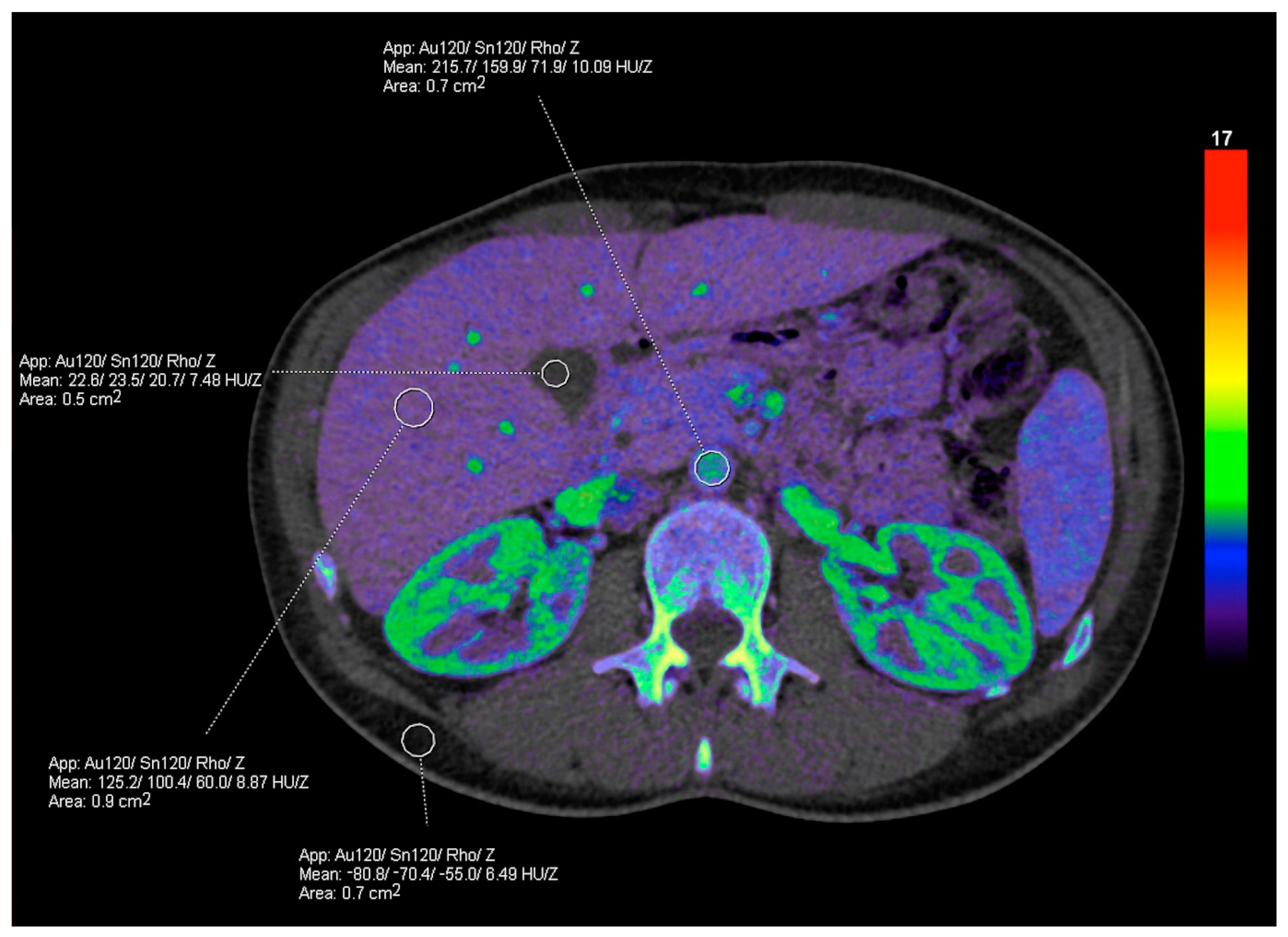
3.1. Postprocessing Techniques
3.2. Liver Diseases
3.2.1. Lesion Detection and Characterization
Hypervascular Lesions
Hypovascular Lesions
3.2.2. Treatment Response Evaluation
3.2.3. Diffuse Liver Diseases
Fat Deposition
Iron Deposition
Fibrosis
3.2.4. Trauma
3.2.5. Vascular Applications
3.3. Limitations
3.4. Future Perspectives
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Furlow, B. Dual-energy computed tomography. Radiol. Technol. 2015, 86, 301ct–321ct. [Google Scholar] [PubMed]
- Marin, D.; Boll, D.T.; Mileto, A.; Nelson, R.C. State of the art: Dual-energy CT of the abdomen. Radiology 2014, 271, 327–342. [Google Scholar] [CrossRef] [PubMed]
- Toia, G.V.; Kim, S.; Dighe, M.K.; Mileto, A. Dual-Energy Computed Tomography in Body Imaging. Semin. Roentgenol. 2018, 53, 132–146. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, Y.; Higaki, T.; Kondo, S.; Kawashita, I.; Takahashi, I.; Awai, K. An introduction to photon-counting detector CT (PCD CT) for radiologists. Jpn. J. Radiol. 2022. Epub ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Cramer, T.W.; Fletcher, J.G.; Paden, R.G.; Boltz, T.F., 2nd; Stiles, W.L.; Pavlicek, W.; Silva, A.C. A primer on the use of dual-energy CT in the evaluation of commonly encountered neoplasms. Abdom. Radiol. 2016, 41, 1618–1631. [Google Scholar] [CrossRef]
- Toia, G.V.; Mileto, A.; Wang, C.L.; Sahani, D.V. Quantitative dual-energy CT techniques in the abdomen. Abdom. Radiol. 2022, 47, 3003–3018. [Google Scholar] [CrossRef]
- Tamm, E.P.; Le, O.; Liu, X.; Layman, R.R.; Cody, D.D.; Bhosale, P.R. “How to” incorporate dual-energy imaging into a high volume abdominal imaging practice. Abdom. Radiol. 2017, 42, 688–701. [Google Scholar] [CrossRef] [Green Version]
- Kulkarni, N.M.; Fung, A.; Kambadakone, A.R.; Yeh, B.M. Computed Tomography Techniques, Protocols, Advancements, and Future Directions in Liver Diseases. Magn. Reason. Imaging Clin. N. Am. 2021, 29, 305–320. [Google Scholar] [CrossRef]
- Lehti, L.; Söderberg, M.; Höglund, P.; Wassélius, J. Comparing Arterial- and Venous-Phase Acquisition for Optimization of Virtual Noncontrast Images from Dual-Energy Computed Tomography Angiography. J. Comput. Assist. Tomogr. 2019, 43, 770–774. [Google Scholar] [CrossRef]
- Durieux, P.; Gevenois, P.A.; Muylem, A.V.; Howarth, N.; Keyzer, C. Abdominal Attenuation Values on Virtual and True Unenhanced Images Obtained with Third-Generation Dual-Source Dual-Energy CT. Am. J. Roentgenol. 2018, 210, 1042–1058. [Google Scholar] [CrossRef]
- Kim, S.; Kang, B.S.; Kwon, W.J.; Bang, M.; Lim, S.; Park, G.M.; Lee, T.Y. Abdominal Organs Attenuation Values and Abdominal Aortic Calcifications on Virtual and True Noncontrast Images Obtained with Third-Generation Dual-Source Dual-Energy Computed Tomography. J. Comput. Assist. Tomogr. 2020, 44, 490–500. [Google Scholar] [CrossRef]
- Wagner-Bartak, N.A.; Toshav, A.M.; Tamm, E.P.; Le, O.; Agarwal, S.; Ng, C.; Qayyum, A. CT Liver Imaging: What is New? Curr. Radiol. Rep. 2015, 3, 7. [Google Scholar] [CrossRef]
- Yamada, Y.; Jinzaki, M.; Hosokawa, T.; Tanami, Y.; Abe, T.; Kuribayashi, S. Abdominal CT: An intra-individual comparison between virtual monochromatic spectral and polychromatic 120-kVp images obtained during the same examination. Eur. J. Radiol. 2014, 83, 1715–1722. [Google Scholar] [CrossRef]
- Rassouli, N.; Chalian, H.; Rajiah, P.; Dhanantwari, A.; Landeras, L. Assessment of 70-keV virtual monoenergetic spectral images in abdominal CT imaging: A comparison study to conventional polychromatic 120-kVp images. Abdom. Radiol. 2017, 42, 2579–2586. [Google Scholar] [CrossRef]
- Hur, S.; Lee, J.M.; Kim, S.J.; Park, J.H.; Han, J.K.; Choi, B.I. 80-kVp CT using Iterative Reconstruction in Image Space algorithm for the detection of hypervascular hepatocellular carcinoma: Phantom and initial clinical experience. Korean J. Radiol. 2012, 13, 152–164. [Google Scholar] [CrossRef] [Green Version]
- Große Hokamp, N.; Höink, A.J.; Doerner, J.; Jordan, D.W.; Pahn, G.; Persigehl, T.; Maintz, D.; Haneder, S. Assessment of arterially hyper-enhancing liver lesions using virtual monoenergetic images from spectral detector CT: Phantom and patient experience. Abdom. Radiol. 2018, 43, 2066–2074. [Google Scholar] [CrossRef]
- Shuman, W.P.; Green, D.E.; Busey, J.M.; Mitsumori, L.M.; Choi, E.; Koprowicz, K.M.; Kanal, K.M. Dual-energy liver CT: Effect of monochromatic imaging on lesion detection, conspicuity, and contrast-to-noise ratio of hypervascular lesions on late arterial phase. Am. J. Roentgenol. 2014, 203, 601–606. [Google Scholar] [CrossRef]
- Mileto, A.; Nelson, R.C.; Samei, E.; Choudhury, K.R.; Jaffe, T.A.; Wilson, J.M.; Marin, D. Dual-energy MDCT in hypervascular liver tumors: Effect of body size on selection of the optimal monochromatic energy level. Am. J. Roentgenol. 2014, 203, 1257–1264. [Google Scholar] [CrossRef]
- De Cecco, C.N.; Caruso, D.; Schoepf, U.J.; De Santis, D.; Muscogiuri, G.; Albrecht, M.H.; Meinel, F.G.; Wichmann, J.L.; Burchett, P.F.; Varga-Szemes, A.; et al. A noise-optimized virtual monoenergetic reconstruction algorithm improves the diagnostic accuracy of late hepatic arterial phase dual-energy CT for the detection of hypervascular liver lesions. Eur. Radiol. 2018, 28, 3393–3404. [Google Scholar] [CrossRef]
- Marin, D.; Ramirez-Giraldo, J.C.; Gupta, S.; Fu, W.; Stinnett, S.S.; Mileto, A.; Bellini, D.; Patel, B.; Samei, E.; Nelson, R.C. Effect of a Noise-Optimized Second-Generation Monoenergetic Algorithm on Image Noise and Conspicuity of Hypervascular Liver Tumors: An In Vitro and In Vivo Study. Am. J. Roentgenol. 2016, 206, 1222–1232. [Google Scholar] [CrossRef]
- Matsuda, M.; Tsuda, T.; Kido, T.; Tanaka, H.; Nishiyama, H.; Itoh, T.; Nakao, K.; Hirooka, M.; Mochizuki, T. Dual-Energy Computed Tomography in Patients with Small Hepatocellular Carcinoma: Utility of Noise-Reduced Monoenergetic Images for the Evaluation of Washout and Image Quality in the Equilibrium Phase. J. Comput. Assist. Tomogr. 2018, 42, 937–943. [Google Scholar] [CrossRef] [PubMed]
- Voss, B.A.; Khandelwal, A.; Wells, M.L.; Inoue, A.; Venkatesh, S.K.; Lee, Y.S.; Johnson, M.P.; Fletcher, J.G. Impact of dual-energy 50-keV virtual monoenergetic images on radiologist confidence in detection of key imaging findings of small hepatocellular carcinomas using multiphase liver CT. Acta Radiol. 2022, 63, 1443–1452. [Google Scholar] [CrossRef] [PubMed]
- Reimer, R.P.; Große Hokamp, N.; Fehrmann Efferoth, A.; Krauskopf, A.; Zopfs, D.; Kröger, J.R.; Persigehl, T.; Maintz, D.; Bunck, A.C. Virtual monoenergetic images from spectral detector computed tomography facilitate washout assessment in arterially hyper-enhancing liver lesions. Eur. Radiol. 2021, 31, 3468–3477. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Shuman, W.P. Clinical Applications of Dual-Energy Computed Tomography in the Liver. Semin. Roentgenol. 2016, 51, 284–291. [Google Scholar] [CrossRef]
- Gordic, S.; Puippe, G.D.; Krauss, B.; Klotz, E.; Desbiolles, L.; Lesurtel, M.; Müllhaupt, B.; Pfammatter, T.; Alkadhi, H. Correlation between Dual-Energy and Perfusion CT in Patients with Hepatocellular Carcinoma. Radiology 2016, 280, 78–87. [Google Scholar] [CrossRef] [Green Version]
- Mulé, S.; Pigneur, F.; Quelever, R.; Tenenhaus, A.; Baranes, L.; Richard, P.; Tacher, V.; Herin, E.; Pasquier, H.; Ronot, M.; et al. Can dual-energy CT replace perfusion CT for the functional evaluation of advanced hepatocellular carcinoma? Eur. Radiol. 2018, 28, 1977–1985. [Google Scholar] [CrossRef]
- Wang, Q.; Shi, G.; Qi, X.; Fan, X.; Wang, L. Quantitative analysis of the dual-energy CT virtual spectral curve for focal liver lesions characterization. Eur. J. Radiol. 2014, 83, 1759–1764. [Google Scholar] [CrossRef]
- Li, J.; Zhao, S.; Ling, Z.; Li, D.; Jia, G.; Zhao, C.; Lin, X.; Dai, Y.; Jiang, H.; Wang, S. Dual-Energy Computed Tomography Imaging in Early-Stage Hepatocellular Carcinoma: A Preliminary Study. Contrast Media Mol. Imaging. 2022, 2022, 2146343. [Google Scholar] [CrossRef]
- Böning, G.; Adelt, S.; Feldhaus, F.; Fehrenbach, U.; Kahn, J.; Hamm, B.; Streitparth, F. Spectral CT in clinical routine imaging of neuroendocrine neoplasms. Clin. Radiol. 2021, 76, 348–357. [Google Scholar] [CrossRef]
- Yang, C.B.; Zhang, S.; Jia, Y.J.; Yu, Y.; Duan, H.F.; Zhang, X.R.; Ma, G.M.; Ren, C.; Yu, N. Dual energy spectral CT imaging for the evaluation of small hepatocellular carcinoma microvascular invasion. Eur. J. Radiol. 2017, 95, 222–227. [Google Scholar] [CrossRef]
- Lewin, M.; Laurent-Bellue, A.; Desterke, C.; Radu, A.; Feghali, J.A.; Farah, J.; Agostini, H.; Nault, J.-C.; Vibert, E.; Guettier, C. Evaluation of perfusion CT and dual-energy CT for predicting microvascular invasion of hepatocellular carcinoma. Abdom. Radiol. 2022, 47, 2115–2127. [Google Scholar] [CrossRef]
- Kim, T.M.; Lee, J.M.; Yoon, J.H.; Joo, I.; Park, S.-J.; Jeon, S.K.; Schmidt, B.; Martin, S. Prediction of microvascular invasion of hepatocellular carcinoma: Value of volumetric iodine quantification using preoperative dual-energy computed tomography. Cancer Imaging 2020, 20, 60. [Google Scholar] [CrossRef]
- Luo, N.; Li, W.; Xie, J.; Fu, D.; Liu, L.; Huang, X.; Su, D.; Jin, G. Preoperative normalized iodine concentration derived from spectral CT is correlated with early recurrence of hepatocellular carcinoma after curative resection. Eur. Radiol. 2021, 31, 1872–1882. [Google Scholar] [CrossRef]
- Tsurusaki, M.; Sofue, K.; Hori, M.; Sasaki, K.; Ishii, K.; Murakami, T.; Kudo, M. Dual-Energy Computed Tomography of the Liver: Uses in Clinical Practices and Applications. Diagnostics 2021, 11, 161. [Google Scholar] [CrossRef]
- Nagayama, Y.; Iyama, A.; Oda, S.; Taguchi, N.; Nakaura, T.; Utsunomiya, D.; Kikuchi, Y.; Yamashita, Y. Dual-layer dual-energy computed tomography for the assessment of hypovascular hepatic metastases: Impact of closing k-edge on image quality and lesion detectability. Eur. Radiol. 2019, 29, 2837–2847. [Google Scholar] [CrossRef]
- Caruso, D.; De Cecco, C.N.; Schoepf, U.J.; Schaefer, A.R.; Leland, P.W.; Johnson, D.; Laghi, A.; Hardie, A.D. Can dual-energy computed tomography improve visualization of hypoenhancing liver lesions in portal venous phase? Assessment of advanced image-based virtual monoenergetic images. Clin. Imaging 2017, 41, 118–124. [Google Scholar] [CrossRef]
- Lenga, L.; Czwikla, R.; Wichmann, J.L.; Leithner, D.; Albrecht, M.H.; Booz, C.; Arendt, C.T.; Yel, I.; D’Angelo, T.; Vogl, T.J.; et al. Dual-energy CT in patients with colorectal cancer: Improved assessment of hypoattenuating liver metastases using noise-optimized virtual monoenergetic imaging. Eur. J. Radiol. 2018, 106, 184–191. [Google Scholar] [CrossRef]
- Ratajczak, P.; Serafin, Z.; Sławińska, A.; Słupski, M.; Leszczyński, W. Improved imaging of colorectal liver metastases using single-source, fast kVp-switching, dual-energy CT: Preliminary results. Pol. J. Radiol. 2018, 83, e643–e649. [Google Scholar] [CrossRef]
- Yamada, Y.; Jinzaki, M.; Tanami, Y.; Abe, T.; Kuribayashi, S. Virtual monochromatic spectral imaging for the evaluation of hypovascular hepatic metastases: The optimal monochromatic level with fast kilovoltage switching dual-energy computed tomography. Investig. Radiol. 2012, 47, 292–298. [Google Scholar] [CrossRef]
- Husarik, D.B.; Gordic, S.; Desbiolles, L.; Krauss, B.; Leschka, S.; Wildermuth, S.; Alkadhi, H. Advanced virtual monoenergetic computed tomography of hyperattenuating and hypoattenuating liver lesions: Ex-vivo and patient experience in various body sizes. Investig. Radiol. 2015, 50, 695–702. [Google Scholar] [CrossRef] [Green Version]
- Agrawal, M.D.; Pinho, D.F.; Kulkarni, N.M.; Hahn, P.F.; Guimaraes, A.R.; Sahani, D.V. Oncologic applications of dual-energy CT in the abdomen. Radiographics 2014, 34, 589–612. [Google Scholar] [CrossRef] [PubMed]
- Patel, B.N.; Rosenberg, M.; Vernuccio, F.; Ramirez-Giraldo, J.C.; Nelson, R.; Farjat, A.; Marin, D. Characterization of Small Incidental Indeterminate Hypoattenuating Hepatic Lesions: Added Value of Single-Phase Contrast-Enhanced Dual-Energy CT Material Attenuation Analysis. Am. J. Roentgenol. 2018, 211, 571–579. [Google Scholar] [CrossRef] [PubMed]
- Sanghavi, P.S.; Jankharia, B.G. Applications of dual energy CT in clinical practice: A pictorial essay. Indian J. Radiol. Imaging 2019, 29, 289–298. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Ju, Y.; Wu, J.; Liu, A.; Chen, A.; Liu, J.; Liu, Y.; Li, J. Differentiation of liver abscess from liver metastasis using dual-energy spectral CT quantitative parameters. Eur. J. Radiol. 2019, 113, 204–208. [Google Scholar] [CrossRef]
- Yu, Y.; Guo, L.; Hu, C.; Chen, K. Spectral CT imaging in the differential diagnosis of necrotic hepatocellular carcinoma and hepatic abscess. Clin. Radiol. 2014, 69, e517–e524. [Google Scholar] [CrossRef]
- Kim, J.E.; Kim, H.O.; Bae, K.; Cho, J.M.; Choi, H.C.; Choi, D.S. Differentiation of small intrahepatic mass-forming cholangiocarcinoma from small liver abscess by dual source dual-energy CT quantitative parameters. Eur. J. Radiol. 2017, 92, 145–152. [Google Scholar] [CrossRef]
- Lv, P.; Liu, J.; Yan, X.; Chai, Y.; Chen, Y.; Gao, J.; Pan, Y.; Li, S.; Guo, H.; Zhou, Y. CT spectral imaging for monitoring the therapeutic efficacy of VEGF receptor kinase inhibitor AG-013736 in rabbit VX2 liver tumours. Eur. Radiol. 2017, 27, 918–926. [Google Scholar] [CrossRef]
- Dai, X.; Schlemmer, H.-P.; Schmidt, B.; Höh, K.; Xu, K.; Ganten, T.M.; Ganten, M.-K. Quantitative therapy response assessment by volumetric iodine-uptake measurement: Initial experience in patients with advanced hepatocellular carcinoma treated with sorafenib. Eur. J. Radiol. 2013, 82, 327–334. [Google Scholar] [CrossRef]
- Dai, X.; Schlemmer, H.-P.; Schmidt, B.; Höh, K.; Xu, K.; Ganten, T.M.; Ganten, M.-K. Application of Gemstone CT Spectroscopy in the Evaluation of Abnormal Enhancement of Lesion Margin After Radiofrequency Ablation of Hepatocellular Carcinoma. Iran. J. Radiol. 2020, 17, e99611. [Google Scholar] [CrossRef]
- Li, J.-P.; Zhao, S.; Jiang, H.-J.; Jiang, H.; Zhang, L.-H.; Shi, Z.-X.; Fan, T.-T.; Wang, S. Quantitative dual-energy computed tomography texture analysis predicts the response of primary small hepatocellular carcinoma to radiofrequency ablation. Hepatobiliary Pancreat. Dis. Int. 2022, 21, 569–576. [Google Scholar] [CrossRef]
- Reimer, R.P.; Hokamp, N.G.; Niehoff, J.; Zopfs, D.; Lennartz, S.; Heidar, M.; Wahba, R.; Stippel, D.; Maintz, D.; dos Santos, D.P.; et al. Value of spectral detector computed tomography for the early assessment of technique efficacy after microwave ablation of hepatocellular carcinoma. PLoS ONE 2021, 16, e0252678. [Google Scholar] [CrossRef]
- Bäumler, W.; Beyer, L.P.; Lürken, L.; Wiggermann, P.; Stroszczynski, C.; Dollinger, M.; Schicho, A. Detection of Incomplete Irreversible Electroporation (IRE) and Microwave Ablation (MWA) of Hepatocellular Carcinoma (HCC) Using Iodine Quantification in Dual Energy Computed Tomography (DECT). Diagnostics 2022, 12, 986. [Google Scholar] [CrossRef]
- Xu, Y.; Xiao, A.; Yang, J.; Zhang, Z.; Zhang, G. Assessment of Lipiodol Deposition and Residual Cancer for Hepatocellular Carcinoma After Transcatheter Arterial Chemoembolization via Iodine-Based Material Decomposition Images with Spectral Computed Tomography Imaging: A Preliminary Study. Iran. J. Radiol. 2015, 12, e26009. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.S.; Chuang, M.T.; Tsai, Y.S.; Tsai, H.M.; Lin, X.Z. Nitroglycerine use in transcatheter arterial (chemo)embolization in patients with hepatocellular carcinoma and dual-energy CT assessment of Lipiodol retention. Eur. Radiol. 2012, 22, 2193–2200. [Google Scholar] [CrossRef]
- Liu, Q.Y.; He, C.D.; Zhou, Y.; Huang, D.; Lin, H.; Wang, Z.; Wang, D.; Wang, J.Q.; Liao, L.P. Application of gemstone spectral imaging for efficacy evaluation in hepatocellular carcinoma after transarterial chemoembolization. World J. Gastroenterol. 2016, 22, 3242–3251. [Google Scholar] [CrossRef]
- Wang, J.; Shen, J.L. Spectral CT in evaluating the therapeutic effect of transarterial chemoembolization for hepatocellular carcinoma: A retrospective study. Medicine 2017, 96, e9236. [Google Scholar] [CrossRef]
- Yue, X.; Jiang, Q.; Hu, X.; Cen, C.; Song, S.; Qian, K.; Lu, Y.; Yang, M.; Li, Q.; Han, P. Quantitative dual-energy CT for evaluating hepatocellular carcinoma after transarterial chemoembolization. Sci. Rep. 2021, 11, 11127. [Google Scholar] [CrossRef]
- Lee, J.A.; Jeong, W.K.; Kim, Y.; Song, S.Y.; Kim, J.; Heo, J.N.; Park, C.K. Dual-energy CT to detect recurrent HCC after TACE: Initial experience of color-coded iodine CT imaging. Eur. J. Radiol. 2013, 82, 569–576. [Google Scholar] [CrossRef]
- Negussie, A.H.; de Ruiter, Q.M.B.; Britton, H.; Donahue, D.R.; Boffi, Q.; Kim, Y.-S.; Pritchard, W.F.; Moonen, C.; Storm, G.; Lewis, A.L.; et al. Synthesis, characterization, and imaging of radiopaque bismuth beads for image-guided transarterial embolization. Sci. Rep. 2021, 11, 533. [Google Scholar] [CrossRef]
- Altenbernd, J.; Wetter, A.; Forsting, M.; Umutlu, L. Treatment response after radioembolisation in patients with hepatocellular carcinoma-An evaluation with dual energy computed-tomography. Eur. J. Radiol. Open 2016, 3, 230–235. [Google Scholar] [CrossRef] [Green Version]
- Bargellini, I.; Crocetti, L.; Turini, F.M.; Lorenzoni, G.; Boni, G.; Traino, A.C.; Caramella, D.; Cioni, R. Response Assessment by Volumetric Iodine Uptake Measurement: Preliminary Experience in Patients with Intermediate-Advanced Hepatocellular Carcinoma Treated with Yttrium-90 Radioembolization. Cardiovasc. Intervent. Radiol. 2018, 41, 1373–1383. [Google Scholar] [CrossRef] [PubMed]
- Apfaltrer, P.; Meyer, M.; Meier, C.; Henzler, T.; Barraza, J.M., Jr.; Dinter, D.J.; Hohenberger, P.; Schoepf, U.J.; Schoenberg, S.O.; Fink, C. Contrast-enhanced dual-energy CT of gastrointestinal stromal tumors: Is iodine-related attenuation a potential indicator of tumor response? Investig. Radiol. 2012, 47, 65–70. [Google Scholar] [CrossRef] [PubMed]
- Meyer, M.; Hohenberger, P.; Overhoff, D.; Bartsch, A.; Henzler, T.; Haubenreisser, H.; Ronald, J.; Schmidt, B.; Flohr, T.; Sedlmair, M.; et al. Dual-Energy CT Vital Iodine Tumor Burden for Response Assessment in Patients with Metastatic GIST Undergoing TKI Therapy: Comparison with Standard CT and FDG PET/CT Criteria. Am. J. Roentgenol. 2022, 218, 659–669. [Google Scholar] [CrossRef] [PubMed]
- Wada, N.; Fujita, N.; Ishimatsu, K.; Takao, S.; Yoshizumi, T.; Miyazaki, Y.; Oda, Y.; Nishie, A.; Ishigami, K.; Ushijima, Y. A novel fast kilovoltage switching dual-energy computed tomography technique with deep learning: Utility for non-invasive assessments of liver fibrosis. Eur. J. Radiol. 2022, 155, 110461. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.J.; Boesen, M.R.; Hansen, S.L.; Ulriksen, P.S.; Holm, S.; Lönn, L.; Hansen, K.L. Assessment of Liver Fat: Dual-Energy CT versus Conventional CT with and without Contrast. Diagnostics 2022, 12, 708. [Google Scholar] [CrossRef]
- Kramer, H.; Pickhardt, P.J.; Kliewer, M.A.; Hernando, D.; Chen, G.H.; Zagzebski, J.A.; Reeder, S.B. Accuracy of Liver Fat Quantification with Advanced CT, MRI, and Ultrasound Techniques: Prospective Comparison with MR Spectroscopy. Am. J. Roentgenol. 2017, 208, 92–100. [Google Scholar] [CrossRef] [Green Version]
- Zheng, X.; Ren, Y.; Phillips, W.T.; Li, M.; Song, M.; Hua, Y.; Zhang, G. Assessment of hepatic fatty infiltration using spectral computed tomography imaging: A pilot study. J. Comput. Assist. Tomogr. 2013, 37, 134–141. [Google Scholar] [CrossRef]
- Corrias, G.; Erta, M.; Sini, M.; Sardu, C.; Saba, L.; Mahmood, U.; Castellanos, S.H.; Bates, D.; Mondanelli, N.; Thomsen, B.; et al. Comparison of Multimaterial Decomposition Fat Fraction with DECT and Proton Density Fat Fraction with IDEAL IQ MRI for Quantification of Liver Steatosis in a Population Exposed to Chemotherapy. Dose Response 2021, 19, 1559325820984938. [Google Scholar] [CrossRef]
- Mendonça, P.R.; Lamb, P.; Kriston, A.; Sasaki, K.; Kudo, M.; Sahani, D.V. Contrast-independent liver-fat quantification from spectral CT exams. Med. Image Comput. Comput. Assist. Interv. 2013, 16 Pt 1, 324–331. [Google Scholar] [CrossRef]
- Molwitz, I.; Campbell, G.M.; Yamamura, J.; Knopp, T.; Toedter, K.D.-C.; Fischer, R.; Wang, Z.J.; Busch, A.; Ozga, A.-K.; Zhang, S.; et al. Fat Quantification in Dual-Layer Detector Spectral Computed Tomography: Experimental Development and First In-Patient Validation. Investig. Radiol. 2022, 57, 463–469. [Google Scholar] [CrossRef]
- Zhang, Q.; Zhao, Y.; Wu, J.; Xie, L.; Chen, A.; Liu, Y.; Song, Q.; Li, J.; Wu, T.; Xie, L.; et al. Quantification of Hepatic Fat Fraction in Patients with Nonalcoholic Fatty Liver Disease: Comparison of Multimaterial Decomposition Algorithm and Fat (Water)-Based Material Decomposition Algorithm Using Single-Source Dual-Energy Computed Tomography. J. Comput. Assist. Tomogr. 2021, 45, 12–17. [Google Scholar] [CrossRef] [PubMed]
- Haji-Momenian, S.; Parkinson, W.; Khati, N.; Brindle, K.; Earls, J.; Zeman, R.K. Single-energy non-contrast hepatic steatosis criteria applied to virtual non-contrast images: Is it still highly specific and positively predictive? Clin. Radiol. 2018, 73, 594.e7–594.e15. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.P.; Choi, H.H.; Ohliger, M.A. Detection of fatty liver using virtual non-contrast dual-energy CT. Abdom. Radiol. 2022, 47, 2046–2056. [Google Scholar] [CrossRef] [PubMed]
- Choi, M.H.; Lee, Y.J.; Choi, Y.J.; Pak, S. Dual-energy CT of the liver: True noncontrast vs. virtual noncontrast images derived from multiple phases for the diagnosis of fatty liver. Eur. J. Radiol. 2021, 140, 109741. [Google Scholar] [CrossRef] [PubMed]
- Niehoff, J.H.; Woeltjen, M.M.; Saeed, S.; Michael, A.E.; Boriesosdick, J.; Borggrefe, J.; Kroeger, J.R. Assessment of hepatic steatosis based on virtual non-contrast computed tomography: Initial experiences with a photon counting scanner approved for clinical use. Eur. J. Radiol. 2022, 149, 110185. [Google Scholar] [CrossRef]
- Kang, H.J.; Lee, D.H.; Park, S.J.; Han, J.K. Virtual noncontrast images derived from dual-energy CT for assessment of hepatic steatosis in living liver donors. Eur. J. Radiol. 2021, 139, 109687. [Google Scholar] [CrossRef]
- Hong, S.B.; Lee, N.K.; Kim, S.; Um, K.; Kim, K.; Kim, I.J. Hepatic Fat Quantification with the Multi-Material Decomposition Algorithm by Using Low-Dose Non-Contrast Material-Enhanced Dual-Energy Computed Tomography in a Prospectively Enrolled Cohort. Medicina 2022, 58, 1459. [Google Scholar] [CrossRef]
- Beck, S.; Jahn, L.; Deniffel, D.; Riederer, I.; Sauter, A.; Makowski, M.R.; Pfeiffer, D. Iodine Images in Dual-energy CT: Detection of Hepatic Steatosis by Quantitative Iodine Concentration Values. J. Digit. Imaging 2022, 35, 1738–1747. [Google Scholar] [CrossRef]
- Zhang, Y.; Xiao, C.; Li, J.; Song, L.X.; Zhao, Y.S.; Han, S.; Li, Z.W.; Guo, C.; Zhao, J.G.; Chang, C.K. Comparative Study on Iron Content Detection by Energy Spectral CT and MRI in MDS Patients. Front. Oncol. 2021, 11, 646946. [Google Scholar] [CrossRef]
- Ma, Q.; Hu, J.; Yang, W.; Hou, Y. Dual-layer detector spectral CT versus magnetic resonance imaging for the assessment of iron overload in myelodysplastic syndromes and aplastic anemia. Jpn. J. Radiol. 2020, 38, 374–381. [Google Scholar] [CrossRef]
- Luo, X.F.; Xie, X.Q.; Cheng, S.; Yang, Y.; Yan, J.; Zhang, H.; Chai, W.M.; Schmidt, B.; Yan, F.H. Dual-Energy CT for Patients Suspected of Having Liver Iron Overload: Can Virtual Iron Content Imaging Accurately Quantify Liver Iron Content? Radiology 2015, 277, 95–103. [Google Scholar] [CrossRef]
- Elbanna, K.Y.; Mansoori, B.; Mileto, A.; Rogalla, P.; Guimarães, L. Dual-energy CT in diffuse liver disease: Is there a role? Abdom. Radiol. 2020, 45, 3413–3424. [Google Scholar] [CrossRef]
- Jiang, X.; Hintenlang, D.E.; White, R.D. Lower limit of iron quantification using dual-energy CT-a phantom study. J. Appl. Clin. Med. Phys. 2021, 22, 299–307. [Google Scholar] [CrossRef]
- Joe, E.; Kim, S.H.; Lee, K.B.; Jang, J.J.; Lee, J.Y.; Lee, J.M.; Han, J.K.; Choi, B.I. Feasibility and accuracy of dual-source dual-energy CT for noninvasive determination of hepatic iron accumulation. Radiology 2012, 262, 126–135. [Google Scholar] [CrossRef]
- Ma, J.; Song, Z.Q.; Yan, F.H. Separation of hepatic iron and fat by dual-source dual-energy computed tomography based on material decomposition: An animal study. PLoS ONE 2014, 9, e110964. [Google Scholar] [CrossRef] [Green Version]
- Sofue, K.; Tsurusaki, M.; Mileto, A.; Hyodo, T.; Sasaki, K.; Nishii, T.; Chikugo, T.; Yada, N.; Kudo, M.; Sugimura, K.; et al. Dual-energy computed tomography for non-invasive staging of liver fibrosis: Accuracy of iodine density measurements from contrast-enhanced data. Hepatol. Res. 2018, 48, 1008–1019. [Google Scholar] [CrossRef]
- Bottari, A.; Silipigni, S.; Carerj, M.L.; Cattafi, A.; Maimone, S.; Marino, M.A.; Mazziotti, S.; Pitrone, A.; Squadrito, G.; Ascenti, G. Dual-source dual-energy CT in the evaluation of hepatic fractional extracellular space in cirrhosis. Radiol. Med. 2020, 125, 7–14. [Google Scholar] [CrossRef]
- Cicero, G.; Mazziotti, S.; Silipigni, S.; Blandino, A.; Cantisani, V.; Pergolizzi, S.; D’angelo, T.; Stagno, A.; Maimone, S.; Squadrito, G.; et al. Dual-energy CT quantification of fractional extracellular space in cirrhotic patients: Comparison between early and delayed equilibrium phases and correlation with oesophageal varices. Radiol. Med. 2021, 126, 761–767. [Google Scholar] [CrossRef]
- Yoon, J.H.; Lee, J.M.; Kim, J.H.; Lee, K.-B.; Kim, H.; Hong, S.K.; Yi, N.-J.; Lee, K.-W.; Suh, K.-S. Hepatic fibrosis grading with extracellular volume fraction from iodine mapping in spectral liver, C.T. Eur. J. Radiol. 2021, 137, 109604. [Google Scholar] [CrossRef]
- Lv, P.; Lin, X.; Gao, J.; Chen, K. Spectral CT: Preliminary studies in the liver cirrhosis. Korean J. Radiol. 2012, 13, 434–442. [Google Scholar] [CrossRef] [Green Version]
- Marri, U.K.; Das, P.; Shalimar Kalaivani, M.; Srivastava, D.N.; Madhusudhan, K.S. Noninvasive Staging of Liver Fibrosis Using 5-Minute Delayed Dual-Energy CT: Comparison with US Elastography and Correlation with Histologic Findings. Radiology 2021, 298, 600–608. [Google Scholar] [CrossRef] [PubMed]
- Morita, K.; Nishie, A.; Ushijima, Y.; Takayama, Y.; Fujita, N.; Kubo, Y.; Ishimatsu, K.; Yoshizumi, T.; Maehara, J.; Ishigami, K. Noninvasive assessment of liver fibrosis by dual-layer spectral detector CT. Eur. J. Radiol. 2021, 136, 109575. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.Q.; He, W.; Yan, B.; Wang, H.Y.; Wang, J. The evaluation of haemodynamics in cirrhotic patients with spectral CT. Br. J. Radiol. 2013, 86, 20130228. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mastrodicasa, D.; Willemink, M.J.; Duran, C.; Pizzi, A.D.; Hinostroza, V.; Molvin, L.; Khalaf, M.; Jeffrey, R.B.; Patel, B.N. Non-invasive assessment of cirrhosis using multiphasic dual-energy CT iodine maps: Correlation with model for end-stage liver disease score. Abdom. Radiol. 2021, 46, 1931–1940. [Google Scholar] [CrossRef]
- Nagayama, Y.; Kato, Y.; Inoue, T.; Nakaura, T.; Oda, S.; Kidoh, M.; Ikeda, O.; Hirai, T. Liver fibrosis assessment with multiphasic dual-energy CT: Diagnostic performance of iodine uptake parameters. Eur Radiol. 2021, 31, 5779–5790, Erratum in Eur Radiol. 2021, 31, 8823–8824. [Google Scholar] [CrossRef]
- Hamid, S.; Nicolaou, S.; Khosa, F.; Andrews, G.; Murray, N.; Abdellatif, W.; Qamar, S.R. Dual-Energy CT: A Paradigm Shift in Acute Traumatic Abdomen. Can. Assoc. Radiol. J. 2020, 71, 371–387. [Google Scholar] [CrossRef] [Green Version]
- Sun, E.X.; Wortman, J.R.; Uyeda, J.W.; Lacson, R.; Sodickson, A.D. Virtual monoenergetic dual-energy CT for evaluation of hepatic and splenic lacerations. Emerg. Radiol. 2019, 26, 419–425. [Google Scholar] [CrossRef]
- Marin, D.; Caywood, D.T.; Mileto, A.; Reiner, C.S.; Seaman, D.M.; Patel, B.N.; Boll, D.T.; Nelson, R.C. Dual-Energy Multidetector-Row Computed Tomography of the Hepatic Arterial System: Optimization of Energy and Material-Specific Reconstruction Techniques. J. Comput. Assist. Tomogr. 2015, 39, 721–729. [Google Scholar] [CrossRef] [Green Version]
- Yin, X.-P.; Gao, B.-L.; Li, C.-Y.; Zhou, H.; Zhao, L.; Zheng, Y.-T.; Zhao, Y.-X. Optimal Monochromatic Imaging of Spectral Computed Tomography Potentially Improves the Quality of Hepatic Vascular Imaging. Korean J. Radiol. 2018, 19, 578–584. [Google Scholar] [CrossRef]
- Majeed, N.F.; Ali, S.M.; Therrien, J.; Wald, C.; Wortman, J.R. Virtual Monoenergetic Spectral Detector CT for Preoperative CT Angiography in Liver Donors. Curr. Probl. Diagn. Radiol. 2022, 51, 517–523. [Google Scholar] [CrossRef]
- Zhao, Y.; Wu, Y.; Zuo, Z.; Suo, H.; Zhao, S.; Han, J.; Chang, X.; Cheng, S. Application of low concentration contrast medium in spectral CT imaging for CT portal venography. J. X-ray Sci. Technol. 2017, 25, 135–143. [Google Scholar] [CrossRef]
- Schabel, C.; Bongers, M.; Sedlmair, M.; Korn, A.; Grosse, U.; Mangold, S.; Claussen, C.D.; Thomas, C. Assessment of the hepatic veins in poor contrast conditions using dual energy CT: Evaluation of a novel monoenergetic extrapolation software algorithm. Rofo 2014, 186, 591–597. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Gao, F.; Shen, J.L. Noninvasive Assessment of Portal Hypertension Using Spectral Computed Tomography. J. Clin. Gastroenterol. 2019, 53, e387–e391. [Google Scholar] [CrossRef]
- Wang, L.; Wang, R.; Zhang, C.; Yue, Z.; Zhao, H.; Fan, Z.; Wu, Y.; Zhang, Y.; Liu, F.; Dong, J. Hepatic parenchyma and vascular blood flow changes after TIPS with spectral CT iodine density in HBV-related liver cirrhosis. Sci. Rep. 2021, 11, 10535. [Google Scholar] [CrossRef]
- Ascenti, G.; Sofia, C.; Mazziotti, S.; Silipigni, S.; D’Angelo, T.; Pergolizzi, S.; Scribano, E. Dual-energy CT with iodine quantification in distinguishing between bland and neoplastic portal vein thrombosis in patients with hepatocellular carcinoma. Clin. Radiol. 2016, 71, 938.e1–938.e9. [Google Scholar] [CrossRef]
- Martin, S.S.; Kolaneci, J.; Czwikla, R.; Booz, C.; Gruenewald, L.D.; Albrecht, M.H.; Thompson, Z.M.; Lenga, L.; Yel, I.; Vogl, T.J.; et al. Dual-Energy CT for the Detection of Portal Vein Thrombosis: Improved Diagnostic Performance Using Virtual Monoenergetic Reconstructions. Diagnostics 2022, 12, 1682. [Google Scholar] [CrossRef]
- George, E.; Wortman, J.R.; Fulwadhva, U.P.; Uyeda, J.W.; Sodickson, A.D. Dual energy CT applications in pancreatic pathologies. Br. J. Radiol. 2017, 90, 20170411. [Google Scholar] [CrossRef]
- Patel, B.N.; Alexander, L.; Allen, B.; Berland, L.; Borhani, A.; Mileto, A.; Moreno, C.; Morgan, D.; Sahani, D.; Shuman, W.; et al. Dual-energy CT workflow: Multi-institutional consensus on standardization of abdominopelvic MDCT protocols. Abdom. Radiol. 2017, 42, 676–687. [Google Scholar] [CrossRef]
- Homayounieh, F.; Singh, R.; Nitiwarangkul, C.; Lades, F.; Schmidt, B.; Sedlmair, M.; Saini, S.; Kalra, M.K. Semiautomatic Segmentation and Radiomics for Dual-Energy CT: A Pilot Study to Differentiate Benign and Malignant Hepatic Lesions. Am. J. Roentgenol. 2020, 215, 398–405. [Google Scholar] [CrossRef]
- Ebrahimian, S.; Singh, R.; Netaji, A.; Madhusudhan, K.S.; Homayounieh, F.; Primak, A.; Lades, F.; Saini, S.; Kalra, M.K.; Sharma, S. Characterization of Benign and Malignant Pancreatic Lesions with DECT Quantitative Metrics and Radiomics. Acad. Radiol. 2022, 29, 705–713. [Google Scholar] [CrossRef]
- Doda Khera, R.; Homayounieh, F.; Lades, F.; Schmidt, B.; Sedlmair, M.; Primak, A.; Saini, S.; Kalra, M.K. Can Dual-Energy Computed Tomography Quantitative Analysis and Radiomics Differentiate Normal Liver from Hepatic Steatosis and Cirrhosis? J. Comput. Assist. Tomogr. 2020, 44, 223–229. [Google Scholar] [CrossRef] [PubMed]
- Meyer, M.; Ronald, J.; Vernuccio, F.; Nelson, R.C.; Ramirez-Giraldo, J.C.; Solomon, J.; Patel, B.N.; Samei, E.; Marin, D. Reproducibility of CT Radiomic Features within the Same Patient: Influence of Radiation Dose and CT Reconstruction Settings. Radiology 2019, 293, 583–591. [Google Scholar] [CrossRef] [PubMed]
- Lee, T.; Lee, J.M.; Yoon, J.H.; Joo, I.; Bae, J.S.; Yoo, J.; Kim, J.H.; Ahn, C.; Kim, J.H. Deep learning-based image reconstruction of 40-keV virtual monoenergetic images of dual-energy CT for the assessment of hypoenhancing hepatic metastasis. Eur. Radiol. 2022, 32, 6407–6417. [Google Scholar] [CrossRef] [PubMed]
- Sato, M.; Ichikawa, Y.; Domae, K.; Yoshikawa, K.; Kanii, Y.; Yamazaki, A.; Nagasawa, N.; Nagata, M.; Ishida, M.; Sakuma, H. Deep learning image reconstruction for improving image quality of contrast-enhanced dual-energy CT in abdomen. Eur. Radiol. 2022, 32, 5499–5507. [Google Scholar] [CrossRef] [PubMed]
- Seo, J.Y.; Joo, I.; Yoon, J.H.; Kang, H.J.; Kim, S.; Kim, J.H.; Ahn, C.; Lee, J.M. Deep learning-based reconstruction of virtual monoenergetic images of kVp-switching dual energy CT for evaluation of hypervascular liver lesions: Comparison with standard reconstruction technique. Eur. J. Radiol. 2022, 154, 110390. [Google Scholar] [CrossRef]
- Shapira, N.; Fokuhl, J.; Schultheiß, M.; Beck, S.; Kopp, F.K.; Pfeiffer, D.; Dangelmaier, J.; Pahn, G.; Sauter, A.P.; Renger, B.; et al. Liver lesion localisation and classification with convolutional neural networks: A comparison between conventional and spectral computed tomography. Biomed. Phys. Eng. Express. 2020, 6, 015038. [Google Scholar] [CrossRef] [Green Version]
- Mileto, A.; Ananthakrishnan, L.; Morgan, D.E.; Yeh, B.M.; Marin, D.; Kambadakone, A.R. Clinical Implementation of Dual-Energy CT for Gastrointestinal Imaging. Am. J. Roentgenol. 2021, 217, 651–663. [Google Scholar] [CrossRef]
- Ng, Y.S.; Xi, Y.; Qian, Y.; Ananthakrishnan, L.; Soesbe, T.C.; Lewis, M.; Lenkinski, R.; Fielding, J.R. Use of Spectral Detector Computed Tomography to Improve Liver Segmentation and Volumetry. J. Comput. Assist. Tomogr. 2020, 44, 197–203. [Google Scholar] [CrossRef]
- Mongan, J.; Rathnayake, S.; Fu, Y.; Wang, R.; Jones, E.F.; Gao, D.W.; Yeh, B.M. In vivo differentiation of complementary contrast media at dual-energy CT. Radiology 2012, 265, 267–272. [Google Scholar] [CrossRef]
- Esquivel, A.; Ferrero, A.; Mileto, A.; Baffour, F.; Horst, K.; Rajiah, P.S.; Inoue, A.; Leng, S.; McCollough, C.; Fletcher, J.G. Photon-Counting Detector CT: Key Points Radiologists Should Know. Korean J. Radiol. 2022, 23, 854–865. [Google Scholar] [CrossRef]
- Muenzel, D.; Daerr, H.; Proksa, R.; Fingerle, A.A.; Kopp, F.K.; Douek, P.; Herzen, J.; Pfeiffer, F.; Rummeny, E.J.; Noël, P.B. Simultaneous dual-contrast multi-phase liver imaging using spectral photon-counting computed tomography: A proof-of-concept study. Eur. Radiol. Exp. 2017, 1, 25. [Google Scholar] [CrossRef] [Green Version]
- Si-Mohamed, S.; Tatard-Leitman, V.; Laugerette, A.; Sigovan, M.; Pfeiffer, D.; Rummeny, E.J.; Coulon, P.; Yagil, Y.; Douek, P.; Boussel, L.; et al. Spectral Photon-Counting Computed Tomography (SPCCT): In-vivo single-acquisition multi-phase liver imaging with a dual contrast agent protocol. Sci. Rep. 2019, 9, 8458. [Google Scholar] [CrossRef] [Green Version]
- Amato, C.; Klein, L.; Wehrse, E.; Rotkopf, L.T.; Sawall, S.; Maier, J.; Ziener, C.H.; Schlemmer, H.; Kachelrieß, M. Potential of contrast agents based on high-Z elements for contrast-enhanced photon-counting computed tomography. Med. Phys. 2020, 47, 6179–6190. [Google Scholar] [CrossRef]
















| Pathology | Application [Reference Number] |
|---|---|
| Lesion detection and characterization |
|
| Treatment response evaluation |
|
| Diffuse liver diseases |
|
| Trauma |
|
| Vascular applications |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Borges, A.P.; Antunes, C.; Caseiro-Alves, F. Spectral CT: Current Liver Applications. Diagnostics 2023, 13, 1673. https://doi.org/10.3390/diagnostics13101673
Borges AP, Antunes C, Caseiro-Alves F. Spectral CT: Current Liver Applications. Diagnostics. 2023; 13(10):1673. https://doi.org/10.3390/diagnostics13101673
Chicago/Turabian StyleBorges, Ana P., Célia Antunes, and Filipe Caseiro-Alves. 2023. "Spectral CT: Current Liver Applications" Diagnostics 13, no. 10: 1673. https://doi.org/10.3390/diagnostics13101673
APA StyleBorges, A. P., Antunes, C., & Caseiro-Alves, F. (2023). Spectral CT: Current Liver Applications. Diagnostics, 13(10), 1673. https://doi.org/10.3390/diagnostics13101673






