Feasibility of Renal Blood Flow Measurement Using 64Cu-ATSM PET/MRI: A Quantitative PET and MRI Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants
2.2. eRBF of the Kidney
2.3. PET/MRI Imaging
2.4. RBF from 64Cu-ATSM-PET
2.5. RBF from ASL-MRI
2.6. Statistical Analysis
3. Results
3.1. RBF Values from the Clinical Parameters and 64Cu-ATSM PET/MRI
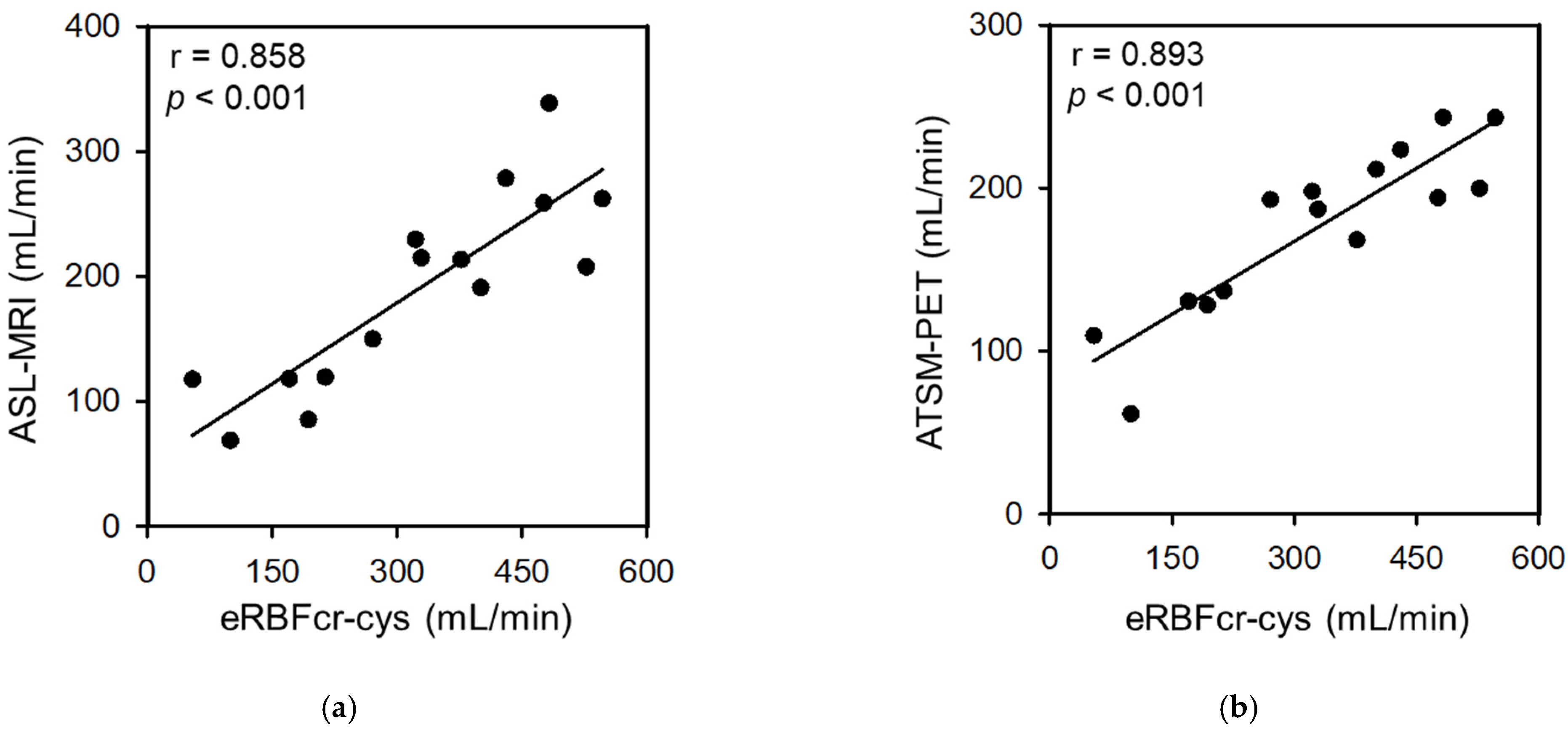
3.2. Correlation between the eRBFs and the RBF from PET/MRI
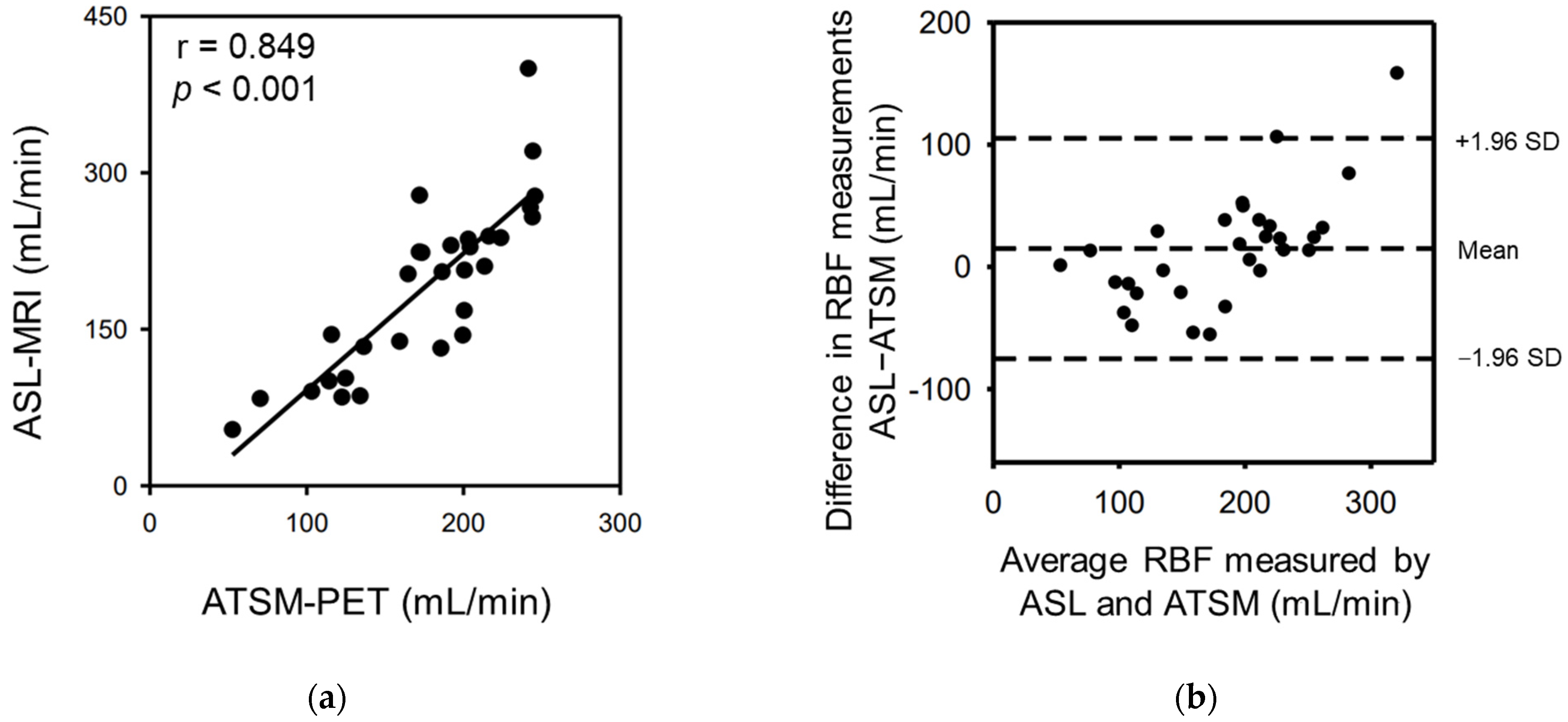
3.3. Correlation between RBF Values from ASL-MRI and 64Cu-ATSM-PET
3.4. Correlation between the Adjusted Body Surface Area eRBFs and RBF from the Images
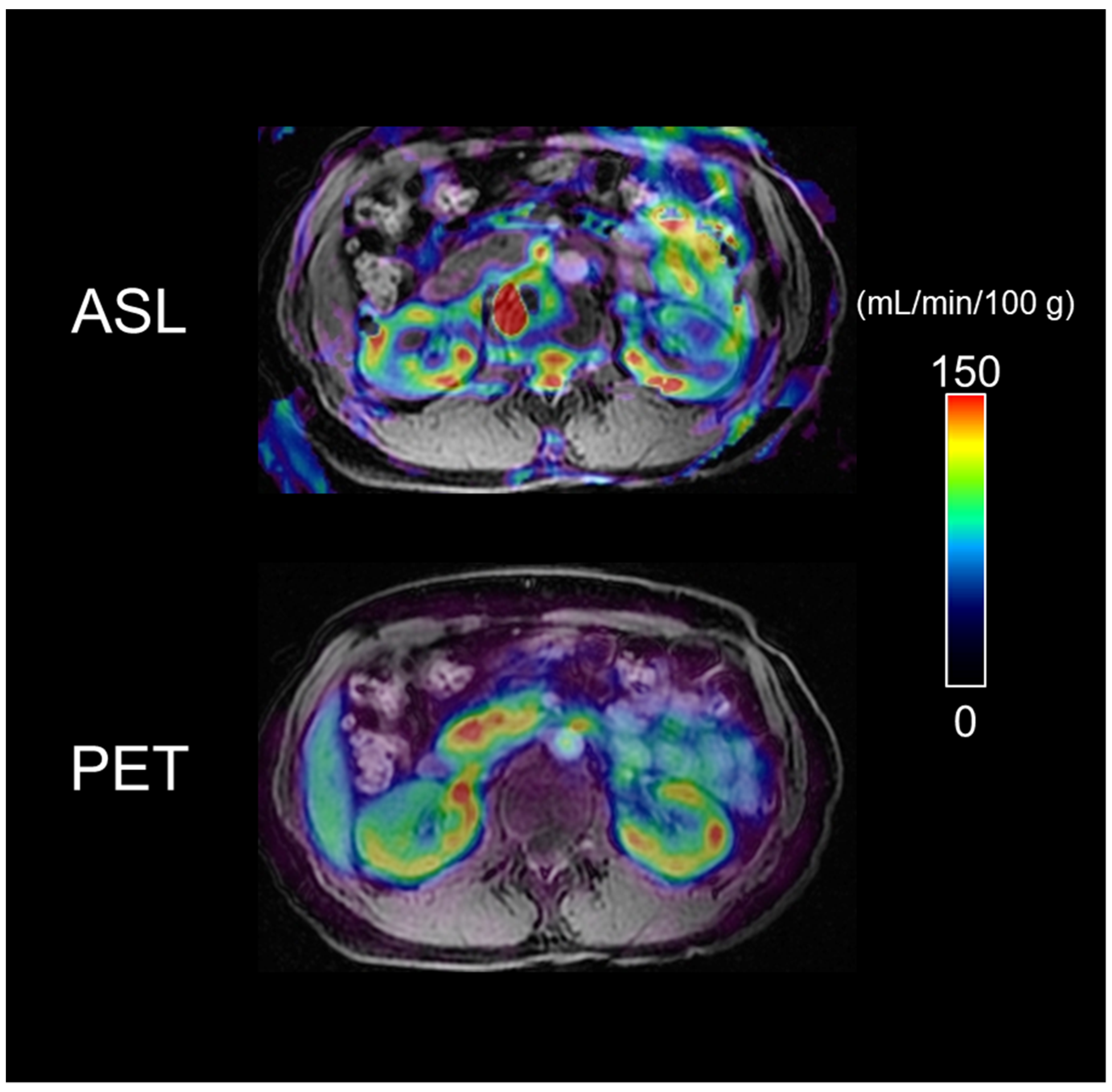
3.5. Representative Images
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cutajar, M.; Thomas, D.L.; Hales, P.W.; Banks, T.; Clark, C.A.; Gordon, I. Comparison of ASL and DCE MRI for the non-invasive measurement of renal blood flow: Quantification and reproducibility. Eur. Radiol. 2014, 24, 1300–1308. [Google Scholar] [CrossRef] [PubMed]
- Gillis, K.A.; McComb, C.; Foster, J.E.; Taylor, A.H.; Patel, R.K.; Morris, S.T.; Jardine, A.G.; Schneider, M.P.; Roditi, G.H.; Delles, C.; et al. Inter-study reproducibility of arterial spin labelling magnetic resonance imaging for measurement of renal perfusion in healthy volunteers at 3 Tesla. BMC Nephrol. 2014, 15, 23. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, K.; Kosaka, N.; Fujiwara, Y.; Matsuda, T.; Yamamoto, T.; Tsuchida, T.; Tsuchiyama, K.; Oyama, N.; Kimura, H. Arterial Transit Time-corrected Renal Blood Flow Measurement with Pulsed Continuous Arterial Spin Labeling MR Imaging. Magn. Reson. Med. Sci. 2017, 16, 38–44. [Google Scholar] [CrossRef] [PubMed]
- Fujibayashi, Y.; Taniuchi, H.; Yonekura, Y.; Ohtani, H.; Konishi, J.; Yokoyama, A. Copper-62-ATSM: A new hypoxia imaging agent with high membrane permeability and low redox potential. J. Nucl. Med. 1997, 38, 1155–1160. [Google Scholar]
- Lohith, T.G.; Kudo, T.; Demura, Y.; Umeda, Y.; Kiyono, Y.; Fujibayashi, Y.; Okazawa, H. Pathophysiologic correlation between 62Cu-ATSM and 18F-FDG in lung cancer. J. Nucl. Med. 2009, 50, 1948–1953. [Google Scholar] [CrossRef]
- Isozaki, M.; Kiyono, Y.; Arai, Y.; Kudo, T.; Mori, T.; Maruyama, R.; Kikuta, K.; Okazawa, H. Feasibility of 62Cu-ATSM PET for evaluation of brain ischaemia and misery perfusion in patients with cerebrovascular disease. Eur. J. Nucl. Med. Mol. Imaging 2011, 38, 1075–1082. [Google Scholar] [CrossRef]
- Kositwattanarerk, A.; Oh, M.; Kudo, T.; Kiyono, Y.; Mori, T.; Kimura, Y.; Maruyama, R.; Fujibayashi, Y.; Fujieda, S.; Okazawa, H. Different distribution of (62)Cu ATSM and (18)F-FDG in head and neck cancers. Clin. Nucl. Med. 2012, 37, 252–257. [Google Scholar] [CrossRef]
- Ikawa, M.; Okazawa, H.; Kudo, T.; Kuriyama, M.; Fujibayashi, Y.; Yoneda, M. Evaluation of striatal oxidative stress in patients with Parkinson’s disease using [62Cu]ATSM PET. Nucl. Med. Biol. 2011, 38, 945–951. [Google Scholar] [CrossRef]
- Ikawa, M.; Okazawa, H.; Tsujikawa, T.; Matsunaga, A.; Yamamura, O.; Mori, T.; Hamano, T.; Kiyono, Y.; Nakamoto, Y.; Yoneda, M. Increased oxidative stress is related to disease severity in the ALS motor cortex: A PET study. Neurology 2015, 84, 2033–2039. [Google Scholar] [CrossRef]
- Ikawa, M.; Okazawa, H.; Nakamoto, Y.; Yoneda, M. PET Imaging for Oxidative Stress in Neurodegenerative Disorders Associated with Mitochondrial Dysfunction. Antioxidants 2020, 9, 861. [Google Scholar] [CrossRef]
- Okazawa, H.; Ikawa, M.; Tsujikawa, T.; Mori, T.; Makino, A.; Kiyono, Y.; Nakamoto, Y.; Kosaka, H.; Yoneda, M. Cerebral Oxidative Stress in Early Alzheimer’s Disease Evaluated by (64)Cu-ATSM PET/MRI: A Preliminary Study. Antioxidants 2022, 11, 1022. [Google Scholar] [CrossRef] [PubMed]
- Matsuo, S.; Imai, E.; Horio, M.; Yasuda, Y.; Tomita, K.; Nitta, K.; Yamagata, K.; Tomino, Y.; Yokoyama, H.; Hishida, A.; et al. Revised equations for estimated GFR from serum creatinine in Japan. Am. J. Kidney Dis. 2009, 53, 982–992. [Google Scholar] [CrossRef] [PubMed]
- Horio, M.; Imai, E.; Yasuda, Y.; Watanabe, T.; Matsuo, S.; Collaborators Developing the Japanese Equation for Estimated GFR. GFR estimation using standardized serum cystatin C in Japan. Am. J. Kidney Dis. 2013, 61, 197–203. [Google Scholar] [CrossRef] [PubMed]
- Inker, L.A.; Eneanya, N.D.; Coresh, J.; Tighiouart, H.; Wang, D.; Sang, Y.; Crews, D.C.; Doria, A.; Estrella, M.M.; Froissart, M.; et al. New Creatinine- and Cystatin C-Based Equations to Estimate GFR without Race. N. Engl. J. Med. 2021, 385, 1737–1749. [Google Scholar] [CrossRef]
- Du Bois, D.; Du Bois, E.F. A formula to estimate the approximate surface area if height and weight be known. 1916. Nutrition 1989, 5, 303–213; discussion 312–303. [Google Scholar]
- Berg, U.B. Differences in decline in GFR with age between males and females. Reference data on clearances of inulin and PAH in potential kidney donors. Nephrol. Dial. Transplant. 2006, 21, 2577–2582. [Google Scholar] [CrossRef]
- Pistrosch, F.; Herbrig, K.; Kindel, B.; Passauer, J.; Fischer, S.; Gross, P. Rosiglitazone improves glomerular hyperfiltration, renal endothelial dysfunction, and microalbuminuria of incipient diabetic nephropathy in patients. Diabetes 2005, 54, 2206–2211. [Google Scholar] [CrossRef]
- Stornello, M.; Valvo, E.V.; Scapellato, L. Hemodynamic, renal, and humoral effects of the calcium entry blocker nicardipine and converting enzyme inhibitor captopril in hypertensive type II diabetic patients with nephropathy. J. Cardiovasc. Pharmacol. 1989, 14, 851–855. [Google Scholar] [CrossRef]
- Coppo, R.; Amore, A.; Gianoglio, B.; Cacace, G.; Picciotto, G.; Roccatello, D.; Peruzzi, L.; Piccoli, G.; De Filippi, P.G. Angiotensin II local hyperreactivity in the progression of IgA nephropathy. Am. J. Kidney Dis. 1993, 21, 593–602. [Google Scholar] [CrossRef]
- Rosansky, S.J.; Harwood, S.J. Relationship of creatinine clearance to orthoiodohippurate measured effective renal plasma flow. Am. J. Kidney Dis. 1983, 3, 229–232. [Google Scholar] [CrossRef]
- Obata, A.; Kasamatsu, S.; McCarthy, D.W.; Welch, M.J.; Saji, H.; Yonekura, Y.; Fujibayashi, Y. Production of therapeutic quantities of (64)Cu using a 12 MeV cyclotron. Nucl. Med. Biol. 2003, 30, 535–539. [Google Scholar] [CrossRef]
- Tanaka, T.; Furukawa, T.; Fujieda, S.; Kasamatsu, S.; Yonekura, Y.; Fujibayashi, Y. Double-tracer autoradiography with Cu-ATSM/FDG and immunohistochemical interpretation in four different mouse implanted tumor models. Nucl. Med. Biol. 2006, 33, 743–750. [Google Scholar] [CrossRef]
- Okazawa, H.; Higashino, Y.; Tsujikawa, T.; Arishima, H.; Mori, T.; Kiyono, Y.; Kimura, H.; Kikuta, K.I. Noninvasive method for measurement of cerebral blood flow using O-15 water PET/MRI with ASL correlation. Eur. J. Radiol. 2018, 105, 102–109. [Google Scholar] [CrossRef] [PubMed]
- Okazawa, H.; Ikawa, M.; Jung, M.; Maruyama, R.; Tsujikawa, T.; Mori, T.; Rahman, M.G.M.; Makino, A.; Kiyono, Y.; Kosaka, H. Multimodal analysis using [(11)C]PiB-PET/MRI for functional evaluation of patients with Alzheimer’s disease. EJNMMI Res. 2020, 10, 30. [Google Scholar] [CrossRef] [PubMed]
- Ohta, S.; Meyer, E.; Fujita, H.; Reutens, D.C.; Evans, A.; Gjedde, A. Cerebral [15O]water clearance in humans determined by PET: I. Theory and normal values. J. Cereb. Blood Flow Metab. 1996, 16, 765–780. [Google Scholar] [CrossRef]
- Islam, M.M.; Tsujikawa, T.; Mori, T.; Kiyono, Y.; Okazawa, H. Estimation of arterial input by a noninvasive image derived method in brain H2(15)O PET study: Confirmation of arterial location using MR angiography. Phys. Med. Biol. 2017, 62, 4514–4524. [Google Scholar] [CrossRef] [PubMed]
- Dai, W.; Garcia, D.; de Bazelaire, C.; Alsop, D.C. Continuous flow-driven inversion for arterial spin labeling using pulsed radio frequency and gradient fields. Magn. Reson. Med. 2008, 60, 1488–1497. [Google Scholar] [CrossRef]
- Alsop, D.C.; Detre, J.A. Reduced transit-time sensitivity in noninvasive magnetic resonance imaging of human cerebral blood flow. J. Cereb. Blood Flow Metab. 1996, 16, 1236–1249. [Google Scholar] [CrossRef]
- Alsop, D.C.; Detre, J.A.; Golay, X.; Gunther, M.; Hendrikse, J.; Hernandez-Garcia, L.; Lu, H.; MacIntosh, B.J.; Parkes, L.M.; Smits, M.; et al. Recommended implementation of arterial spin-labeled perfusion MRI for clinical applications: A consensus of the ISMRM perfusion study group and the European consortium for ASL in dementia. Magn. Reson. Med. 2015, 73, 102–116. [Google Scholar] [CrossRef]
- Mora-Gutierrez, J.M.; Garcia-Fernandez, N.; Slon Roblero, M.F.; Paramo, J.A.; Escalada, F.J.; Wang, D.J.; Benito, A.; Fernandez-Seara, M.A. Arterial spin labeling MRI is able to detect early hemodynamic changes in diabetic nephropathy. J. Magn. Reson. Imaging 2017, 46, 1810–1817. [Google Scholar] [CrossRef]
- Cai, Y.Z.; Li, Z.C.; Zuo, P.L.; Pfeuffer, J.; Li, Y.M.; Liu, F.; Liu, R.B. Diagnostic value of renal perfusion in patients with chronic kidney disease using 3D arterial spin labeling. J. Magn. Reson. Imaging 2017, 46, 589–594. [Google Scholar] [CrossRef] [PubMed]
| HCs | CKD | p | |
|---|---|---|---|
| Age | 56.2 ± 3.7 | 63.3 ± 9.0 | 0.12 |
| eRBF (mL/min) | |||
| eRBFcr | 295.6 ± 30.9 | 174.9 ± 82.7 | <0.05 |
| eRBFcys | 485.4 ± 92.1 | 225.5 ± 105.5 | <0.001 |
| eRBFcr-cys | 486.5 ± 56.4 | 246.0 ± 121.2 | <0.001 |
| RBF (mL/min) | |||
| 64Cu-ATSM-PET (n = 30) | 218.5 ± 26.0 | 153.7 ± 49.3 | <0.001 |
| ASL-MRI (n = 30) | 251.7 ± 66.2 | 159.6 ± 71.0 | <0.01 |
| RBF (mL/min/100 g) | |||
| 64Cu-ATSM-PET (n = 30) | 151.0 ± 20.2 | 123.5 ± 21.5 | <0.05 |
| ASL-MRI (n = 30) | 172.2 ± 38.3 | 124.8 ± 29.7 | <0.001 |
| 64Cu-ATSM-PET SUV | 3.2 ± 1.0 | 3.1 ± 0.9 | 0.89 |
| Intercept (95% CI) | Slope (95% CI) | r | p | ||
|---|---|---|---|---|---|
| PET/MRI-RBF vs. eRBF (unadjusted BSA) (mL/min) | ASL-RBF vs. eRBFcr | 36.6 (−31.8 to 105.1) | 0.714 (0.419 to 1.009) | 0.823 | <0.001 |
| ASL-RBF vs. eRBFcys | 63.9 (6.9 to 120.9) | 0.405 (0.241 to 0.569) | 0.829 | <0.001 | |
| ASL-RBF vs. eRBFcr-cys | 49.4 (−6.2 to 105.1) | 0.432 (0.277 to 0.587) | 0.858 | <0.001 | |
| 64Cu-ATSM-RBF vs. eRBFcr | 67.1 (27.6 to 106.5) | 0.503 (0.333 to 0.672) | 0.871 | <0.001 | |
| 64Cu-ATSM-RBF vs. eRBFcys | 87.4 (53.5 to 121.3) | 0.281 (0.184 to 0.379) | 0.866 | <0.001 | |
| 64Cu-ATSM-RBF vs. eRBFcr-cys | 77.7 (45.2 to 110.2) | 0.299 (0.209 to 0.390) | 0.893 | <0.001 | |
| PET/MRI-RBF vs. eRBF (adjusted BSA) (mL/min/1.73 m2) | ASL-RBF vs. eRBFcr | 38.6 (−38.2 to 115.4) | 0.722 (0.382 to 1.063) | 0.786 | <0.001 |
| ASL-RBF vs. eRBFcys | 57.9 (−2.6 to 118.3) | 0.437 (0.256 to 0.617) | 0.823 | <0.001 | |
| ASL-RBF vs. eRBFcr-cys | 48.6 (−12.9 to 110.1) | 0.446 (0.269 to 0.623) | 0.834 | <0.001 | |
| 64Cu-ATSM-RBF vs. eRBFcr | 66.5 (22.5 to 110.6) | 0.518 (0.322 to 0.713) | 0.846 | <0.001 | |
| 64Cu-ATSM-RBF vs. eRBFcys | 82.3 (47.1 to 117.5) | 0.307 (0.202 to 0.412) | 0.868 | <0.001 | |
| 64Cu-ATSM-RBF vs. eRBFcr-cys | 75.8 (40.5 to 111.1) | 0.313 (0.212 to 0.415) | 0.879 | <0.001 | |
| MRI vs. PET-RBF | ASL-RBF vs. 64Cu-ATSM-RBF | −39.5 (−97.3 to 18.3) | 1.311 (0.995 to 1.627) | 0.849 | <0.001 |
| Intercept (95% CI) | Slope (95% CI) | r | p | |
|---|---|---|---|---|
| ASL-RBF vs. eGFRcr | 33.2 (−56.6 to 122.9) | 3.147 (1.454 to 4.839) | 0.744 | <0.01 |
| ASL-RBF vs. eGFRcys | 48.9 (−18.3 to 116.0) | 1.973 (1.113 to 2.832) | 0.809 | <0.001 |
| ASL-RBF vs. eGFRcr-cys | 39.4 (−29.9 to 108.7) | 2.007 (1.154 to 2.860) | 0.816 | <0.001 |
| 64Cu-ATSM-RBF vs. eGFRcr | 55.2 (8.7 to 101.7) | 2.405 (1.529 to 3.281) | 0.854 | <0.001 |
| 64Cu-ATSM-RBF vs. eGFRcys | 72.6 (36.8 to 108.4) | 1.432 (0.974 to 1.890) | 0.882 | <0.001 |
| 64Cu-ATSM-RBF vs. eGFRcr-cys | 64.8 (29.6 to 100.0) | 1.470 (1.036 to 1.903) | 0.897 | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nishikawa, Y.; Takahashi, N.; Nishikawa, S.; Shimamoto, Y.; Nishimori, K.; Kobayashi, M.; Kimura, H.; Tsujikawa, T.; Kasuno, K.; Mori, T.; et al. Feasibility of Renal Blood Flow Measurement Using 64Cu-ATSM PET/MRI: A Quantitative PET and MRI Study. Diagnostics 2023, 13, 1685. https://doi.org/10.3390/diagnostics13101685
Nishikawa Y, Takahashi N, Nishikawa S, Shimamoto Y, Nishimori K, Kobayashi M, Kimura H, Tsujikawa T, Kasuno K, Mori T, et al. Feasibility of Renal Blood Flow Measurement Using 64Cu-ATSM PET/MRI: A Quantitative PET and MRI Study. Diagnostics. 2023; 13(10):1685. https://doi.org/10.3390/diagnostics13101685
Chicago/Turabian StyleNishikawa, Yudai, Naoki Takahashi, Sho Nishikawa, Yuki Shimamoto, Kazuhisa Nishimori, Mamiko Kobayashi, Hideki Kimura, Tetsuya Tsujikawa, Kenji Kasuno, Tetsuya Mori, and et al. 2023. "Feasibility of Renal Blood Flow Measurement Using 64Cu-ATSM PET/MRI: A Quantitative PET and MRI Study" Diagnostics 13, no. 10: 1685. https://doi.org/10.3390/diagnostics13101685
APA StyleNishikawa, Y., Takahashi, N., Nishikawa, S., Shimamoto, Y., Nishimori, K., Kobayashi, M., Kimura, H., Tsujikawa, T., Kasuno, K., Mori, T., Kiyono, Y., Okazawa, H., & Iwano, M. (2023). Feasibility of Renal Blood Flow Measurement Using 64Cu-ATSM PET/MRI: A Quantitative PET and MRI Study. Diagnostics, 13(10), 1685. https://doi.org/10.3390/diagnostics13101685








