Hemodynamic Performance of Transcatheter Aortic Valves: A Comprehensive Review
Abstract
:1. How to Assess THV Function
2. Hemodynamic Performance of Transcatheter vs. Surgical Aortic Bioprosthesis
3. Hemodynamic Performance of Transcatheter Aortic Bioprosthesis
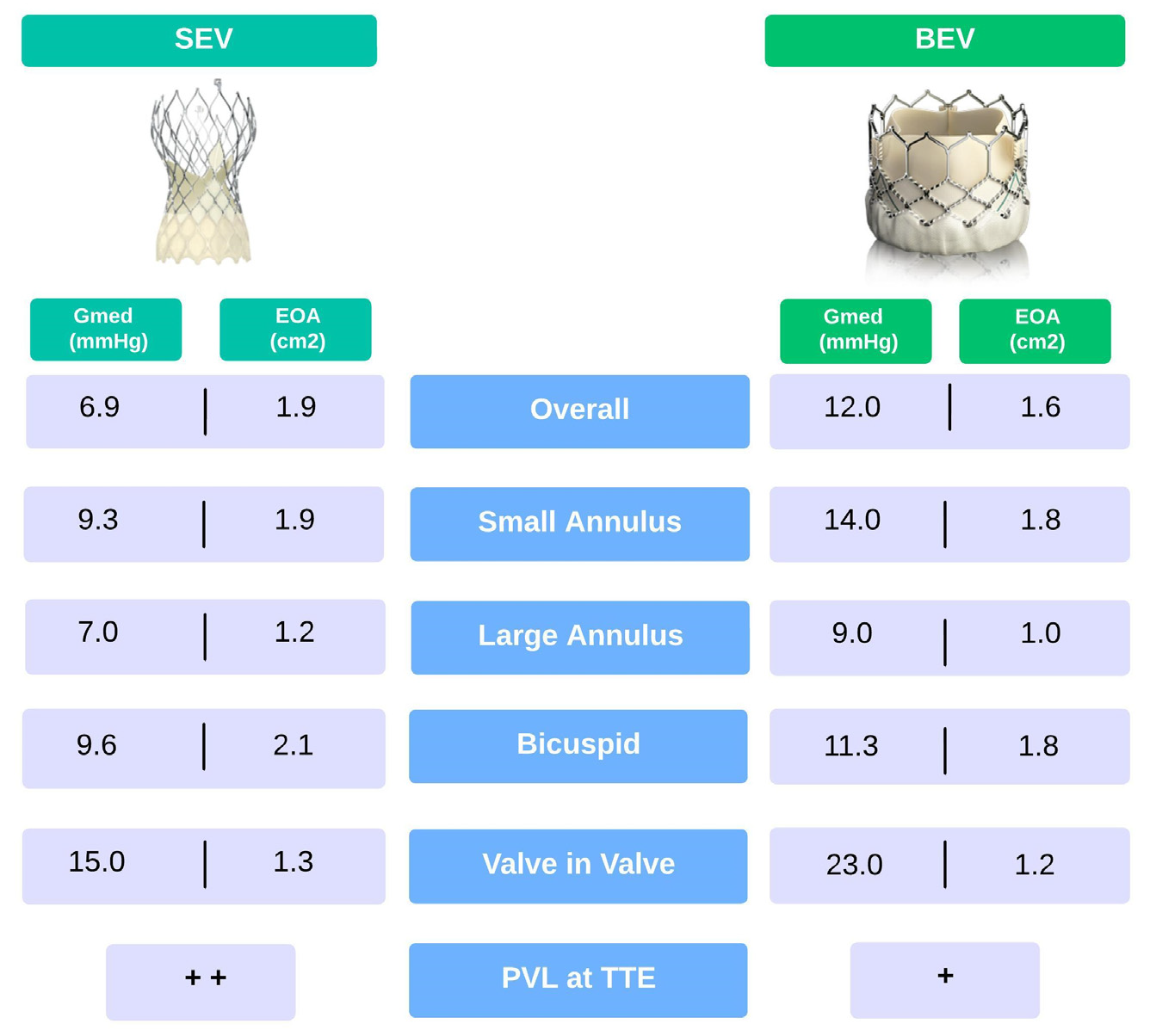
4. Balloon- vs. Self-Expandable THV
5. Valve-in-Valve
6. Structural Valve Deterioration
7. Paravalvular Regurgitation
8. Patient Prosthesis Mismatch
9. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Brennan, J.M.; Edwards, F.H.; Zhao, Y.; O’Brien, S.; Booth, M.E.; Dokholyan, R.S.; Douglas, P.S.; Peterson, E.D. Long-Term Safety and Effectiveness of Mechanical Versus Biologic Aortic Valve Prostheses in Older Patients. Circulation 2013, 127, 1647–1655. [Google Scholar] [CrossRef]
- Leon, M.B.; Smith, C.R.; Mack, M.J.; Makkar, R.R.; Svensson, L.G.; Kodali, S.K.; Thourani, V.H.; Tuzcu, E.M.; Miller, D.C.; Herrmann, H.C.; et al. Transcatheter or Surgical Aortic-Valve Replacement in Intermediate-Risk Patients. N. Engl. J. Med. 2016, 374, 1609–1620. [Google Scholar] [CrossRef] [PubMed]
- Hahn, R.T.; Leipsic, J.; Douglas, P.S.; Jaber, W.A.; Weissman, N.J.; Pibarot, P.; Blanke, P.; Oh, J.K. Comprehensive Echocardiographic Assessment of Normal Transcatheter Valve Function. JACC Cardiovasc. Imaging. 2019, 12, 25–34. [Google Scholar] [CrossRef]
- Lancellotti, P.; Pibarot, P.; Chambers, J.; Edvardsen, T.; Delgado, V.; Dulgheru, R.; Pepi, M.; Cosyns, B.; Dweck, M.R.; Garbi, M.; et al. Recommendations for the imaging assessment of prosthetic heart valves: A report from the European Association of Cardiovascular Imaging endorsed by the Chinese Society of Echocardiography, the Inter-American Society of Echocardiography, and the Brazilian Department of Cardiovascular Imaging. Eur. Heart J. Cardiovasc. Imaging. 2016, 17, 589–590. [Google Scholar] [PubMed]
- Herrmann, H.C.; Pibarot, P.; Wu, C.; Hahn, R.T.; Tang, G.H.L.; Abbas, A.E.; Playford, D.; Ruel, M.; Jilaihawi, H.; Sathananthan, J.; et al. Bioprosthetic Aortic Valve Hemodynamics: Definitions, Outcomes, and Evidence Gaps: JACC State-of-the-Art Review. J. Am. Coll. Cardiol. 2022, 80, 527–544. [Google Scholar] [CrossRef]
- Johnston, D.R.; Soltesz, E.G.; Vakil, N.; Rajeswaran, J.; Roselli, E.E.; Sabik, J.F.; Smedira, N.G.; Svensson, L.G.; Lytle, B.W.; Blackstone, E.H. Long-term durability of bioprosthetic aortic valves: Implications from 12,569 implants. Ann. Thorac. Surg. 2015, 99, 1239–1247. [Google Scholar] [CrossRef]
- Yankah, C.A.; Pasic, M.; Musci, M.; Stein, J.; Detschades, C.; Siniawski, H.; Hetzer, R. Aortic valve replacement with the Mitroflow pericardial bioprosthesis: Durability results up to 21 years. J. Thorac. Cardiovasc. Surg. 2008, 136, 688–696. [Google Scholar] [CrossRef]
- Douglas, P.S.; Leon, M.B.; Mack, M.J.; Svensson, L.G.; Webb, J.G.; Hahn, R.T.; Pibarot, P.; Weissman, N.J.; Miller, D.C.; Kapadia, S.; et al. Longitudinal Hemodynamics of Transcatheter and Surgical Aortic Valves in the PARTNER Trial. JAMA Cardiol. 2017, 2, 1197–1206. [Google Scholar] [CrossRef] [PubMed]
- Thourani, V.H.; Kodali, S.; Makkar, R.R.; Herrmann, H.C.; Williams, M.; Babaliaros, V.; Smalling, R.; Lim, S.; Malaisrie, S.C.; Kapadia, S.; et al. Transcatheter aortic valve replacement versus surgical valve replacement in intermediate-risk patients: A propensity score analysis. Lancet 2016, 387, 2218–2225. [Google Scholar] [CrossRef]
- Dayan, V.; Vignolo, G.; Soca, G.; Paganini, J.J.; Brusich, D.; Pibarot, P. Predictors and Outcomes of Prosthesis-Patient Mismatch After Aortic Valve Replacement. JACC Cardiovasc. Imaging. 2016, 9, 924–933. [Google Scholar] [CrossRef]
- O’Hair, D.; Yakubov, S.J.; Grubb, K.J.; Oh, J.K.; Ito, S.; Deeb, G.M.; Van Mieghem, N.M.; Adams, D.H.; Bajwa, T.; Kleiman, N.S.; et al. Structural Valve Deterioration After Self-Expanding Transcatheter or Surgical Aortic Valve Implantation in Patients at Intermediate or High Risk. JAMA Cardiol. 2023, 8, 111–119. [Google Scholar] [CrossRef]
- D’Onofrio, A.; Salizzoni, S.; Rubino, A.S.; Besola, L.; Filippini, C.; Alfieri, O.; Colombo, A.; Agrifoglio, M.; Fischlein, T.; Rapetto, F.; et al. The Rise of New Technologies for Aortic Valve Stenosis: A Comparison of Sutureless and Transcatheter Aortic Valve Implantation. J. Thorac. Cardiovasc. Surg. 2016, 152, 99–109. [Google Scholar] [CrossRef]
- Muneretto, C.; Alfieri, O.; Cesana, B.M.; Bisleri, G.; De Bonis, M.; Di Bartolomeo, R.; Savini, C.; Folesani, G.; Di Bacco, L.; Rambaldini, M.; et al. A Comparison of Conventional Surgery, Transcatheter Aortic Valve Replacement, and Sutureless Valves in “Real-World” Patients with Aortic Stenosis and Intermediate- to High-Risk Profile. J. Thorac. Cardiovasc. Surg 2015, 150, 1570–1577. [Google Scholar] [CrossRef]
- Kamperidis, V.; van Rosendael, P.J.; de Weger, A.; Katsanos, S.; Regeer, M.; van der Kley, F.; Mertens, B.; Sianos, G.; Ajmone Marsan, N.; Bax, J.J.; et al. Surgical sutureless and transcatheter aortic valves: Hemodynamic performance and clinical outcomes in propensity score-matched high-risk populations with severe aortic stenosis. JACC Cardiovasc. Interv. 2015, 8, 670–677. [Google Scholar] [CrossRef]
- Dionne, P.O.; Poulin, F.; Bouchard, D.; Généreux, P.; Ibrahim, R.; Cartier, R.; Lamarche, Y.; Demers, P. Early Hemodynamic Results in Patients With Small Aortic Annulus After Aortic Valve Replacement. Innovations 2017, 12, 254–258. [Google Scholar] [CrossRef] [PubMed]
- Meco, M.; Miceli, A.; Montisci, A.; Donatelli, F.; Cirri, S.; Ferrarini, M.; Lio, A.; Glauber, M. Sutureless aortic valve replacement versus transcatheter aortic valve implantation: A meta-analysis of comparative matched studies using propensity score matching. Interact. Cardiovasc. Thorac. Surg. 2018, 26, 202–209. [Google Scholar] [CrossRef]
- Tamburino, C.; Barbanti, M.; D’Errigo, P.; Ranucci, M.; Onorati, F.; Covello, R.D.; Santini, F.; Rosato, S.; Santoro, G.; Fusco, D.; et al. 1-Year Outcomes After Transfemoral Transcatheter or Surgical Aortic Valve Replacement: Results From the Italian OBSERVANT Study. J. Am. Coll. Cardiol. 2015, 66, 804–812. [Google Scholar] [CrossRef]
- Barbanti, M.; Tamburino, C.; D’Errigo, P.; Biancari, F.; Ranucci, M.; Rosato, S.; Santoro, G.; Fusco, D.; Seccareccia, F.; OBSERVANT Research Group. Five-Year Outcomes of Transfemoral Transcatheter Aortic Valve Replacement or Surgical Aortic Valve Replacement in a Real World Population: Final Results from the OBSERVANT Study. Circ. Cardiovasc. Interv. 2019, 12, e007825. [Google Scholar] [CrossRef] [PubMed]
- del Trigo, M.; Muñoz-Garcia, A.J.; Wijeysundera, H.C.; Nombela-Franco, L.; Cheema, A.N.; Gutierrez, E.; Serra, V.; Kefer, J.; Amat-Santos, I.J.; Benitez, L.M.; et al. Incidence, timing, and predictors of valve hemodynamic deterioration after transcatheter aortic valve replacement multicenter registry. J. Am. Coll. Cardiol. 2016, 67, 644–655. [Google Scholar] [CrossRef] [PubMed]
- Rodés-Cabau, J.; Webb, J.G.; Cheung, A.; Ye, J.; Dumont, E.; Osten, M.; Feindel, C.M.; Natarajan, M.K.; Velianou, J.L.; Martucci, G.; et al. Long-Term Outcomes After Transcatheter Aortic Valve Implantation: Insights on Prognostic Factors and Valve Durability From the Canadian Multicenter Experience. J. Am. Coll. Cardiol. 2012, 60, 1864–1875. [Google Scholar] [CrossRef] [PubMed]
- Toggweiler, S.; Humphries, K.H.; Lee, M.; Binder, R.K.; Moss, R.R.; Freeman, M.; Ye, J.; Cheung, A.; Wood, D.A.; Webb, J.G. 5-Year Outcome After Transcatheter Aortic Valve Implantation. J. Am. Coll. Cardiol. 2013, 61, 413–419. [Google Scholar] [CrossRef]
- Abdel-Wahab, M.; Landt, M.; Neumann, F.J.; Massberg, S.; Frerker, C.; Kurz, T.; Kaur, J.; Toelg, R.; Sachse, S.; Jochheim, D.; et al. 5-Year Outcomes After TAVR With Balloon-Expandable Versus Self-Expanding Valves: Results From the CHOICE Randomized Clinical Trial. JACC Cardiovasc. Interv. 2020, 13, 1071–1082. [Google Scholar] [CrossRef]
- Lanz, J.; Kim, W.K.; Walther, T.; Burgdorf, C.; Möllmann, H.; Linke, A.; Redwood, S.; Thilo, C.; Hilker, M.; Joner, M.; et al. Safety and efficacy of a self-expanding versus a balloon-expandable bioprosthesis for transcatheter aortic valve replacement in patients with symptomatic severe aortic stenosis: A randomised non-inferiority trial. Lancet 2019, 394, 1619–1628. [Google Scholar] [CrossRef]
- Thiele, H.; Kurz, T.; Feistritzer, H.J.; Stachel, G.; Hartung, P.; Eitel, I.; Marquetand, C.; Nef, H.; Doerr, O.; Lauten, A.; et al. Comparison of newer generation self-expandable vs. balloon-expandable valves in transcatheter aortic valve implantation: The randomized SOLVE-TAVI trial. Eur. Heart J. 2020, 41, 1890–1899. [Google Scholar] [CrossRef] [PubMed]
- van Belle, E.; Vincent, F.; Labreuche, J.; Auffret, V.; Debry, N.; Lefèvre, T.; Eltchaninoff, H.; Manigold, T.; Gilard, M.; Verhoye, J.P.; et al. Balloon-Expandable Versus Self-Expanding Transcatheter Aortic Valve Replacement: A Propensity-Matched Comparison from the FRANCE-TAVI Registry. Circulation 2020, 141, 243–259. [Google Scholar] [CrossRef] [PubMed]
- Regazzoli, D.; Chiarito, M.; Cannata, F.; Pagnesi, M.; Miura, M.; Ziviello, F.; Picci, A.; Reifart, J.; De Marco, F.; Bedogni, F.; et al. Transcatheter Self-Expandable Valve Implantation for Aortic Stenosis in Small Aortic Annuli: The TAVI-SMALL Registry. JACC Cardiovasc. Interv. 2020, 13, 196–206. [Google Scholar] [CrossRef]
- Mauri, V.; Kim, W.K.; Abumayyaleh, M.; Walther, T.; Moellmann, H.; Schaefer, U.; Conradi, L.; Hengstenberg, C.; Hilker, M.; Wahlers, T.; et al. Short-Term Outcome and Hemodynamic Performance of Next-Generation Self-Expanding Versus Balloon-Expandable Transcatheter Aortic Valves in Patients with Small Aortic Annulus: A Multicenter Propensity-Matched Comparison. Circ Cardiovasc. Interv. 2017, 10, e005013. [Google Scholar] [CrossRef] [PubMed]
- Abdelghani, M.; Mankerious, N.; Allali, A.; Landt, M.; Kaur, J.; Sulimov, D.S.; Merten, C.; Sachse, S.; Mehilli, J.; Neumann, F.J.; et al. Bioprosthetic Valve Performance After Transcatheter Aortic Valve Replacement With Self-Expanding Versus Balloon-Expandable Valves in Large Versus Small Aortic Valve Annuli: Insights From the CHOICE Trial and the CHOICE-Extend Registry. JACC Cardiovasc. Interv. 2018, 11, 2507–2518. [Google Scholar] [CrossRef] [PubMed]
- Yoon, S.H.; Bleiziffer, S.; de Backer, O.; Delgado, V.; Arai, T.; Ziegelmueller, J.; Barbanti, M.; Sharma, R.; Perlman, G.Y.; Khalique, O.K.; et al. Outcomes in Transcatheter Aortic Valve Replacement for Bicuspid Versus Tricuspid Aortic Valve Stenosis. J. Am. Coll. Cardiol. 2017, 69, 2579–2589. [Google Scholar] [CrossRef]
- Mangieri, A.; Tchetchè, D.; Kim, W.K.; Pagnesi, M.; Sinning, J.M.; Landes, U.; Kornowski, R.; De Backer, O.; Nickenig, G.; Ielasi, A.; et al. Balloon Versus Self-Expandable Valve for the Treatment of Bicuspid Aortic Valve Stenosis: Insights from the BEAT International Collaborative Registrys. Circ. Cardiovasc. Interv. 2020, 13, e008714. [Google Scholar] [CrossRef] [PubMed]
- Rotman, O.M.; Bianchi, M.; Ghosh, R.P.; Kovarovic, B.; Bluestein, D. Principles of TAVR valve design, modelling, and testing. Expert Rev. Med. Devices. 2018, 15, 771–791. [Google Scholar] [CrossRef]
- Dvir, D.; Webb, J.; Brecker, S.; Bleiziffer, S.; Hildick-Smith, D.; Colombo, A.; Descoutures, F.; Hengstenberg, C.; Moat, N.E.; Bekeredjian, R.; et al. Transcatheter aortic valve replacement for degenerative bioprosthetic surgical valves: Results from the global valve-in-valve registry. Circulation 2012, 126, 2335–2344. [Google Scholar] [CrossRef] [PubMed]
- Rodés-Cabau, J.; Abbas, A.E.; Serra, V.; Vilalta, V.; Nombela-Franco, L.; Regueiro, A.; Al-Azizi, K.M.; Iskander, A.; Conradi, L.; Forcillo, J.; et al. Balloon- vs. Self-Expanding Valve Systems for Failed Small Surgical Aortic Valve Bioprostheses. J. Am. Coll. Cardiol. 2022, 80, 681–693. [Google Scholar] [CrossRef] [PubMed]
- Brinkmann, C.; Abdel-Wahab, M.; Bedogni, F.; Bhadra, O.D.; Charbonnier, G.; Conradi, L.; Hildick-Smith, D.; Kargoli, F.; Latib, A.; Van Mieghem, N.M.; et al. Outcomes of valve-in-valve transcatheter aortic valve implantation with and without bioprosthetic valve fracture. EuroIntervention 2021, 17, 848–855. [Google Scholar] [CrossRef] [PubMed]
- Matta, A.; Levai, L.; Roncalli, J.; Elbaz, M.; Bouisset, F.; Nader, V.; Blanco, S.; Campelo Parada, F.; Carrié, D.; Lhermusier, T. Comparison of in-hospital outcomes and long-term survival for valve-in-valve transcatheter aortic valve replacement versus the benchmark native valve transcatheter aortic valve replacement procedure. Front. Cardiovasc. Med. 2023, 10, 1113012. [Google Scholar] [CrossRef]
- Ribeiro, H.B.; Rodés-Cabau, J.; Blanke, P.; Leipsic, J.; Kwan Park, J.; Bapat, V.; Makkar, R.; Simonato, M.; Barbanti, M.; Schofer, J.; et al. Incidence, predictors, and clinical outcomes of coronary obstruction following transcatheter aortic valve replacement for degenerative bioprosthetic surgical valves: Insights from the VIVID registry. Eur. Heart J. 2018, 39, 687–695. [Google Scholar] [CrossRef]
- Malvindi, P.G.; Kattach, H.; Luthra, S.; Ohri, S. Modes of failure of Trifecta aortic valve prosthesis. Interact. Cardiovasc. Thorac. Surg. 2022, 35, ivac086. [Google Scholar] [CrossRef]
- Capodanno, D.; Petronio, A.S.; Prendergast, B.; Eltchaninoff, H.; Vahanian, A.; Modine, T.; Lancellotti, P.; Sondergaard, L.; Ludman, P.F.; Tamburino, C.; et al. Standardized definitions of structural deterioration and valve failure in assessing long-term durability of transcatheter and surgical aortic bioprosthetic valves: A consensus statement from the European Association of Percutaneous Cardiovascular Interventions (EAPCI) endorsed by the European Society of Cardiology (ESC) and the European Association for Cardio-Thoracic Surgery (EACTS). Eur. J. Cardiothorac. Surg. 2017, 52, 408–417. [Google Scholar] [PubMed]
- Dvir, D.; Bourguignon, T.; Otto, C.M.; Hahn, R.T.; Rosenhek, R.; Webb, J.G.; Treede, H.; Sarano, M.E.; Feldman, T.; Wijeysundera, H.C.; et al. Standardized Definition of Structural Valve Degeneration for Surgical and Transcatheter Bioprosthetic Aortic Valves. Circulation 2018, 137, 388–399. [Google Scholar] [CrossRef]
- Généreux, P.; Piazza, N.; Alu, M.C.; Nazif, T.; Hahn, R.T.; Pibarot, P.; Bax, J.J.; Leipsic, J.A.; Blanke, P.; Blackstone, E.H.; et al. Valve Academic Research Consortium 3: Updated Endpoint Definitions for Aortic Valve Clinical Research. J. Am. Coll. Cardiol. 2021, 77, 2717–2746. [Google Scholar] [CrossRef]
- Rodriguez-Gabella, T.; Voisine, P.; Puri, R.; Pibarot, P.; Rodés-Cabau, J. Aortic Bioprosthetic Valve Durability: Incidence, Mechanisms, Predictors, and Management of Surgical and Transcatheter Valve Degeneration. J. Am. Coll. Cardiol. 2017, 70, 1013–1028. [Google Scholar] [CrossRef]
- Capodanno, D.; Søndergaard, L.; Tamburino, C. Durability of transcatheter bioprosthetic aortic valves: The story so far. EuroIntervention 2019, 15, 846–849. [Google Scholar] [CrossRef]
- Barbanti, M.; Costa, G.; Zappulla, P.; Todaro, D.; Picci, A.; Rapisarda, G.; Di Simone, E.; Sicuso, R.; Buccheri, S.; Gulino, S.; et al. Incidence of long-term structural valve dysfunction and bioprosthetic valve failure after transcatheter aortic valve replacement. J. Am. Heart Assoc. 2018, 7, 008440. [Google Scholar] [CrossRef]
- Testa, L.; Latib, A.; Brambilla, N.; De Marco, F.; Fiorina, C.; Adamo, M.; Giannini, C.; Angelillis, M.; Barbanti, M.; Sgroi, C.; et al. Long-term clinical outcome and performance of transcatheter aortic valve replacement with a self-expandable bioprosthesis. Eur. Heart J. 2020, 41, 1876–1886. [Google Scholar] [CrossRef] [PubMed]
- Ruel, M.; Kulik, A.; Rubens, F.D.; Bédard, P.; Masters, R.G.; Pipe, A.L.; Mesana, T.G. Late incidence and determinants of reoperation in patients with prosthetic heart valves. Eur. J. Cardiothorac. Surg. 2004, 25, 364–370. [Google Scholar] [CrossRef]
- Mahjoub, H.; Mathieu, P.; Larose, E.; Dahou, A.; Sénéchal, M.; Dumesnil, J.G.; Després, J.P.; Pibarot, P. Determinants of aortic bioprosthetic valve calcification assessed by multidetector CT. Heart 2015, 101, 472–477. [Google Scholar] [CrossRef]
- Côté, N.; Pibarot, P.; Clavel, M.A. Incidence, risk factors, clinical impact, and management of bioprosthesis structural valve degeneration. Curr. Opin. Cardiol. 2017, 32, 123–129. [Google Scholar] [CrossRef]
- Rheude, T.; Pellegrini, C.; Cassese, S.; Wiebe, J.; Wagner, S.; Trenkwalder, T.; Alvarez, H.; Mayr, N.P.; Hengstenberg, C.; Schunkert, H.; et al. Predictors of haemodynamic structural valve deterioration following transcatheter aortic valve implantation with latest generation balloon-expandable valves. EuroIntervention 2020, 15, 1233–1239. [Google Scholar] [CrossRef]
- Rallidis, L.S.; Moyssakis, I.E.; Ikonomidis, I.; Nihoyannopoulos, P. Natural history of early aortic paraprosthetic regurgitation: A five-year follow-up. Am. Heart 1999, 138, 351–357. [Google Scholar] [CrossRef] [PubMed]
- Okuno, T.; Dangas, G.D.; Hengstenberg, C.; Sartori, S.; Herrmann, H.C.; de Winter, R.; Gilard, M.; Tchétché, D.; Möllmann, H.; Makkar, R.R.; et al. Two-year clinical outcomes after successful transcatheter aortic valve implantation with balloon-expandable versus self-expanding valves: A subanalysis of the GALILEO trial. Catheter. Cardiovasc. Interv. 2022, 100, 636–645. [Google Scholar] [CrossRef] [PubMed]
- Zoghbi, W.A.; Asch, F.M.; Bruce, C.; Gillam, L.D.; Grayburn, P.A.; Hahn, R.T.; Inglessis, I.; Islam, A.M.; Lerakis, S.; Little, S.H.; et al. Guidelines for the Evaluation of Valvular Regurgitation After Percutaneous Valve Repair or Replacement: A Report from the American Society of Echocardiography Developed in Collaboration with the Society for Cardiovascular Angiography and Interventions, Japanese Society of Echocardiography, and Society for Cardiovascular Magnetic Resonance. J. Am. Soc. Echocardiogr. 2019, 32, 431–475. [Google Scholar] [PubMed]
- Zamorano, J.L.; Badano, L.P.; Bruce, C.; Chan, K.L.; Gonalves, A.; Hahn, R.T.; Keane, M.G.; La Canna, G.; Monaghan, M.J.; Nihoyannopoulos, P.; et al. EAE/ASE recommendations for the use of echocardiography in new transcatheter interventions for valvular heart disease. Eur. Heart J. 2011, 32, 2189–2214. [Google Scholar] [CrossRef] [PubMed]
- Yokoyama, H.; Sugiyama, Y.; Miyashita, H.; Jalanko, M.; Ochiai, T.; Shishido, K.; Yamanaka, F.; Vähäsilta, T.; Saito, S.; Laine, M.; et al. Impact of Mild Paravalvular Regurgitation on Long-Term Clinical Outcomes After Transcatheter Aortic Valve Implantation. Am. J. Cardiol. 2023, 191, 14–22. [Google Scholar] [CrossRef] [PubMed]
- Hahn, R.T.; Pibarot, P.; Weissman, N.J.; Rodriguez, L.; Jaber, W.A. Assessment of paravalvular aortic regurgitation after transcatheter aortic valve replacement: Intra-core laboratory variability. J. Am. Soc. Echocardiogr. 2015, 28, 415–422. [Google Scholar] [CrossRef]
- Pibarot, P.; Hahn, R.T.; Weissman, N.J.; Monaghan, M.J. Assessment of Paravalvular Regurgitation Following TAVR A Proposal of Unifying Grading Scheme. JACC Cardiovasc. Imaging 2015, 8, 340–360. [Google Scholar] [CrossRef] [PubMed]
- Kasel, A.M.; Cassese, S.; Bleiziffer, S.; Amaki, M.; Hahn, R.T.; Kastrati, A.; Sengupta, P.P. Standardized imaging for aortic annular sizing: Implications for transcatheter valve selection. JACC Cardiovasc. Imaging. 2013, 6, 249–262. [Google Scholar] [CrossRef]
- Wendt, D.; Shehada, S.E.; König, L.; Kahlert, P.; Frey, U.; Mourad, F.; Jakob, H.; Thielmann, M.; El Gabry, M. Modified implantation height of the Sapien3™ transcatheter heart valve. Minim. Invasive Ther. Allied Technol. 2020, 29, 70–77. [Google Scholar] [CrossRef]
- Breitbart, P.; Minners, J.; Hein, M.; Schröfel, H.; Neumann, F.J.; Ruile, P. Implantation depth and its influence on complications after TAVI with self-expanding valves. Int. J. Cardiovasc. Imaging. 2021, 37, 3081–3092. [Google Scholar] [CrossRef]
- Pibarot, P.; Weissman, N.J.; Stewart, W.J.; Hahn, R.T.; Lindman, B.R.; Mcandrew, T.; Kodali, S.K.; Mack, M.J.; Thourani, V.H.; Miller, D.C.; et al. Incidence and Sequelae of Prosthesis-Patient Mismatch in Transcatheter Versus Surgical Valve Replacement in High-Risk Patients With Severe Aortic Stenosis A PARTNER Trial Cohort-A Analysis. J. Am. Coll. Cardiol. 2014, 64, 1323–1334. [Google Scholar] [CrossRef]
- Okuno, T.; Khan, F.; Asami, M.; Praz, F.; Heg, D.; Winkel, M.G.; Lanz, J.; Huber, A.; Gräni, C.; Räber, L.; et al. Prosthesis-Patient Mismatch Following Transcatheter Aortic Valve Replacement With Supra-Annular and Intra-Annular Prostheses. JACC Cardiovasc. Interv. 2019, 12, 2173–2182. [Google Scholar] [CrossRef]
- Hahn, R.T.; Pibarot, P.; Stewart, W.J.; Weissman, N.J.; Gopalakrishnan, D.; Keane, M.G.; Anwaruddin, S.; Wang, Z.; Bilsker, M.; Lindman, B.R.; et al. Comparison of transcatheter and surgical aortic valve replacement in severe aortic stenosis: A longitudinal study of echocardiography parameters in cohort a of the PARTNER trial (Placement of aortic transcatheter valves). J. Am. Coll. Cardiol. 2013, 61, 2514–2521. [Google Scholar] [CrossRef] [PubMed]
- Lang, R.M.; Badano, L.P.; Victor, M.A.; Afilalo, J.; Armstrong, A.; Ernande, L.; Flachskampf, F.A.; Foster, E.; Goldstein, S.A.; Kuznetsova, T.; et al. Recommendations for cardiac chamber quantification by echocardiography in adults: An update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J. Am. Soc. Echocardiogr. 2015, 28, 1–39.14. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Angellotti, D.; Manzo, R.; Castiello, D.S.; Immobile Molaro, M.; Mariani, A.; Iapicca, C.; Nappa, D.; Simonetti, F.; Avvedimento, M.; Leone, A.; et al. Hemodynamic Performance of Transcatheter Aortic Valves: A Comprehensive Review. Diagnostics 2023, 13, 1731. https://doi.org/10.3390/diagnostics13101731
Angellotti D, Manzo R, Castiello DS, Immobile Molaro M, Mariani A, Iapicca C, Nappa D, Simonetti F, Avvedimento M, Leone A, et al. Hemodynamic Performance of Transcatheter Aortic Valves: A Comprehensive Review. Diagnostics. 2023; 13(10):1731. https://doi.org/10.3390/diagnostics13101731
Chicago/Turabian StyleAngellotti, Domenico, Rachele Manzo, Domenico Simone Castiello, Maddalena Immobile Molaro, Andrea Mariani, Cristina Iapicca, Dalila Nappa, Fiorenzo Simonetti, Marisa Avvedimento, Attilio Leone, and et al. 2023. "Hemodynamic Performance of Transcatheter Aortic Valves: A Comprehensive Review" Diagnostics 13, no. 10: 1731. https://doi.org/10.3390/diagnostics13101731






