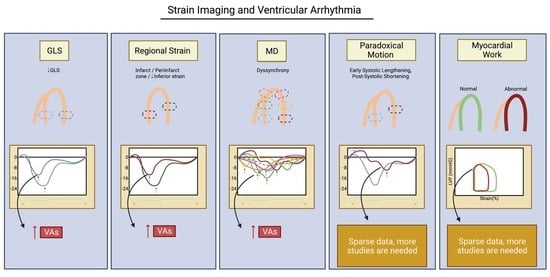
Strain Imaging and Ventricular Arrhythmia
Abstract
:1. Introduction
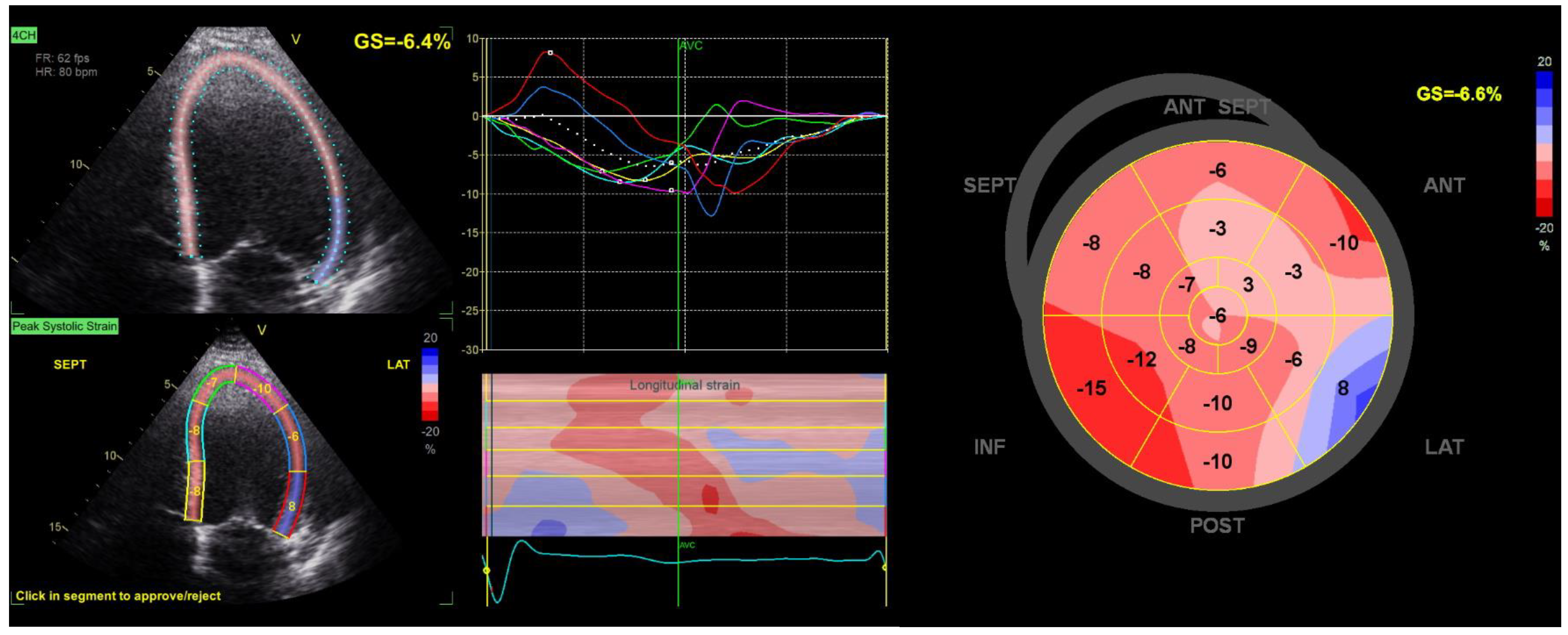
2. Concept of Myocardial Strain Imaging
3. Global Longitudinal Strain
3.1. Value of GLS
3.2. GLS as a Marker of Ventricular Arrhythmia
| Study | Year | Design | Sample Size | Arrhythmia Outcome | Arrhythmia Monitoring | No. of Events | Follow-Up | Key Strain Findings |
|---|---|---|---|---|---|---|---|---|
| Myocardial infarction | ||||||||
| Haugaa et al. [58] | 2010 | Prosp. | 85 | Appropriate ICD therapy | ICD monitoring | 38 | 2.3 (range: 0.6–5.5) years | MD but not GLS was an independent predictor of appropriate ICD therapy. |
| Haugaa et al. [57] | 2013 | Prosp. | 569 | Composite: Sustained VT VF SCD | Not specified | 15 | 30 (IQR: 18) months | GLS was a univariate predictor of VA but not an independent predictor of VA when adjusted for MD. MD was an independent predictor of VA. |
| Ersbøll et al. [56] | 2013 | Prosp. | 988 | Composite: VA Appropriate ICD therapy Definite/suspected SCD | Admission with documented VA ICD monitoring in subgroup SCD based on hospital and prehospital records. | 34 | 29.7 (IQR: 23.5–32.7) months | GLS and MD were independent predictors of VA. In patients with LVEF < 35%, both GLS and MD were independent predictors of VAs, but only GLS was an independent predictor of VAs in patients with LVEF > 35%. |
| Sjøli et al. [62] | 2011 | Prosp. | 77 | Composite: Cardiac death Reinfarction Hospitalization for HF UAP Life-threatening arrhythmia | Not specified | 17 | 3.29 ± 1.59 (range: 0–5.22) years | GLS measured in both the acute phase and after 10 days was an independent predictor of the composite outcome. |
| Nguyen et al. [63] | 2015 | Not specified | 467 | VT | Documented on 24 h ambulatory ECG monitoring during hospitalizationEP study | 51 | Median: 25 (range: 6–43) months | In multivariate analysis, MD was significantly associated with VT, and GLS was borderline significantly associated with VT. |
| Choi et al. [64] | 2022 | Retrosp. | 545 | Composite: All-cause death Rehospitalization for acute HF VA | National database and electrical medical records | 55 | Median: 49.5 months | Reduced 3D and 2D GLS were both independently associated with the composite outcome. |
| Leong et al. [65] | 2015 | Retrosp. | 206 | Appropriate ICD therapy | ICD monitoring | 75 | Median: 24 (IQR: 7.8–24) months | GLS and MD were independently associated with VT. |
| Structural heart disease | ||||||||
| Guerra et al. [66] | 2020 | Prosp. | 203 | Any VA detected by ICD | ICD monitoring | 74 | 817 (IQR: 440–1105) days | GLS Ws an independent predictor of the first VA episode but not recurrent episodes. MD was not associated with VAs. |
| Heart failure with reduced ejection fraction | ||||||||
| Nikoo et al. [67] | 2020 | Prosp. | 70 | Appropriate ICD therapy | ICD monitoring | 30 | 1.8 ± 0.6 (1–3) years | Reduced GLS was a predictor of VAs. Better diagnostic performance than LVEF. MD was not reported. |
| Hasselberg et al. [68] | 2016 | Prosp. | 170 | Composite: VT VF SCA Appropriate ATP Appropriate defibrillator shock therapy | CRT-D monitoring | 18 | 1.9 ± 0.3 years | GLS and MD at baseline were not independent predictors of the VA endpoint. MD at 6 months was an independent predictor of the VA endpoint. |
| Mornoş et al. [69] | 2017 | Prosp. | 340 | Composite: VT VF SCD | Hospital documentationDeath certificate | 48 | 36 ± 9 months | GLS, MD, and the ratio of GLS to MD (GLS/MD) were univariate predictors of VAs, but only GLS/MD was an independent predictor of VAs. |
| Matsuzoe et al. [70] | 2016 | Retrosp. | 72 | Appropriate ICD therapy | ICD monitoring | 34 | 17 (IQR: 0.2–72.5) months | GLS and MD were not independently associated with the VA endpoint. Only LV dyssynergy (SD of peak strain) was independently associated with the VA endpoint. |
| Biering-Sørensen et al. [61] | 2017 | RCT substudy | 1064 | Appropriate ICD/CRT-D therapy | ICD/CRT-D monitoring Adjudicated events | 254 | 2.9 (IQR: 2.0–3.7) years | GLS and all regional (anterior and inferior) strain were associated with VT/VF, whereas MD was not. |
| Bax et al. [71] | 2017 | RCT substudy | 755 | Composite: Appropriate ICD/CRT-D therapy Arrhythmic death Atrial tachyarrhythmias | ICD/CRT-D monitoring Adjudicated events | 72 | 19.4 months | GLS was not independently associated with the arrhythmic endpoint. MD was not investigated. |
| Biering-Sørensen et al. [72] | 2016 | Retrosp. | 151 | Composite: CVD Appropriate ICD therapy | ICD monitoring CVD from the national cause of death registry | 40 | 2.3 (IQR: 1.5–3.1) years | Neither MD nor GLS was associated with VAs. |
| Winsløw et al. [73] | 2023 | RCT substudy | 401 | Composite: SCD Appropriate ICD therapy Admission with sustained ventricular arrhythmia Resuscitated cardiac arrest | ICD monitoring ECG Hospital/source documentation Adjudicated events | 52 | 4.0 (IQR:2.8–5) years | Neither GLS nor LVEF was associated with the VA endpoint. Only inferior strain was independently associated with the VA endpoint. |
| Non-ischemic dilated cardiomyopathy | ||||||||
| Haugaa et al. [74] | 2012 | Prosp. | 94 | Composite: Appropriate ICD therapy Sustained VT Cardiac arrest Cardiac syncope | Not specified | 12 | 22 (Range:1–46) months | Both GLS and MD were independent predictors of the VA endpoint. |
| Melichova et al. [75] | 2021 | Prosp. | 290 | Composite: SCD Shock from ICD Sustained VT | Medical records (ICD therapy, ECG, Holter, aborted cardiac arrest) Cause of death registry | 32 | 22 ± 12 months | Both GLS and MD were independent predictors of the VA endpoint. |
| Negishi et al. [76] | 2016 | Retrosp. | 124 | Appropriate ICD therapy | ICD monitoring | 36 | 3.8 (IQR: 2.2–6.0) years | GLS, but not MD, was an independent predictor of VAs. |
| Hypertrophic cardiomyopathy | ||||||||
| Haland et al. [77] | 2016 | Prosp. | 150 HCM | Composite: Sustained and non-sustained VT Previous aborted cardiac arrest | 24–48 h Holter monitoring ICD monitoring | 37 | Not specified | GLS and MD were univariate predictors of the VA endpoint, but only MD was an independent predictor. |
| Candan et al. [78] | 2017 | Prosp. | 63 | Appropriate ICD therapy | ICD monitoring | 17 | 3 years (21.5 ± 6.9 months) | GLS and MD were independent predictors of VAs. |
| Debonnaire et al. [79] | 2014 | Retrosp. | 92 | Appropriate ICD therapy | ICD monitoring | 21 | 4.7 (2.2–8.2) years | GLS was independently associated with VAs. MD was not investigated. |
| Candan et al. [80] | 2019 | Prosp. | 59 | Non-sustained VT | 24–72 h Holter monitoring | 17 | N/A | LV Twist and GLS were independent predictors for non-sustained VT. MD was not investigated. |
| Popa-Fotea et al. [81] | 2020 | Prosp. | 47 | Non-sustained VT | 24 h Holter monitoring | 16 | N/A | GLS, RV and LV MD were univariate predictors of non-sustained VT, but only RV and LV MD were independent predictors of non-sustained VT. |
| Hiemstra et al. [82] | 2017 | Prosp. | 427 | Composite: Aborted SCD Appropriate ICD therapy | Medical chart review Contact with general practitioner | 53 | 6.7 (IQR: 3.3–10.0) years | GLS was independently associated with the VA endpoint. MD was not investigated. |
| Jalanko et al. [83] | 2016 | Prosp. | 31 | Non-sustained VT | 24 h Holter monitoring | 11 | N/A | Both GLS and MD were associated with non-sustained VT in univariate analysis, but only MD was independently associated with non-sustained VT. |
| Chagas cardiomyopathy | ||||||||
| Barros et al. [84] | 2016 | Retrosp., case-control study | 62 | Clinically indicated implantation of ICD. | N/A | 28 | N/A | MD and GLS were more abnormal in the group with ICD, and both were independent markers of previous events precipitating ICD. |
| Azevedo et al. [85] | 2021 | Prosp. | 77 | Composite: VES Non-sustained VT | 24 h Holter | Not specified | N/A | Both GLS and MD were associated with non-sustained VT in univariate analysis, but only MD was independently associated with non-sustained VT, paired VES, and VES in bigeminy. |
| Long QT syndrome | ||||||||
| Haugaa et al. [86] | 2010 | Prosp. | 101 LQTS 35 healthy individuals | History of either: Documented arrhythmia Syncope Cardiac arrest | N/A | 48 | N/A | LQTS patients with a history of arrhythmia had higher MD but similar GLS compared to those without arrhythmia. |
| Lamin A/C mutation | ||||||||
| Haugaa et al. [87] | 2015 | Prosp. | 33 | Composite: Non-sustained VT VT VF | Not specified | 11 | Not specified | Patients with any ventricular arrhythmia had higher MD but similar GLS compared to those without ventricular arrhythmia. |
| Tetralogy of Fallot | ||||||||
| Diller et al. [88] | 2012 | Retrosp. | 413 | Composite: SCD Sustained VTResuscitated SCD Appropriate ICD discharge | ICD monitoring | 19 | 2.9 (IQR:1.4–4.4) years | GLS was an independent predictor of the VA endpoint. MD was not investigated. |
| Van Grootel et al. [89] | 2019 | Prosp. | 151 ToF | Composite: Death HF Reintervention Hospitalization for cardiac reasons Symptomatic ventricular and supraventricular arrhythmias | Regularly checked at an outpatient clinic | 62 | 71.5 (IQR: 64–75.3) months | GLS, RV strain, and apical rotation were univariate predictors of the composite outcome. Only apical rotation was independently associated with the composite outcome. MD was not investigated. |
| Cardiac amyloidosis | ||||||||
| Hamon et al. [90] | 2016 | Prosp. | 45 | Appropriate ICD therapy | ICD monitoring | 12 | 17 ± 13.7 months | GLS was not associated with VAs. MD was not investigated. |
| Brugada syndrome | ||||||||
| Scheirlynck et al. [91] | 2020 | Case-control study | 175 BrS | History of either: VT VF Aborted cardiac arrest | Medical records | 19 | N/A | Patients with a history of VAs or aborted cardiac arrest had higher MD than, but similar GLS to, those who had not had VAs or aborted cardiac arrest. |
| Elite Athletes | ||||||||
| Lie et al. [92] | 2021 | Cross-sectional study | 43 athletes with VT and 30 healthy athletes | Composite of life-threatening VAs: VF Sustained VT Aborted cardiac arrest Appropriate ICD therapy | 24 h Holter monitoring ECG Telemetry ILR monitoring Intracardiac device monitoring | 23 | N/A | MD was higher and GLS was lower in VA patients. Only MD was independently associated with life-threatening VAs. |
| Arrhythmogenic cardiomyopathy | ||||||||
| Lie et al. [93] | 2018 | Prosp. | 117 | VT Cardiac arrest Appropriate ICD shock | ECG Holter monitoring ICD monitoring | 18 | 2.0 (IQR:0.5–3.5) years | Patients with VAs had reduced LV and RV strain and higher LV and RV MD. RV strain and LV MD were independently associated with VAs. |
| Lie et al. [94] | 2021 | LCS | 168 | Composite: Aborted cardiac arrest Sustained VT Appropriate ICD shock | Not specified | 54 | 1.3 (IQR: 0.4–3.5) years | LV GLS was independently associated with VAs. MD was not reported. |
| Sarvari et al. [95] | 2011 | Prosp. Case-control study | 42 symptomatic 27 asymptomatic 30 healthy | History of either: VT VF | N/A | 42 | N/A | Patients with a history of VAs had lower LV and RV strain and higher LV and RV MD. Only RV MD was independently associated with a history of VAs. |
| Kirkels et al. [96] | 2021 | Retrosp. | 160 | History of either: Sustained VT Appropriate ICD therapy Aborted cardiac arrest | N/A | 47 | N/A | Patients with a history of VAs had reduced LV GLS and RV strain and higher RV MD than those without VA history. RV MD was independently associated with VAs. |
| Mitral valve prolapse | ||||||||
| Ermakov et al. [97] | 2019 | Retrosp. | 59 MVP | History of: Ventricular couplets Ventricular bigeminy Non-sustained VT VT ICD for aborted cardiac arrest | N/A | 32 | N/A | MD higher but similar GLS in patients with a history of VA compared to those without arrhythmia. MD was independently associated with a history of VA. |
| Unexplained syncope | ||||||||
| Falsing et al. [98] | 2021 | Retrosp. | 288 | VT | ILR monitoring | 36 | 2.9 (IQR:1.3–3.5) years | GLS was independently associated with VT. MD was not associated with VT. |
| Idiopathic ventricular fibrillation | ||||||||
| Groeneveld et al. [99] | 2021 | Retrosp. Case-control study | 47 IVF 47 healthy individuals | VF | N/A | N/A | N/A | IVF patients had lower GLS, higher MD, and higher post-systolic index than matched controls. No adjusted analyses were performed. |
| Acute myocarditis | ||||||||
| Pruitt et al. [100] | 2021 | Retrosp. | 66 | Composite: VT VF SVT High-grade or complete heart block Any arrhythmia requiring antiarrhythmic medication | Medical records | 23 | During hospitalization | GLS was independently associated with the composite arrhythmia outcome. |
4. Regional Strain
4.1. Is Regional Strain Worthwhile to Consider?
4.2. Regional Strain and Risk of Ventricular Arrhythmia
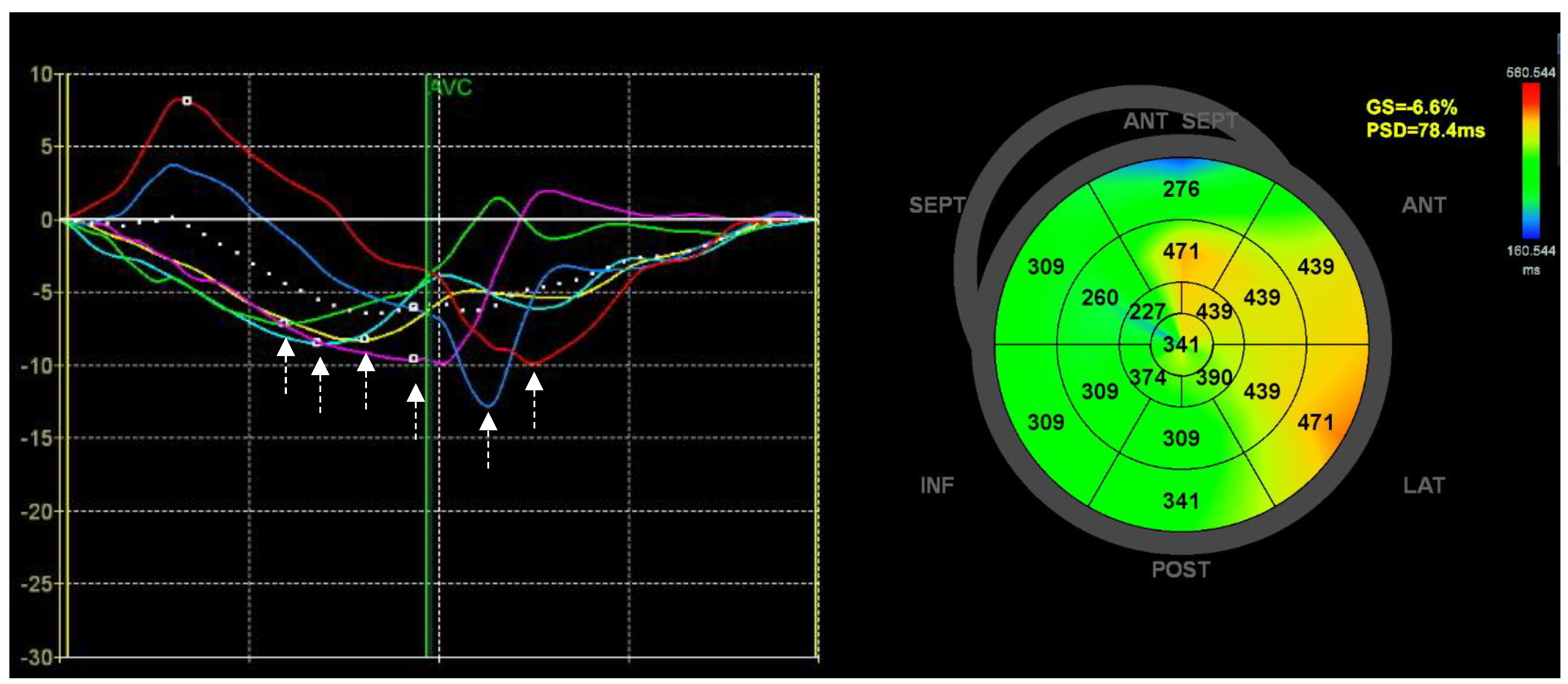
5. Mechanical Dyssynchrony
5.1. Mechanical Dispersion Fundamentals
5.2. Mechanical Dispersion and Ventricular Arrhythmias
| Study | Year | Design | Sample Size | Arrhythmia Outcome | Arrhythmia Monitoring | No. of Events | Follow-Up | Key Strain Findings |
|---|---|---|---|---|---|---|---|---|
| Myocardial infarction | ||||||||
| Ersbøll et al. [56] | 2013 | Prosp. | 988 | Composite: VA Appropriate ICD therapy Definite/suspected SCD | Admission with documented VA ICD monitoring in subgroup SCD based on hospital and prehospital records | 34 | 29.7 (IQR: 23.5–32.7) months | GLS and MD were independent predictors of VA. In patients with LVEF < 35%, both GLS and MD were independent predictors of VAs, but only GLS was an independent predictor of VAs in patients with LVEF > 35%. |
| Haugaa et al. [57] | 2013 | Prosp. | 569 | Composite: Sustained VT VF SCD | Not specified | 15 | 30 (IQR: 18) months | GLS was a univariate predictor of VA but not an independent predictor of VA when adjusted for MD. MD was an independent predictor of VA. |
| Nguyen et al. [63] | 2015 | Not specified | 467 | VT | Documented on 24 h ambulatory ECG Monitoring during hospitalization EP study | 51 | 25 (range: 6–43) months | In multivariate analysis, MD was significantly associated with VT, and GLS was borderline significantly associated with VT. |
| Leong et al. [65] | 2015 | Retrosp. | 206 | Appropriate ICD therapy | ICD monitoring | 75 | 24 (IQR: 7.8–24) months | GLS and MD were independently associated with VT. |
| Haugaa et al. [58] | 2010 | Prosp. | 85 | Appropriate ICD therapy | ICD monitoring | 38 | 2.3 (range: 0.6–5.5) years | MD, but not GLS, was an independent predictor of appropriate ICD therapy. |
| Structural heart disease | ||||||||
| Guerra et al. [66] | 2020 | Prosp. | 203 | Any VA detected by ICD | ICD monitoring | 74 | 817 (IQR: 440–1105) days | GLS was an independent predictor of the first VA episode but not recurrent episodes. MD was not associated with VAs. |
| Heart failure with reduced ejection fraction | ||||||||
| Matsuzoe et al. [70] | 2016 | Retrosp. | 72 | Appropriate ICD therapy | ICD monitoring | 34 | 17 (IQR: 0.2–72.5) months | GLS and MD were not independently associated with the VA endpoint. Only LV dyssynergy (SD of peak strain) was independently associated with the VA endpoint. |
| Hasselberg et al. [68] | 2016 | Prosp. | 170 | Composite: VT VF SCA Appropriate ATP Appropriate defibrillator shock therapy | CRT-D monitoring | 18 | 1.9 ± 0.3 years | GLS and MD at baseline were not independent predictors of the VA endpoint. MD at 6 months was an independent predictor of the VA endpoint. |
| Mornoş et al. [69] | 2017 | Prosp. | 340 | Composite: VT VF SCD | Hospital documentationDeath certificate | 48 | 36 ± 9 months | GLS, MD, and the ratio of GLS to MD (GLS/MD) were univariate predictors of VAs, but only GLS/MD was an independent predictor of VAs. |
| Banasik et al. [130] | 2016 | Retrosp. | 47 | Appropriate CRT-D therapy | CRT-D monitoring | 29 | 4 years | MD was greater in patients experiencing VAs. GLS was not reported. No multivariate analyses were performed. |
| Van der Bijl et al. [131] | 2018 | Retrosp. | 1185 | Appropriate CRT-D therapy | CRT-D monitoring | 403 | 55 ± 36 months | No difference in VA events between high vs. low baseline MD but more frequent VA events in those with high MD at 6 months. MD at 6 months was independently associated with VAs. GLS was not reported. |
| Biering-Sørensen et al. [61] | 2017 | RCT substudy | 1064 | Appropriate ICD/CRT-D therapy | ICD/CRT-D monitoring Adjudicated events | 254 | 2.9 (IQR:2.0–3.7) years | GLS and all regional (anterior and inferior) strains were associated with VT/VF, whereas MD was not. |
| Kutyifa et al. [133]. | 2013 | RCT substudy | 1077 | VT/VF | ICD/CRT-D monitoring Adjudicated events | - 205 (for baseline associations) - 90 (for associations after 12 months) | 2.3 ± 0.9 years | Baseline MD was not associated with VAs. Patients with LBBB who had >15% improvement in MD had a lower risk of VAs. |
| Biering-Sørensen et al. [72] | 2016 | Retrosp. | 151 | Composite: CVD Appropriate ICD therapy | ICD monitoring CVD from the national cause of death registry | 40 | 2.3 (IQR: 1.5–3.1) years | Neither MD nor GLS was associated with VAs. |
| Non-ischemic dilated cardiomyopathy | ||||||||
| Haugaa et al. [74] | 2012 | Prosp. | 94 | Composite: Appropriate ICD therapy Sustained VT Cardiac arrest Cardiac syncope | Not specified | 12 | 22 (Range:1–46) months | Both GLS and MD were independent predictors of the VA endpoint. |
| Kosiuk et al. [134] | 2015 | Prosp. | 20 | Composite: VT VF | Holter, duration not specified ICD monitoring | 11 | 70 ± 40 months | Greater MD in patients with VAs and MD was independently associated with the VA endpoint. |
| Negishi et al. [76] | 2016 | Retrosp. | 124 | Appropriate ICD therapy | ICD monitoring | 36 | 3.8 (IQR: 2.2–6.0) years | GLS but not MD was an independent predictor of VAs. |
| Melichova et al. [75] | 2021 | Prosp. | 290 | Composite: SCD Shock from ICD Sustained VT | Medical records (ICD therapy, ECG, Holter, aborted cardiac arrest) Cause of death registry | 32 | 22 ± 12 months | Both GLS and MD were independent predictors of VA endpoint. |
| Hypertrophic cardiomyopathy | ||||||||
| Haland et al. [77] | 2016 | Prosp. | 150 HCM | Composite: Sustained and non-sustained VT Previous aborted cardiac arrest | 24–48 h Holter monitoring ICD monitoring | 37 | Not specified | GLS and MD were univariate predictors of the VA endpoint, but only MD was an independent predictor. |
| Candan et al. [78] | 2017 | Prosp. | 63 | Appropriate ICD therapy | ICD monitoring | 17 | 3 years (21.5 ± 6.9 months) | GLS and MD were independent predictors of VAs. |
| Jalanko et al. [83] | 2016 | Prosp. | 31 | Non-sustained VT | 24 h Holter monitoring | 11 | N/A | Both GLS and MD were associated with non-sustained VT in univariate analysis, but only MD was independently associated with non-sustained VT. |
| Popa-Fotea et al. [81] | 2020 | Prosp. | 47 | Non-sustained VT | 24 h Holter monitoring | 16 | N/A | GLS, RV, and LV MD were univariate predictors of non-sustained VT, but only RV and LV MD were independent predictors of non-sustained VT. |
| Chagas cardiomyopathy | ||||||||
| Barros et al. [84] | 2016 | Retrosp., case-control study | 62 | Clinically indicated implantation of ICD. | N/A | 28 | N/A | MD and GLS were more abnormal in the group with ICD, and both were independent markers of previous events precipitating ICD. |
| Azevedo et al. [85] | 2021 | Prosp. | 77 | Composite: VES Non-sustained VT | 24 h Holter | Not specified | N/A | Both GLS and MD were associated with non-sustained VT in univariate analysis, but only MD was independently associated with non-sustained VT, paired VES, and VES in bigeminy. |
| Long QT syndrome | ||||||||
| Haugaa et al. [132] | 2008 | Prosp. | 73 LQTS 20 healthy individuals | History of either: Documented arrhythmia Syncope Cardiac arrest | N/A | 33 | Not specified | LQTS patients with a history of arrhythmia had a higher MD than those without arrhythmia. GLS not reported. |
| Haugaa et al. [86] | 2010 | Prosp. | 101 LQTS 35 healthy individuals | History of either: Documented arrhythmia Syncope Cardiac arrest | N/A | 48 | N/A | LQTS patients with a history of arrhythmia had a higher MD but similar GLS compared to those without arrhythmia. |
| Lamin A/C mutation | ||||||||
| Haugaa et al. [87] | 2015 | Prosp. | 33 | Composite: Non-sustained VT VT VF | Not specified | 11 | Not specified | Patients with any ventricular arrhythmia had higher MD but similar GLS compared to those without ventricular arrhythmia. |
| Arrhythmogenic cardiomyopathy | ||||||||
| Lie et al. [93] | 2018 | Prosp. | 117 | VT Cardiac arrest Appropriate ICD shock | ECG Holter monitoring ICD monitoring | 18 | 2.0 (IQR:0.5–3.5) years | Patients with VAs had reduced LV and RV strain and higher LV and RV MD. RV strain and LV MD were independently associated with VAs. |
| Kirkels et al. [96] | 2021 | Retrosp. | 160 | History of either: Sustained VT Appropriate ICD therapy Aborted cardiac arrest | N/A | 47 | N/A | Patients with a history of VAs had reduced LV GLS and RV strain and higher RV MD than those without VA history. RV MD was independently associated with VAs. |
| Sarvari et al. [95] | 2011 | Prosp. Case-control study | 42 symptomatic 27 asymptomatic 30 healthy | History of either: VT VF | N/A | 42 | N/A | Patients with a history of VAs had lower LV and RV strain and higher LV and RV MD. Only RV MD was independently associated with a history of VAs. |
| Mitral valve prolapse | ||||||||
| Ermakov et al. [97] | 2019 | Retrosp. | 59 MVP | History of: Ventricular couplets Ventricular bigeminy Non-sustained VT VT ICD for aborted cardiac arrest | N/A | 32 | N/A | MD was higher but similar GLS was seen in patients with a history of VA compared to those without arrhythmia. MD was independently associated with a history of VA. |
| Brugada syndrome | ||||||||
| Scheirlynck et al. [91] | 2020 | Prosp. | 175 BrS | History of: Sustained VT VF Aborted cardiac arrest | Medical records | 19 | Not specified | Patients with a history of VAs had higher LV MD but similar LV GLS, RV strain, and RV MD compared to those without VA history. High LV MD was independently associated with VA history. |
| Elite Athletes | ||||||||
| Lie et al. [92] | 2021 | Cross-sectional study | 43 athletes with VT and 30 healthy athletes | Composite of life-threatening VAs: VF Sustained VT Aborted cardiac arrest Appropriate ICD therapy | 24 h Holter monitoring ECG Telemetry ILR monitoring Intracardiac device monitoring | 23 | N/A | MD was higher and GLS was lower in VA patients. Only MD was independently associated with life-threatening VAs. |
| Idiopathic ventricular fibrillation | ||||||||
| Groeneveld et al. [99] | 2021 | Retrosp. Case-control study | 47 IVF 47 healthy individuals | VF | N/A | N/A | N/A | IVF patients had lower GLS, higher MD, and higher post-systolic index than matched controls. No adjusted analyses were performed. |
| Repaired Tetralogy of Fallot | ||||||||
| Van Grootel et al. [89] | 2019 | Prosp. | 151 ToF | Composite: Death HF Reintervention Hospitalization for cardiac reasons Symptomatic ventricular and supraventricular arrhythmias. | Regularly checked at an outpatient clinic | 62 | 71.5 (IQR: 64–75.3) months | GLS, RV strain, and apical rotation were univariate predictors of the composite outcome. Only apical rotation was independently associated with the composite outcome. MD was not investigated. |
| Unexplained syncope | ||||||||
| Falsing et al. [98] | 2021 | Retrosp. | 288 | VT | ILR monitoring | 36 | 2.9 (IQR:1.3–3.5) years | GLS was independently associated with VT. MD was not associated with VT. |
6. Future Directions
6.1. An Unexplored World of Strain Measures
6.2. Extending Current Findings into Clinical Practice
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Singh, S.N. Antiarrhythmic Drug Therapy of Ventricular Tachycardia in the Elderly: Lessons From Clinical Trials. Am. J. Geriatr. Cardiol. 1998, 7, 56–59. [Google Scholar] [PubMed]
- Lo, R.; Chia, K.K.M.; Hsia, H.H. Ventricular Tachycardia in Ischemic Heart Disease. Card. Electrophysiol. Clin. 2017, 9, 25–46. [Google Scholar] [CrossRef] [PubMed]
- Zeppenfeld, K.; Tfelt-Hansen, J.; de Riva, M.; Winkel, B.G.; Behr, E.R.; Blom, N.A.; Charron, P.; Corrado, D.; Dagres, N.; de Chillou, C.; et al. 2022 ESC Guidelines for the management of patients with ventricular arrhythmias and the prevention of sudden cardiac death. Eur. Heart J. 2022, 43, 3997–4126. [Google Scholar] [CrossRef] [PubMed]
- Heidenreich, P.A.; Bozkurt, B.; Aguilar, D.; Allen, L.A.; Byun, J.J.; Colvin, M.M.; Deswal, A.; Drazner, M.H.; Dunlay, S.M.; Evers, L.R.; et al. 2022 AHA/ACC/HFSA Guideline for the Management of Heart Failure: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation 2022, 145, e895–e1032. [Google Scholar] [CrossRef]
- Malm, S.; Frigstad, S.; Sagberg, E.; Larsson, H.; Skjaerpe, T. Accurate and reproducible measurement of left ventricular volume and ejection fraction by contrast echocardiography: A comparison with magnetic resonance imaging. J. Am. Coll. Cardiol. 2004, 44, 1030–1035. [Google Scholar] [CrossRef]
- Thavendiranathan, P.; Popović, Z.B.; Flamm, S.D.; Dahiya, A.; Grimm, R.A.; Marwick, T.H. Improved interobserver variability and accuracy of echocardiographic visual left ventricular ejection fraction assessment through a self-directed learning program using cardiac magnetic resonance images. J. Am. Soc. Echocardiogr. 2013, 26, 1267–1273. [Google Scholar] [CrossRef]
- Larsen, L.; Markham, J.; Haffajee, C.I. Sudden death in idiopathic dilated cardiomyopathy: Role of ventricular arrhythmias. Pacing Clin. Electrophysiol. 1993, 16 Pt 1, 1051–1059. [Google Scholar] [CrossRef]
- Bonou, M.; Mavrogeni, S.; Kapelios, C.J.; Markousis-Mavrogenis, G.; Aggeli, C.; Cholongitas, E.; Protoerou, A.D.; Barbetseas, J. Cardiac Adiposity and Arrhythmias: The Role of Imaging. Diagnostics 2021, 11, 362. [Google Scholar] [CrossRef]
- Kukavica, D.; Guglielmo, M.; Baggiano, A.; Muscogiuri, G.; Fusini, L.; Muratori, M.; Tamborini, G.; Mantegazza, V.; Trancuccio, A.; Arnò, C.; et al. Arrhythmic Mitral Valve Prolapse: Introducing an Era of Multimodality Imaging-Based Diagnosis and Risk Stratification. Diagnostics 2021, 11, 467. [Google Scholar] [CrossRef]
- Pavon, A.G.; Monney, P.; Schwitter, J. Mitral Valve Prolapse, Arrhythmias, and Sudden Cardiac Death: The Role of Multimodality Imaging to Detect High-Risk Features. Diagnostics 2021, 11, 683. [Google Scholar] [CrossRef]
- Nikolaidou, C.; Kotanidis, C.P.; Wijesurendra, R.; Leal-Palado, J.; Kouskouras, K.; Vassilikos, V.P.; Karvounis, H.; Ntusi, N.; Antoniades, C.; Neubauer, S.; et al. Cardiac Magnetic Resonance to Detect the Underlying Substrate in Patients with Frequent Idiopathic Ventricular Arrhythmias. Diagnostics 2021, 11, 1109. [Google Scholar] [CrossRef]
- Crescenzi, C.; Zorzi, A.; Vessella, T.; Martino, A.; Panattoni, G.; Cipriani, A.; De Lazzari, M.; Marra, M.P.; Fusco, A.; Sciarra, L.; et al. Predictors of Left Ventricular Scar Using Cardiac Magnetic Resonance in Athletes with Apparently Idiopathic Ventricular Arrhythmias. J. Am. Heart Assoc. 2021, 10, e018206. [Google Scholar] [CrossRef]
- Wacker-Gussmann, A.; Strasburger, J.F.; Wakai, R.T. Contribution of Fetal Magnetocardiography to Diagnosis, Risk Assessment, and Treatment of Fetal Arrhythmia. J. Am. Heart Assoc. 2022, 11, e025224. [Google Scholar] [CrossRef]
- Fesas, A.; Giannoula, E.; Vrachimis, A.; Doumas, A.; Wenning, C.; Didagelos, M.; Iakovou, I. Cardiac Autonomic Nervous System and Ventricular Arrhythmias: The Role of Radionuclide Molecular Imaging. Diagnostics 2021, 11, 1273. [Google Scholar] [CrossRef]
- Zhou, S.; AbdelWahab, A.; Sapp, J.L.; Sung, E.; Aronis, K.N.; Warren, J.W.; MacInnis, P.J.; Shah, R.; Horácek, B.M.; Berger, R.; et al. Assessment of an ECG-Based System for Localizing Ventricular Arrhythmias in Patients with Structural Heart Disease. J. Am. Heart Assoc. 2021, 10, e022217. [Google Scholar] [CrossRef]
- Saramet, E.E.; Negru, R.D.; Oancea, A.; Constantin, M.M.L.; Ancuta, C. 24 h Holter ECG Monitoring of Patients with Rheumatoid Arthritis-A Potential Role for a Precise Evaluation of QT Interval Duration and Associated Arrhythmic Complications. Diagnostics 2022, 12, 638. [Google Scholar] [CrossRef]
- Ramírez, J.; Kiviniemi, A.; van Duijvenboden, S.; Tinker, A.; Lambiase, P.D.; Juntilla, J.; Perkiömäki, J.S.; Huikuri, H.V.; Orini, M.; Munroe, P.B. ECG T-Wave Morphologic Variations Predict Ventricular Arrhythmic Risk in Low- and Moderate-Risk Populations. J. Am. Heart Assoc. 2022, 11, e025897. [Google Scholar] [CrossRef]
- Gehi, A.K. High-Resolution ECG for Predicting Ventricular Arrhythmia in Hypertrophic Cardiomyopathy: Another Tool in the Toolbox. J. Am. Heart Assoc. 2022, 11, e028095. [Google Scholar] [CrossRef]
- Suszko, A.M.; Chakraborty, P.; Viswanathan, K.; Barichello, S.; Sapp, J.; Talajic, M.; Laksman, Z.; Yee, R.; Woo, A.; Spears, D.; et al. Automated Quantification of Abnormal QRS Peaks from High-Resolution ECGs Predicts Late Ventricular Arrhythmias in Hypertrophic Cardiomyopathy: A 5-Year Prospective Multicenter Study. J. Am. Heart Assoc. 2022, 11, e026025. [Google Scholar] [CrossRef]
- Martini, C.; Di Maria, B.; Reverberi, C.; Tuttolomondo, D.; Gaibazzi, N. Commercially Available Heart Rate Monitor Repurposed for Automatic Arrhythmia Detection with Snapshot Electrocardiographic Capability: A Pilot Validation. Diagnostics 2022, 12, 712. [Google Scholar] [CrossRef]
- Guarracini, F.; Testolina, M.; Giacopelli, D.; Martin, M.; Triglione, F.; Coser, A.; Quintarelli, S.; Bonmassari, R.; Marini, M. Programming Optimization in Implantable Cardiac Monitors to Reduce False-Positive Arrhythmia Alerts: A Call for Research. Diagnostics 2022, 12, 994. [Google Scholar] [CrossRef] [PubMed]
- Biering-Sørensen, T.; Biering-Sørensen, S.R.; Olsen, F.J.; Sengeløv, M.; Jørgensen, P.G.; Mogelvang, R.; Shah, A.M.; Jensen, J.S. Global Longitudinal Strain by Echocardiography Predicts Long-Term Risk of Cardiovascular Morbidity and Mortality in a Low-Risk General Population: The Copenhagen City Heart Study. Circ. Cardiovasc. Imaging 2017, 10, e005521. [Google Scholar] [CrossRef] [PubMed]
- Shah, A.M.; Claggett, B.; Loehr, L.R.; Chang, P.P.; Matsushita, K.; Kitzman, D.; Konety, S.; Kucharska-Newton, A.; Sueta, C.A.; Mosley, T.H.; et al. Heart Failure Stages among Older Adults in the Community: The Atherosclerosis Risk in Communities Study. Circulation 2017, 135, 224–240. [Google Scholar] [CrossRef] [PubMed]
- Cheng, S.; McCabe, E.L.; Larson, M.G.; Merz, A.A.; Osypiuk, E.; Lehman, B.T.; Stantchev, P.; Aragam, J.; Solomon, S.D.; Benjamin, E.J.; et al. Distinct Aspects of Left Ventricular Mechanical Function Are Differentially Associated with Cardiovascular Outcomes and All-Cause Mortality in the Community. J. Am. Heart Assoc. 2015, 4, e002071. [Google Scholar] [CrossRef] [PubMed]
- Russo, C.; Jin, Z.; Elkind, M.S.V.; Rundek, T.; Homma, S.; Sacco, R.L.; Di Tullio, M.R. Prevalence and prognostic value of subclinical left ventricular systolic dysfunction by global longitudinal strain in a community-based cohort. Eur. J. Heart Fail. 2014, 16, 1301–1309. [Google Scholar] [CrossRef]
- Wong, C.Y.; O’Moore-Sullivan, T.; Leano, R.; Byrne, N.; Beller, E.; Marwick, T.H. Alterations of left ventricular myocardial characteristics associated with obesity. Circulation 2004, 110, 3081–3087. [Google Scholar] [CrossRef]
- Leitman, M.; Lysyansky, P.; Sidenko, S.; Shir, V.; Peleg, E.; Binenbaum, M.; Kaluski, E.; Krakover, R.; Vered, Z. Two-dimensional strain-a novel software for real-time quantitative echocardiographic assessment of myocardial function. J. Am. Soc. Echocardiogr. 2004, 17, 1021–1029. [Google Scholar] [CrossRef]
- Reisner, S.A.; Lysyansky, P.; Agmon, Y.; Mutlak, D.; Lessick, J.; Friedman, Z. Global longitudinal strain: A novel index of left ventricular systolic function. J. Am. Soc. Echocardiogr. 2004, 17, 630–633. [Google Scholar] [CrossRef]
- Smiseth, O.A.; Torp, H.; Opdahl, A.; Haugaa, K.H.; Urheim, S. Myocardial strain imaging: How useful is it in clinical decision making? Eur. Heart J. 2016, 37, 1196–1207. [Google Scholar] [CrossRef]
- Stöhr, E.J.; Shave, R.E.; Baggish, A.L.; Weiner, R.B. Left ventricular twist mechanics in the context of normal physiology and cardiovascular disease: A review of studies using speckle tracking echocardiography. Am. J. Physiol. Heart Circ. Physiol. 2016, 311, H633–H644. [Google Scholar] [CrossRef]
- Negishi, K.; Negishi, T.; Kurosawa, K.; Hristova, K.; Popescu, B.A.; Vinereanu, D.; Yuda, S.; Marwick, T.H. Practical guidance in echocardiographic assessment of global longitudinal strain. JACC Cardiovasc. Imaging 2015, 8, 489–492. [Google Scholar] [CrossRef] [PubMed]
- Reimer, K.A.; Lowe, J.E.; Rasmussen, M.M.; Jennings, R.B. The wavefront phenomenon of ischemic cell death. 1. Myocardial infarct size vs duration of coronary occlusion in dogs. Circulation 1977, 56, 786–794. [Google Scholar] [CrossRef] [PubMed]
- Tan, Y.T.; Wenzelburger, F.; Lee, E.; Heatlie, G.; Leyva, F.; Patel, K.; Frenneaux, M.; Sanderson, J.E. The pathophysiology of heart failure with normal ejection fraction: Exercise echocardiography reveals complex abnormalities of both systolic and diastolic ventricular function involving torsion, untwist, and longitudinal motion. J. Am. Coll. Cardiol. 2009, 54, 36–46. [Google Scholar] [CrossRef] [PubMed]
- Shah, A.M.; Claggett, B.; Sweitzer, N.K.; Shah, S.J.; Anand, I.S.; Liu, L.; Pitt, B.; Pfeffer, M.A.; Solomon, S.D. Prognostic Importance of Impaired Systolic Function in Heart Failure with Preserved Ejection Fraction and the Impact of Spironolactone. Circulation 2015, 132, 402–414. [Google Scholar] [CrossRef] [PubMed]
- DeVore, A.D.; McNulty, S.; Alenezi, F.; Ersboll, M.; Vader, J.M.; Oh, J.K.; Lin, G.; Redfield, M.M.; Lewis, G.; Semigran, M.J.; et al. Impaired left ventricular global longitudinal strain in patients with heart failure with preserved ejection fraction: Insights from the RELAX trial. Eur. J. Heart Fail. 2017, 19, 893–900. [Google Scholar] [CrossRef]
- Geer, J.C.; Crago, C.A.; Little, W.C.; Gardner, L.L.; Bishop, S.P. Subendocardial ischemic myocardial lesions associated with severe coronary atherosclerosis. Am. J. Pathol. 1980, 98, 663–680. [Google Scholar]
- Duncker, D.J.; Traverse, J.H.; Ishibashi, Y.; Bache, R.J. Effect of NO on transmural distribution of blood flow in hypertrophied left ventricle during exercise. Am. J. Physiol. 1999, 276, H1305–H1312. [Google Scholar] [CrossRef]
- Ono, S.; Waldman, L.K.; Yamashita, H.; Covell, J.W.; Ross, J. Effect of coronary artery reperfusion on transmural myocardial remodeling in dogs. Circulation 1995, 91, 1143–1153. [Google Scholar] [CrossRef]
- Le, T.T.; Huang, W.; Singh, G.K.; Toh, D.-F.; Ewe, S.H.; Tang, H.C.; Loo, G.; Bryant, J.A.; Ang, B.; Tay, E.L.-W.; et al. Echocardiographic Global Longitudinal Strain Is Associated with Myocardial Fibrosis and Predicts Outcomes in Aortic Stenosis. Front. Cardiovasc. Med. 2021, 8, 750016. [Google Scholar] [CrossRef]
- Munk, K.; Andersen, N.H.; Nielsen, S.S.; Bibby, B.M.; Bøtker, H.E.; Nielsen, T.T.; Poulsen, S.H. Global longitudinal strain by speckle tracking for infarct size estimation. Eur. J. Echocardiogr. 2011, 12, 156–165. [Google Scholar] [CrossRef]
- Ersbøll, M.; Valeur, N.; Mogensen, U.M.; Andersen, M.; Greibe, R.; Møller, J.E.; Hassager, C.; Søgaard, P.; Køber, L. Global left ventricular longitudinal strain is closely associated with increased neurohormonal activation after acute myocardial infarction in patients with both reduced and preserved ejection fraction: A two-dimensional speckle tracking study. Eur. J. Heart Fail. 2012, 14, 1121–1129. [Google Scholar] [CrossRef]
- Risum, N.; Ali, S.; Olsen, N.T.; Jons, C.; Khouri, M.G.; Lauridsen, T.K.; Samad, Z.; Velazquez, E.J.; Søgaard, P.; Kisslo, J. Variability of global left ventricular deformation analysis using vendor dependent and independent two-dimensional speckle-tracking software in adults. J. Am. Soc. Echocardiogr. 2012, 25, 1195–1203. [Google Scholar] [CrossRef]
- Farsalinos, K.E.; Daraban, A.M.; Ünlü, S.; Thomas, J.D.; Badano, L.P.; Voigt, J.U. Head-to-Head Comparison of Global Longitudinal Strain Measurements among Nine Different Vendors: The EACVI/ASE Inter-Vendor Comparison Study. J. Am. Soc. Echocardiogr. 2015, 28, 1171–1181.e2. [Google Scholar] [CrossRef]
- Voigt, J.U.; Pedrizzetti, G.; Lysyansky, P.; Marwick, T.H.; Houle, H.; Baumann, R.; Pedri, S.; Ito, Y.; Abe, Y.; Metz, S.; et al. Definitions for a common standard for 2D speckle tracking echocardiography: Consensus document of the EACVI/ASE/Industry Task Force to standardize deformation imaging. Eur. Heart J. Cardiovasc. Imaging. 2015, 16, 1–11. [Google Scholar] [CrossRef]
- Yingchoncharoen, T.; Agarwal, S.; Popović, Z.B.; Marwick, T.H. Normal ranges of left ventricular strain: A meta-analysis. J. Am. Soc. Echocardiogr. 2013, 26, 185–191. [Google Scholar] [CrossRef]
- Yang, H.; Wright, L.; Negishi, T.; Negishi, K.; Liu, J.; Marwick, T.H. Research to Practice: Assessment of Left Ventricular Global Longitudinal Strain for Surveillance of Cancer Chemotherapeutic-Related Cardiac Dysfunction. JACC Cardiovasc. Imaging 2018, 11, 1196–1201. [Google Scholar] [CrossRef]
- Skaarup, K.G.; Lassen, M.C.H.; Johansen, N.D.; Olsen, F.J.; Lind, J.N.; Jørgensen, P.G.; Jensen, G.; Schnohr, P.; Prescott, E.; Søgaard, P.; et al. Age- and sex-based normal values of layer-specific longitudinal and circumferential strain by speckle tracking echocardiography: The Copenhagen City Heart Study. Eur. Heart J. Cardiovasc. Imaging 2022, 23, 629–640. [Google Scholar] [CrossRef]
- Karlsen, S.; Dahlslett, T.; Grenne, B.; Sjøli, B.; Smiseth, O.; Edvardsen, T.; Brunvand, H. Global longitudinal strain is a more reproducible measure of left ventricular function than ejection fraction regardless of echocardiographic training. Cardiovasc. Ultrasound 2019, 17, 18. [Google Scholar] [CrossRef]
- Lyon, A.R.; López-Fernández, T.; Couch, L.S.; Asteggiano, R.; Aznar, M.C.; Bergler-Klein, J.; Boriani, G.; Cardinale, D.; Cordoba, R.; Cosyns, B.; et al. 2022 ESC Guidelines on cardio-oncology developed in collaboration with the European Hematology Association (EHA), the European Society for Therapeutic Radiology and Oncology (ESTRO) and the International Cardio-Oncology Society (IC-OS). Eur. Heart J. 2022, 43, 4229–4361. [Google Scholar] [CrossRef]
- Nagueh, S.F.; Smiseth, O.A.; Appleton, C.P.; Byrd, B.F., 3rd; Dokainish, H.; Edvardsesn, T.; Flachskampf, F.A.; Gillebert, T.C.; Klein, A.L.; Lancellotti, P.; et al. Recommendations for the Evaluation of Left Ventricular Diastolic Function by Echocardiography: An Update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J. Am. Soc. Echocardiogr. 2016, 29, 277–314. [Google Scholar] [CrossRef]
- Olsen, F.J.; Lindberg, S.; Pedersen, S.; Iversen, A.; Davidovski, F.S.; Galatius, S.; Fritz-Hansen, T.; Gislason, G.H.; Søgaard, P.; Møgelvang, R.; et al. Global longitudinal strain predicts cardiovascular events after coronary artery bypass grafting. Heart 2021, 107, 814–821. [Google Scholar] [CrossRef] [PubMed]
- Ersbøll, M.; Valeur, N.; Mogensen, U.M.; Andersen, M.J.; Møller, J.E.; Velazquez, E.J.; Hassager, C.; Søgaard, P.; Køber, L. Prediction of all-cause mortality and heart failure admissions from global left ventricular longitudinal strain in patients with acute myocardial infarction and preserved left ventricular ejection fraction. J. Am. Coll Cardiol. 2013, 61, 2365–2373. [Google Scholar] [CrossRef]
- Stecker, E.C.; Vickers, C.; Waltz, J.; Socoteanu, C.; John, B.T.; Mariani, R.; McAnulty, J.H.; Gunson, K.; Jui, J.; Chugh, S.S. Population-based analysis of sudden cardiac death with and without left ventricular systolic dysfunction: Two-year findings from the Oregon Sudden Unexpected Death Study. J. Am. Coll Cardiol. 2006, 47, 1161–1166. [Google Scholar] [CrossRef] [PubMed]
- Santangeli, P.; Dello Russo, A.; Casella, M.; Pelargonio, G.; Di Biase, L.; Natale, A. Left ventricular ejection fraction for the risk stratification of sudden cardiac death: Friend or foe? Intern. Med. J. 2011, 41, 55–60. [Google Scholar] [CrossRef] [PubMed]
- Buxton, A.E.; Lee, K.L.; Hafley, G.E.; Pirex, L.A.; Fisher, J.D.; Gold, M.R.; Josephson, M.E.; Lehmann, M.H.; Prystowsky, E.N.; MUSTT Investigators. Limitations of ejection fraction for prediction of sudden death risk in patients with coronary artery disease: Lessons from the MUSTT study. J. Am. Coll. Cardiol. 2007, 50, 1150–1157. [Google Scholar] [CrossRef]
- Ersbøll, M.; Valeur, N.; Andersen, M.J.; Mogensen, U.M.; Vinther, M.; Svendsen, J.H.; Møller, J.E.; Kisslo, J.; Velazquez, E.J.; Hassager, C.; et al. Early echocardiographic deformation analysis for the prediction of sudden cardiac death and life-threatening arrhythmias after myocardial infarction. JACC Cardiovasc. Imaging 2013, 6, 851–860. [Google Scholar] [CrossRef]
- Haugaa, K.H.; Grenne, B.L.; Eek, C.H.; Ersbøll, M.; Valeur, N.; Svendsen, J.H.; Florian, A.; Sjøli, B.; Brunvand, H.; Køber, L.; et al. Strain echocardiography improves risk prediction of ventricular arrhythmias after myocardial infarction. JACC Cardiovasc. Imaging 2013, 6, 841–850. [Google Scholar] [CrossRef]
- Haugaa, K.H.; Smedsrud, M.K.; Steen, T.; Kongsgaard, E.; Loennechen, J.P.; Skjaerpe, T.; Voigt, J.-U.; Willems, R.; Smith, G.; Smiseth, O.A.; et al. Mechanical dispersion assessed by myocardial strain in patients after myocardial infarction for risk prediction of ventricular arrhythmia. JACC Cardiovasc. Imaging 2010, 3, 247–256. [Google Scholar] [CrossRef]
- Kawakami, H.; Nerlekar, N.; Haugaa, K.H.; Edvardsen, T.; Marwick, T.H. Prediction of Ventricular Arrhythmias with Left Ventricular Mechanical Dispersion. JACC Cardiovasc. Imaging 2020, 13, 562–572. [Google Scholar] [CrossRef]
- Harapoz, M.; Zada, M.; Matthews, J.; Kumar, S.; Thomas, L. Echocardiographic predictors of ventricular arrhythmias in patients with non-ischemic cardiomyopathy. Int. J. Cardiol. Heart Vasc. 2022, 39, 100962. [Google Scholar] [CrossRef]
- Biering-Sørensen, T.; Knappe, D.; Pouleur, A.C.; Claggett, B.; Wang, P.J.; Moss, A.J.; Solomon, S.D.; Kutyifa, V. Regional Longitudinal Deformation Improves Prediction of Ventricular Tachyarrhythmias in Patients with Heart Failure with Reduced Ejection Fraction: A MADIT-CRT Substudy (Multicenter Automatic Defibrillator Implantation Trial-Cardiac Resynchronization Therapy). Circ. Cardiovasc. Imaging 2017, 10, e005096. [Google Scholar] [CrossRef]
- Sjøli, B.; Grenne, B.; Smiseth, O.A.; Edvardsen, T.; Brunvand, H. The advantage of global strain compared to left ventricular ejection fraction to predict outcome after acute myocardial infarction. Echocardiography 2011, 28, 556–563. [Google Scholar] [CrossRef]
- Nguyen, B.L.; Capotosto, L.; Persi, A.; Placanica, A.; Rafique, A.; Piccirillo, G.; Gaudio, C.; Gang, E.S.; Siegel, R.J.; Vitarelli, A. Global and regional left ventricular strain indices in post-myocardial infarction patients with ventricular arrhythmias and moderately abnormal ejection fraction. Ultrasound Med. Biol. 2015, 41, 407–417. [Google Scholar] [CrossRef]
- Choi, W.; Kim, C.H.; Hwang, I.C.; Yoon, C.-H.; Choi, H.-M.; Yoon, Y.E.; Chae, I.-H.; Cho, G.-Y. Three-Dimensional Myocardial Strain for the Prediction of Clinical Events in Patients with ST-Segment Elevation Myocardial Infarction. J. Cardiovasc. Imaging 2022, 30, 185–196. [Google Scholar] [CrossRef]
- Leong, D.P.; Hoogslag, G.E.; Piers, S.R.D.; Höke, U.; Thijssen, J.; Marsan, N.A.; Schalij, M.J.; Zeppenfeld, K.; Bax, J.J.; Delgado, V. The relationship between time from myocardial infarction, left ventricular dyssynchrony, and the risk for ventricular arrhythmia: Speckle-tracking echocardiographic analysis. J. Am. Soc. Echocardiogr. 2015, 28, 470–477. [Google Scholar] [CrossRef]
- Guerra, F.; Malagoli, A.; Contadini, D.; Baiocco, E.; Menditto, A.; Bonelli, P.; Rossi, L.; Sticozzi, C.; Zanni, A.; Cai, J.; et al. Global Longitudinal Strain as a Predictor of First and Subsequent Arrhythmic Events in Remotely Monitored ICD Patients with Structural Heart Disease. JACC Cardiovasc. Imaging 2020, 13 Pt 1, 1–9. [Google Scholar] [CrossRef]
- Nikoo, M.H.; Naeemi, R.; Moaref, A.; Attar, A. Global longitudinal strain for prediction of ventricular arrhythmia in patients with heart failure. ESC Heart Fail. 2020, 7, 2956–2961. [Google Scholar] [CrossRef]
- Hasselberg, N.E.; Haugaa, K.H.; Bernard, A.; Ribe, M.P.; Kongsgaard, E.; Donal, E.; Edvardsen, T. Left ventricular markers of mortality and ventricular arrhythmias in heart failure patients with cardiac resynchronization therapy. Eur. Heart J. Cardiovasc. Imaging 2016, 17, 343–350. [Google Scholar] [CrossRef]
- Mornoş, C.; Muntean, D.; Mornoş, A.; Crişan, S.; Petrescu, L.; Ionac, A.; Sosdean, R.; Cozma, D. Risk stratification in patients with heart failure: The value of considering both global longitudinal left ventricular strain and mechanical dispersion. Can. J. Physiol. Pharmacol. 2017, 95, 1360–1368. [Google Scholar] [CrossRef]
- Matsuzoe, H.; Tanaka, H.; Matsumoto, K.; Toki, H.; Shimoura, H.; Ooka, J.; Sano, H.; Sawa, T.; Motoji, Y.; Mochizuki, Y.; et al. Left ventricular dyssynergy and dispersion as determinant factors of fatal ventricular arrhythmias in patients with mildly reduced ejection fraction. Eur. Heart J. Cardiovasc. Imaging 2016, 17, 334–342. [Google Scholar] [CrossRef]
- Bax, J.J.; Delgado, V.; Sogaard, P.; Singh, J.P.; Abraham, W.T.; Borer, J.S.; Dickstein, K.; Gras, D.; Brugada, J.; Robertsen, M.; et al. Prognostic implications of left ventricular global longitudinal strain in heart failure patients with narrow QRS complex treated with cardiac resynchronization therapy: A subanalysis of the randomized EchoCRT trial. Eur. Heart J. 2017, 38, 720–726. [Google Scholar] [CrossRef] [PubMed]
- Biering-Sørensen, T.; Olsen, F.J.; Storm, K.; Fritz-Hansen, T.; Olsen, N.T.; Jøns, C.; Vinther, M.; Søgaard, P.; Risum, N. Prognostic value of tissue Doppler imaging for predicting ventricular arrhythmias and cardiovascular mortality in ischaemic cardiomyopathy. Eur. Heart J. Cardiovasc. Imaging 2016, 17, 66. [Google Scholar] [CrossRef] [PubMed]
- Winsløw, U.; Elming, M.B.; Thune, J.J.; Haarbo, J.; Philbert, B.T.; Svendsen, J.H.; Pehrson, S.; Jøns, C.; Bundgaard, H.; Køber, L.; et al. Reduced inferior wall longitudinal strain is associated with malignant arrhythmias in non-ischemic heart failure. Pacing Clin. Electrophysiol. 2023. Epub Ahead of Print. [Google Scholar] [CrossRef] [PubMed]
- Haugaa, K.H.; Goebel, B.; Dahlslett, T.; Meyer, K.; Jung, C.; Lauten, A.; Figulla, H.R.; Poerner, T.C.; Edvardsen, T. Risk assessment of ventricular arrhythmias in patients with nonischemic dilated cardiomyopathy by strain echocardiography. J. Am. Soc. Echocardiogr. 2012, 25, 667–673. [Google Scholar] [CrossRef]
- Melichova, D.; Nguyen, T.M.; Salte, I.M.; Klaeboe, L.G.; Sjøli, B.; Karlsen, S.; Dahlslett, T.; Leren, I.S.; Edvardsen, T.; Brunvand, H.; et al. Strain echocardiography improves prediction of arrhythmic events in ischemic and non-ischemic dilated cardiomyopathy. Int. J. Cardiol. 2021, 342, 56–62. [Google Scholar] [CrossRef]
- Negishi, K.; Negishi, T.; Zardkoohi, O.; Ching, E.A.; Basu, N.; Wilkoff, B.L.; Popovic, Z.B.; Marwick, T.H. Left atrial booster pump function is an independent predictor of subsequent life-threatening ventricular arrhythmias in non-ischaemic cardiomyopathy. Eur. Heart J. Cardiovasc. Imaging 2016, 17, 1153–1160. [Google Scholar] [CrossRef]
- Haland, T.F.; Almaas, V.M.; Hasselberg, N.E.; Saberniak, J.; Leren, I.S.; Hopp, E.; Edvardsen, T.; Haugaa, K.H. Strain echocardiography is related to fibrosis and ventricular arrhythmias in hypertrophic cardiomyopathy. Eur. Heart J. Cardiovasc. Imaging 2016, 17, 613–621. [Google Scholar] [CrossRef]
- Candan, O.; Gecmen, C.; Bayam, E.; Guner, A.; Celik, M.; Doğan, C. Mechanical dispersion and global longitudinal strain by speckle tracking echocardiography: Predictors of appropriate implantable cardioverter defibrillator therapy in hypertrophic cardiomyopathy. Echocardiography 2017, 34, 835–842. [Google Scholar] [CrossRef]
- Debonnaire, P.; Thijssen, J.; Leong, D.P.; Joyce, E.; Katsanos, S.; Hoogslag, G.E.; Schalij, M.J.; Atsma, D.E.; Bax, J.J.; Delgado, V.; et al. Global longitudinal strain and left atrial volume index improve prediction of appropriate implantable cardioverter defibrillator therapy in hypertrophic cardiomyopathy patients. Int. J. Cardiovasc. Imaging 2014, 30, 549–558. [Google Scholar] [CrossRef]
- Candan, O.; Gecmen, C.; Kalaycı, A.; Bayam, E.; Guner, A.; Gunduz, S.; Cersit, S.; Ozkan, M. Left ventricular twist in hypertrophic cardiomyopathy: Predictor of nonsustained ventricular tachycardia. Herz 2019, 44, 238–246. [Google Scholar] [CrossRef]
- Popa-Fotea, N.M.; Micheu, M.M.; Onciul, S.; Zamfir, D.; Dorobanţu, M. Combined right and left ventricular mechanical dispersion enhance the arrhythmic risk stratification in hypertrophic cardiomyopathy. J. Cardiol. 2020, 76, 364–370. [Google Scholar] [CrossRef]
- Hiemstra, Y.L.; Debonnaire, P.; Bootsma, M.; van Zwet, E.W.; Delgado, V.; Schalij, M.J.; Atsma, D.E.; Bax, J.J.; Marsan, N.A. Global Longitudinal Strain and Left Atrial Volume Index Provide Incremental Prognostic Value in Patients with Hypertrophic Cardiomyopathy. Circ. Cardiovasc. Imaging 2017, 10, e005706. [Google Scholar] [CrossRef]
- Jalanko, M.; Tarkiainen, M.; Sipola, P.; Jääskeläinen, P.; Lauerma, K.; Laine, M.; Nieminen, M.S.; Laakso, M.; Heliö, T.; Kuusisto, J. Left ventricular mechanical dispersion is associated with nonsustained ventricular tachycardia in hypertrophic cardiomyopathy. Ann. Med. 2016, 48, 417–427. [Google Scholar] [CrossRef]
- Barros, M.V.L.; Leren, I.S.; Edvardsen, T.; Haugaa, K.H.; Carmo, A.A.L.; Lage, T.A.R.; Nunes, M.C.P.; Rocha, M.O.C.; Ribeiro, A.L. Mechanical Dispersion Assessed by Strain Echocardiography Is Associated with Malignant Arrhythmias in Chagas Cardiomyopathy. J. Am. Soc. Echocardiogr. 2016, 29, 368–374. [Google Scholar] [CrossRef]
- Azevedo, A.C.A.; Barros, M.V.L.; Klaboe, L.G.; Edvardsen, T.; Costa, H.S.; Paixao, G.M.M.; Santos Junior, O.R.; Nunes, M.C.P.; Rocha, M.O.C. Association between myocardial mechanical dispersion and ventricular arrhythmogenicity in chagas cardiomyopathy. Int. J. Cardiovasc. Imaging 2021, 37, 2727–2734. [Google Scholar] [CrossRef] [PubMed]
- Haugaa, K.H.; Amlie, J.P.; Berge, K.E.; Leren, T.P.; Smiseth, O.A.; Edvardsen, T. Transmural differences in myocardial contraction in long-QT syndrome: Mechanical consequences of ion channel dysfunction. Circulation 2010, 122, 960377. [Google Scholar] [CrossRef]
- Haugaa, K.H.; Hasselberg, N.E.; Edvardsen, T. Mechanical dispersion by strain echocardiography: A predictor of ventricular arrhythmias in subjects with lamin A/C mutations. JACC Cardiovasc. Imaging 2015, 8, 104–106. [Google Scholar] [CrossRef]
- Diller, G.P.; Kempny, A.; Liodakis, E.; Alonso-Gonzalez, R.; Inuzuka, R.; Uebing, A.; Orwat, S.; Dimopoulos, K.; Swan, L.; Li, W.; et al. Left ventricular longitudinal function predicts life-threatening ventricular arrhythmia and death in adults with repaired tetralogy of fallot. Circulation 2012, 125, 2440–2446. [Google Scholar] [CrossRef]
- Van Grootel, R.W.J.; van den Bosch, A.E.; Baggen, V.J.M.; Menting, M.E.; Baart, S.J.; Cuypers, J.A.A.E.; Witsenburg, M.; Roos-Hesselink, J.W. The Prognostic Value of Myocardial Deformation in Adult Patients with Corrected Tetralogy of Fallot. J. Am. Soc. Echocardiogr. 2019, 32, 866–875.e2. [Google Scholar] [CrossRef] [PubMed]
- Hamon, D.; Algalarrondo, V.; Gandjbakhch, E.; Extramiana, F.; Marijon, E.; Elbaz, N.; Selhane, D.; Dubois-Rande, J.-L.; Teiger, E.; Plante-Bordeneuve, V.; et al. Outcome and incidence of appropriate implantable cardioverter-defibrillator therapy in patients with cardiac amyloidosis. Int. J. Cardiol. 2016, 222, 562–568. [Google Scholar] [CrossRef]
- Scheirlynck, E.; Van Malderen, S.; Motoc, A.; Lie, Ø.H.; de Asmundis, C.; Sieira, J.; Chierchia, G.-B.; Brugada, P.; Cosyns, B.; Droogmans, S. Contraction alterations in Brugada syndrome; association with life-threatening ventricular arrhythmias. Int. J. Cardiol. 2020, 299, 147–152. [Google Scholar] [CrossRef] [PubMed]
- Lie, Ø.H.; Klaboe, L.G.; Dejgaard, L.A.; Skjølsvik, E.T.; Grimsmo, J.; Bosse, G.; Hopp, E.; Edvardsen, T.; Haugaa, K.H. Cardiac Phenotypes and Markers of Adverse Outcome in Elite Athletes with Ventricular Arrhythmias. JACC Cardiovasc. Imaging 2021, 14, 148–158. [Google Scholar] [CrossRef] [PubMed]
- Lie, Ø.H.; Rootwelt-Norberg, C.; Dejgaard, L.A.; Leren, I.S.; Stokke, M.K.; Edvardsen, T.; Haugaa, K.H. Prediction of Life-Threatening Ventricular Arrhythmia in Patients with Arrhythmogenic Cardiomyopathy: A Primary Prevention Cohort Study. JACC Cardiovasc. Imaging 2018, 11, 1377–1386. [Google Scholar] [CrossRef] [PubMed]
- Lie, Ø.H.; Chivulescu, M.; Rootwelt-Norberg, C.; Ribe, M.; Bogsrud, M.P.; Lyseggen, E.; Beitnes, J.O.; Almaas, V.; Haugaa, K.H. Left Ventricular Dysfunction in Arrhythmogenic Cardiomyopathy: Association with Exercise Exposure, Genetic Basis, and Prognosis. J. Am. Heart Assoc. 2021, 10, e018680. [Google Scholar] [CrossRef] [PubMed]
- Sarvari, S.I.; Haugaa, K.H.; Anfinsen, O.G.; Leren, T.P.; Smiseth, O.A.; Kongsgaard, E.; Amlie, J.P.; Edvardsen, T. Right ventricular mechanical dispersion is related to malignant arrhythmias: A study of patients with arrhythmogenic right ventricular cardiomyopathy and subclinical right ventricular dysfunction. Eur. Heart J. 2011, 32, 1089–1096. [Google Scholar] [CrossRef]
- Kirkels, F.P.; Lie, Ø.H.; Cramer, M.J.; Chivulescu, M.; Rootwelt-Norberg, C.; Asselbergs, F.W.; Teske, A.J.; Haugaa, K.H. Right Ventricular Functional Abnormalities in Arrhythmogenic Cardiomyopathy: Association with Life-Threatening Ventricular Arrhythmias. JACC Cardiovasc. Imaging 2021, 14, 900–910. [Google Scholar] [CrossRef]
- Ermakov, S.; Gulhar, R.; Lim, L.; Bibby, D.; Fang, Q.; Nah, G.; Abraham, T.P.; Schiller, N.B.; Delling, F.N. Left ventricular mechanical dispersion predicts arrhythmic risk in mitral valve prolapse. Heart 2019, 105, 1063–1069. [Google Scholar] [CrossRef]
- Falsing, M.M.; Brainin, P.; Andersen, D.M.; Larroudé, C.E.; Lindhardt, T.B.; Modin, D.; Ravnkilde, K.; Karsum, E.H.; Gislason, G.; Biering-Sørensen, T. Usefulness of echocardiography for predicting ventricular tachycardia detected by implantable loop recorder in syncope patients. Int. J. Cardiovasc. Imaging 2021, 37, 3157–3166. [Google Scholar] [CrossRef]
- Groeneveld, S.A.; van der Ree, M.H.; Taha, K.; de Bruin-Bon, R.H.A.; Cramer, M.J.; Teske, A.J.; Bouma, B.J.; Amin, A.S.; Wilde, A.A.M.; Postema, P.G.; et al. Echocardiographic deformation imaging unmasks global and regional mechanical dysfunction in patients with idiopathic ventricular fibrillation: A multicenter case-control study. Heart Rhythm. 2021, 18, 1666–1672. [Google Scholar] [CrossRef]
- Pruitt, C.R.; Menon, S.; Lal, A.K.; Eckhauser, A.W.; Ou, Z.; Presson, A.; Miller, T.; Niu, M. Usefulness of Left Ventricular Myocardial Deformation in Children Hospitalized for Acute Myocarditis who Develop Arrhythmias. Am. J. Cardiol. 2021, 152, 113–119. [Google Scholar] [CrossRef]
- Yu, S.; Zhou, J.; Yang, K.; Chen, X.; Zheng, Y.; Zhao, K.; Song, J.; Ji, K.; Zhou, P.; Yan, H.; et al. Correlation of Myocardial Strain and Late Gadolinium Enhancement by Cardiac Magnetic Resonance after a First Anterior ST-Segment Elevation Myocardial Infarction. Front. Cardiovasc. Med. 2021, 8, 705487. [Google Scholar] [CrossRef]
- Wang, N.; Hung, C.L.; Shin, S.H.; Claggett, B.; Skali, H.; Thune, J.J.; Køber, L.; Shah, A.M.; McMurray, J.J.V.; Pfeffer, M.A.; et al. Regional cardiac dysfunction and outcome in patients with left ventricular dysfunction, heart failure, or both after myocardial infarction. Eur. Heart J. 2016, 37, 466–472. [Google Scholar] [CrossRef]
- Biering-Sørensen, T.; Jensen, J.S.; Pedersen, S.H.; Galatius, S.; Fritz-Hansen, T.; Bech, J.; Olsen, F.J.; Mogelvang, R. Regional Longitudinal Myocardial Deformation Provides Incremental Prognostic Information in Patients with ST-Segment Elevation Myocardial Infarction. PLoS ONE 2016, 11, e0158280. [Google Scholar] [CrossRef]
- Olsen, F.J.; Pedersen, S.; Galatius, S.; Fritz-Hansen, T.; Gislason, G.; Biering-Sørensen, T. Association between regional longitudinal strain and left ventricular thrombus formation following acute myocardial infarction. Int. J. Cardiovasc. Imaging 2020, 36, 1271–1281. [Google Scholar] [CrossRef]
- Amzulescu, M.S.; Langet, H.; Saloux, E.; Manrique, A.; Boileau, L.; Slimani, A.; Allain, P.; Roy, C.; de Meester, C.; Pasquet, A.; et al. Head-to-Head Comparison of Global and Regional Two-Dimensional Speckle Tracking Strain Versus Cardiac Magnetic Resonance Tagging in a Multicenter Validation Study. Circ. Cardiovasc. Imaging 2017, 10, e006530. [Google Scholar] [CrossRef]
- Mirea, O.; Pagourelias, E.D.; Duchenne, J.; Bogaert, J.; Thomas, J.D.; Badano, L.P.; Voigt, J.-U.; EACVI-ASE-Industry Standardization Task Force. Variability and Reproducibility of Segmental Longitudinal Strain Measurement: A Report from the EACVI-ASE Strain Standardization Task Force. JACC Cardiovasc. Imaging 2018, 11, 15–24. [Google Scholar] [CrossRef]
- Moss, A.J.; Wilber, D.J.; Brown, M.W. Prophylactic Implantation of a Defibrillator in Patients with Myocardial Infarction and Reduced Ejection Fraction. N. Engl. J. Med. 2002, 346, 877–883. [Google Scholar] [CrossRef]
- Bardy, G.H.; Lee, K.L.; Mark, D.B.; Poole, J.E.; Packer, D.L.; Boineau, R.; Domanski, M.; Troutman, C.; Anderson, J.; Johnson, G.; et al. Amiodarone or an implantable cardioverter-defibrillator for congestive heart failure. N. Engl. J. Med. 2005, 352, 225–237. [Google Scholar] [CrossRef]
- Verma, A.; Marrouche, N.F.; Schweikert, R.A.; Saliba, W.; Wazni, O.; Cummings, J.; Abdul-Karim, A.; Bhargava, M.; Burkhardt, J.D.; Kilicaslan, F.; et al. Relationship between successful ablation sites and the scar border zone defined by substrate mapping for ventricular tachycardia post-myocardial infarction. J. Cardiovasc. Electrophysiol. 2005, 16, 465–471. [Google Scholar] [CrossRef]
- Fernandes, V.R.S.; Wu, K.C.; Rosen, B.D.; Schmidt, A.; Lardo, A.C.; Osman, N.; Halperin, H.R.; Tomaselli, G.; Berger, R.; Bluemke, D.A.; et al. Enhanced infarct border zone function and altered mechanical activation predict inducibility of monomorphic ventricular tachycardia in patients with ischemic cardiomyopathy. Radiology 2007, 245, 712–719. [Google Scholar] [CrossRef]
- Yan, A.T.; Shayne, A.J.; Brown, K.A.; Gupta, S.N.; Chan, C.W.; Luu, T.M.; Di Carli, M.F.; Reynolds, H.G.; Stevenson, W.G.; Kwong, R.Y. Characterization of the peri-infarct zone by contrast-enhanced cardiac magnetic resonance imaging is a powerful predictor of post-myocardial infarction mortality. Circulation 2006, 114, 32–39. [Google Scholar] [CrossRef] [PubMed]
- Roes, S.D.; Borleffs, C.J.W.; van der Geest, R.J.; Westenberg, J.J.M.; Marsan, N.A.; Kaandorp, T.A.M.; Reiber, J.H.C.; Zeppenfeld, K.; Lamb, H.J.; de Roos, A.; et al. Infarct tissue heterogeneity assessed with contrast-enhanced MRI predicts spontaneous ventricular arrhythmia in patients with ischemic cardiomyopathy and implantable cardioverter-defibrillator. Circ. Cardiovasc. Imaging 2009, 2, 183–190. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, A.; Azevedo, C.F.; Cheng, A.; Gupta, S.N.; Bluemke, D.A.; Foo, T.K.; Gerstenblith, G.; Weiss, R.G.; Marbán, E.; Tomaselli, G.F.; et al. Infarct tissue heterogeneity by magnetic resonance imaging identifies enhanced cardiac arrhythmia susceptibility in patients with left ventricular dysfunction. Circulation 2007, 115, 2006–2014. [Google Scholar] [CrossRef] [PubMed]
- Bertini, M.; Ng, A.C.T.; Borleffs, C.J.W.; Delgado, V.; Wijnmaalen, A.P.; Nucifora, G.; Ewe, S.H.; Shanks, M.; Thijssen, J.; Zeppenfeld, K.; et al. Longitudinal mechanics of the periinfarct zone and ventricular tachycardia inducibility in patients with chronic ischemic cardiomyopathy. Am. Heart J. 2010, 160, 729–736. [Google Scholar] [CrossRef] [PubMed]
- Ng, A.C.T.; Bertini, M.; Borleffs, C.J.W.; Delgado, V.; Boersma, E.; Piers, S.R.D.; Thijssen, J.; Nucifora, G.; Shanks, M.; Ewe, S.H.; et al. Predictors of death and occurrence of appropriate implantable defibrillator therapies in patients with ischemic cardiomyopathy. Am. J. Cardiol. 2010, 106, 1566–1573. [Google Scholar] [CrossRef]
- Hoogslag, G.E.; Thijssen, J.; Höke, U.; Boden, H.; Antoni, M.L.; Debonnaire, P.; Haeck, M.L.A.; Holman, E.R.; Bax, J.J.; Marsan, N.A.; et al. Prognostic implications of left ventricular regional function heterogeneity assessed with two-dimensional speckle tracking in patients with ST-segment elevation myocardial infarction and depressed left ventricular ejection fraction. Heart Vessel. 2014, 29, 619–628. [Google Scholar] [CrossRef]
- Mancini, G.B.; DeBoe, S.F.; Anselmo, E.; Simon, S.B.; LeFree, M.T.; Vogel, R.A. Quantitative regional curvature analysis: An application of shape determination for the assessment of segmental left ventricular function in man. Am. Heart J. 1987, 113 Pt 1, 326–334. [Google Scholar] [CrossRef]
- Greenbaum, R.A.; Gibson, D.G. Regional non-uniformity of left ventricular wall movement in man. Br. Heart J. 1981, 45, 29–34. [Google Scholar] [CrossRef]
- Inoue, H.; Zipes, D.P. Increased afferent vagal responses produced by epicardial application of nicotine on the canine posterior left ventricle. Am. Heart J. 1987, 114 Pt 1, 757–764. [Google Scholar] [CrossRef]
- Boogers, M.J.; Borleffs, C.J.W.; Henneman, M.M.; van Bommel, R.J.; van Ramshorst, J.; Boersma, E.; Dibbets-Schneider, P.; Stokkel, M.P.; van der Wall, E.E.; Schalij, M.J.; et al. Cardiac sympathetic denervation assessed with 123-iodine metaiodobenzylguanidine imaging predicts ventricular arrhythmias in implantable cardioverter-defibrillator patients. J. Am. Coll. Cardiol. 2010, 55, 2769–2777. [Google Scholar] [CrossRef]
- Sasano, T.; Abraham, M.R.; Chang, K.C.; Ashikaga, H.C.; Mills, K.J.; Holt, D.P.; Hilton, J.; Nekolla, S.G.; Dong, J.; Lardo, A.C.; et al. Abnormal sympathetic innervation of viable myocardium and the substrate of ventricular tachycardia after myocardial infarction. J. Am. Coll. Cardiol. 2008, 51, 2266–2275. [Google Scholar] [CrossRef]
- Tayal, B.; Gorcsan, J.; Delgado-Montero, A.; Marek, J.J.; Haugaa, K.H.; Ryo, K.; Goda, A.; Olsen, N.T.; Saba, S.; Risum, N.; et al. Mechanical Dyssynchrony by Tissue Doppler Cross-Correlation is Associated with Risk for Complex Ventricular Arrhythmias after Cardiac Resynchronization Therapy. J. Am. Soc. Echocardiogr. 2015, 28, 1474–1481. [Google Scholar] [CrossRef]
- Olsen, N.T.; Mogelvang, R.; Jons, C.; Fritz-Hansen, T.; Sogaard, P. Predicting response to cardiac resynchronization therapy with cross-correlation analysis of myocardial systolic acceleration: A new approach to echocardiographic dyssynchrony evaluation. J. Am. Soc. Echocardiogr. 2009, 22, 657–664. [Google Scholar] [CrossRef]
- Risum, N.; Tayal, B.; Hansen, T.F.; Bruun, N.E.; Jensen, M.T.; Lauridsen, T.K.; Saba, S.; Kisslo, J.; Gorcsan, J., 3rd; Sogaard, P. Identification of Typical Left Bundle Branch Block Contraction by Strain Echocardiography Is Additive to Electrocardiography in Prediction of Long-Term Outcome After Cardiac Resynchronization Therapy. J. Am. Coll. Cardiol. 2015, 66, 631–641. [Google Scholar] [CrossRef]
- Rodríguez-Zanella, H.; Haugaa, K.; Boccalini, F.; Secco, E.; Edvardsen, T.; Badano, L.P.; Muraru, D. Physiological Determinants of Left Ventricular Mechanical Dispersion: A 2-Dimensional Speckle Tracking Echocardiographic Study in Healthy Volunteers. JACC Cardiovasc. Imaging 2018, 11, 650–651. [Google Scholar] [CrossRef]
- Nagueh, S.F. Mechanical dyssynchrony in congestive heart failure: Diagnostic and therapeutic implications. J. Am. Coll. Cardiol. 2008, 51, 18–22. [Google Scholar] [CrossRef]
- Han, J.; Moe, G.K. Nonuniform Recovery of Excitability in Ventricular Muscle. Circ. Res. 1964, 14, 44–60. [Google Scholar] [CrossRef]
- Vassallo, J.A.; Cassidy, D.M.; Kindwall, K.E.; Marchlinski, F.E.; Josephson, M.E. Nonuniform recovery of excitability in the left ventricle. Circulation 1988, 78, 1365–1372. [Google Scholar] [CrossRef]
- Aagaard, E.N.; Kvisvik, B.; Pervez, M.O.; Lyngbakken, M.N.; Berge, T.; Enger, S.; Orstad, E.B.; Smith, P.; Omland, T.; Tveit, A.; et al. Left ventricular mechanical dispersion in a general population: Data from the Akershus Cardiac Examination 1950 study. Eur. Heart J. Cardiovasc. Imaging 2020, 21, 183–190. [Google Scholar] [CrossRef]
- Banasik, G.; Segiet, O.; Elwart, M.; Szulik, M.; Lenarczyk, R.; Kalarus, Z.; Kukulski, T. LV mechanical dispersion as a predictor of ventricular arrhythmia in patients with advanced systolic heart failure: A pilot study. Herz 2016, 41, 599–604. [Google Scholar] [CrossRef]
- Van der Bijl, P.; Khidir, M.J.H.; Leung, M.; Yilmaz, D.; Mertens, B.; Marsan, N.A.; Delgado, V.; Bax, J.J. Reduced left ventricular mechanical dispersion at 6 months follow-up after cardiac resynchronization therapy is associated with superior long-term outcome. Heart Rhythm. 2018, 15, 1683–1689. [Google Scholar] [CrossRef] [PubMed]
- Haugaa, K.H.; Edvardsen, T.; Leren, T.P.; Gran, J.M.; Smiseth, O.A.; Amlie, J.P. Left ventricular mechanical dispersion by tissue Doppler imaging: A novel approach for identifying high-risk individuals with long QT syndrome. Eur. Heart J. 2009, 30, 330–337. [Google Scholar] [CrossRef] [PubMed]
- Kutyifa, V.; Pouleur, A.C.; Knappe, D.; Al-Ahmad, A.; Gibinski, M.; Wang, P.J.; McNitt, S.; Merkely, B.; Goldenberg, I.; Solomon, S.D.; et al. Dyssynchrony and the risk of ventricular arrhythmias. JACC Cardiovasc. Imaging 2013, 6, 432–444. [Google Scholar] [CrossRef] [PubMed]
- Kosiuk, J.; Dinov, B.; Bollmann, A.; Koutalas, E.; Mussigbrodt, A.; Sommer, P.; Arya, A.; Richter, S.; Hindricks, G.; Breithardt, O.A. Association between ventricular arrhythmias and myocardial mechanical dispersion assessed by strain analysis in patients with nonischemic cardiomyopathy. Clin. Res. Cardiol. 2015, 104, 1072–1077. [Google Scholar] [CrossRef] [PubMed]
- Brainin, P. Myocardial Postsystolic Shortening and Early Systolic Lengthening: Current Status and Future Directions. Diagnostics 2021, 11, 1428. [Google Scholar] [CrossRef]
- Eek, C.; Grenne, B.; Brunvand, H.; Aakhus, S.; Endresen, K.; Smiseth, O.A.; Edvardsen, T.; Skulstad, H. Postsystolic shortening is a strong predictor of recovery of systolic function in patients with non-ST-elevation myocardial infarction. Eur. J. Echocardiogr. 2011, 12, 483–489. [Google Scholar] [CrossRef]
- Kahyaoglu, M.; Gecmen, C.; Candan, O.; İzgi, I.A.; Kirma, C. The duration of early systolic lengthening may predict ischemia from scar tissue in patients with chronic coronary total occlusion lesions. Int. J. Cardiovasc. Imaging 2019, 35, 1823–1829. [Google Scholar] [CrossRef]
- Ring, M.; Persson, H.; Mejhert, M.; Edner, M. Post-systolic motion in patients with heart failure--a marker of left ventricular dyssynchrony? Eur. J. Echocardiogr. 2007, 8, 352–359. [Google Scholar] [CrossRef]
- Lee, A.P.W.; Jin, C.N.; Fan, Y.; Wong, R.H.L.; Underwood, M.J.; Wan, S. Functional Implication of Mitral Annular Disjunction in Mitral Valve Prolapse: A Quantitative Dynamic 3D Echocardiographic Study. JACC Cardiovasc. Imaging 2017, 10, 1424–1433. [Google Scholar] [CrossRef]
- Voigt, J.U.; Lindenmeier, G.; Exner, B.; Regenfus, M.; Werner, D.; Reulbach, U.; Nixdorff, U.; Flachskampf, F.A.; Daniel, W.G. Incidence and characteristics of segmental postsystolic longitudinal shortening in normal, acutely ischemic, and scarred myocardium. J. Am. Soc. Echocardiogr. 2003, 16, 415–423. [Google Scholar] [CrossRef]
- Brainin, P.; Biering-Sørensen, S.R.; Møgelvang, R.; de Knegt, M.C.; Olsen, F.J.; Galatius, S.; Gislason, G.H.; Jensen, J.S.; Biering-Sørensen, T. Post-systolic shortening: Normal values and association with validated echocardiographic and invasive measures of cardiac function. Int. J. Cardiovasc. Imaging 2019, 35, 327–337. [Google Scholar] [CrossRef]
- Russell, K.; Eriksen, M.; Aaberge, L.; Wilhelmsen, N.; Skulstad, H.; Remme, E.W.; Haugaa, K.H.; Opdahl, A.; Fjeld, J.G.; Gjesdal, O.; et al. A novel clinical method for quantification of regional left ventricular pressure-strain loop area: A non-invasive index of myocardial work. Eur. Heart J. 2012, 33, 724–733. [Google Scholar] [CrossRef]
- Russell, K.; Eriksen, M.; Aaberge, L.; Wilhelmsen, N.; Skulstad, H.; Gjesdal, O.; Edvardsen, T.; Smiseth, O.A. Assessment of wasted myocardial work: A novel method to quantify energy loss due to uncoordinated left ventricular contractions. Am. J. Physiol. Heart Circ. Physiol. 2013, 305, H996–H1003. [Google Scholar] [CrossRef]
- Galli, E.; John-Matthwes, B.; Rousseau, C.; Schnell, F.; Leclercq, C.; Donal, E. Echocardiographic reference ranges for myocardial work in healthy subjects: A preliminary study. Echocardiography 2019, 36, 1814–1824. [Google Scholar] [CrossRef]
- Edwards, N.F.A.; Scalia, G.M.; Shiino, K.; Sabapathy, S.; Anderson, B.; Chamberlain, R.; Khandheria, B.K.; Chan, J. Global Myocardial Work Is Superior to Global Longitudinal Strain to Predict Significant Coronary Artery Disease in Patients with Normal Left Ventricular Function and Wall Motion. J. Am. Soc. Echocardiogr. 2019, 32, 947–957. [Google Scholar] [CrossRef]
- Hiemstra, Y.L.; van der Bijl, P.; El Mahdiui, M.; Bax, J.J.; Delgado, V.; Marsan, N.A. Myocardial Work in Nonobstructive Hypertrophic Cardiomyopathy: Implications for Outcome. J. Am. Soc. Echocardiogr. 2020, 33, 1201–1208. [Google Scholar] [CrossRef]
- Smiseth, O.A.; Donal, E.; Penicka, M.; Sletten, O.J. How to measure left ventricular myocardial work by pressure-strain loops. Eur. Heart J. Cardiovasc. Imaging 2021, 22, 259–261. [Google Scholar] [CrossRef]
- Manganaro, R.; Marchetta, S.; Dulgheru, R.; Ilardi, F.; Sugimoto, T.; Robinet, S.; Cimino, S.; Go, Y.Y.; Bernard, A.; Kacharava, G.; et al. Echocardiographic reference ranges for normal non-invasive myocardial work indices: Results from the EACVI NORRE study. Eur. Heart J. Cardiovasc. Imaging 2019, 20, 582–590. [Google Scholar] [CrossRef]
- Morbach, C.; Sahiti, F.; Tiffe, T.; Cejka, V.; Eichner, F.A.; Gelbrich, G.; Heuschmann, P.U.; Störk, S.; STAAB consortium. Myocardial work—Correlation patterns and reference values from the population-based STAAB cohort study. PLoS ONE 2020, 15, e0239684. [Google Scholar] [CrossRef]
- Olsen, F.J.; Skaarup, K.G.; Lassen, M.C.H.; Johansen, N.D.; Sengeløv, M.; Jensen, G.B.; Schnohr, P.; Marott, J.L.; Søgaard, P.; Gislason, G.; et al. Normal Values for Myocardial Work Indices Derived From Pressure-Strain Loop Analyses: From the CCHS. Circ. Cardiovasc. Imaging 2022, 15, e013712. [Google Scholar] [CrossRef]
- Truong, V.T.; Vo, H.Q.; Ngo, T.N.M.; Mazur, J.; Nguyen, T.T.H.; Pham, T.T.M.; Le, T.K.; Phan, H.; Palmer, C.; Nagueh, S.F.; et al. Normal Ranges of Global Left Ventricular Myocardial Work Indices in Adults: A Meta-Analysis. J. Am. Soc. Echocardiogr. 2022, 35, 369–377. [Google Scholar] [CrossRef] [PubMed]
- Papadopoulos, K.; Özden Tok, Ö.; Mitrousi, K.; Ikonomidis, I. Myocardial Work: Methodology and Clinical Applications. Diagnostics 2021, 11, 573. [Google Scholar] [CrossRef] [PubMed]
- Roemer, S.; Jaglan, A.; Santos, D.; Umland, M.; Jain, R.; Tajik, A.J.; Khandheria, B.K. The Utility of Myocardial Work in Clinical Practice. J. Am. Soc. Echocardiogr. 2021, 34, 807–818. [Google Scholar] [CrossRef] [PubMed]
- Aalen, J.M.; Donal, E.; Larsen, C.K.; Duchenne, J.; Lederlin, M.; Cvijic, M.; Hubert, A.; Voros, G.; Leclercq, C.; Bogaert, J.; et al. Imaging predictors of response to cardiac resynchronization therapy: Left ventricular work asymmetry by echocardiography and septal viability by cardiac magnetic resonance. Eur. Heart J. 2020, 41, 3813–3823. [Google Scholar] [CrossRef]
- Jaworski, K.; Firek, B.; Syska, P.; Lewandowski, M.; Śpiewak, M.; Dąbrowski, R. Malignant arrhythmia associated with mitral annular disjunction: Myocardial work as a potential tool in the search for a substrate. Kardiol. Pol. 2022, 80, 93–94. [Google Scholar] [CrossRef]


| Strengths |
|---|
| On the verge of guideline implementation |
| Direct tissue measure |
| Can investigate each fiber aspect |
| High reproducibility, automatic options |
| Angle independent (compared to Doppler) |
| Can provide regional details |
| Can provide measures of diastolic function and dyssynchrony |
| Changes typically precede changes in LVEF |
| Limitations |
| Vendor dependency Loading dependency |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bjerregaard, C.L.; Skaarup, K.G.; Lassen, M.C.H.; Biering-Sørensen, T.; Olsen, F.J. Strain Imaging and Ventricular Arrhythmia. Diagnostics 2023, 13, 1778. https://doi.org/10.3390/diagnostics13101778
Bjerregaard CL, Skaarup KG, Lassen MCH, Biering-Sørensen T, Olsen FJ. Strain Imaging and Ventricular Arrhythmia. Diagnostics. 2023; 13(10):1778. https://doi.org/10.3390/diagnostics13101778
Chicago/Turabian StyleBjerregaard, Caroline Løkke, Kristoffer Grundtvig Skaarup, Mats Christian Højbjerg Lassen, Tor Biering-Sørensen, and Flemming Javier Olsen. 2023. "Strain Imaging and Ventricular Arrhythmia" Diagnostics 13, no. 10: 1778. https://doi.org/10.3390/diagnostics13101778
APA StyleBjerregaard, C. L., Skaarup, K. G., Lassen, M. C. H., Biering-Sørensen, T., & Olsen, F. J. (2023). Strain Imaging and Ventricular Arrhythmia. Diagnostics, 13(10), 1778. https://doi.org/10.3390/diagnostics13101778