Strain Rate Changes during Stress Echocardiography Are the Most Accurate Predictors of Significant Coronary Artery Disease in Patients with Previously Treated Acute Coronary Syndrome
Abstract
1. Introduction
2. Methods
2.1. Study Protocol
2.2. Statistical Analysis
3. Results
Demographic and Clinical Indices of the Patients
4. Coronary Angiography Findings
4.1. Echocardiographic Indices
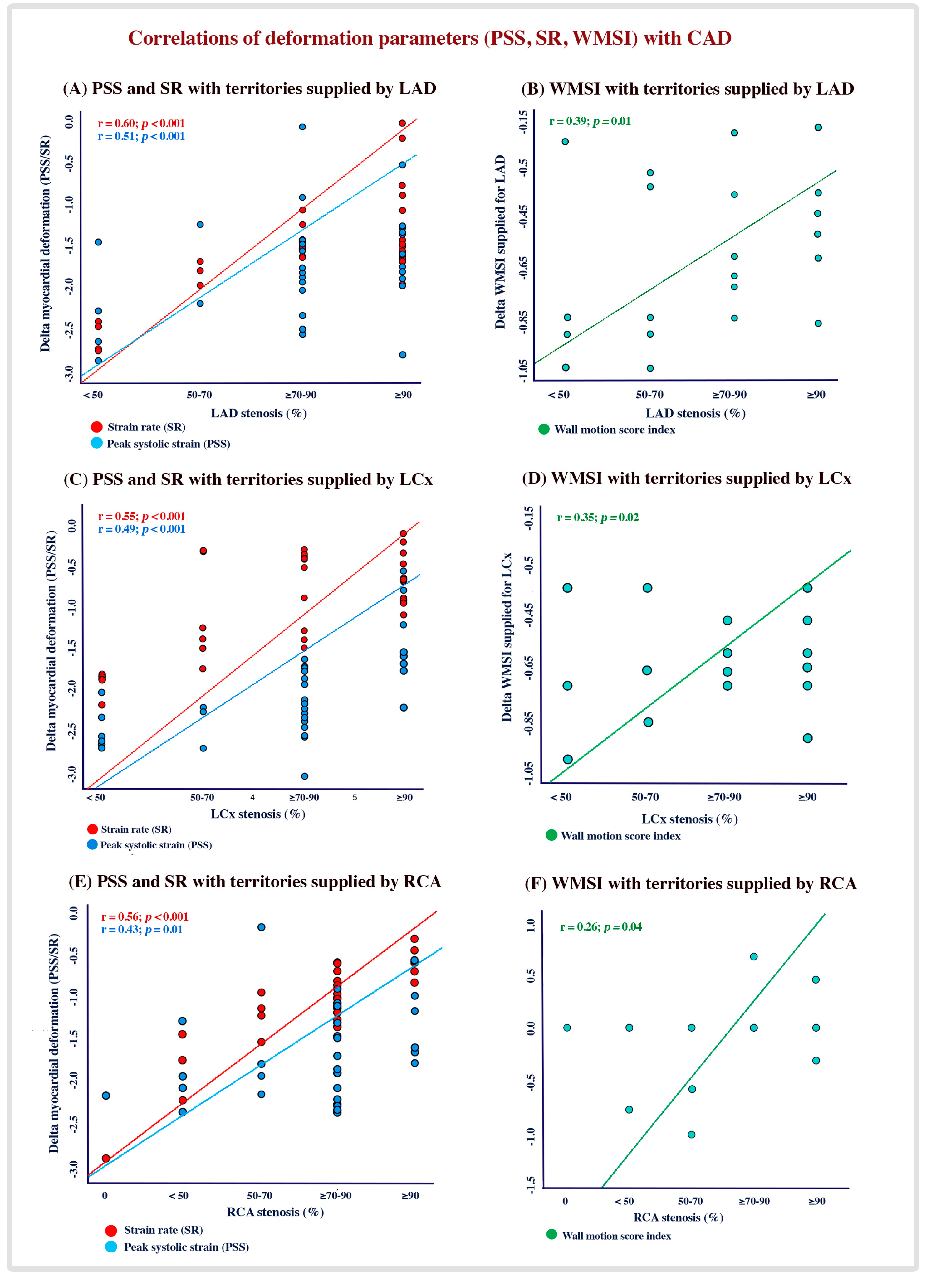
4.2. Correlation of CAD Significance with Global and Regional LV Systolic Function
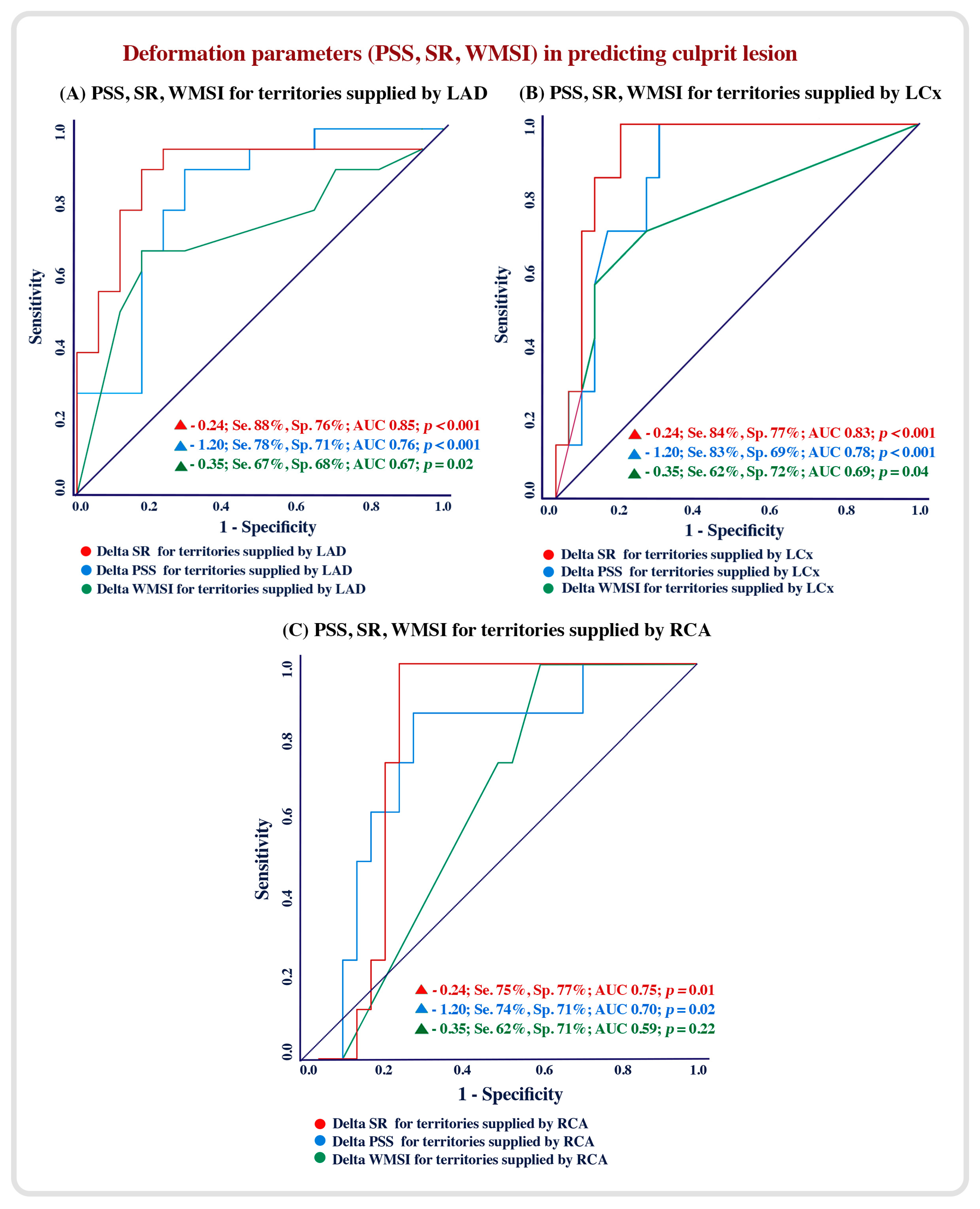
4.3. Echocardiographic Predictors of Culprit Lesions
5. Discussion
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviation
| △ | Delta (change in) |
| µg/kg/min | Microgram/kilogram/minute |
| ACS | Acute coronary syndrome |
| CABG | Coronary artery bypass graft surgery |
| CAD | Coronary artery disease |
| CRT | Cardiac resynchronization therapy devices |
| DSE | Dobutamine stress echocardiography |
| EF | Ejection fraction |
| ICD | Intra cardiac defibrillator |
| IHD | Ischemic heart disease |
| LAD | Left anterior descending artery |
| LCx | Left circumflex artery |
| LV | Left ventricle |
| NT-pro BNP | N-terminal brain naturetic peptide |
| PCI | Percutaneous coronary intervention |
| PPM | Permanent pacemaker |
| PSS | Peak systolic strain |
| RCA | Right coronary artery |
| ROC | Receiver operating characteristic curve |
| SR | Peak systolic strain rate |
| STE | Speckle tracking echocardiography |
| WMSI | Wall motion score index |
References
- Dai, H.; Much, A.A.; Maor, E.; Asher, E.; Younis, A.; Xu, Y.; Lu, Y.; Liu, X.; Shu, J.; Bragazzi, N.L. Global, regional, and national burden of ischaemic heart disease and its attributable risk factors, 1990–2017: Results 129 from the Global Burden of Disease Study 2017. Eur. Heart J.-Qual. Care Clin. Outcomes 2022, 8, 50–60. [Google Scholar] [CrossRef]
- Hess, E.P.; Brison, R.J.; Perry, J.J.; Calder, L.A.; Thiruganasambandamoorthy, V.; Agarwal, D.; Sadosty, A.T.; Silvilotti, M.L.; Jaffe, A.S.; Montori, V.M.; et al. Development of a clinical prediction rule for 30-day cardiac events in emergency department patients with chest pain and possible acute coronary syndrome. Ann. Emerg. Med. 2012, 59, 115–125. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Cannon, C.P. Acute coronary syndromes: Diagnosis and management, part I. Mayo Clin. Proc. 2009, 84, 917–938. [Google Scholar] [CrossRef] [PubMed]
- Hoit, B.D. Strain and strain rate echocardiography and coronary artery disease. Circ. Cardiovasc. Imaging 2011, 4, 179–190. [Google Scholar] [CrossRef] [PubMed]
- Chaichuum, S.; Chiang, S.J.; Daimon, M.; Chang, S.C.; Chan, C.L.; Hsu, C.Y.; Chen, H.H.; Tseng, C.L. Segmental Tissue Speckle Tracking Predicts the Stenosis Severity in Patients with Coronary Artery Disease. Front. Cardiovasc. Med. 2022, 8, 832096. [Google Scholar] [CrossRef] [PubMed]
- Zuo, H.J.; Yang, X.T.; Liu, Q.G.; Zhang, Y.; Zeng, H.S.; Yan, J.T.; Wang, D.W.; Wang, H. Global Longitudinal Strain at Rest for Detection of Coronary Artery Disease in Patients without Diabetes Mellitus. Curr. Med. Sci. 2018, 38, 413–421. [Google Scholar] [CrossRef]
- Zuo, H.; Yan, J.; Zeng, H.; Li, W.; Li, P.; Liu, Z.; Cui, G.; Lv, J.; Wang, D.; Wang, H. Diagnostic power of longitudinal strain at rest for the detection of obstructive coronary artery disease in patients with type 2 diabetes mellitus. Ultrasound Med. Biol. 2015, 41, 89–98. [Google Scholar] [CrossRef]
- Rumbinaitė, E.; Žaliaduonytė-Pekšienė, D.; Vieželis, M.; Čeponienė, I.; Lapinskas, T.; Žvirblytė, R.; Venclovienė, J.; Morkūnaitė, K.; Bielinis, A.; Šlapikas, R.; et al. Dobutamine-stress echocardiography speckle-tracking imaging in the assessment of hemodynamic significance of coronary artery stenosis in patients with moderate and high probability of coronary artery disease. Medicina 2016, 52, 331–339. [Google Scholar] [CrossRef]
- Steeds, R.P.; Wheeler, R.; Bhattacharyya, S.; Reiken, J.; Nihoyannopoulos, P.; Senior, R.; Monaghan, M.J.; Sharma, V. Stress echocardiography in coronary artery disease: A practical guideline from the British Society of Echocardiography. Echo Res. Pract. 2019, 6, G17–G33. [Google Scholar] [CrossRef]
- Olmos, L.I.; Dakik, H.; Gordon, R.; Dunn, J.K.; Verani, M.S.; Quiñones, M.A.; Zoghbi, W.A. Long-term prognostic value of exercise echocardiography compared with exercise 201Tl, ECG, and clinical variables in patients evaluated for coronary artery disease. Circulation 1998, 98, 2679–2686. [Google Scholar] [CrossRef]
- Çalapkorur, B.; Özdoğru, İ.; Baran, O.; Keleşoğlu, Ş.; Oğuzhan, A.; Kaya, M.G. Two methods for increasing sensitivity of dobutamine stress echocardiography: Strain imaging and heart-type fatty acid-binding protein levels. Anatol. J. Cardiol. 2016, 16, 210–211. [Google Scholar] [CrossRef] [PubMed]
- Lancellotti, P.; Pellikka, P.A.; Budts, W.; Chaudhry, F.A.; Donal, E.; Dulgheru, R.; Edvardsen, T.; Garbi, M.; Ha, J.W.; Kane, G.C.; et al. The clinical use of stress echocardiography in non-ischaemic heart disease: Recommendations fromthe European Association of CardiovascularImaging andthe American Society of Echocardiography. Eur. Heart J. Cardiovasc. Imaging 2016, 17, 1191–1229. [Google Scholar] [CrossRef] [PubMed]
- Vasey, C.G.; Usedom, J.E.; Woodard, S.M.; Bhapkar, M.; Schwartz, T.; Koch, G.G. Prediction of cardiac mortality after myocardial infarction: The role of maximal treadmill stress echocardiography. Am. Soc. Echocardiogr. 2001, 14, 38–43. [Google Scholar] [CrossRef]
- Picano, E.; Lattanzi, F.; Sicari, R.; Silvestri, O.; Polimeno, S.; Pingitore, A.; Petix, N.; Margaria, F.; Magaia, O.; Mathias, W., Jr.; et al. Role of stress echocardiography in risk stratification early after an acute myocardial infarction. EPIC (Echo Persantin International Cooperative) and EDIC (Echo Dobutamine International Cooperative) Study Groups. Eur. Heart J. 1997, 18 (Suppl. D), D78–D85. [Google Scholar] [CrossRef] [PubMed]
- Lancellotti, P.; Dulgheru, R.; Go, Y.Y.; Sugimoto, T.; Marchetta, S.; Oury, C.; Garbi, M. Stress echocardiography in patients with native valvular heart disease. Heart 2018, 104, 807–813. [Google Scholar] [CrossRef] [PubMed]
- Pavasini, R.; Fabbri, G.; Bianchi, N.; Deserio, M.A.; Sanguettoli, F.; Zanarelli, L.; Tonet, E.; Passarini, G.; Serenelli, M.; Campo, G. The role of stress echocardiography in transcatheter aortic valve implantation and transcatheter edge-to-edge repair era: A systematic review. Front. Cardiovasc. Med. 2022, 9, 964669. [Google Scholar] [CrossRef]
- O’Sullivan, C.A.; Henein, M.Y.; Sutton, R.; Coats, A.J.; Sutton, G.C.; Gibson, D.G. Abnormal ventricular activation and repolarisation during dobutamine stress echocardiography in coronary artery disease. Heart 1998, 79, 468–473. [Google Scholar] [CrossRef] [PubMed]
- Pellikka, P.A.; Arruda-Olson, A.; Chaudhry, F.A.; Chen, M.H.; Marshall, J.E.; Porter, T.R.; Sawada, S.G. Guidelines for Performance, Interpretation, and Application of Stress Echocardiography in Ischemic Heart Disease: From the American Society of Echocardiography. J. Am. Soc. Echocardiogr. 2020, 33, 1–41.e8. [Google Scholar] [CrossRef]
- Voigt, J.U.; Exner, B.; Schmiedehausen, K.; Huchzermeyer, C.; Reulbach, U.; Nixdorff, U.; Platsch, G.; Kuwert, T.; Daniel, W.G.; Flachskampf, F.A. Strain-rate imaging during dobutamine stress echocardiography provides objective evidence of inducible ischemia. Circulation 2003, 107, 2120–2126. [Google Scholar] [CrossRef]
- Pathan, F.; D’Elia, N.; Nolan, M.T.; Marwick, T.H.; Negishi, K. Normal Ranges of Left Atrial Strain by Speckle-Tracking Echocardiography: A Systematic Review and Meta-Analysis. J. Am. Soc. Echocardiogr. 2017, 30, 59–70.e8. [Google Scholar] [CrossRef]
- Khemka, A.; Sawada, S.G. Dobutamine echocardiography for assessment of viability in the current era. Curr. Opin. Cardiol. 2019, 34, 484–489. [Google Scholar] [CrossRef] [PubMed]
- Lang, R.M.; Badano, L.P.; Mor-Avi, V.; Afilalo, J.; Armstrong, A.; Ernande, L.; Flachskampf, F.A.; Foster, E.; Goldstein, S.A.; Kuznetsova, T.; et al. Recommendations for cardiac chamber quantification by echocardiography in adults: An update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J. Am. Soc. Echocardiogr. 2015, 16, 233–271. [Google Scholar]
- Neglia, D.; Rovai, D.; Caselli, C.; Pietila, M.; Teresinska, A.; Aguadé-Bruix, S.; Pizzi, M.N.; Todiere, G.; Gimelli, A.; Schroeder, S.; et al. Detection of significant coronary artery disease by noninvasive anatomical and functional imaging. Circ. Cardiovasc. Imaging 2015, 8, e002179. [Google Scholar] [CrossRef] [PubMed]
- Jurado-Román, A.; Agudo-Quílez, P.; Rubio-Alonso, B.; Molina, J.; Díaz, B.; García-Tejada, J.; Martín, R.; Tello, R. Superiority of wall motion score index over left ventricle ejection fraction in predicting cardiovascular events after an acute myocardial infarction. Eur. Heart J. Acute Cardiovasc. Care 2019, 8, 78–85. [Google Scholar] [CrossRef] [PubMed]
- Nagueh, S.F.; Smiseth, O.A.; Appleton, C.P.; Gillebert, T.C.; Marino, P.N.; Oh, J.K.; Smiseth, O.A.; Waggoner, A.D.; Flachskampf, F.A.; Pellikka, P.A.; et al. Recommendations for the evaluation of left ventricular diastolic function by echocardiography: An update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J. Am. Soc. Echocardiogr. 2016, 29, 277–314. [Google Scholar] [CrossRef]
- Hamzaraj, K.; Kammerlander, A.; Gyöngyösi, M.; Frey, B.; Distelmaier, K.; Graf, S. Patient Selection and Clinical Indication for Chronic Total Occlusion Revascularization—A Workflow Focusing on Non-Invasive Cardiac Imaging. Life 2022, 13, 4. [Google Scholar] [CrossRef]
- Shenouda, R.B.; Bytyçi, I.; Sobhy, M.; Henein, M.Y. Reduced regional strain rate is the most accurate dysfunction in predicting culprit lesions in patients with acute coronary syndrome. Clin. Physiol. Funct. Imaging 2020, 40, 21–29. [Google Scholar] [CrossRef]
- Shenouda, R.; Bytyçi, I.; Sobhy, M.; Henein, M.Y. Early Recovery of Left Ventricular Function After Revascularization in Acute Coronary Syndrome. J. Clin. Med. 2019, 9, 24. [Google Scholar] [CrossRef]
- Algranati, D.; Kassab, G.S.; Lanir, Y. Why is the subendocardium more vulnerable to ischemia? A new paradigm. Am. J. Physiol. Heart Circ. Physiol. 2011, 300, H1090–H1100. [Google Scholar] [CrossRef]
- Bytyçi, I.; Alves, L.; Alves, O.; Lopes, C.; Bajraktari, G.; Henein, M.Y. Left Ventricular Myocardial and Cavity Velocity Disturbances Are Powerful Predictors of Significant Coronary Artery Stenosis. J. Clin. Med. 2022, 11, 6185. [Google Scholar] [CrossRef]
- Henein, M.Y.; Gibson, D.G. Long axis function in disease. Heart 1999, 81, 229–231. [Google Scholar] [CrossRef] [PubMed]
- Henein, M.Y.; O’Sullivan, C.; Davies, S.W.; Sigwart, U.; Gibson, D.G. Effects of acute coronary occlusion and previous ischaemic injury on left ventricular wall motion in humans. Heart 1997, 77, 338–345. [Google Scholar] [CrossRef] [PubMed]
- Henein, M.Y.; Priestley, K.; Davarashvili, T.; Buller, N.; Gibson, D.G. Early changes in left ventricular subendocardial function after successful coronary angioplasty. Br. Heart J. 1993, 69, 501–506. [Google Scholar] [CrossRef] [PubMed]
- Penicka, M.; Bartunek, J.; Wijns, W.; De Wolf, I.; Heyndrickx, G.R.; De Raedt, H.; Barbato, E.; De Bruyne, B. Tissue doppler imaging predicts recovery of left ventricular function after recanalization of an occluded coronary artery. J. Am. Coll. Cardiol. 2004, 43, 85–91. [Google Scholar] [CrossRef] [PubMed]
- Voigt, J.U.; Pedrizzetti, G.; Lysyansky, P.; Marwick, T.H.; Houle, H.; Baumann, R.; Pedri, S.; Ito, Y.; Abe, Y.; Metz, S.; et al. Definitions for a common standard for 2D speckle tracking echocardiography: Consensus document of the EACVI/ASE/Industry Task Force to standardize deformation imaging. J. Am. Soc. Echocardiogr. 2015, 28, 183–193. [Google Scholar] [CrossRef] [PubMed]
- Mirea, O.; Pagourelias, E.D.; Duchenne, J.; Bogaert, J.; Thomas, J.D.; Badano, L.P.; Voigt, J.U. EACVI-ASE-Industry Standardization Task Force. Intervendor Differences in the Accuracy of Detecting Regional Functional Abnormalities: A Report From the EACVI-ASE Strain Standardization Task Force. JACC Cardiovasc. Imaging 2018, 11, 25–34. [Google Scholar] [CrossRef]
- Sonaglioni, A.; Nicolosi, G.L.; Rigamonti, E.; Lombardo, M.; La Sala, L. Molecular Approaches and Echocardiographic Deformation Imaging in Detecting Myocardial Fibrosis. Int. J. Mol. Sci. 2022, 23, 10944. [Google Scholar] [CrossRef]
| Variable | Patients |
|---|---|
| (n = 33) | |
| Demographics and clinical indices | |
| Age (years) | 59 ± 11 |
| Males (n, %) | 24 (72.7) |
| Hypertension (n, %) | 18 (54.5) |
| Diabetes (n, %) | 17 (51.5) |
| Dyslipidemia (n, %) | 16 (48.5) |
| Obesity (n, %) | 7 (21.2) |
| Smokers (n, %) | 14 (42.4) |
| Previous PCI (n, %) | 18 (54.5) |
| Previous CABG (n, %) | 9 (27.3) |
| Clinical presentation | |
| Chest pain (n, %) | 18 (54.5) |
| Dyspnea (n, %) | 9 (27.2) |
| Mixed symptoms (n, %) | 6 (18.2) |
| Outcome data | |
| Time since first ACS (years) | 6.10 ± 3.9 |
| Culprit (occluded) lesion (n, %) | 23 (69.7) |
| LAD occluded (n, %) | 17 (51.5) |
| LCx occluded (n, %) | 8 (24.2) |
| RCA occluded (n, %) | 8 (24.2) |
| Variable | All Patients | |
|---|---|---|
| (n = 33) | ||
| LV systolic function | ||
| LV EF (%) | Δ (%) | −6.03 ± 4.2 (16.6) |
| Global PSS | Δ (%) | −1.81 ± 0.9 (17.3) |
| Global SR | Δ (%) | −0.23 ± 0.6 (41.8) |
| Territories supplied by LAD | ||
| Longitudinal PSS | Δ (%) | −1.37 ± 1.5 (13.9) |
| Strain rate | Δ (%) | −0.13 ± 0.4 (23.2) |
| Territories supplied by LCx | ||
| Longitudinal PSS | Δ (%) | −1.83 ± 1.1 (16.9) |
| Strain rate | Δ (%) | −0.28 ± 0.2 (54.7) |
| Territories supplied by RCA | ||
| Longitudinal PSS | Δ (%) | −1.65 ± 1.2 (15.4) |
| Strain rate | Δ (%) | −0.24 ± 0.4 (44.4) |
| Wall motion score index | ||
| Global WMSI | Δ (%) | 0.22 ± 0.2 (10.6) |
| WMSI supplied by LAD | Δ (%) | 0.27 ± 0.3 (12.0) |
| WMSI supplied by LCx | Δ (%) | 0.20 ± 0.4 (11.8) |
| WMSI supplied by RCA | Δ (%) | 0.11 ± 0.3 (4.84) |
| Variable | Patients | Patients | Patients | Patients | Patients | Patients | |
|---|---|---|---|---|---|---|---|
| LAD − | LAD + | LCx − | LCx + | RCA − | RCA + | ||
| (n = 16) | (n = 17) | (n = 25) | (n = 8) | (n = 25) | (n = 8) | ||
| LV systolic function | |||||||
| LV EF (%) | Δ (%) | 8.9 ± 4.3 (21.0) | 3.2 ± 3.5 (10.6) | 6.7 ± 4.3 (17.3) | 4.1 ± 3.3 (13.6) | 8.5 ± 4.6 (20.7) | 5.9 ± 4.2 (18.7) |
| Global PSS | Δ (%) | −2.9 ± 0.9 (25.4) | −0.9 ± 1.1 (9.9) | −2.1 ± 1.2 (19.2) | −0.9 ± 1.1 (9.8) | −2.30 ± 0.9 (19.8) | −1.10 ± 1.2 (11.1) |
| Global SR | Δ (%) | −0.29 ± 1.1 (48.3) | −0.17 ± 0.2 (34.1) | −0.33 ± 0.2 (55.9) | −0.14 ± 0.2 (37.8) | −0.29 ± 0.2 (42.6) | −0.14 ± 0.2 (27.4) |
| Territories supplied by LAD | |||||||
| Longitudinal PSS | Δ (%) | −1.73 ± 0.9 (15.4) | −1.0 ± 0.9 (11.8) | −1.62 ± 1.4 (16.2) | −0.86 ± 0.9 (9.42) | −1.9 ± 1.4 (17.9) | −1.17 ± 1.6 (12.1) |
| Strain rate | Δ (%) | −0.17 ± 0.2 (28.3) | −0.02 ± 0.3 (3.9) | 0.14 ± 0.3 (25.4) | 0.14 ± 0.3 (22.4) | −0.06 ± 0.4 (10.3) | −0.08 ± 0.2 (13.1) |
| Territories supplied by LCx | |||||||
| Longitudinal PSS | Δ (%) | −1.89 ± 1.1 (16.3) | −1.77 ± 1.1 (17.9) | −2.3 ± 1.1 (19.9) | −0.7 ± 0.3 (8.2) | −1.80 ± 1.0 (14.7) | −1.70 ± 1.1 (15.6) |
| Strain rate | Δ (%) | −0.33 ± 0.3 (61.1) | −0.29 ± 0.3 (60.3) | −0.31 ± 0.3 (50.1) | −0.10 ± 0.4 (24.3) | −0.31 ± 1.6 (58.4) | −0.27 ± 0.9 (43.5) |
| Territories supplied by RCA | |||||||
| Longitudinal PSS | Δ (%) | −0.31 ± 0.3 (20.8) | −1.2 ± 1.1 (12.2) | −1.21 ± 1.7 (9.52) | −1.20 ± 1.6 (10.4) | 2.70 ± 1.6 (22.3) | −0.66 ± 0.9 (6.8) |
| Strain rate | Δ (%) | −0.24 ± 0.1 (42.1) | −0.24 ± 0.2 (46.1) | −0.13 ± 0.2 (18.1) | −0.08 ± 0.2 (11.3) | −0.26 ± 0.5 (37.1) | −0.05 ± 0.2 (8.9) |
| Wall motion score index | |||||||
| Global WMSI | Δ (%) | 0.43 ± 0.2 (33.5) | 0.16 ± 0.2 (8.18) | 0.27 ± 0.2 (13.4) | 0.21 ± 0.2 (9.21) | 0.27 ± 0.2 (13.1) | 0.40 ± 0.2 (18.1) |
| WMSI supplied by LAD | Δ (%) | 0.32 ± 0.2 (17.4) | 0.22 ± 0.3 (8.43) | 0.24 ± 0.3 (10.7) | 0.27 ± 0.4 (12.1) | 0.28 ± 0.2 (12.4) | 0.21 ± 0.3 (9.9) |
| WMSI supplied by LCx | Δ (%) | 0.44 ± 0.3 (26.6) | 0.43 ± 0.4 (24.5) | 0.37 ± 0.3 (22.8) | 0.52 ± 0.3 (26.6) | 0.38 ± 0.4 (29.6) | 0.66 ± 0.5 (56.4) |
| WMSI supplied by RCA | Δ (%) | 0.06 ± 0.3 (2.69) | 0.09 ± 0.2 (3.89) | 0.08 ± 0.3 (3.70) | 0.04 ± 0.2 (1.50) | 0.02 ± 0.2 (0.93) | 0.37 ± 0.5 (13.7) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shenouda, R.; Bytyçi, I.; El Sharkawy, E.; Hisham, N.; Sobhy, M.; Henein, M.Y. Strain Rate Changes during Stress Echocardiography Are the Most Accurate Predictors of Significant Coronary Artery Disease in Patients with Previously Treated Acute Coronary Syndrome. Diagnostics 2023, 13, 1796. https://doi.org/10.3390/diagnostics13101796
Shenouda R, Bytyçi I, El Sharkawy E, Hisham N, Sobhy M, Henein MY. Strain Rate Changes during Stress Echocardiography Are the Most Accurate Predictors of Significant Coronary Artery Disease in Patients with Previously Treated Acute Coronary Syndrome. Diagnostics. 2023; 13(10):1796. https://doi.org/10.3390/diagnostics13101796
Chicago/Turabian StyleShenouda, Rafik, Ibadete Bytyçi, Eman El Sharkawy, Noha Hisham, Mohamed Sobhy, and Michael Y. Henein. 2023. "Strain Rate Changes during Stress Echocardiography Are the Most Accurate Predictors of Significant Coronary Artery Disease in Patients with Previously Treated Acute Coronary Syndrome" Diagnostics 13, no. 10: 1796. https://doi.org/10.3390/diagnostics13101796
APA StyleShenouda, R., Bytyçi, I., El Sharkawy, E., Hisham, N., Sobhy, M., & Henein, M. Y. (2023). Strain Rate Changes during Stress Echocardiography Are the Most Accurate Predictors of Significant Coronary Artery Disease in Patients with Previously Treated Acute Coronary Syndrome. Diagnostics, 13(10), 1796. https://doi.org/10.3390/diagnostics13101796







