Medical Imaging of Inflammations and Infections of Breast Implants
Abstract
:1. Introduction
2. Radiological Imaging
2.1. Mammography (MX)
2.2. Ultrasound
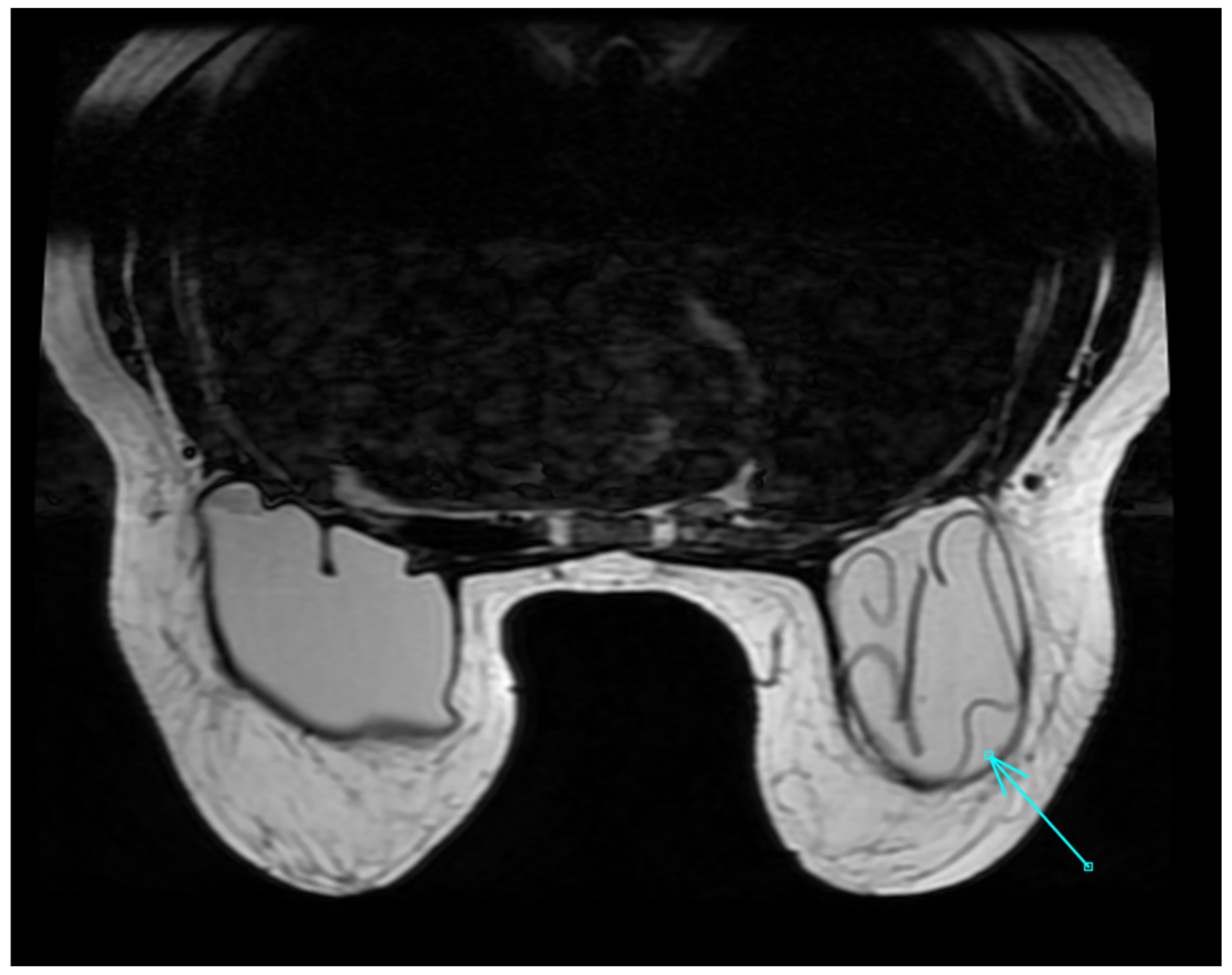
2.3. MRI
3. Nuclear Medicine Imaging
3.1. Scintigraphic Imaging
3.2. PET
4. Discussion and Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Stefura, T.; Rusinek, J.; Wątor, J.; Zagórski, A.; Zając, M.; Libondi, G.; Wysocki, W.M.; Koziej, M. Implant vs. autologous tissue-based breast reconstruction: A systematic review and meta-analysis of the studies comparing surgical approaches in 55,455 patients. J. Plast. Reconstr. Aesthetic Surg. 2023, 77, 346–358. [Google Scholar] [CrossRef]
- Kaoutzanis, C.; Winocour, J.; Unger, J.; Gabriel, A.; Maxwell, G.P. The Evolution of Breast Implants. Semin. Plast. Surg. 2019, 33, 217–223. [Google Scholar] [CrossRef]
- American Society of Plastic Surgeons (ASPS). 2018 Plastic Surgery Statistics Report. 2018. Available online: https://www.plasticsurgery.org/documents/News/Statistics/2018/plastic-surgery-statistics-report-2018.pdf (accessed on 1 March 2023).
- Saldanha, I.J.; Broyles, J.M.; Adam, G.P.; Cao, W.; Bhuma, M.R.; Mehta, S.; Pusic, A.L.; Dominici, L.S.; Balk, E.M. Implant-based Breast Reconstruction after Mastectomy for Breast Cancer: A Systematic Review and Meta-analysis. Plast. Reconstr. Surg. Glob. Open 2022, 10, e4179. [Google Scholar] [CrossRef]
- Cohen Tervaert, J.W.; Mohazab, N.; Redmond, D.; van Eeden, C.; Osman, M. Breast implant illness: Scientific evidence of its existence. Expert Rev. Clin. Immunol. 2022, 18, 15–29. [Google Scholar] [CrossRef]
- Hu, Y.; Zhou, X.; Tong, X.; Chen, X.; Wang, M.; Wu, X.; Li, P.; Tang, F.; Zhou, J.; Li, P. Postoperative antibiotics and infection rates after implant-based breast reconstruction: A systematic review and meta-analysis. Front. Surg. 2022, 9, 926936. [Google Scholar] [CrossRef]
- Kanapathy, M.; Faderani, R.; Arumugam, V.; Haque, S.; Mosahebi, A. Management of periprosthetic breast infection: A systematic review and meta-analysis. J. Plast. Reconstr. Aesthetic Surg. 2021, 74, 2831–2845. [Google Scholar] [CrossRef] [PubMed]
- Pyfer, B.; Chatterjee, A.; Chen, L.; Nigriny, J.; Czerniecki, B.; Tchou, J.; Fisher, C. Early Postoperative Outcomes in Breast Conservation Surgery Versus Simple Mastectomy with Implant Reconstruction: A NSQIP Analysis of 11,645 Patients. Ann. Surg. Oncol. 2016, 23, 92–98. [Google Scholar] [CrossRef] [PubMed]
- Washer, L.L.; Gutowski, K. Breast implant infections. Infect. Dis. Clin. N. Am. 2012, 26, 111–125. [Google Scholar] [CrossRef]
- Sinha, I.; Pusic, A.L.; Wilkins, E.G.; Hamill, J.B.; Chen, X.; Kim, H.M.; Guldbrandsen, G.; Chun, Y.S. Late Surgical-Site Infection in Immediate Implant-Based Breast Reconstruction. Plast. Reconstr. Surg. 2017, 139, 20–28. [Google Scholar] [CrossRef]
- Pittet, B.; Montandon, D.; Pittet, D. Infection in breast implants. Lancet Infect. Dis. 2005, 5, 94–106. [Google Scholar] [CrossRef] [PubMed]
- Drury, K.E.; Lanier, S.T.; Khavanin, N.; Hume, K.M.; Gutowski, K.A.; Thornton, B.P.; Hansen, N.M.; Murphy, R.X., Jr.; Fine, N.A.; Kim, J.Y. Impact of Postoperative Antibiotic Prophylaxis Duration on Surgical Site Infections in Autologous Breast Reconstruction. Ann. Plast. Surg. 2016, 76, 174–179. [Google Scholar] [CrossRef] [PubMed]
- Samreen, N.; Glazebrook, K.N.; Bhatt, A.; Venkatesh, S.K.; McMenomy, B.P.; Chandra, A.; Leng, S.; Adler, K.E.; McCollough, C.H. Imaging findings of mammary and systemic silicone deposition secondary to breast implants. Br. J. Radiol. 2018, 91, 20180098. [Google Scholar] [CrossRef]
- Georgieva, M.; Kammerer, S.; Prantl, L.; Jung, F.; Stroszczynski, C.; Jung, E.M. Imaging of breast implant and implant-associated complications: Capsular contracture and intra- or extracapsular rupture. Clin. Hemorheol. Microcirc. 2020, 76, 221–231. [Google Scholar] [CrossRef]
- Bachour, Y. Capsular Contracture in Breast Implant Surgery: Where are We Now and Where are We Going? Aesthetic Plast. Surg. 2021, 45, 1328–1337. [Google Scholar] [CrossRef]
- Haran, O.; Bracha, G.; Tiosano, A.; Menes, T.; Madah, E.; Gur, E.; Barnea, Y.; Arad, E. Postirradiation Capsular Contracture in Implant-Based Breast Reconstruction: Management and Outcome. Plast. Reconstr. Surg. 2021, 147, 11–19. [Google Scholar] [CrossRef]
- Gunawardana, R.T.; Dessauvagie, B.F.; Taylor, D.B. Breast implant-associated anaplastic large cell lymphoma, an under-recognised entity. J. Med. Imaging Radiat. Oncol. 2019, 63, 630–638. [Google Scholar] [CrossRef] [PubMed]
- Sánchez Rubio, N.; Lannegrand Menéndez, B.; Duque Muñoz, M.; Montes Fernández, M.; Ciudad Fernández, M.J. Uncommon complications of breast prostheses. Radiologia 2020, 62, 266–279, (In English, Spanish). [Google Scholar] [CrossRef] [PubMed]
- Hudson, A.; Brown, R.K.J.; Minoshima, S.; Dunn, D. An Unusual Presentation of Breast Implant Rupture. Clin. Nucl. Med. 2022, 47, e271–e273. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Jiang, L.; Xu, Y.; Zhang, X.; Liu, B. An Unusual False-Positive Uptake of Radioiodine Caused by Breast Implants. Clin. Nucl. Med. 2022, 47, 646–647. [Google Scholar] [CrossRef] [PubMed]
- Vedala, K.; Sobash, P.T.; Johnson, D.; Kakkera, K. Not All That Shines on a PET Scan Is Cancer: A Silicone-Induced Granuloma Masquerading as Malignancy. Clin. Pract. 2020, 11, 8–12. [Google Scholar] [CrossRef] [PubMed]
- Khakbaz, E.; Lang, C.; Lelkaitis, G.; Grønhøj, C. Late migration of silicon as a complication to breast transplant rupture: Case report and literature review. Int. J. Surg. Case Rep. 2021, 85, 106241. [Google Scholar] [CrossRef] [PubMed]
- Verde, F.; Vigliar, E.; Romeo, V.; Campanino, M.R.; Accurso, A.; Canta, L.; Garbino, N.; Basso, L.; Cavaliere, C.; Nicolai, E.; et al. Breast implant associated anaplastic large cell lymphoma (BIA-ALCL): A challenging cytological diagnosis with hybrid PET/MRI staging and follow-up. Breast Cancer 2021, 28, 527–532. [Google Scholar] [CrossRef]
- Pandika, V.; Covington, M.F. FDG PET/CT and Ultrasound Evaluation of Breast Implant-Associated Anaplastic Large Cell Lymphoma. Clin. Nucl. Med. 2020, 45, 68–73. [Google Scholar] [CrossRef]
- Mescam, L.; Camus, V.; Schiano, J.M.; Adélaïde, J.; Picquenot, J.M.; Guille, A.; Bannier, M.; Ruminy, P.; Viailly, P.J.; Jardin, F.; et al. EBV+ diffuse large B-cell lymphoma associated with chronic inflammation expands the spectrum of breast implant-related lymphomas. Blood 2020, 135, 2004–2009. [Google Scholar] [CrossRef]
- Phan Sy, O.; Rouchy, R.C.; De Leiris, N.; Nika, E.; Djaileb, L. FDG PET/CT of a Supraclavicular Silicone Granuloma at Follow-up of a Breast Carcinoma. Clin. Nucl. Med. 2020, 45, e169–e170. [Google Scholar] [CrossRef]
- Montes Fernández, M.; Ciudad Fernández, M.J.; de la Puente Yagüe, M.; Brenes Sánchez, J.; Benito Arjonilla, E.; Moreno Domínguez, L.; Lannegrand Menéndez, B.; Ruiz Rodríguez, J.; Herrera de la Muela, M.; Cabeza Martinez, B.; et al. Breast implant-associated Anaplastic large cell lymphoma (BIA-ALCL): Imaging findings. Breast J. 2019, 25, 728–730. [Google Scholar] [CrossRef]
- Siminiak, N.; Czepczyński, R. PET-CT for the staging of breast implant- -associated anaplastic large cell lymphoma. Nucl. Med. Rev. Cent. East. Eur. 2019, 22, 90–91. [Google Scholar]
- Palot Manzil, F.F.; Bhambhvani, P.G. 18F-FDG PET/CT Unveiling of Implant Rupture and Clinically Unsuspected Silicone Granuloma in Treated Breast Cancer. J. Nucl. Med. Technol. 2018, 46, 394–395. [Google Scholar] [CrossRef] [PubMed]
- Domínguez, M.L.; Salvat, C.; F-Llana, B.; Vigil, C.; Suárez, J.P.; González, F.M. Extracapsular breast implant rupture mimicking local cancer recurrence on 18F-FDG PET/CT. Rev. Esp. Med. Nucl. Imagen Mol. 2018, 37, 392–394, (In English, Spanish). [Google Scholar] [CrossRef] [PubMed]
- D’hulst, L.; Nicolaij, D.; Beels, L.; Gheysens, O.; Alaerts, H.; Van de Wiele, C.; Maes, A. False-Positive Axillary Lymph Nodes Due to Silicone Adenitis on (18)F-FDG PET/CT in an Oncological Setting. J. Thorac. Oncol. 2016, 11, e73–e75. [Google Scholar] [CrossRef] [PubMed]
- Acevedo-Báñez, I.; García-Gomez, F.J.; Jiménez-Granero, P.; Carrillo-Cruz, E.; Perez-Lopez, O.; Borrego-Dorado, I. 18F-FDG-PET/CT in implant-associated anaplastic large cell lymphoma of the breast. Br. J. Haematol. 2015, 169, 1. [Google Scholar] [CrossRef] [PubMed]
- Karnatovskaia, L.V.; Khoor, A.; Johnson, M.M.; Kaplan, J. A 60-year-old woman with PET scan-avid lung nodules and a history of a ruptured silicone breast implant. Chest 2014, 146, e138–e142. [Google Scholar] [CrossRef]
- Ulaner, G.A.; D’Andrea, G.; Cody, H.S., 3rd. Breast implant foreign body reaction mimicking breast cancer recurrence on FDG PET/CT. Clin. Nucl. Med. 2013, 38, 480–481. [Google Scholar] [CrossRef] [PubMed]
- Soudack, M.; Yelin, A.; Simansky, D.; Ben-Nun, A. Fluorodeoxyglucose--positive internal mammary lymph node in breast cancer patients with silicone implants: Is it always metastatic cancer? Eur. J. Cardiothorac. Surg. 2013, 44, 79–82. [Google Scholar] [CrossRef]
- Ho, L.; Wassef, H.; Seto, J. FDG PET/CT imaging in granulomatous changes secondary to breast silicone injection. Clin. Radiol. 2010, 65, 659–661. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.J.; Lee, B.F.; Yao, W.J.; Wu, P.S.; Chen, W.C.; Peng, S.L.; Chiu, N.T. A false positive F-FDG PET/CT scan caused by breast silicone injection. Korean J. Radiol. 2009, 10, 194–196. [Google Scholar] [CrossRef]
- Bhargava, P.; Glass, E.; Ghesani, M. Inflammatory F-18 FDG uptake secondary to ruptured breast prosthesis. Clin. Nucl. Med. 2006, 31, 227–228. [Google Scholar] [CrossRef] [PubMed]
- Hurwitz, R. F-18 FDG positron emission tomographic imaging in a case of ruptured breast implant: Inflammation or recurrent tumor? Clin. Nucl. Med. 2003, 28, 755–756. [Google Scholar] [CrossRef]
- Leslie, K.; Buscombe, J.; Davenport, A. Implant infection in a transsexual with renal failure. Nephrol. Dial. Transplant. 2000, 15, 436–437. [Google Scholar] [CrossRef]
- Ellenberger, P.; Graham, W.P., 3rd; Manders, E.K.; Basarab, R.M. Labeled leukocyte scans for detection of retained polyurethane foam. Plast. Reconstr. Surg. 1986, 77, 77–79. [Google Scholar] [CrossRef]
- Hartshorne, M.F.; Maragh, H.A.; Telepak, R.J.; Bunker, S.R. Ga-67 uptake in capsular contracture around a breast implant. Clin. Nucl. Med. 1982, 7, 572–573. [Google Scholar] [CrossRef] [PubMed]
- Eklund, G.W.; Busby, R.C.; Miller, S.H.; Job, J.S. Improved imaging of the augmented breast. AJR Am. J. Roentgenol. 1988, 151, 469–473. [Google Scholar] [CrossRef]
- Silverstein, M.J.; Handel, N.; Gamagami, P.; Waisman, E.; Gierson, E.D. Mammographic measurements before and after augmentation mammaplasty. Plast. Reconstr. Surg. 1990, 86, 1126–1130. [Google Scholar] [CrossRef] [PubMed]
- Couto, L.S.; Freitas-Junior, R.; Corrêa, R.S.; Lauar, M.V.; Bauab, S.P.; Urban, L.A.B.D.; Cruvinel-Filho, J.L.O.; Soares, L.R.; Savaris, R.F. Are All Views with and without Displacement Maneuver Necessary in Augmentation Mammography? Putting Numbers Into Perspective. Asian Pac. J. Cancer Prev. 2022, 23, 233–239. [Google Scholar] [CrossRef]
- Jensen, S.R.; Mackey, J.K. Xeromammography after augmentation mammoplasty. AJR Am. J. Roentgenol. 1985, 144, 629–633. [Google Scholar] [CrossRef] [PubMed]
- Steinbach, B.G.; Hardt, N.S.; Abbitt, P.L. Mammography: Breast implants--types, complications, and adjacent breast pathology. Curr. Probl. Diagn Radiol. 1993, 22, 39–86. [Google Scholar] [CrossRef] [PubMed]
- Venkataraman, S.; Hines, N.; Slanetz, P.J. Challenges in mammography: Part 2, multimodality review of breast augmentation--imaging findings and complications. AJR Am. J. Roentgenol. 2011, 197, W1031–W1045. [Google Scholar] [CrossRef]
- Lin, D.J.; Wong, T.T.; Ciavarra, G.A.; Kazam, J.K. Adventures and Misadventures in Plastic Surgery and Soft-Tissue Implants. Radiographics 2017, 37, 2145–2163. [Google Scholar] [CrossRef] [PubMed]
- O’Toole, M.; Caskey, C.I. Imaging spectrum of breast implant complications: Mammography, ultrasound, and magnetic resonance imaging. Semin. Ultrasound CT MR 2000, 21, 351–361. [Google Scholar] [CrossRef] [PubMed]
- Dixon, J.M.; Walsh, J.; Paterson, D.; Chetty, U. Colour Doppler ultrasonography studies of benign and malignant breast lesions. Br. J. Surg. 1992, 79, 259–260. [Google Scholar] [CrossRef]
- Xiao, X.Y.; Chen, X.; Guan, X.F.; Wu, H.; Qin, W.; Luo, B.M. Superb microvascular imaging in diagnosis of breast lesions: A comparative study with contrast-enhanced ultrasonographic microvascular imaging. Br. J. Radiol. 2016, 89, 20160546. [Google Scholar] [CrossRef] [PubMed]
- Balleyguier, C.; Opolon, P.; Mathieu, M.C.; Athanasiou, A.; Garbay, J.R.; Delaloge, S.; Dromain, C. New potential and applications of contrast-enhanced ultrasound of the breast: Own investigations and review of the literature. Eur. J. Radiol. 2009, 69, 14–23. [Google Scholar] [CrossRef] [PubMed]
- Rzymski, P.; Kubasik, M.; Gaca, M.; Opala, T. Is the shear wave sonographic elastography correlated with pain after breast augmentation with silicone implants an indication of inflammatory activity? A preliminary report. Videosurgery Other Miniinvasive Tech. 2011, 6, 217–225. [Google Scholar] [CrossRef] [PubMed]
- Expert Panel on Breast Imaging; Lourenco, A.P.; Moy, L.; Baron, P.; Didwania, A.D.; diFlorio, R.M.; Heller, S.L.; Holbrook, A.I.; Lewin, A.A.; Mehta, T.S.; et al. ACR Appropriateness Criteria® Breast Implant Evaluation. J. Am. Coll. Radiol. 2018, 15, S13–S25. [Google Scholar]
- Liu, L.; Long, M.; Wang, J.; Liu, N.; Ge, X.; Hu, Z.; Shen, W. Quantitative Analysis of Diffusion-Weighted Imaging for Diagnosis of Puerperal Breast Abscess After Polyacrylamide Hydrogel Augmentation Mammoplasty: Compared with Other Conventional Modalities. Aesthetic Plast. Surg. 2015, 39, 84–90. [Google Scholar] [CrossRef]
- Lee, C.J.; Kim, S.G.; Kim, L.; Choi, M.S.; Lee, S.I. Unfavorable findings following breast augmentation using injected polyacrylamide hydrogel. Plast. Reconstr. Surg. 2004, 114, 1967–1968. [Google Scholar] [CrossRef]
- Steinbach, B.G.; Hardt, N.S.; Abbitt, P.L.; Lanier, L.; Caffee, H.H. Breast implants, common complications, and concurrent breast disease. Radiographics 1993, 13, 95–118. [Google Scholar] [CrossRef] [PubMed]
- Krisnan, R.N.K.; Chotai, N. Imaging Spectrum of Augmented Breast and Post-Mastectomy Reconstructed Breast with Common Complications: A Pictorial Essay. Korean J. Radiol. 2021, 22, 1005–1020. [Google Scholar] [CrossRef] [PubMed]
- Noreña-Rengifo, B.D.; Sanín-Ramírez, M.P.; Adrada, B.E.; Luengas, A.B.; Martínez de Vega, V.; Guirguis, M.S.; Saldarriaga-Uribe, C. MRI for Evaluation of Complications of Breast Augmentation. Radiographics 2022, 42, 929–946. [Google Scholar] [CrossRef] [PubMed]
- Sutton, E.J.; Dashevsky, B.Z.; Watson, E.J.; Tyagi, N.; Bernard-Davila, B.; Martinez, D.; Dogan, A.; Horwitz, S.M.; Cordeiro, P.G.; Morris, E.A. Incidence of benign and malignant peri-implant fluid collections and masses on magnetic resonance imaging in women with silicone implants. Cancer Med. 2020, 9, 3261–3267. [Google Scholar] [CrossRef]
- Shah, A.T.; Jankharia, B.B. Imaging of common breast implants and implant-related complications: A pictorial essay. Indian J. Radiol. Imaging 2016, 26, 216–225. [Google Scholar] [CrossRef] [PubMed]
- Prasad, K.; Zhou, R.; Zhou, R.; Schuessler, D.; Ostrikov, K.K.; Bazaka, K. Cosmetic reconstruction in breast cancer patients: Opportunities for nanocomposite materials. Acta Biomater. 2019, 86, 41–65. [Google Scholar] [CrossRef] [PubMed]
- Goldberg, M.T.; Llaneras, J.; Willson, T.D.; Boyd, J.B.; Venegas, R.J.; Dauphine, C.; Kalantari, B.N. Squamous Cell Carcinoma Arising in Breast Implant Capsules. Ann. Plast. Surg. 2021, 86, 268–272. [Google Scholar] [CrossRef]
- Bewtra, C.; Gharde, P. Current Understanding of Breast Implant-Associated Anaplastic Large Cell Lymphoma. Cureus 2022, 14, e30516. [Google Scholar] [CrossRef]
- Lee, K.T.; Kim, S.; Jeon, B.J.; Pyon, J.K.; Mun, G.H.; Ryu, J.M.; Lee, S.K.; Yu, J.; Kim, S.W.; Lee, J.E.; et al. Association of the Implant Surface Texture Used in Reconstruction With Breast Cancer Recurrence. JAMA Surg. 2020, 155, 1132–1140. [Google Scholar] [CrossRef]
- Mehdi, A.S.; Bitar, G.; Sharma, R.K. RMH BIA-ALCL Working Group; Iyengar, S.; El-Sharkawi, D.; Tasoulis, M.K.; Attygalle, A.D.; Cunningham, D.; Sharma, B. Breast implant-associated anaplastic large cell lymphoma (BIA-ALCL): A good practice guide, pictorial review, and new perspectives. Clin. Radiol. 2022, 77, 79–87. [Google Scholar] [CrossRef] [PubMed]
- Chacko, A.; Lloyd, T. Breast implant-associated anaplastic large cell lymphoma: A pictorial review. Insights Imaging 2018, 9, 683–686. [Google Scholar] [CrossRef]
- FDA. FDA Issues Safety Alert for Squamous Cell Carcinoma and Various Lymphomas in Scar Tissue around Breast Implants 2022. Available online: https://www.fda.gov/news-events/press-announcements/fda-issues-safety-alert-squamous-cell-carcinoma-and-various-lymphomas-scar-tissue-around-breast (accessed on 8 September 2022).
- Casali, M.; Lauri, C.; Altini, C.; Bertagna, F.; Cassarino, G.; Cistaro, A.; Erba, A.P.; Ferrari, C.; Mainolfi, C.G.; Palucci, A.; et al. State of the art of 18F-FDG PET/CT application in inflammation and infection: A guide for image acquisition and interpretation. Clin. Transl. Imaging 2021, 9, 299–339. [Google Scholar] [CrossRef] [PubMed]
- Schillaci, O. Use of dual-point fluorodeoxyglucose imaging to enhance sensitivity and specificity. Semin. Nucl. Med. 2012, 42, 267–280. [Google Scholar] [CrossRef] [PubMed]
- Pijl, J.P.; Nienhuis, P.H.; Kwee, T.C.; Glaudemans, A.W.J.M.; Slart, R.H.J.A.; Gormsen, L.C. Limitations and Pitfalls of FDG-PET/CT in Infection and Inflammation. Semin. Nucl. Med. 2021, 51, 633–645. [Google Scholar] [CrossRef]
- Ichiya, Y.; Kuwabara, Y.; Sasaki, M.; Yoshida, T.; Akashi, Y.; Murayama, S.; Nakamura, K.; Fukumura, T.; Masuda, K. FDG-PET in infectious lesions: The detection and assessment of lesion activity. Ann. Nucl. Med. 1996, 10, 185–191. [Google Scholar] [CrossRef] [PubMed]
- Schillaci, O.; Calabria, F.; Tavolozza, M.; Cicciò, C.; Carlani, M.; Caracciolo, C.R.; Danieli, R.; Orlacchio, A.; Simonetti, G. 18F-choline PET/CT physiological distribution and pitfalls in image interpretation: Experience in 80 patients with prostate cancer. Nucl. Med. Commun. 2010, 31, 39–45. [Google Scholar] [CrossRef] [PubMed]
- Leskinen-Kallio, S.; Någren, K.; Lehikoinen, P.; Ruotsalainen, U.; Joensuu, H. Uptake of 11C-methionine in breast cancer studied by PET. An association with the size of S-phase fraction. Br. J. Cancer 1991, 64, 1121–1124. [Google Scholar] [CrossRef] [PubMed]
- Bentestuen, M.; Al-Obaydi, N.; Zacho, H.D. FAPI-avid nonmalignant PET/CT findings: An expedited systematic review. Semin. Nucl. Med. 2023; in press. [Google Scholar] [CrossRef] [PubMed]




| Authors | Publication Year | Nuclear Medicine Imaging Technique | No. of Patients | Main Findings |
|---|---|---|---|---|
| Hudson A et al. [19] | 2022 | [99mTc]Tc-diphosphonates Scintigraphy | 1 | Implant rupture |
| Wang Y et al. [20] | 2022 | 131Iodine Scintigraphy | 1 | Aspecific implant uptake |
| Vedala K et al. [21] | 2021 | [18F]FDG PET/CT | 1 | Granulomatosis |
| Khakbaz E et al. [22] | 2021 | [18F]FDG PET/CT | 1 | Granulomatosis |
| Verde F et al. [23] | 2020 | [18F]FDG PET/MRI | 1 | BIA-ALCL |
| Pandika V et al. [24] | 2020 | [18F]FDG PET/CT | 4 | BIA-ALCL |
| Mescam L et al. [25] | 2020 | [18F]FDG PET/CT | 3 | BIA-ALCL |
| Phan S et al. [26] | 2020 | [18F]FDG PET/CT | 1 | Granulomatosis |
| Montes Fernandez M et al. [27] | 2019 | [18F]FDG PET/CT | 1 | BIA-ALCL |
| Siminiak N et al. [28] | 2019 | [18F]FDG PET/CT | 2 | BIA-ALCL |
| Palot Manzil FF et al. [29] | 2018 | [18F]FDG PET/CT | 1 | Granulomatosis |
| Dominguez ML et al. [30] | 2018 | [18F]FDG PET/CT | 1 | Granulomatosis |
| D’hulst L et al. [31] | 2016 | [18F]FDG PET/CT | 1 | Granulomatosis |
| Acevedo-Banez I et al. [32] | 2015 | [18F]FDG PET/CT | 1 | BIA-ALCL |
| Karnatovskaia LV et al. [33] | 2014 | [18F]FDG PET/CT | 1 | Nodular Lymphoid Hyperplasia |
| Ulaner GA et al. [34] | 2013 | [18F]FDG PET/CT | 1 | Granulomatosis |
| Soudack M. et al. [35] | 2013 | [18F]FDG PET/CT | 12 | Granulomatosis |
| Ho L et al. [36] | 2010 | [18F]FDG PET/CT | 1 | Granulomatosis |
| Chen C et al. [37] | 2009 | [18F]FDG PET/CT | 1 | Granulomatosis |
| Bhargava P et al. [38] | 2006 | [18F]FDG PET/CT | 1 | Capsular rupture |
| Hurwitz R et al. [39] | 2003 | [18F]FDG PET/CT | 1 | Granulomatosis |
| Leslie K et al. [40] | 2000 | [67Ga] Gallium-citrate Scintigraphy | 1 | Implant infection |
| Ellenberger P et al. [41] | 1985 | [99mTc] Tc-HMPAO-labeled leukocytes Scintigraphy | 1 | Implant infection |
| Hartshorne MF et al. [42] | 1982 | [67Ga] Gallium-citrate Scintigraphy | 1 | Capsular contracture |
| Imaging Technique | Normal Findings | Abnormal Findings | Comments |
|---|---|---|---|
| Mammography (MX) | - Regular-shape opacities; - Density dependent on the used material, up to radiolucent in liquid material. | - Regular round or oval opacities; - Double-implant contour, sign of pericapsular fluid collection; - Calcified irregular opacities, sign of granulomas; - Lobulated dense opacities, sign of siliconomas with surrounding inflammatory reaction; - Calcifications with or without mass opacity, signs of parasitic infections; - Asymmetrical dense fat tissue, sign of mastitis, with or without cutaneous thickening; - Cutaneous thickening, common sign of mastitis and/or after radiosurgery; - Periprosthetic fluid collection, glandular edema, and cutaneous thickening, signs of late infection; - Capsular contracture may mimic infection, specific signs of contracture include implants deformation, capsular thickening, and presence of calcifications. | Worldwide diagnostic technique for breast assessment, but in case with breast implants the accuracy is reduced. Combination of standard and projections with Eklund’s maneuvers increases the diagnostic accuracy. Not possible in patients with large or extra-large breast implants. |
| Ultrasound (US) | -Regular, linear echogenic implant wall, oval or round in shape; -A second chamber is always found in breast expanders and in double-lumen implants; -The peri-prosthetic capsule is depicted as two parallel echogenic lines; -Implant wall folding “ripples” can be normally present as regular wall waves; - Minimal layers of peri-capsular hypoechoic liquid can be normally present; -A single round regular interruption of the parallel lines consists in the valve, present in all breast expanders on the upper-external side. A single-lumen implant valve is positioned on the posterior side and usually not visible on US. | - Abnormally echoic or abundant peri-prosthetic fluid collection is a sign of inflammation, infection, or implant rejection; - Large peri-prosthetic focal seromas or hematomas can be commonly found in the immediate post-surgical period; -Signs of capsular contracture such as inhomogeneous thickened capsule and irregular ripples are uncommonly visible on US; - Siliconomas are shown as hyperechoic regular/oval-shaped nodules; - Fat edema or fat necrosis can be identifiable on US as signs of liponecrosis, infection, or post-radiation changes; - The “snowstorm sign” is a rare but typical sign of extracapsular rupture; - Intracapsular rupture can be seen as regular hyperechoic intra-prosthetic lines; - Capsular hypervascularization is always a sign of active inflammation or malignancy; - Contrast-enhanced ultrasound (CEUS) can improve the demonstration of hypervascularization; - Elastosonography can detect the presence and estimate the degree of capsular fibrosis, for example in capsular contracture; - In the differential diagnosis between infection and inflammation, US-guided fine-needle aspiration biopsy (FNAB) can play a crucial role. | Widely available diagnostic technique, considerably operator-dependent: a high level of expertise in the evaluation of breast implant abnormalities and breast focal lesions is required. Ancillary techniques, such as color/power-Doppler, US contrast agent administration, and elastosonography, can significantly improve the diagnostic accuracy. US is the most simple and affordable tool to be used as a guide for diagnostic invasive procedures, such as FNAB and core biopsy. |
| MRI | Breast implants show different intensity signals due to their composition: -Silicone single lumen has an intermediate-to-high signal on T2W images, a high signal on the silicone-specific sequence, and a loss of signal in the silicone suppressed sequence; -Saline, single lumen has a high signal on T2W images; -Standard double lumen (outer saline, inner silicone); -Reverse double lumen (outer silicone, inner saline). A fibrous capsule hypointense in all sequences and a small periprosthetic fluid amount are paraphysiological findings. | Acute complications: -Hematoma —hyperintense on T1W images, decreasing over time; -Seroma—intermediate-to-hyperintense on T2W images; -Abscess—fluid collection with irregular, thick peripheral enhancement; -Ancillary signs—edema, skin thickening, and adenopathy. Late complications: -Capsular contraction—prosthetic contour alterations, peripheral enhancement; -Intracapsular breast implant rupture (uncollapsed rupture “keyhole sign”, minimal collapse “subcapsular line sign”, and partial-to-full collapse “linguine sign”); -Extracapsular breast implant rupture; -Rare, breast implant-associated anaplastic large-cell lymphoma, ALCL (peri-implant collection with an enhancing mass and lymphadenopathy). | Breast magnetic resonance imaging is the most accurate technique to assess prosthetic integrity in the clinical or ultrasound suspicion of rupture, but is not justified as a pure screening examination in asymptomatic women of all ages and with any type of prosthesis. Its parametric nature allows the typing of the content of periprosthetic fluid collections (seroma, hematoma) and, combined with the administration of contrast medium, the detection of periprosthetic neoplastic recurrences or complications (breast implant-associated anaplastic large-cell lymphoma, ALCL). |
| [67Ga]Ga-citrate Scintigraphy | -No uptake around the implant. | -Different degree of radiopharmaceutical uptake in inflammatory/infected foci. | Since the introduction of [18F]FDG PET/CT, [67Ga]Ga-citrate scintigraphy can be proposed where PET/CT is not available. |
| Radiolabeled leukocytes’ Scintigraphy | -No uptake around the implant. | -Increasing uptake over time in areas of leukocyte-mediated infection. | Radiolabeled leukocytes scintigraphy still represents a possible diagnostic option for breast implant infections and should be considered as a second-line imaging tool in cases that remain equivocal after first-line imaging. |
| [18F]FDG PET/CT | -No uptake or only faint uptake around the breast implant. -No axillary lymph node uptake, or just faint uptake in normally-sized nodes, vascular hilum well-visible. | -Focal uptake around the implant and in axillary, mediastinal (usually internal mammary), and supraclavicular enlarged lymph nodes; -Pericapsular fluid collection may be present, with detectable faint activity; -Fluid effusion between the breast implant and the host fibrous capsule causing asymmetry and swelling of the breast can be a sign of breast implant-associated anaplastic large-cell lymphoma. | Even bearing in mind the clinical history of each patient, both visual and semiquantitative analysis (SUVmax) do not discriminate among inflammation, infection, and neoplastic foci, because they take up glucose similarly. The clinical setting of each focal uptake (implantation for oncological versus aesthetic reasons) and any morphological findings (see above) may lead the clinician to follow-up or to collect a biopsy specimen, and eventually fluid culturing, to rule out granuloma/infection versus node metastases or lymphoma or SCC associated with breast implants. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Giovannini, E.; Travascio, L.; Follacchio, G.A.; Bauckneht, M.; Criscuoli, B.; De Cataldo, C.; Iozzelli, A.; Cimini, A.; Ricci, M. Medical Imaging of Inflammations and Infections of Breast Implants. Diagnostics 2023, 13, 1807. https://doi.org/10.3390/diagnostics13101807
Giovannini E, Travascio L, Follacchio GA, Bauckneht M, Criscuoli B, De Cataldo C, Iozzelli A, Cimini A, Ricci M. Medical Imaging of Inflammations and Infections of Breast Implants. Diagnostics. 2023; 13(10):1807. https://doi.org/10.3390/diagnostics13101807
Chicago/Turabian StyleGiovannini, Elisabetta, Laura Travascio, Giulia Anna Follacchio, Matteo Bauckneht, Benedetta Criscuoli, Camilla De Cataldo, Andrea Iozzelli, Andrea Cimini, and Maria Ricci. 2023. "Medical Imaging of Inflammations and Infections of Breast Implants" Diagnostics 13, no. 10: 1807. https://doi.org/10.3390/diagnostics13101807
APA StyleGiovannini, E., Travascio, L., Follacchio, G. A., Bauckneht, M., Criscuoli, B., De Cataldo, C., Iozzelli, A., Cimini, A., & Ricci, M. (2023). Medical Imaging of Inflammations and Infections of Breast Implants. Diagnostics, 13(10), 1807. https://doi.org/10.3390/diagnostics13101807








