Autoptic Findings in Cases of Sudden Death Due to Kawasaki Disease
Abstract
:1. Introduction
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Burns, J.C.; Glodé, M.P. Kawasaki syndrome. Lancet 2004, 364, 533–544. [Google Scholar] [CrossRef] [PubMed]
- Jennette, J.C.; Falk, R.J.; Bacon, P.A.; Basu, N.; Cid, M.C.; Ferrario, F.; Flores-Suarez, L.F.; Gross, W.L.; Guillevin, L.; Hagen, E.C.; et al. Overview of the 2012 Revised International Chapel Hill Consensus Conference Nomenclature of Vasculitides. Arthritis Rheum. 2013, 65, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Younger, D.S. Epidemiology of the Vasculitides. Neurol. Clin. 2019, 37, 201–217. [Google Scholar] [CrossRef] [PubMed]
- Kawasaki, T. Acute febrile mucocutaneous syndrome with lymphoid involvement with specific desquamation of the fingers and toes in children. Arerugi 1967, 16, 178–222. [Google Scholar]
- Burns, J.C. Commentary: Translation of Dr. Tomisaku Kawasaki’s original report of fifty patients in 1967. Pediatr. Infect. Dis. J. 2002, 21, 993–995. [Google Scholar] [CrossRef]
- Takahashi, K.; Oharaseki, T.; Yokouchi, Y. Pathogenesis of Kawasaki disease. Clin. Exp. Immunol. 2011, 164 (Suppl. 1), 20–22. [Google Scholar] [CrossRef]
- Nigro, G.; Krzysztofiak, A.; Porcaro, M.; Mango, T.; Zerbini, M.; Gentilomi, G.; Musiani, M. Active or recent parvovirus B19 infection in children with Kawasaki disease. Lancet 1994, 343, 1260–1261. [Google Scholar] [CrossRef]
- Kato, H.; Inoue, O.; Koga, Y.; Shingu, M.; Fujimoto, T.; Kondo, M.; Yamamoto, S.; Tominaga, K.; Sasaguri, Y. Variant Strain of Propionibacterium Acnes: A Clue to the Aetiology of Kawasaki Disease. Lancet 1983, 322, 1383–1388. [Google Scholar] [CrossRef]
- Catalano-Pons, C.; Giraud, C.; Rozenberg, F.; Meritet, J.-F.; Lebon, P.; Gendrel, D. Detection of human bocavirus in children with Kawasaki disease. Clin. Microbiol. Infect. 2007, 13, 1220–1222. [Google Scholar] [CrossRef]
- Meissner, H.C.; Leung, D.Y.M. Superantigens, conventional antigens and the etiology of Kawasaki syndrome. Pediatr. Infect. Dis. J. 2000, 19, 91–94. [Google Scholar] [CrossRef]
- Uehara, R.; Yashiro, M.; Nakamura, Y.; Yanagawa, H. Clinical Features of Patients With Kawasaki Disease Whose Parents Had the Same Disease. Arch. Pediatr. Adolesc. Med. 2004, 158, 1166–1169. [Google Scholar] [CrossRef]
- Fujita, Y.; Nakamura, Y.; Sakata, K.; Hara, N.; Kobayashi, M.; Nagai, M.; Yanagawa, H.; Kawasaki, T. Kawasaki Disease in Families. Pediatrics 1989, 84, 666–669. [Google Scholar] [CrossRef]
- Furukawa, S.; Matsubara, T.; Jujoh, K.; Yone, K.; Sugawara, T.; Sasai, K.; Kato, H.; Yabuta, K. Peripheral blood monocyte/macrophages and serum tumor necrosis factor in Kawasaki disease. Clin. Immunol. Immunopathol. 1988, 48, 247–251. [Google Scholar] [CrossRef]
- Matsubara, T.; Furukawa, S.; Yabuta, K. Serum levels of tumor necrosis factor, interleukin 2 receptor, and interferon-γ in Kawasaki disease involved coronary-artery lesions. Clin. Immunol. Immunopathol. 1990, 56, 29–36. [Google Scholar] [CrossRef]
- Maury, C.P.; Salo, E.; Pelkonen, P. Circulating Interleukin-1β in Patients with Kawasaki Disease. N. Engl. J. Med. 1988, 319, 1670–1671. [Google Scholar] [CrossRef]
- Ueno, Y.; Takano, N.; Kanegane, H.; Yokoi, T.; Yachie, A.; Miyawaki, T.; Taniguchi, N. The acute phase nature of interleukin 6: Studies in Kawasaki disease and other febrile illnesses. Clin. Exp. Immunol. 1989, 76, 337–342. [Google Scholar]
- Eberhard, B.A.; Andersson, U.; Laxer, R.M.; Rose, V.; Silverman, E.D. Evaluation of the cytokine response in Kawasaki disease. Pediatr. Infect. Dis. J. 1995, 14, 199–202. [Google Scholar] [CrossRef]
- Manlhiot, C.; Mueller, B.; O’shea, S.; Majeed, H.; Bernknopf, B.; Labelle, M.; Westcott, K.V.; Bai, H.; Chahal, N.; Birken, C.S.; et al. Environmental epidemiology of Kawasaki disease: Linking disease etiology, pathogenesis and global distribution. PLoS ONE 2018, 13, e0191087. [Google Scholar] [CrossRef]
- Ayusawa, M.; Sonobe, T.; Uemura, S.; Ogawa, S.; Nakamura, Y.; Kiyosawa, N.; Ishii, M.; Harada, K. Revision of diagnostic guidelines for Kawasaki disease (the 5th revised edition). Pediatr. Int. 2005, 47, 232–234. [Google Scholar] [CrossRef]
- Kato, H.; Sugimura, T.; Akagi, T.; Sato, N.; Hashino, K.; Maeno, Y.; Kazue, T.; Eto, G.; Yamakawa, R. Long-term consequences of Kawasaki disease. A 10- to 21-year follow-up study of 594 patients. Circulation 1996, 94, 1379–1385. [Google Scholar] [CrossRef]
- McCrindle, B.W.; Manlhiot, C.; Newburger, J.W.; Harahsheh, A.S.; Giglia, T.M.; Dallaire, F.; Friedman, K.; Low, T.; Runeckles, K.; Mathew, M.; et al. Medium-Term Complications Associated With Coronary Artery Aneurysms After Kawasaki Disease: A Study From the International Kawasaki Disease Registry. J. Am. Heart Assoc. 2020, 9, e016440. [Google Scholar] [CrossRef] [PubMed]
- Lin, M.-T.; Sun, L.-C.; Wu, E.-T.; Wang, J.-K.; Lue, H.-C.; Wu, M.-H. Acute and late coronary outcomes in 1073 patients with Kawasaki disease with and without intravenous γ-immunoglobulin therapy. Arch. Dis. Child. 2015, 100, 542–547. [Google Scholar] [CrossRef] [PubMed]
- Kanegaye, J.T.; Wilder, M.S.; Molkara, D.; Frazer, J.R.; Pancheri, J.; Tremoulet, A.H.; Watson, V.E.; Best, B.M.; Burns, J.C. Recognition of a Kawasaki Disease Shock Syndrome. Pediatrics 2009, 123, e783–e789. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, Y.; Yanagawa, H.; Kato, H.; Harada, K.; Kawasaki, T. Mortality among patients with a history of Kawasaki disease: The third look. The Kawasaki Disease Follow-up Group. Pediatr. Int. 1998, 40, 419–423. [Google Scholar] [CrossRef] [PubMed]
- Liberati, A.; Altman, D.G.; Tetzlaff, J.; Mulrow, C.; Gøtzsche, P.C.; Ioannidis, J.P.A.; Clarke, M.; Devereaux, P.J.; Kleijnen, J.; Moher, D. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: Explanation and elaboration. J. Clin. Epidemiol. 2009, 62, e1–e34. [Google Scholar] [CrossRef]
- Aterman, K.; Dische, M.R.; Franke, J.; Fraser, G.M.; Meyer, W.W. Aneurysms of the coronary arteries in infants and children. A review, and report of six cases. Virchows Arch. A 1977, 374, 27–44. [Google Scholar] [CrossRef]
- Kegel, S.M.; Dorsey, T.J.; Rowen, M.; Taylor, W.F. Cardiac death in mucocutaneous lymph node syndrome. Am. J. Cardiol. 1977, 40, 282–286. [Google Scholar] [CrossRef] [PubMed]
- Wilson, D.A.; Luckstead, E.F.; Stuemky, J.H. Echocardiographic findings in a fatal case of Kawasaki’s disease. Am. J. Dis. Child. 1979, 133, 1028–1030. [Google Scholar] [CrossRef] [PubMed]
- Tanimoto, T.; Kamiya, T.; Misawa, H.; Manabe, H.; Go, S.; Yutani, C. An autopsied case of an elementary school boy with sudden death four years after Kawasaki disease: On the problem of present method of cardiac mass screening of school children. Jpn. Circ. J. 1981, 45, 1438–1442. [Google Scholar] [CrossRef]
- Font, R.L.; Mehta, R.S.; Streusand, S.B.; O’Boyle, T.E.; Kretzer, F.L. Bilateral retinal ischemia in Kawasaki disease. Postmortem findings and electron microscopic observations. Ophthalmology 1983, 90, 569–577. [Google Scholar] [CrossRef]
- Imakita, M.; Sasaki, Y.; Misugi, K.; Miyazawa, Y.; Hyodo, Y. Kawasaki disease complicated with mitral insufficiency. Autopsy findings with special reference to valvular lesion. Acta Pathol. Jpn. 1984, 34, 605–616. [Google Scholar] [CrossRef]
- Embil, J.A.; McFarlane, E.S.; Murphy, D.M.; Krause, V.W.; Stewart, H.B. Adenovirus type 2 isolated from a patient with fatal Kawasaki disease. Can. Med. Assoc. J. 1985, 132, 1400. [Google Scholar]
- Nakano, H.; Saito, A.; Ueda, K.; Nojima, K. Clinical characteristics of myocardial infarction following Kawasaki disease: Report of 11 cases. J. Pediatr. 1986, 108, 198–203. [Google Scholar] [CrossRef]
- Cloney, D.L.; Teja, K.; Lohr, J.A. Fatal case of atypical Kawasaki syndrome. Pediatr. Infect. Dis. J. 1987, 6, 297–299. [Google Scholar] [CrossRef]
- Fujiwara, T.; Fujiwara, H.; Nakano, H. Pathological features of coronary arteries in children with Kawasaki disease in which coronary arterial aneurysm was absent at autopsy. Quantitative analysis. Circulation 1988, 78, 345–350. [Google Scholar] [CrossRef]
- McCowen, C.; Henderson, D.C. Sudden death in incomplete Kawasaki’s disease. Arch. Dis. Child. 1988, 63, 1254–1256. [Google Scholar] [CrossRef]
- Sakai, Y.; Takayanagi, K.; Inoue, T.; Yamaguchi, H.; Hayashi, T.; Morooka, S.; Takabatake, Y.; Sato, Y. Coronary artery aneurysms and congestive heart failure--possible long-term course of Kawasaki disease in an adult--a case report. Angiology 1988, 39, 625–630. [Google Scholar] [CrossRef]
- Loubser, M.D.; Hoh, M.C.; Sreeram, N. Sudden death in incomplete Kawasaki disease. Arch. Dis. Child. 1989, 64, 637–638. [Google Scholar] [CrossRef]
- Burke, A.P.; Farb, A.; Virmani, R.; Goodin, J.; Smialek, J.E. Sports-related and non-sports-related sudden cardiac death in young adults. Am. Heart J. 1991, 121, 568–575. [Google Scholar] [CrossRef]
- Corrado, D.; Thiene, G.; Cocco, P.; Frescura, C. Non-atherosclerotic coronary artery disease and sudden death in the young. Br. Heart J. 1992, 68, 601–607. [Google Scholar] [CrossRef]
- Naganuma, H.; Kyogoku, M.; Abe, J.; Fukui, K.; Inoue, S.; Yamamoto, K.; Kato, S.; Nakagawa, H. An autopsy case of Kawasaki disease with reference to occurrence of acute coronary thrombosis in the convalescent stage. Acta Pathol. Jpn. 1992, 42, 604–613. [Google Scholar] [CrossRef] [PubMed]
- Shaukat, N.; Ashraf, S.; Mebewu, A.; Freemont, A.; Keenan, D. Myocardial infarction in a young adult due to Kawasaki disease. A case report and review of the late cardiological sequelae of Kawasaki disease. Int. J. Cardiol. 1993, 39, 222–226. [Google Scholar] [CrossRef] [PubMed]
- Smith, B.A.; Grider, D.J. Sudden death in a young adult: Sequelae of childhood Kawasaki disease. Am. J. Emerg. Med. 1993, 11, 381–383. [Google Scholar] [CrossRef] [PubMed]
- Kristensen, I.B.; Kristensen, B.O. Sudden death caused by thrombosed coronary artery aneurysm. Two unusual cases of Kawasaki disease. Int. J. Leg. Med. 1994, 106, 277–280. [Google Scholar] [CrossRef]
- Lie, J.T.; Sanders, J.A. Kawasaki disease: Sudden death in early infancy from accelerated late sequelae of coronary artery aneurysms. Cardiovasc. Pathol. 1997, 6, 175–178. [Google Scholar] [CrossRef]
- Burke, A.P.; Virmani, R.; Perry, L.W.; Li, L.; King, T.M.; Smialek, J. Fatal Kawasaki disease with coronary arteritis and no coronary aneurysms. Pediatrics 1998, 101, 108–112. [Google Scholar] [CrossRef]
- McConnell, M.E.; Hannon, D.W.; Steed, R.D.; Gilliland, M.G. Fatal obliterative coronary vasculitis in Kawasaki disease. J. Pediatr. 1998, 133, 259–261. [Google Scholar] [CrossRef]
- Fineschi, V.; Paglicci Reattelli, L.; Baroldi, G. Coronary artery aneurysms in a young adult: A case of sudden death. A late sequelae of Kawasaki disease? Int. J. Leg. Med. 1999, 112, 120–123. [Google Scholar] [CrossRef]
- Suzuki, N.; Seguchi, M.; Kouno, C.; Inukai, K.; Kito, H.; Kobayashi, H. Rupture of coronary aneurysm in Kawasaki disease. Pediatr. Int. 1999, 41, 318–320. [Google Scholar] [CrossRef]
- Kazuma, N.; Tatara, K.; Murata, M. Can Heart Rate Variability Predict Sudden Death? A Case of Sudden Death in a Child with Severe Coronary Sequelae of Kawasaki Disease. Pediatr. Cardiol. 2000, 21, 403–406. [Google Scholar] [CrossRef]
- Maresi, E.; Passantino, R.; Midulla, R.; Ottoveggio, G.; Orlando, E.; Becchina, G.; Meschis, L.; Amato, G. Sudden infant death caused by a ruptured coronary aneurysm during acute phase of atypical Kawasaki disease. Hum. Pathol. 2001, 32, 1407–1409. [Google Scholar] [CrossRef] [PubMed]
- Heaton, P.; Wilson, N. Fatal Kawasaki disease caused by early occlusive coronary artery disease. Arch. Dis. Child. 2002, 87, 145–146. [Google Scholar] [CrossRef] [PubMed]
- Bartoloni, G.; Salvatrice, D.M.; Carlo, R. Sudden death in a 21-year-old man caused by thrombosed coronary aneurysm: Late sequelae or a very late onset of Kawasaki disease? Cardiovasc. Pathol. 2002, 11, 318–321. [Google Scholar] [CrossRef] [PubMed]
- Rozin, L.; Koehler, S.A.; Shakir, A.; Ladham, S.; Wecht, C.H. Kawasaki disease: A review of pathologic features of stage IV disease and two cases of sudden death among asymptotic young adults. Am. J. Forensic. Med. Pathol. 2003, 24, 45–50. [Google Scholar] [CrossRef] [PubMed]
- Freeman, A.F.; Crawford, S.E.; Cornwall, M.L.; Garcia, F.L.; Shulman, S.T.; Rowley, A.H. Angiogenesis in fatal acute Kawasaki disease coronary artery and myocardium. Pediatr. Cardiol. 2005, 26, 578–584. [Google Scholar] [CrossRef]
- Tsuda, E.; Arakaki, Y.; Shimizu, T.; Sakaguchi, H.; Yoshimura, S.; Yazaki, S.; Echigo, S. Changes in causes of sudden deaths by decade in patients with coronary arterial lesions due to Kawasaki disease. Cardiol. Young 2005, 15, 481–488. [Google Scholar] [CrossRef]
- Ozdogu, H.; Boga, C. Fatal cardiac tamponade in a patient with Kawasaki disease. Heart Lung. 2005, 34, 257–259. [Google Scholar] [CrossRef]
- Diana, M.C.; Villa, G.; Gattorno, M.; Ottonello, G.; Costabel, S.; Savioli, C.; Di Pietro, P. Sudden death in an infant revealing atypical Kawasaki disease. Pediatr. Emerg. Care 2006, 22, 35–37. [Google Scholar] [CrossRef]
- Sunagawa, K.; Mitsumata, M.; Ayusawa, M.; Kusumi, Y. Ruptured giant aneurysm of the left anterior descending coronary artery in Kawasaki disease. Pediatr. Cardiol. 2008, 29, 1115–1119. [Google Scholar] [CrossRef]
- Papadodima, S.A.; Sakelliadis, E.I.; Goutas, N.D.; Vlachodimitropoulos, D.G.; Spiliopoulou, C.A. Atypical kawasaki disease presenting with symptoms from the genitourinary system: An autopsy report. J. Trop. Pediatr. 2009, 55, 55–57. [Google Scholar] [CrossRef]
- Yokouchi, Y.; Oharaseki, T.; Ihara, F.; Naoe, S.; Sugawara, S.; Takahashi, K. Repeated stent thrombosis after DES implantation and localized hypersensitivity to a stent implanted in the distal portion of a coronary aneurysm thought to be a sequela of Kawasaki disease: Autopsy report. Pathol. Int. 2010, 60, 112–118. [Google Scholar] [CrossRef]
- Pucci, A.; Martino, S.; Tibaldi, M.; Bartoloni, G. Incomplete and atypical Kawasaki disease: A clinicopathologic paradox at high risk of sudden and unexpected infant death. Pediatr. Cardiol. 2012, 33, 802–805. [Google Scholar] [CrossRef]
- Ponniah, U. Coronary artery thrombus resulting in sudden cardiac death in an infant with Kawasaki disease and giant coronary artery aneurysms. Ann. Pediatr. Cardiol. 2013, 6, 197–199. [Google Scholar] [CrossRef]
- Okura, N.; Okuda, T.; Shiotani, S.; Kohno, M.; Hayakawa, H.; Suzuki, A.; Kawasaki, T. Sudden death as a late sequel of Kawasaki disease: Postmortem CT demonstration of coronary artery aneurysm. Forensic Sci. Int. 2013, 225, 85–88. [Google Scholar] [CrossRef]
- Miyamoto, T.; Ikeda, K.; Ishii, Y.; Kobayashi, T. Rupture of a coronary artery aneurysm in Kawasaki disease: A rare case and review of the literature for the past 15 years. J. Thorac. Cardiovasc. Surg. 2014, 147, e67–e69. [Google Scholar] [CrossRef]
- Shimizu, C.; Sood, A.; Lau, H.D.; Oharaseki, T.; Takahashi, K.; Krous, H.F.; Campman, S.; Burns, J.C. Cardiovascular pathology in 2 young adults with sudden, unexpected death due to coronary aneurysms from Kawasaki disease in childhood. Cardiovasc. Pathol. 2015, 24, 310–316. [Google Scholar] [CrossRef]
- Parsons, S.; Lynch, M. Sudden cardiac death while playing Australian Rules football: A retrospective 14 year review. Forensic Sci. Med. Pathol. 2016, 12, 158–162. [Google Scholar] [CrossRef]
- Chang, H.K.; Fernandes, J.; Nair, V. Kawasaki Disease: An Autopsy Case Series and Review of the Literature. Am. J. Forensic Med. Pathol. 2016, 37, 183–186. [Google Scholar] [CrossRef]
- Wei, Y.J.; Zhao, X.L.; Liu, B.M.; Niu, H.; Li, Q. Cardiac Complications in 38 Cases of Kawasaki Disease with Coronary Artery Aneurysm Diagnosed by Echocardiography. Echocardiography 2016, 33, 764–770. [Google Scholar] [CrossRef]
- Yajima, D.; Shimizu, K.; Oka, K.; Asari, M.; Maseda, C.; Okuda, K.; Shiono, H.; Ohtani, S.; Ogawa, K. A Case of Sudden Infant Death Due to Incomplete Kawasaki Disease. J. Forensic Sci. 2016, 61 (Suppl. 1), S259–S264. [Google Scholar] [CrossRef]
- Fukazawa, R.; Kobayashi, T.; Mikami, M.; Saji, T.; Hamaoka, K.; Kato, H.; Suzuki, H.; Tsuda, E.; Ayusawa, M.; Miura, M.; et al. Nationwide Survey of Patients With Giant Coronary Aneurysm Secondary to Kawasaki Disease 1999–2010 in Japan. Circ. J. 2017, 82, 239–246. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.; Han, M.H.; Lee, S. Sudden Child Death due to Thrombotic Giant Coronary Artery Aneurysms Complicated by Atypical Kawasaki Disease: An Autopsy Case. J. Pathol. Transl. Med. 2018, 52, 248–251. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Tuokan, T.; Shi, Y. Sudden Death as a Sequel of Ruptured Giant Coronary Artery Aneurysm in Kawasaki Disease. Am. J. Forensic Med. Pathol. 2018, 39, 375–377. [Google Scholar] [CrossRef] [PubMed]
- Pacheco, D.A.; Miller, C.R.; Boor, P.J.; Mambo, N.C. Incomplete Kawasaki disease with development of fatal coronary artery thrombosis in a 13-year-old male. Cardiovasc. Pathol. 2019, 42, 54–58. [Google Scholar] [CrossRef]
- Flossdorf, S.; Schiwy-Bochat, K.H.; Teifel, D.; Fries, J.W.U.; Rothschild, M.A. Sudden death of a young adult with coronary artery vasculitis, coronary aneurysms, parvovirus B19 infection and Kawasaki disease. Forensic Sci. Med. Pathol. 2020, 16, 498–503. [Google Scholar] [CrossRef]
- Zhang, Q.; Wang, L. Sudden death due to incomplete Kawasaki disease: A case report. Technol. Health Care 2021, 29, 351–355. [Google Scholar] [CrossRef]
- Staats, K.; Tremoulet, A.H.; Harvey, H.; Burns, J.C.; Donofrio-Odmann, J.J. A Four-Year-Old with History of Kawasaki Disease Presenting in Acute Shock. Prehosp. Emerg. Care 2021, 25, 281–288. [Google Scholar] [CrossRef]
- Maeda, K.; Chong, P.F.; Akamine, S.; Yamashita, F.; Morooka, Y.; Mori, H.; Lee, S.; Mizuno, Y.; Kira, R. Case Report: Acute Fulminant Cerebral Edema With Perivascular Abnormalities Related to Kawasaki Disease. Front. Pediatr. 2021, 9, 732110. [Google Scholar] [CrossRef]
- Ayusawa, M.; Namiki, H.; Abe, Y.; Ichikawa, R.; Morioka, I. Sudden Death in Patients with a History of Kawasaki Disease under School Supervision. Children 2022, 9, 1593. [Google Scholar] [CrossRef]
- Kawasaki, T.; Kosaki, F.; Okawa, S.; Shigematsu, I.; Yanagawa, H. A new infantile acute febrile mucocutaneous lymph node syndrome (MLNS) prevailing in Japan. Pediatrics 1974, 54, 271–276. [Google Scholar] [CrossRef]
- Giaconi, C.; Manetti, A.C.; Turco, S.; Coppola, M.; Forni, D.; Marra, D.; La Russa, R.; Karaboue, M.; Maiese, A.; Papi, L.; et al. Post-mortem computer tomography in ten cases of death while diving: A retrospective evaluation. Radiol. Med. 2022, 127, 318–329. [Google Scholar] [CrossRef]
- Ferrara, M.; Bertozzi, G.; Zanza, C.; Longhitano, Y.; Piccolella, F.; Lauritano, C.E.; Volonnino, G.; Manetti, A.C.; Maiese, A.; La Russa, R. Traumatic Brain Injury and Gut Brain Axis: The Disruption of an Alliance. Rev. Recent Clin. Trials. 2022, 17, 268–279. [Google Scholar] [CrossRef]
- Makino, N.; Nakamura, Y.; Yashiro, M.; Sano, T.; Ae, R.; Kosami, K.; Kojo, T.; Aoyama, Y.; Kotani, K.; Yanagawa, H. Epidemiological observations of Kawasaki disease in Japan, 2013–2014. Pediatr. Int. 2018, 60, 581–587. [Google Scholar] [CrossRef]
- Manlhiot, C.; O’Shea, S.; Bernknopf, B.; LaBelle, M.; Chahal, N.; Dillenburg, R.F.; Lai, L.S.; Bock, D.; Lew, B.; Masood, S.; et al. Epidemiology of Kawasaki Disease in Canada 2004 to 2014: Comparison of Surveillance Using Administrative Data vs Periodic Medical Record Review. Can. J. Cardiol. 2018, 34, 303–309. [Google Scholar] [CrossRef]
- Suda, K.; Kudo, Y.; Higaki, T.; Nomura, Y.; Miura, M.; Matsumura, M.; Ayusawa, M.; Ogawa, S.; Matsuishi, T. Multicenter and Retrospective Case Study of Warfarin and Aspirin Combination Therapy in Patients With Giant Coronary Aneurysms Caused by Kawasaki Disease. Circ. J. 2009, 73, 1319–1323. [Google Scholar] [CrossRef]
- Taubert, K.A.; Rowley, A.H.; Shulman, S.T. Nationwide survey of Kawasaki disease and acute rheumatic fever. J. Pediatr. 1991, 119, 279–282. [Google Scholar] [CrossRef]
- Shain, B.N.; American Academy of Pediatrics; Committee on Adolescence. Suicide and suicide attempts in adolescents. Pediatrics 2007, 120, 669–676. [Google Scholar] [CrossRef]
- King, W.J.; Schlieper, A.; Birdi, N.; Cappelli, M.; Korneluk, Y.; Rowe, P.C. The effect of Kawasaki disease on cognition and behavior. Arch. Pediatr. Adolesc. Med. 2000, 154, 463–468. [Google Scholar] [CrossRef]
- Carlton-Conway, D.; Ahluwalia, R.; Henry, L.; Michie, C.; Wood, L.; Tulloh, R. Behaviour sequelae following acute Kawasaki disease. BMC Pediatr. 2005, 5, 14. [Google Scholar] [CrossRef]
- Alves, N.R.; Magalhães, C.M.; Almeida, R.d.F.; Santos, R.C.; Gandolfi, L.; Pratesi, R. Prospective study of Kawasaki disease complications: Review of 115 cases. Rev. Assoc. Med. Bras. 2011, 57, 295–300. [Google Scholar] [CrossRef]
- Kato, H.; Ichinose, E.; Yoshioka, F.; Takechi, T.; Matsunaga, S.; Suzuki, K.; Rikitake, N. Fate of coronary aneurysms in Kawasaki disease: Serial coronary angiography and long-term follow-up study. Am. J. Cardiol. 1982, 49, 1758–1766. [Google Scholar] [CrossRef] [PubMed]
- Burns, J.C.; Hiroko, S.; Gordon, J.B.; Malhotra, B.S.; Schoenwetter, M.; Kawasaki, T. Sequelae of Kawasaki disease in adolescents and young adults. J. Am. Coll. Cardiol. 1996, 28, 253–257. [Google Scholar] [CrossRef] [PubMed]
- Sasaguri, Y.; Kato, H. Regression of aneurysms in Kawasaki syndrome: A pathologic study. J. Pediatr. 1982, 100, 225–231. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, Y.; Yanagawa, H.; Harada, K.; Kato, H.; Kawasaki, T. Mortality among persons with a history of Kawasaki disease in Japan: The fifth look. Arch. Pediatr. Adolesc. Med. 2002, 156, 162–165. [Google Scholar] [CrossRef] [PubMed]
- Tsuda, E.; Tsujii, N.; Kimura, K.; Suzuki, A. Distribution of Kawasaki disease coronary artery aneurysms and the relationship to coronary artery diameter. Pediatr. Cardiol. 2017, 38, 932–940. [Google Scholar] [CrossRef]
- Zhang, D.; Liu, L.; Huang, X.; Tian, J. Insights Into Coronary Artery Lesions in Kawasaki Disease. Front. Pediatr. 2020, 8, 493. [Google Scholar] [CrossRef]
- Yonesaka, S.; Takahashi, T.; Eto, S.; Sato, T.; Otani, K.; Ueda, T.; Sato, A.; Kitagawa, Y.; Konno, Y.; Kinjo, M. Biopsy-proven myocardial sequels in Kawasaki disease with giant coronary aneurysms. Cardiol. Young 2010, 20, 602–609. [Google Scholar] [CrossRef]
- Aquaro, G.D.; Guidi, B.; Emdin, M.; Pucci, A.; Chiti, E.; Santurro, A.; Scopetti, M.; Biondi, F.; Maiese, A.; Turillazzi, E.; et al. Post-Mortem Cardiac Magnetic Resonance in Explanted Heart of Patients with Sudden Death. Int. J. Environ. Res. Public Health 2022, 19, 13395. [Google Scholar] [CrossRef]
- Ross, B.A. Kawasaki disease: Unsafe at any age? J. Am. Coll. Cardiol. 1995, 25, 1425–1427. [Google Scholar] [CrossRef]
- Türkuçar, S.; Yıldız, K.; Acarı, C.; Dundar, H.A.; Kır, M.; Ünsal, E. Risk factors of intravenous immunoglobulin resistance and coronary arterial lesions in Turkish children with Kawasaki disease. Turk. J. Pediatr. 2020, 62, 1–9. [Google Scholar] [CrossRef]
- Xie, T.; Wang, Y.; Fu, S.; Wang, W.; Xie, C.; Zhang, Y.; Gong, F. Predictors for intravenous immunoglobulin resistance and coronary artery lesions in Kawasaki disease. Pediatr. Rheumatol. Online J. 2017, 15, 17. [Google Scholar] [CrossRef]
- Newburger, J.W.; Takahashi, M.; Burns, J.C. Kawasaki Disease. J. Am. Coll. Cardiol. 2016, 67, 1738–1749. [Google Scholar] [CrossRef]
- Furusho, K.; Kamiya, T.; Nakano, H.; Kiyosawa, N.; Shinomiya, K.; Hayashidera, T.; Tamura, T.; Hirose, O.; Manabe, Y.; Yokoyama, T.; et al. High-dose intravenous gammaglobulin for Kawasaki disease. Lancet 1984, 2, 1055–1058. [Google Scholar] [CrossRef]
- McCrindle, B.W. Kawasaki disease: A childhood disease with important consequences into adulthood. Circulation 2009, 120, 6–8. [Google Scholar] [CrossRef]
- Taslakian, E.N.; Wi, C.I.; Seol, H.Y.; Boyce, T.G.; Johnson, J.N.; Ryu, E.; King, K.S.; Juhn, Y.J.; Choi, B.S. Long-term Incidence of Kawasaki Disease in a North American Community: A Population-Based Study. Pediatr. Cardiol. 2021, 42, 1033–1040. [Google Scholar] [CrossRef]
- Burgner, D.; Harnden, A. Kawasaki disease: What is the epidemiology telling us about the etiology? Int. J. Infect. Dis. 2005, 9, 185–194. [Google Scholar] [CrossRef]
- Gitto, L.; Serinelli, S.; Busardò, F.P.; Panebianco, V.; Bolino, G.; Maiese, A. Can post-mortem computed tomography be considered an alternative for autopsy in deaths due to hemopericardium? J. Geriatr. Cardiol. 2014, 11, 363–367. [Google Scholar] [CrossRef]

| Ref. | Num. | Age and Sex | In-Life/Postmortem Diagnosis and Treatment | Brief Case Description | Autopsy Data | Histological Analysis | Cause of Death |
|---|---|---|---|---|---|---|---|
| Aterman et al. 1977 [26] | 5 | 4 m.o. M | Postmortem diagnosis. | Hospitalized with BT of 38 °C, respiratory distress, and signs of congestive heart failure, he suddenly died from cardiovascular collapse. | Heart: considerably enlarged. Several CAAs, rounded or fusiform, of variable size and filled with thrombi. Other viscera: marked congestion. | Heart: the CAA wall showed fibrous tissue with loss of smooth muscle and elastic fibers. Fresh and organized thrombi within the CAA lumen. Extensive loss of muscle mass with eosinophilic necrosis, disappearance of fibrils, and a scanty mononuclear response in the myocardium. | ACF |
| 5 m.o. M | Postmortem diagnosis. | Hospitalized for fever up to 40 °C, not responding to penicillin. During hospital stay, he suffered acute pericarditis and died suddenly. | Heart: fibrinous exudate in the pericardial sac. CAAs of all proximal segments. | CAs showed thinning and destruction of the media, inflammatory infiltration, marked periarteritis, focal fibrinoid changes, and the presence of fibroblasts. Thrombi in the lumen of the arteries. Other vessels: arteritis in the renal arteries and in some branches of the hepatic, peritesticular, and mesenteric arteries. | Pericarditis | ||
| 5 m.o. M | Postmortem diagnosis. | Two months after an upper respiratory infection, he underwent intermittent congestive heart failure. | Heart: enlarged with hypertrophied LV. RCA aneurysm. | Heart: CAAs showed severe intimal fibrous thickening and thrombosis with recanalization. Small foci of fibrosis in the myocardium. Kidneys: obliterative changes in the renal arteries and recent infarct in the right kidney. | ACF | ||
| 2 y.o. F | Postmortem diagnosis. | Hospitalized for fever and cervical lymphadenopathy. During the hospital stay, the patient developed heart failure and suddenly died. | Heart: fibrinous exudate in the pericardial sac. Dilated and thrombosed CAAs (located in the LCA, LAD, and RCA). | Heart: CAAs showed focal thinning and necrosis of the walls and thrombi in the lumen. CAs had focal acute vasculitis, chronic inflammation with destruction of the media, and fresh proliferating tissue. Focal eosinophilia and a few areas of frank necrosis with neutrophils in the myocardium. | ACF | ||
| 3 y.o. M | Postmortem diagnosis. | The patient suddenly died from cardiac arrest after being hospitalized for grunting respirations, bronchial breathing, rales, and rhonchi. | Heart: dilatation of both ventricles and the right atrium. CAAs (located at the LAD and one CX branch). | Heart: CAAs showed fibrous tissue in the wall and fresh thrombi in the lumen. Severe uniform thickening and subsequent marked narrowing of the lumen of the CAs. Markedly scarred myocardium and myocardial infarction with coagulation necrosis and demarcation by neutrophils. Other arteries: severe intimal fibrous scarring, splitting of the internal elastic tunica, and focal scarring of the media with occasional calcification. The abdominal aorta wall had severe fibrous scarring of the intima and media with focal calcium deposition. | AMI | ||
| Kegel et al. 1977 [27] | 1 | 12 y.o. M | Postmortem diagnosis. | The patient was found dead in bed 1 week before a scheduled bypass surgery. One day before death, 2 h after playing soccer, he experienced vomiting and chest pain. | Heart: all chambers were dilated. Marked dilatation of the LV. The LV apex was tinged with fibrosis. Numerous pericardial adhesions. Severe sclerosis of the endocardium. The CAA of the LCA contained a thrombus. The LAD, CX, and RCA were thickened and the lumens were narrowed with complete obstruction of the proximal RCA. | Heart: the CAAs demonstrated thickening of the media, subintimal fibrosis with marked hyalinization, and extensive calcification, and the lumen contained a recent thrombus. Severe nuclear hypertrophy and fibrosis in the myocardium. The LV septum demonstrated severe endocardial sclerosis. | AMI |
| Wilson et al. 1979 [28] | 1 | 4 y.o. M | In-life diagnosis. Treated with ASA. | Sudden death of a child who was hospitalized after three weeks of cervical lymphadenopathy. | Heart: moderate dilation of the RV and LV. Extensive organized thrombus in the CAs. | Heart: changes related to severe acute infarction. Necrotizing vasculitis of the CAs. | AMI |
| Tanimoto et al. 1981 [29] | 1 | 8 y.o. M | In-life diagnosis. No treatment. | Sudden death 4 years after the onset of KD symptoms. The patient died while running. | Heart: LV wall showed white and dark-brown discoloration. Six CAAs at the LCA. After cutting the largest aneurysm, a red-brown fresh thrombus was found. | Heart: CAs arteriosclerotic changes with a thin elastic layer and intima thickening. CAs showed thrombosis. CAAs with fresh and organized thrombi. Old and acute LV infarction. | AMI |
| Font et al. 1983 [30] | 1 | 4 m.o. M | In-life diagnosis. No treatment. | At the age of 3 months, the patient was hospitalized for otitis media, fever, and conjunctivitis. After discharge, he was hospitalized again because of pericardial effusion and some days later, after improvement, he became lethargic and suddenly died. | Heart: serosanguineous pericardial effusion. Vasculitis with thrombosis in the epicardial vessels. CAAs filled with thrombi. Other vessels: several, diffuse aneurysmal dilatations, some filled with thrombi. Other viscera: multiple infarcts of the spleen and kidneys, splenomegaly. Lymphoid hyperplasia of cervical axillary, and periportal lymph nodes. Subarachnoid hemorrhage, multifocal cerebellar hemorrhages, and microthrombi in several vessels of the thalamus. Necrosis of distal digits of the hands and feet. | Heart: vasculitis with thrombosis in the epicardial vessels. CAAs filled with thrombi. Other viscera: subarachnoid hemorrhage. | Cerebral hemorrhage |
| Imakita et al. 1984 [31] | 3 | 3 m.o. F | In-life diagnosis. Treated with ASA. | She had a fever, bilateral conjunctival congestion, redness of the lips, and macular erythema. Three days later, she was hospitalized with a suspected diagnosis of KD. During her hospital stay, she was diagnosed with mitral insufficiency and irregularity of the coronary artery. Her persistent fever and general condition appeared to be improving during the hospital stay. The patient suddenly died 10 days after the onset of symptoms. | Heart: hypertrophy. Thickness of the LV wall. Multiple small, dome-like elevations on the mitral valve. The aortic valve was elongated and showed severe waving. Three CAAs (located at the proximal segments of the LCA and the RCA) filled with red-brown thrombi. Other viscera/vessels: fusiform aneurysms in the right internal iliac artery. | Heart: severe pancarditis. Edema and infiltration of lymphocytes and plasma cells around epicardial coronary arteries. Fibroblast proliferation and severe inflammatory infiltration in the endocardium. Edema and prominent infiltration of lymphocytes and large histiocytic cells in the myocardium, as well as small foci of degeneration and scarring. Valves: lesion composed of inflammatory infiltration with fibrous connective tissue, proliferation of small capillaries, and severe chronic inflammation of the cardiac valves. Perivascular inflammation and different stages of pathological changes in other visceral vessels. | Pancarditis |
| 4 m.o. M | In-life diagnosis. Treatment N/A. | Sudden death 21 days after the onset of KD. | Heart: hypertrophy. Serosanguinous hydropericardium. CA thrombosis. Other viscera: kidney infarction, microthrombosis in the lungs, liver and spleen congestion, brain edema. Other vessels: thrombosis in the iliac arteries. Other: hydrothorax, slightly turbid ascites. | Heart: myocarditis, pericarditis, valvulitis. CA thrombosis. Mild inflammatory infiltration, increment of fibrous connective tissue, and proliferation of small capillaries at the mitral valve. Mild inflammatory cell infiltration at the tricuspid valve. | Pancarditis | ||
| 2 m.o. M | In-life diagnosis. Treatment N/A. | Sudden death 33 days after the onset of KD. | Heart: hypertrophy. CA thrombosis. Other viscera: perivascular inflammation in the liver, kidneys, and lymph nodes. Infarction of the spleen and kidneys. Other vessels: diffuse artery thrombosis. Other: Ascites, dry gangrene of the fingers and toes. | Heart: pericarditis, endocarditis. CA thrombosis. Mild inflammatory infiltration and increment of fibrous connective tissue in the aortic and pulmonary valves. Other viscera: perivascular inflammation Other vessels: thrombosis. | Pericarditis | ||
| Embil et al. 1985 [32] | 1 | 7 m.o. M | In-life diagnosis. Treatment N/A. | Four weeks after admission for aseptic meningitis (with fever, irritability, and maculopapular rash), the infant suffered from severe cardiac arrest. | Heart: multiple CAAs and thrombosis of the right coronary artery and the main branches of the LCA. | CA thrombosis. | AMI |
| Nakano et al. 1986 [33] | 2 | 1 y.o. M | In-life diagnosis. Treatment N/A. | Sudden death 19 days after the onset of KD symptoms. | NA | NA | Arrhythmia |
| 1 y.o. M | In-life diagnosis. Treatment N/A. | Sudden death four months after the onset of KD symptoms. He died from severe hypoxic brain damage after cardiac arrest. | NA | NA | Hypoxic brain damage | ||
| Cloney et al. 1987 [34] | 1 | 5 y.o. F | Postmortem diagnosis. | Hospitalized for fever and lymphadenitis. On the 21st day of hospitalization, she suddenly died. | Heart: all chambers were dilatated. Macroscopic evidence of myocardial infarction. CAAs (located at the LCA), filled with a nonoccluding thrombus. | Heart: recent (2–4-day-old) transmural myocardial infarction. Severe CA arteritis. Microemboli within the CA branches. | AMI |
| Fujiwara et al. 1988 [35] | 5 | 10 m.o. M | In-life diagnosis. Treatment N/A. | Sudden death 9 days after the onset of KD. | Heart: Acute myocarditis. | Heart: acute myocarditis. Acute CA inflammation. | Myocarditis |
| 3 m.o. M | In-life diagnosis. Treatment N/A. | Sudden death 18 days after the onset of KD. | Heart: Acute myocarditis. | Heart: acute myocarditis. Acute CA inflammation. Slight dilatation of the major CAs. | Myocarditis | ||
| 1 y.o. M | In-life diagnosis. Treatment N/A. | Sudden death 19 days after the onset of KD. | Heart: Acute myocarditis. | Heart: acute myocarditis. Severe panvasculitis and severe inflammation of the perivascular area. Slight dilatation of the major CAs. | Myocarditis | ||
| 3 m.o. F | In-life diagnosis. Treatment N/A. | Sudden death 22 days after the onset of KD. | Heart: Acute myocarditis. | Heart: acute myocarditis. Slight microscopic mononuclear cell infiltration in the intima and adventitia of the CAs. | Myocarditis | ||
| 4 y.o. M | In-life diagnosis. Treatment N/A. | He died 2 days after the onset of a spasm of the LAD followed by acute myocardial infarction that occurred during cineangiography. | NA | Heart: Acute anteroseptal myocardial infarction. Abnormal fibrous intimal thickening of the CAs. | AMI | ||
| McCowen and Henderson 1988 [36] | 1 | 12 y.o. M | Postmortem diagnosis. | Sudden death while walking. | Heart: CAAs of the LAD filled with thrombi. The distal LAD and the proximal portion of the RCA showed complete obliteration of the lumen by firm grayish tissue. | Heart: CAA wall showed a loss of smooth muscle and replacement by fibrous connective tissue. An organized and recanalized thrombus within the distal LAD. Other CAs showed extensive media fibrosis and elastic lamella disarray. Lungs: edema and congestion. | AMI |
| Sakai et al. 1988 [37] | 1 | 39 y.o. M | In-life diagnosis. Treatment N/A. | Sudden death while carrying a refrigerator. | Heart: enlarged, LCA aneurysm, partly filled with a gelatin-like substance. Healed myocardial infarction. LAD occlusion. | Heart: the CAAs showed organized thrombi and calcification. Myocardial cells were highly fragmented and atrophied in the noninfarcted area. Healed myocardial infarction was confirmed. | ACF |
| Loubser et al. 1989 [38] | 1 | 2 y.o. F | Postmortem diagnosis. | Hospitalized for palmar erythema, cervical lymphadenopathy, stomatitis, and mild hepatomegaly. Forty-eight hours later, the patient collapsed suddenly and could not be resuscitated. | Heart: RCA occluded by a recent thrombus; LAD wall thickened with a narrowed lumen. | Heart: Coronary arteritis. | AMI |
| Burke et al. 1990 [39] | 1 | 17 y.o. M | Postmortem diagnosis. | Sudden death during exercise. | Heart: healed transmural posteroseptal infarction. Coronary arteries: aneurysm in the proximal segment of the RCA and in the LAD. | Heart: signs of myocardial infarction. CAA with mixed chronic inflammatory infiltrate in the wall. | AMI |
| Corradoet al. 1992 [40] | 1 | 6 y.o. M | Postmortem diagnosis. | Sudden death 3 years after myocardial infarction. | Heart: CAAs and thrombosis, leading to severe ischemic cardiomyopathy. | NA | AMI |
| Naganuma et al. 1992 [41] | 1 | 1 y.o. M | In-life diagnosis. Treated with ASA and IVIG. | Hospitalized for persistent fever, erythematous exanthema, convulsions, and vomiting. The patient recovered. Sudden death occurred 2 months after hospital discharge while the patient was asymptomatic. | Coronary arteries: in the RCA, two defective areas with fresh thrombi and marked stenosis in the RCA. Gradual narrowing of the LCA. Cervical lymph node and thymus swelling. | Heart: The CAs showed marked intima fibrocellular thickening, destruction of the internal elastic lamina and media, and marked periarterial fibrosis of the CAAs. Fresh thrombi within the CA lumen. The LV myocardium showed diffuse and transmural fresh myocardial necrosis. Other vessels: marked perivascular fibrosis with intimal thickening Immunohistochemical staining for desmin in the lesion of the left CAA showed many desmin-positive cells in the intima. | AMI |
| Shauka et al. 1993 [42] | 1 | 24 y.o. M | Postmortem diagnosis. | He had chest pain, and myocardial infarction was diagnosed. | Heart: enlarged with LV hypertrophy. CAAs with an organized thrombus. Mural fibrosis. | NA | AMI |
| Smith and Grider 1993 [43] | 1 | 18 y.o. M | Postmortem diagnosis. | He collapsed during a physical conditioning run. | Heart: thrombosed, fusiform CAAs. | Heart: CAAs with remote and recent thrombosis, calcification, and fibrotic changes. Remote transmural myocardial infarction of the LV and recent myocardial infarction of the RV. | Arrhythmia |
| Kristensen and Kristensen 1994 [44] | 2 | 11 y.o. M | Postmortem diagnosis. | Found dead, no symptoms reported. | Heart: fibrous infarct in the septum. CAAs filled with thrombi. | Heart: CAAs showed intimal proliferative fibrosis, medial thinning with a loss of elastic membranes, and occlusive luminal thromboses. Extensive septal fibrosis. | Arrhythmia |
| 29 y.o. M | Postmortem diagnosis. | Sudden death. | Heart: enlarged with endocardial and myocardial fibrosis. Calcific CAAs (located at the LCA and RCA) with lumen occlusion. | Heart: CAAs with organized thrombus. Myocardium: extensive fibrosis | Arrhythmia | ||
| Lie and Sanders 1997 [45] | 1 | 11 m.o. M | Postmortem diagnosis. | He presented at the ER with fever, irritability, difficulty breathing, and arching of the back. Cardiac arrest occurred within 1 h. | Heart: heavier than average. CAAs with luminal occlusion. | Heart: CAAs with intimal fibrocellular proliferation of organized thrombosis. Other CAs showed cord-like wall thickening. Recent multifocal and healed infarcts in the myocardium. Other vessels: iliac artery aneurysm- | AMI |
| Burke et al. 1998 [46] | 2 | 4 y.o. M | Postmortem diagnosis. | Multiple hospital admissions for fever and abdominal pain. On the day of death, he complained of abdominal pain and became unresponsive. | Heart: diffuse CA thickening and stenosis. | Heart: fibrosis or necrosis of the LV lateral wall. Fibrointimal proliferation with focal destruction of the media and scattered inflammatory infiltrates in the intima, media, and adventitia of the CAs. Focal acute, organized thrombus. | AMI |
| 20 m.o. M | In-life diagnosis. Treated with ASA and IVIG. | At 8 m.o., he was diagnosed with KD. He had cyanosis and limpness, an ECG showed slight ST segment elevation or depression in most leads, and he died suddenly. | Heart: diffuse mottling. Diffuse intimal thickening and mild ectasia with pinpoint lumens of the CAs. | Heart: ongoing CAs arteritis with extensive fibrointimal proliferation and mixed inflammatory infiltrate. Occasional giant cells. Acute subendocardial infarction of the LV. Viscera: arteritis of the hepatic and renal arteries. | AMI | ||
| McConnell et al. 1998 [47] | 1 | 3 y.o. M | In-life diagnosis. Treated with IVIG. | Death occurred 7 months after the onset of KD symptoms. Cardiac arrest during daily maintenance asthma treatment. | Heart: enlarged. Narrowing of the LCA, LAD, and RCA due to fibrosis. | Heart: fibrointimal proliferation with some smooth muscle proliferation of the CAs. Active inflammation with lymphocytes, plasma cells, and occasional eosinophils in all sections of the epicardial arteries. | ACS |
| Fineschi et al. 1999 [48] | 1 | 21 y.o. M | Postmortem diagnosis. | Sudden death while playing soccer. | Heart: small white area of the internal part of the anterior wall of the LV. Calcified saccular CAAs (LAD and RCA) with pronounced stenosis distal to the aneurysms. | Heart: CAA walls showed an internal fibrocalcified layer and an external thin tunica media with an advanced vascularized and organized thrombus. The CA wall distal to the aneurysm showed obliterative intimal thickening (smooth muscle cell proliferation, elastic and fibrous network, and interstitial proteoglycan accumulation) with lumen narrowing. Old myocardial fibrosis with a few small islands of fatty tissue in the center. Foci of myocardial contraction band necrosis. | Arrhythmia |
| Suzuki et al. 1999 [49] | 1 | 4 y.o. F | In-life diagnosis. Treatment NA. | Hospitalized for high fever with swollen lymph nodes, exanthema, strawberry tongue, and conjunctivitis. She died 18 days later, following the rupture of a large aneurysm of the LCA. | Heart: hemopericardium. Dilated LCA and RCA. Giant CAAs. | Heart: CAs vasculitis with proliferation of fibroblasts and myofibroblasts. The ruptured CAA showed panvasculitis accompanied by the destruction of elastic laminae. | CAA rupture |
| Kazuma et al. 2000 [50] | 1 | 11 y.o. M | In-life diagnosis. | Sudden death while playing soccer. | Heart: thin at the apex of the LV. Ventricular wall fibrosis. Old myocardial infarction. Giant calcified CAA (LAD). | Heart: fibrosis, old myocardial infarction. Giant calcified aneurysm. | Arrhythmia |
| Maresi E. et al. 2001 [51] | 1 | 2 m.o. M | Postmortem diagnosis. | Hospitalized for rhinitis, coughing, conjunctival hyperemia, and allergic exanthema. Sudden death after seven days. | Heart: LV hypertrophy. Hemopericardium. Perforated CAA of the LAD. | Heart: transmural lymphomonocytic inflammation of the CAA wall with some eosinophils, edema, and necrosis at the site of rupture. Chronic inflammation in the proximity of the epicardial coronary vessels. Small foci of active lymphocytic myocarditis. | CAA rupture |
| Heaton and Wilson 2002 [52] | 2 | 9 m.o. M | In-life diagnosis. Treated with ASA and IVIG. | On day 95 after the beginning of KD symptoms, the patient died from myocardial infarction. | Heart: pale endocardium of the LV free wall and interventricular septum. Prominent segmental mural thickening and luminal stenosis of the CAs with mild CAAs of the LAD and RCA. | Heart: extensive patchy, acute subendocardial myocardial infarction. Circumferential mural fibrosis with chronic inflammatory cell infiltrate of the epicardial CAs, causing luminal stenosis. | AMI |
| 4 y.o. M | In-life diagnosis. Treated with ASA and IVIG. | LV function impairment after ten weeks of KD symptoms. He died after the induction of general anesthesia for cardiac catheterization. | Heart: thickening of the CA wall, LAD dilation. | Heart: fibrocellular intimal proliferation, causing severe luminal stenosis of all CAs. Myocardial infarction. Viscera: widespread systemic arteritis affecting other large- and middle-sized vessels with significant stenoses of the splenic, renal, superior, and distal mesenteric arteries. Duodenal infarction. | AMI | ||
| Bartoloni et al. 2002 [53] | 1 | 21 y.o. M | Postmortem diagnosis. | Found dead on his bed after some days of fever, arthralgia, and emesis. | Heart: CAA of the CX with an occlusive recent thrombosis in the distal half of the artery. | Heart: foci of myocarditis. Posterior myocardial infarction. CA vasculitis and perivasculitis. The CAA wall showed polymorphous inflammatory infiltrate. | AMI |
| Rozin et al. 2003 [54] | 1 | 21 y.o. M | Postmortem diagnosis | Found unresponsive on his bed. | Heart: LV hypertrophy. Focal myocarditis. Calcified CAA of the LAD, filled with a thrombus. Similar CAA in the CX. Minimal RCA aneurysmal dilation. Other organs: congestion and edema of the lungs, hepatosplenomegaly. | Heart: focal myocarditis. Large calcified fusiform CAAs filled with thrombi plus mural thickening. | ACS |
| Freema et al. 2005 [55] | 8 | 10 y.o. M | In-life diagnosis. Treated with IVIG/ASA. | Sudden death 13 days after the onset of KD symptoms. | NA | Heart: microvessel density count showed grade 3 or 4 inflammation in the myocardium and CAA. The CAA wall was disrupted. VEGF, PDGF-A, and bFGF were detected diffusely in the myocardium. Angiostatin was detected predominantly in inflammatory cells in the CAA adventitia. Mast cells were present in the CAA adventitia and myocardium. | CAA rupture |
| 3 m.o. F | In-life diagnosis. No treatment. | Sudden death 14 days after the onset of KD symptoms. | NA | Myocarditis | |||
| 11 m.o. M | Postmortem diagnosis. | Sudden death 2 weeks after the onset of KD symptoms. | NA | CAA rupture | |||
| 4 m.o. M | In-life diagnosis. No treatment. | Sudden death 17–18 days after the onset of KD symptoms. | NA | AMI | |||
| 4 m.o. M | In-life diagnosis. Treated with IVIG/ASA. | Sudden death 3–4 weeks after the onset of KD symptoms. | NA | Myocarditis | |||
| 4 m.o. M | Postmortem diagnosis. | Sudden death 4 weeks after the onset of KD symptoms. | NA | ACF | |||
| 7 m.o. M | In-life diagnosis. No treatment. | Sudden death 4 weeks days after the onset of KD symptoms. | NA | AMI | |||
| 10 m.o. F | In-life diagnosis. No treatment. | Sudden death 5 weeks after the onset of KD symptoms. | NA | AMI | |||
| Tsuda et al. 2005 [56] | 12 | 8 y.o. M | In-life diagnosis. No treatment. | Sudden death 4 years after KD onset. | Heart: coronary arterial lesions, acute and old myocardial infarction, CAAs, and severe localized stenosis of the LAD. | Heart: acute and old myocardial infarction. CA stenosis. | AMI |
| 1 y.o. M | In-life diagnosis. Treated with ASA and dipyridamole | Sudden death some months after KD onset and five days after cardiac catheterization. | Heart: acute inferior myocardial infarction with occlusion of the RCA. | Heart: acute and old myocardial infarction. CA stenosis. | AMI | ||
| 1 y.o. M | In-life diagnosis. Treated with ASA, nitrates, and CA antagonist | Sudden death more than 1 year after KD onset and 2 months after CA bypass grafting due to a distal aneurysm at the LAD. | Heart: patency of the grafts, but occlusion of the distal LAD. | CA stenosis. | AMI | ||
| 1 y.o. M | In-life diagnosis. Treated with flurbiprofen. | Sudden death some months after KD onset. | NA | NA | Undefined | ||
| 15 y.o. M | In-life diagnosis. Treated with ASA and dipyridamole. | Sudden death while playing soccer 4 years after KD onset. Diagnosed with segmental stenosis of the RCA and localized stenosis of the LAD. | NA | NA | Undefined | ||
| 18 y.o. M | In-life diagnosis. Treated with flurbiprofen. | Sudden death 12 years after KD onset. | NA | NA | AMI | ||
| 16 y.o. M | In-life diagnosis. Treated with ASA and warfarin. | Sudden death 5 years after KD onset. Diagnosed with a giant aneurysm of the LCA and occlusion of the RCA. He previously suffered from an inferior myocardial infarction. | NA | NA | Undefined | ||
| 22 y.o. M | In-life diagnosis. Treated with ASA. | Sudden death some months after KD onset. He previously suffered from myocardial infarction and was diagnosed with CX stenosis. | NA | NA | Undefined | ||
| 22 y.o. M | In-life diagnosis. Treated with nitrates and CA antagonist. | Sudden death six months after KD onset. Diagnosed with segmental stenosis of the LAD, CX, and RCA. | NA | NA | Undefined | ||
| 19 y.o. F | In-life diagnosis. Treated with flurbiprofen, a diuretic, and digoxin. | Sudden death six months after KD onset. She previously had a myocardial infarction. | NA | NA | Undefined | ||
| 27 y.o. F | In-life diagnosis. Treated with ASA, warfarin, ACE inhibitor, and beta-blocker D. | Sudden death about two years after the onset of KD symptoms. Diagnosed with myocardial infarction and mitral regurgitation and previously underwent coronary artery bypass grafting surgery and mitral annuloplasty. | NA | NA | Undefined | ||
| 26 y.o. M | In-life diagnosis. Treated with ASA and ACE inhibitor. | Found dead about 3 years after KD onset. He suffered from a previous myocardial infarction and LV dysfunction. He was diagnosed with segmental stenosis of the RCA. | NA | NA | Undefined | ||
| Ozdoguand Boga 2005 [57] | 1 | 18 y.o. M | In-life diagnosis. Treated with IVIG. | He suffered from inflammatory myositis, lymphadenopathy, and mucosal changes. He died from massive hemorrhagic pericardial effusion and cardiac tamponade. | NA | NA | Cardiac tamponade |
| Diana et al. 2006 [58] | 1 | 4 m.o. F | Postmortem diagnosis. | Previous episode of heart block. Sudden death shortly after recovery. | Heart: arteritis with a significant degree of aneurysmal dilatation of the LAD and RCA. Thrombosis. | CA arteritis. CAA with thrombosis. | ACS |
| Sunagawa et al. 2008 [59] | 1 | 5 y.o. M | In-life diagnosis. Treatment N/A. | Sudden death following the rupture of a giant aneurysm of the LAD. | Heart: enlarged. Hemopericardium. CAA of the LAD with a fissure. Thrombus attached to the aneurysmal wall. CX and RCA dilation. | Heart: CAA and LAD CAA rupture, panvasculitis with inflammatory cell infiltration (monocytes, lymphocytes, plasmacytes, and macrophages), fibrinoid-like necrosis, and massive destruction of elastic fibers and smooth muscle cells. A small thrombus with focal organization in the aneurysmal wall. Inflammatory cells were seen in the immunohistochemical examination. | CAA rupture |
| Papadodima et al. 2009 [60] | 1 | 11 y.o. M | Postmortem diagnosis. | Admitted to the hospital complaining from 5 days of fever and hematuria. After almost 2 weeks of hospitalization, he showed melena and intense abdominal pain and then suddenly died. | Heart: enlarged, intermediate edema. Multiple thrombi within the CAs. | Heart: intramural dense, polymorphonuclear inflammatory infiltration and necrosis of the CAs. CA thrombosis. | ACS |
| Yokouchi et al. 2010 [61] | 1 | 40 y.o. F | Postmortem diagnosis. | Sudden death. He was a smoker and previously had an acute myocardial infarction that was treated with stent implantation. He developed multiple stent reocclusions. | Heart: Extensive fibrosis, fresh infarction in the anterior and posterior walls. CAAs in the RCA and LCA. | Heart: rupture of the internal elastic lamina of the LCA aneurysm, which had thrombi within the lumen. The RCA aneurysm was calcified with an organized thrombus. The lumen of each CA stent was occluded by thrombi and extensive inflammatory infiltration. Other CAs showed diffuse concentric intimal thickening, extension, and focal rupture of the internal elastic lamina, and focal destruction of the tunica media. Aorta: slight fibrotic thickening of the intima, few atherosclerotic changes. | Arrhythmia |
| Pucci et al. 2012 [62] | 2 | 3 m.o. F | Postmortem diagnosis. | Sudden cardiac arrest after eight days of intermittant fever, red and cracked lips, maculopapular rash. | Heart: pericardial effusion. CAAs with occlusive thrombosis of the LAD. | Heart: CA thrombosis associated with inflammatory infiltration in the CAA wall. Contraction bands and multiple foci of T-lymphocytic myocarditis. | AMI |
| 3 m.o. M | Postmortem diagnosis. | Sudden cardiac arrest after ten days of intermittant fever, bronchiolitis, and exanthema. | Heart: moderate pericardial effusion. Ectatic cord-like shaped coronary arteries with subocclusive or laminar thrombosis and multiple CAAs. | Heart: CAA thrombosis associated with inflammatory infiltrate. Multiple foci of coagulative necrosis and diffuse T-lymphocytic infiltrates. | AMI | ||
| Ponniah 2013 [63] | 1 | 6 m.o. M | Postmortem diagnosis. | Diagnosed with CAA due to KD, died from extensive CA thrombosis. | Heart: multifocal myocardial scars. CAAs with occluding thrombus. | Heart: CAAs with thrombi and intimal wall thickening. | ACS |
| Okura et al. 2013 [64] | 1 | 30 y.o. M | In-life diagnosis. Treatment N/A. | Sudden death. Previously diagnosed with acute KD and a complicated CAA. | Postmortem CT: coarse calcification of the CA aneurysm | Thin CAA wall with myointimal proliferation associated with a disrupted internal elastic lamina and medial smooth muscle necrosis with replacement by fibrocalcification. | ACS |
| Miyamoto et al. 2014 [65] | 1 | 3 m.o. M | In-life diagnosis. Treated with IVIG and prednisolone sodium metazoate. | Sudden death due to CAA rupture. | RCA aneurysm rupture combined with multiple CAAs in the CX and LAD. | CAA: rupture. Thickened aneurysmal wall with the presence of inflammation. | CAA rupture |
| Shimiz et al. 2015 [66] | 2 | 22 y.o. M | In-life diagnosis. Treated with IVIG twice, followed by ASA and dipyridamole | Sudden death | Heart: enlarged, smooth epicardium. Pale myocardial areas. CAA of the LAD. The distal artery was very small and partially occluded. | Heart: Adventitial sparse cellular fibrosis, extensive dystrophic calcification, and regions of chondro-ossification of the LAD. Patchy myocardium, widespread fibrosis with compensatory hypertrophic changes, acute ischemia areas. Contraction band necrosis. Other viscera: congestion with marked pulmonary edema and congestion. | AMI |
| 30 y.o. M | Postmortem diagnosis. | Found unresponsive. | Heart: enlarged, smooth epicardium, posterior apical myocardium infarct with patchy interstitial fibrosis and pink discoloration. CAA of the LAD, which had a thin and calcified wall and a thrombus within the lumen. Re-canalized CAA with multiple lumina of the RCA. | Heart: focal interstitial fibrosis and scattered thickened myocytes with enlarged nuclei. Asymmetric transmural fibrosis of the LAD with dystrophic calcification, causing eccentric narrowing of the lumen that was occluded by an acute thrombus. The CAA wall showed a small focus of calcification, diffuse fibrosis, and a disrupted internal elastic lamina. | AMI | ||
| Parsons and Lynch 2016 [67] | 1 | 22 y.o. M | In-life diagnosis. Treatment N/A. | Sudden death. | Heart: enlarged, LV hypertrophy, myocardial fibrosis, probe patent foramen ovale. CAA of the LAD. | Heart: myocardial fibrosis. CA stenosis. | ACS |
| Chang et al. 2016 [68] | 1 | 6 y.o. M | Postmortem diagnosis. | Suddenly died while experiencing nausea, feeling unwell, and dizziness. | Heart: CAAs. A recent thrombus within a CAA lumen. | CAA: focal but minimal lymphoplasmacytic infiltration in the LCA and myofibroblastic proliferation within the LAD, CX, and RCA. Heart: acute myocardial infarction, partly transmural, affecting the anteroseptal and anterolateral LV. An old healed subendocardial infarct along the basal half of the lateral wall of the LV and patchy subendocardial fibrosis affecting the posteroseptal wall of the LV. | AMI |
| Wei et al. 2016 [69] | 7 | 7 y.o. M | In-life diagnosis. Treated. | Chest pain, abdominal pain, vomiting, and dyspnea and then died four years after the onset of KD symptoms. | 4 CAs involved. | NA | AMI |
| 9 y.o. M | In-life diagnosis. Treated. | Chest pain, abdominal pain, vomiting, and dyspnea and then died two years after the onset of KD symptoms. | 3 CAs involved. | NA | AMI | ||
| 8 y.o. F | In-life diagnosis. Treated. | Chest pain, abdominal pain, vomiting, and dyspnea and then died four years after the onset of KD symptoms. | 2 Cas involved. | NA | AMI | ||
| 4 y.o. M | In-life diagnosis. Treated. | Sudden death 3 years after the onset of KD symptoms. | 2 CAs involved. | NA | Undefined | ||
| 17 y.o. F | In-life diagnosis. Treated. | Sudden death 13 years after the onset of KD symptoms. | 3 CAs involved. | NA | Undefined | ||
| 10 m.o. M | In-life diagnosis. Treated. | Sudden death 5 months after the onset of KD symptoms. | 1 CA involved. | NA | CAA rupture | ||
| 1 y.o. M | In-life diagnosis. Treated. | Sudden death 29 days after the onset of KD symptoms. | Heart: pancarditis and hemopericardium. CAAs with thrombosis. Other organs: multiple small arteries and veins were involved. | Heart: pancarditis. CAA rupture. CA thrombosis. | CAA rupture | ||
| Yajima et al. 2016 [70] | 1 | 5 m.o. M | Postmortem diagnosis. | Hospitalized for fever and suppuration at the site of (BCG) vaccination. He suddenly died after hospital discharge. | Heart: fluid in the pericardium. Epicardium petechiae. Other viscera: pleural fluid, fluid in thetracheal space, pleural petechiae. Small subcapsular liver hemorrhages. Brain edema. | Heart: myocardial and CA inflammatory infiltrations (mononuclear cells, mainly lymphocytes). Emboli within the CAs. Other viscera: inflammation of the lungs, liver, and kidneys. | AMI |
| Fukazawa et al. 2017 [71] | 11 | N/A | 5 cases, in-life diagnosis. Treated with IVIG. | Death due to CA rupture within 1 month from the onset of KD symptoms. | NA | NA | 5 CAA rupture |
| N/A | 6 cases, in-life diagnosis. Treatment N/A. | Most deaths due to MI occurred from 6 months to 2 years from the onset of KD symptoms., | NA | NA | 6 AMI | ||
| Kim et al. 2018 [72] | 1 | 23 m.o. M | Postmortem diagnosis. | Sudden death 6 weeks after an episode of mild fever, general myalgia, and diarrhea. | Heart: pericardial yellowish effusion. Myocardium infarction, both recent and old. CAAs. | Heart: foci of acute and old infarction. The LAD wall showed mural lymphoplasmacytic infiltration, adventitial fibrosis, and neovascularization. Polymorphonuclear leukocyte and lymphocyte infiltration with focal acute necrosis, which resulted in the destruction of the internal elastic lamina at the RCA. | AMI |
| Zhang et al. 2018 [73] | 1 | 5 y.o. M | Postmortem diagnosis | Sudden death due to the rupture of a CAA during the acute phase of KD. | Heart: hemopericardium. Ruptured CAA of the LAD. | Heart: granulation tissue in cardiac adventitia. Necrotic cardiac muscle fibers in the myocardium. The CAA showed transmural inflammation, disruptions of the internal elastic lamina, smooth muscle, and inflammatory infiltrates with edema and variable necrosis of the intima, media, and adventitia. Mixed thrombosis in the aneurysmal lumen. | CAA rupture |
| Pachec et al. 2019 [74] | 1 | 13 y.o. M | Postmortem diagnosis | Sudden death 2 weeks after streptococcal pharyngitis. | Heart: enlarged, mild epicarditis, pale myocardium. CA luminal stenosis. | Heart: globally edematous myocardium. CA wall showed inflammatory infiltrates, muscular-layer destruction, and a loss of elastic tissue. Some vessels had recent thrombi. | ACS |
| Flossdorf et al. 2020 [75] | 1 | 20 y.o. M | Postmortem diagnosis. | Found dead after vomiting the day before. | Heart: ventricular aneurysm below the aortic valve. Multiple myocardial scars. CAAs filled with thrombi. | Heart: the CAA wall showed thickening of the intima, thin media with only a small residual amount of smooth muscle cells, and transmural inflammation with circumscribed calcified plaques at the border to the innermost layer of the arteries. Neoangiogenesis around the vessels. CA thrombosis and stenosis. | ACS |
| Zhang and Wang 2021 [76] | 1 | 1 y.o. M | Postmortem diagnosis | Hospitalized for fever and cough with a preliminary diagnosis of acute severe bronchial pneumonia but no typical KD characteristics. After antibiotics and supportive treatment, the condition worsened and then he died. | Heart: cable-shaped bulge at the anterior area of the heart. | Coronary artery: inflammatory granulation tissue. Wall thrombosis and LCA malformation accompanied by vasculitis. | ACS |
| Staats et al. 2021 [77] | 1 | 4 y.o. F | In-life diagnosis. Treated with warfarin, atorvastatin, and ASA. | Sudden death. Previously diagnosed with CAAs. | Heart: diffuse patchy, pale areas of scar tissue in the interventricular septum, associated with severe ischemic changes of cardiomyocytes. Luminal obliteration of her coronary aneurysms with organized thrombi. | Heart: myocardial fibrosis, ischemic changes. CA stenosis and thrombosis. | Arrhythmia |
| Maeda et al. 2021 [78] | 1 | 2 y.o. F | In-life diagnosis. Treated with ASA and IVIG. | Hospitalized for fever and cervical lymphadenopathy. On day 12 after the beginning of symptoms, she vomited and had generalized tonic–clonic seizures without recovery of consciousness. Cranial MRI showed vasogenic edema. She died in the following hours. | NA | NA | Acute encephalitis |
| Ayusawa et al. 2022 [79] | 14 | School-aged F | In-life diagnosis. | Sudden death. Previously diagnosed with KD and CABG. | NA | NA | Undefined |
| School-aged F | In-life diagnosis. | Sudden death. Previously diagnosed with KD and AMI. | Heart: fibrosis at the posterior and lateral walls of the LV. Dilative hypertrophy of the RV, Weight of the heart: 340 g. Coronary arteries: LCA aneurysm with total occlusion, RCA aneurysm. | Heart: myocardial fibrosis. | AMI | ||
| School-aged M | In-life diagnosis. | Sudden death. Previously diagnosed with KD and CAA. | NA | NA | Undefined | ||
| School-aged F | In-life diagnosis. | Sudden death. Previously diagnosed with KD and CAAs. | NA | NA | Undefined | ||
| School-aged M | In-life diagnosis. | Sudden death. Previously diagnosed with KD and CAA. | NA | NA | AMI | ||
| School-aged M | In-life diagnosis. | Sudden death. Previously diagnosed with KD. | NA | NA | ACF | ||
| School-aged M | In-life diagnosis. | Sudden death. Previously diagnosed with KD. | NA | NA | AMI | ||
| School-aged M | In-life diagnosis. | Sudden death. Previously diagnosed with KD. | NA | NA | AMI | ||
| School-aged M | In-life diagnosis. | Sudden death. Previously diagnosed with KD and CAA. | Heart: ischemic heart disease. CA atherosclerosis, RCA aneurysm. | NA | Undefined. | ||
| School-aged M | In-life diagnosis. | Sudden death. Previously diagnosed with KD. | NA | NA | ACF | ||
| School-aged M | In-life diagnosis. | Sudden death. Previously diagnosed with KD. | NA | NA | AMI | ||
| School-aged M | In-life diagnosis. | Sudden death. Previously diagnosed with KD. | NA | NA | Arrhythmia | ||
| School-aged M | In-life diagnosis. | Sudden death. Previously diagnosed with KD and CAA. | NA | NA | AMI | ||
| School-aged M | In-life diagnosis. | Sudden death. Previously diagnosed with KD. | NA | NA | Arrhythmia |
| Characteristics | Number of Cases (tot = 117) | ||
|---|---|---|---|
| Sex | Male | 86 (73.50%) | |
| Female | 20 (17.09%) | ||
| NA | 11 (9.40%) | ||
| Age (y.o.) | <5 | 28 (23.93%) | |
| 6–12 | 26 (22.22%) | ||
| 13–20 | 27 (23.08%) | ||
| >20 | 10 (8.55%) | ||
| NA | 15 (12.82%) | ||
| Diagnosis | In-life (n = 80) | Treated | 37 (31.63%) |
| Not treated | 7 (5.98%) | ||
| Unknown | 36 (30.77%) | ||
| Postmortem | 37 (31.62%) | ||
| Characteristics | Cause of Death | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| AMI | ACF | Peri-carditis | Pan-carditis | Myo-Carditis | Arrhythmia | ACS | CAA Rupture | Cardiac Tamponade | Acute Encephalitis | Cerebral Hemorrhage | Cerebral Hypoxia | Undefined | ||
| Sex | Male | 36 | 6 | 2 | 1 | 4 | 8 | 9 | 8 | 1 | - | 1 | 1 | 9 |
| Female | 6 | 1 | - | 1 | 2 | 2 | 1 | 1 | - | 1 | - | - | 5 | |
| Unknown | 6 | - | - | - | - | - | - | 5 | - | - | - | - | - | |
| Age (y.o.) | <5 | 9 | 3 | 2 | 2 | 5 | - | 2 | 4 | - | - | 1 | - | - |
| 6–12 | 12 | 1 | - | - | 1 | 2 | 2 | 4 | - | 1 | - | 1 | 2 | |
| 13–20 | 15 | 2 | - | - | - | 4 | 1 | 1 | - | - | - | - | 4 | |
| >20 | 2 | - | - | - | - | 1 | 2 | - | 1 | - | - | - | 4 | |
| N/A | 4 | 1 | - | - | - | 3 | 3 | - | - | - | - | - | 4 | |
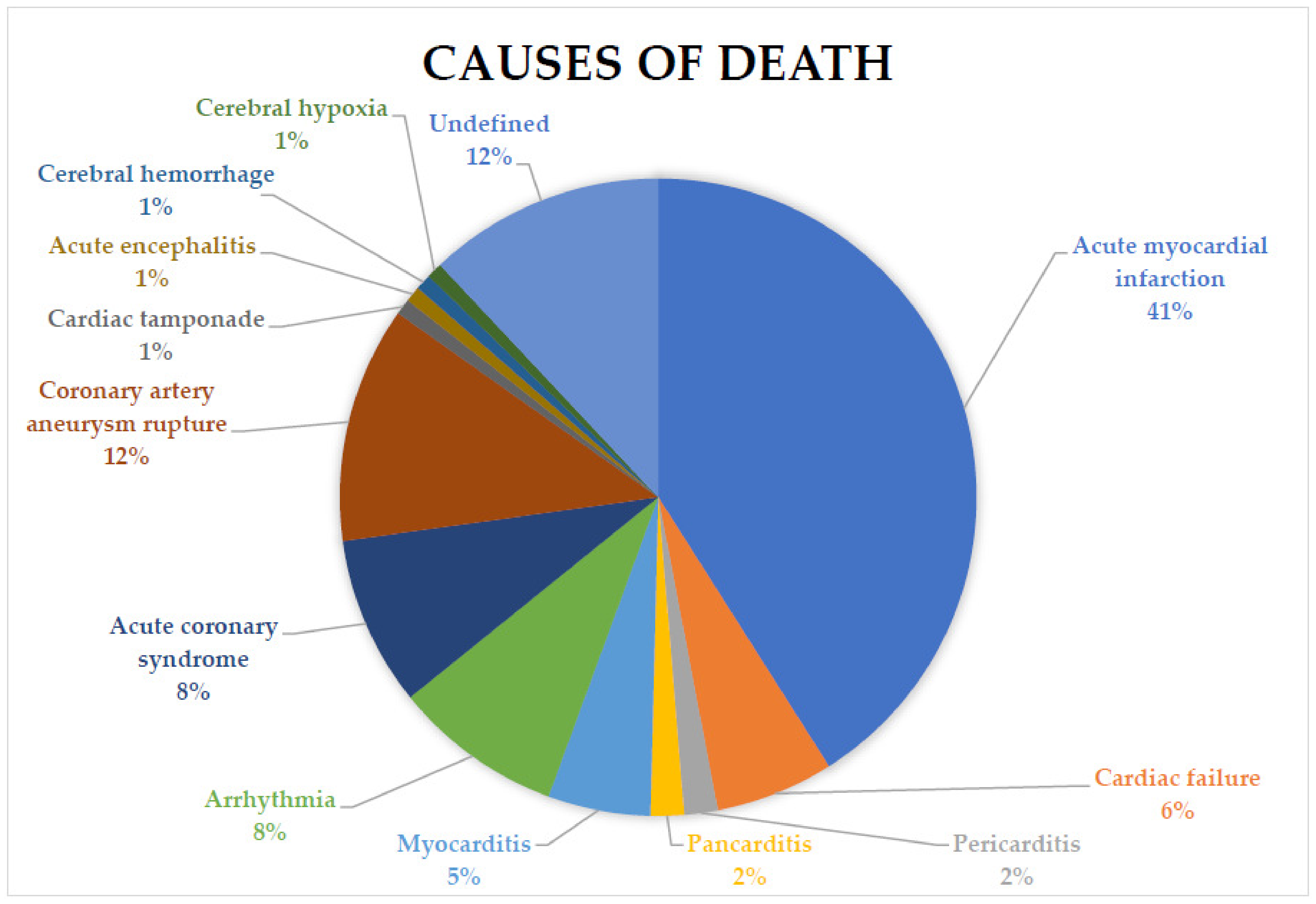
| Total | 48 (41.03%) | 7 (5.98%) | 2 (1.71%) | 2 (1.71%) | 6 (5.13%) | 10 (8.55%) | 10 (8.55%) | 14 (11.97%) | 1 (0.85%) | 1 (0.85%) | 1 (0.85%) | 1 (0.85%) | 14 (11.97%) | |
| Autoptic Data Gross Findings | |||
|---|---|---|---|
| Cardiac Death | Cerebral Death | Undefined Death | |
| Enlarged heart | 19 | - | - |
| Heart hypertrophy | 4 | - | - |
| Hemopericardium | 3 | - | - |
| Pericardial effusion | 7 | 1 | - |
| Myocardial fibrosis | 21 | - | - |
| Coronary thrombosis * | 7 | 1 | - |
| Coronary stenosis * | 18 | - | - |
| CAA ** | 17 | - | 1 |
| CAAs *** | 29 | 1 | - |
| CAA filled with thrombus | 22 | 1 | - |
| CAA rupture | 14 | - | - |
| Aneurysms of other vessels | 1 | - | - |
| Thrombosis of other vessels | 2 | 1 | - |
| Other visceral involvement | 1 | 1 | - |
| Other visceral congestion | 4 | - | - |
| Subarachnoid hemorrhage | - | 1 | - |
| Total cases | 65 | 1 | 1 |
| Histopatological Findings | ||
|---|---|---|
| Cardiac Death | Cerebral Death | |
| Acute myocardial infarction | 17 | - |
| Myocardial fibrosis | 17 | - |
| Myocardial necrosis | 11 | - |
| Myocardial inflammatory infiltrates | 14 | - |
| Pericardial inflammatory infiltrates | 1 | - |
| Peri-, myo-, and endocardium inflammatory infiltrates | 3 | - |
| Coronary artery stenosis | 16 | - |
| Coronary artery fibrosis | 13 | - |
| Coronary artery thrombosis | 19 | 1 |
| Coronary thrombus recanalization | 1 | - |
| Coronary artery wall inflammatory infiltrates | 26 | - |
| Coronary artery wall thickening | 9 | - |
| Coronary artery wall necrosis | 4 | - |
| Coronary wall thinning | 3 | - |
| CAA thrombosis | 17 | 1 |
| CAA wall fibrosis | 10 | - |
| CAA wall calcification | 7 | - |
| CAA thrombus recanalization | 2 | - |
| CAA rupture | 7 | - |
| Neoangiogenesis | 4 | - |
| Systemic vessels wall inflammatory infiltrates | 7 | - |
| Systemic vessels thrombosis | 2 | 1 |
| Kidney infarction | 1 | - |
| Renal artery stenosis | 1 | - |
| Cerebral hemorrhage | - | 1 |
| Total cases | 71 | 1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Visi, G.; Spina, F.; Del Duca, F.; Manetti, A.C.; Maiese, A.; La Russa, R.; Frati, P.; Fineschi, V. Autoptic Findings in Cases of Sudden Death Due to Kawasaki Disease. Diagnostics 2023, 13, 1831. https://doi.org/10.3390/diagnostics13111831
Visi G, Spina F, Del Duca F, Manetti AC, Maiese A, La Russa R, Frati P, Fineschi V. Autoptic Findings in Cases of Sudden Death Due to Kawasaki Disease. Diagnostics. 2023; 13(11):1831. https://doi.org/10.3390/diagnostics13111831
Chicago/Turabian StyleVisi, Giacomo, Federica Spina, Fabio Del Duca, Alice Chiara Manetti, Aniello Maiese, Raffaele La Russa, Paola Frati, and Vittorio Fineschi. 2023. "Autoptic Findings in Cases of Sudden Death Due to Kawasaki Disease" Diagnostics 13, no. 11: 1831. https://doi.org/10.3390/diagnostics13111831
APA StyleVisi, G., Spina, F., Del Duca, F., Manetti, A. C., Maiese, A., La Russa, R., Frati, P., & Fineschi, V. (2023). Autoptic Findings in Cases of Sudden Death Due to Kawasaki Disease. Diagnostics, 13(11), 1831. https://doi.org/10.3390/diagnostics13111831







