Positive p53 Expression Is Associated with Primary Endocrine Therapy Resistance in Locally Advanced Stage Luminal B HER2-Negative Breast Cancer Patients: A Cross-Sectional Study in Indonesia
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patients and Samples
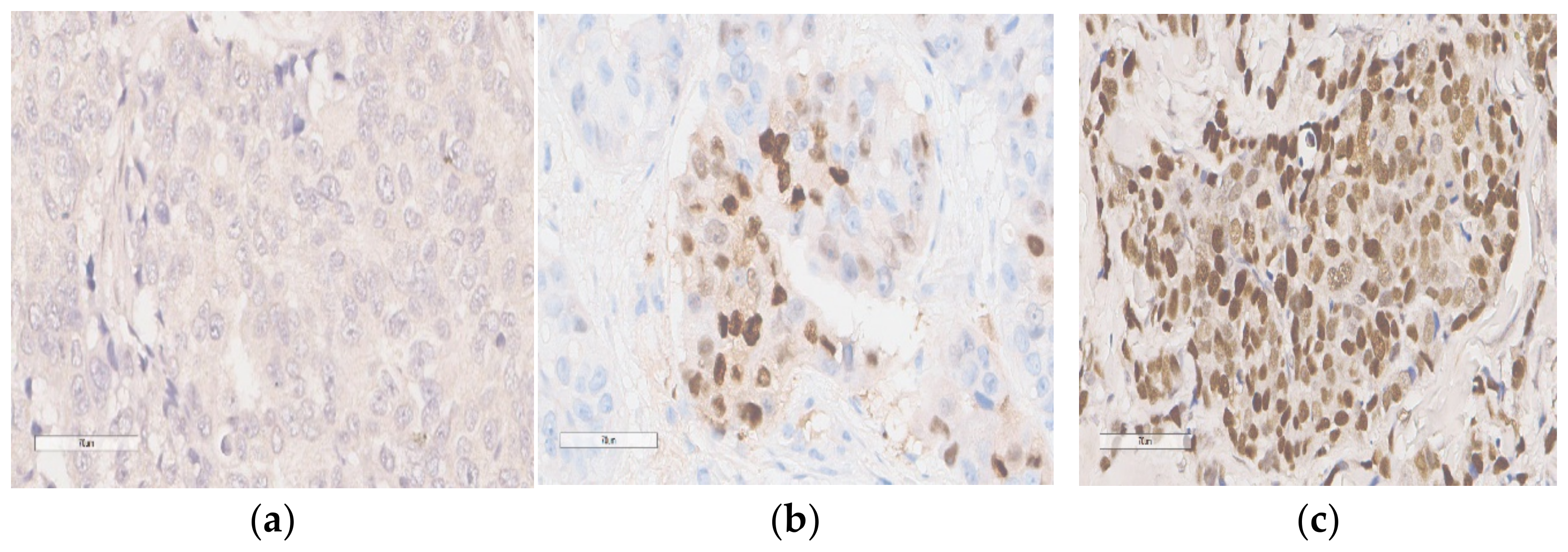
2.2. Immunohistochemical Analysis for p53 Expression
2.3. Statistical Analysis
3. Results
Figures and Tables
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- WHO. GLOBOCAN 2020: New Global Cancer Data [Internet]. 2020. Available online: https://www.who.int/publications/m/item/cancer-idn-2020 (accessed on 12 May 2021).
- Nasional IPDB dan JK Profil Kanker Timja Payudara RS Kanker Dharmais [Internet]. Jakarta. 2020. Available online: https://dharmais.co.id/srikandi/wp-content/uploads/2021/01/Format-Website_Timja-Kanker-Payudara.pdf (accessed on 8 May 2023).
- Widiana, I.K.; Irawan, H. Clinical and Subtypes of Breast Cancer in Indonesia. Asian Pac. J. Cancer Care 2020, 5, 281–285. [Google Scholar] [CrossRef]
- Robinson, M.; Atmakusumah, T.D.; Irawan, C.; Shatri, H. Immunohistochemistry Profile of Breast Cancer Patients that Get Anthracyclin—Based Chemotherapy in RSUD Kota Bogor. J. Penyakit Dalam Indones. 2019, 6, 173–177. [Google Scholar] [CrossRef]
- Meilani, I.T.; Minhajat, R. Hubungan Faktor Risiko dengan Ekspresi ER, PR, HER2 pada Pasien Kanker Payudara di RSUP Wahidin Sudirohusodo.Makassar. 2017. Available online: http://digilib.unhas.ac.id/uploaded_files/temporary/DigitalCollection/OGQyNjkxNGY3Y2FjYTBlODk3ZmFhMGM3ZWE2ZWRiZmI1ZWE4M2Q0ZQ==.pdf (accessed on 19 May 2023).
- Metzger-Filho, O.; Sun, Z.; Viale, G.; Price, K.N.; Crivellari, D.; Snyder, R.D.; Gelber, R.D.; Castiglione-Gertsch, M.; Coates, A.S.; Goldhirsch, A.; et al. Patterns of recurrence outcome according to breast cancer subtypes in lymph node-negative disease: Results from international breast cancer study group trials VIII and IX. J. Clin Oncol. 2013, 31, 3083–3090. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.H.; Hu, P.H.; Tu, J.H.; Yu, N.S. Luminal B breast cancer: Patterns of recurrence and clinical outcome. Oncotarget 2016, 7, 65024–65033. [Google Scholar] [CrossRef]
- Yang, Z.J.; Yu, Y.; Hou, X.W.; Chi, J.R.; Ge, J.; Wang, X.; Cao, X.C. The prognostic value of node status in different breast cancer subtypes. Oncotarget 2017, 8, 4563–4571. [Google Scholar] [CrossRef]
- Hanker, A.B.; Sudhan, D.R.; Arteaga, C.L. Overcoming Endocrine Resistance in Breast Cancer. Cancer Cell 2020, 37, 496–513. [Google Scholar] [CrossRef]
- CCardoso, F.; Paluch-Shimon, S.; Senkus, E.; Curigliano, G.; Aapro, M.S.; André, F.; Barrios, C.H.; Bergh, J.; Bhattacharyya, G.S.; Biganzoli, L.; et al. 5th ESO-ESMO international consensus guidelines for advanced breast cancer (ABC 5). Ann. Oncol. 2020, 31, 1623–1649. [Google Scholar] [CrossRef]
- Krauss, K.; Stickeler, E. Endocrine Therapy in Early Breast Cancer. Breast Care 2020, 15, 337–346. [Google Scholar] [CrossRef]
- Luque-Bolivar, A.; Pérez-Mora, E.; Villegas, V.E.; Rondón-Lagos, M. Resistance and overcoming resistance in breast cancer. Breast Cancer Targets Ther. 2020, 12, 211–229. [Google Scholar] [CrossRef]
- Haque, M.M.; Desai, K.V. Pathways to Endocrine Therapy Resistance in Breast Cancer. Front. Endocrinol. 2019, 10, 573. [Google Scholar] [CrossRef]
- Halim, F.; Azhar, Y.; Suwarman, S.; Hernowo, B. p53 Mutation as Plausible Predictor for Endocrine Resistance Therapy in Luminal Breast Cancer. F1000Research 2022, 11, 330. [Google Scholar] [CrossRef]
- Coates, A.S.; Millar, E.K.; O’Toole, S.A.; Molloy, T.J.; Viale, G.; Goldhirsch, A.; Regan, M.M.; Gelber, R.D.; Sun, Z.; Castiglione-Gertsch, M.; et al. Prognostic interaction between expression of p53 and estrogen receptor in patients with node-negative breast cancer: Results from IBCSG Trials VIII and IX. Breast Cancer Res. 2012, 14, R143. [Google Scholar] [CrossRef]
- Ahn, S.H.; Kim, H.J.; Han, W.; Cho, J.; Gong, G.; Jung, K.H.; Kim, S.B.; Son, B.H.; Lee, J.W. Breast Cancer Effect Modification of Hormonal Therapy by p53 Status in Invasive Breast Cancer. J. Breast Cancer 2013, 16, 386–394. [Google Scholar] [CrossRef]
- Jia, X.Q.; Hong, Q.; Cheng, J.Y.; Li, J.W.; Wang, Y.J.; Mo, M.; Shao, Z.M.; Shen, Z.Z.; Liu, G.Y. Accumulation of p53 is prognostic for aromatase inhibitor resistance in early-stage postmenopausal patients with ER-positive breast cancer. Onco Targets Ther. 2015, 8, 549–555. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.K.; Bae, S.Y.; Lee, J.H.; Lee, H.; Yi, H.; Kil, W.H. Distinguishing Low-Risk Luminal A Breast Cancer Subtypes with Ki-67 and p53 Is More Predictive of Long-Term Survival. PLoS ONE 2015, 10, e0124658. [Google Scholar] [CrossRef]
- Abubakar, M.; Guo, C.; Koka, H.; Sung, H.; Shao, N.; Guida, J.; Deng, J.; Li, M.; Hu, N.; Zhou, B.; et al. Clinicopathological and epidemiological significance of breast cancer subtype reclassification based on p53 immunohistochemical expression. Npj Breast Cancer 2019, 5, 20. [Google Scholar] [CrossRef]
- Chuangsuwanich, T.; Pongpruttipan, T.; O-charoenrat, P.; Komoltri, C.; Watcharahirun, S.; Sa-nguanraksa, D. Clinicopathologic features of breast carcinomas classified by biomarkers and correlation with microvessel density and VEGF expression: A study from Thailand. Asian Pac. J Cancer Prev. 2014, 15, 1187–1192. [Google Scholar] [CrossRef]
- Noah, N. The STROBE Initiative STrengthening the Reporting of OBservational studies in Epidemiology (STROBE) [Internet]. Epidemiol. Infect. 2008, 136, 865. [Google Scholar] [CrossRef]
- Kemenkes, R.I. Panduan Penatalaksanaan Kanker Payudara [Internet]. 2018, pp. 7–11. Available online: http://kanker.kemkes.go.id/guidelines/PPKPayudara.pdf (accessed on 18 May 2021).
- Kikuchi, S.; Nishimura, R.; Osako, T.; Okumura, Y.; Nishiyama, Y.; Toyozumi, Y.; Arima, N. Definition of p53 overexpression and its association with the clinicopathological features in luminal/HER2-negative breast cancer. Anticancer Res. 2013, 33, 3891–3898. [Google Scholar] [PubMed]
- Yamashita, H.; Toyama, T.; Nishio, M.; Ando, Y.; Hamaguchi, M.; Zhang, Z.; Kobayashi, S.; Fujii, Y.; Iwase, H. P53 Protein Accumulation Predicts Resistance To Endocrine Therapy and Decreased Post-Relapse Survival in Metastatic Breast Cancer. Breast Cancer Res. 2006, 8, R48. [Google Scholar] [PubMed]
- Ades, F.; Zardavas, D.; Bozovic-Spasojevic, I.; Pugliano, L.; Fumagalli, D.; De Azambuja, E.; Viale, G.; Sotiriou, C.; Piccart, M. Luminal B breast cancer: Molecular characterization, clinical management, and future perspectives. J. Clin. Oncol. 2014, 32, 2794–2803. [Google Scholar] [CrossRef]
- Nelson, D.J.; Clark, B.; Munyard, K.; Williams, V.; Groth, D.; Gill, J.; Preston, H.; Chan, A. A review of the importance of immune responses in luminal B breast cancer. Oncoimmunology 2017, 6, e1282590. [Google Scholar] [CrossRef]
- Yang, Z.J.; Liu, Y.X.; Huang, Y.; Chen, Z.J.; Zhang, H.Z.; Yu, Y.; Wang, X.; Cao, X.C. The regrouping of Luminal B (HER2 negative), a better discriminator of outcome and recurrence score. Cancer Med. 2023, 12, 2493–2504. [Google Scholar] [CrossRef]
- Lei, J.T.; Anurag, M.; Haricharan, S.; Gou, X.; Ellis, M.J. Endocrine therapy resistance: New insights. Breast 2019, 48 (Suppl. 1), S26–S30. [Google Scholar] [CrossRef]
- Rasha, F.; Sharma, M.; Pruitt, K. Mechanisms of endocrine therapy resistance in breast cancer. Mol. Cell. Endocrinol. 2021, 532, 111322. [Google Scholar] [CrossRef]
- Wang, L.W.; Li, L.; Zhang, H.Y.; Chen, Y.Y.; Zhong, Y.H. Patterns of chest wall recurrence and suggestions on the clinical target volume of breast cancer: A retrospective analysis of 121 postmastectomy patients. Cancer Manag. Res. 2020, 12, 5909–5918. [Google Scholar] [CrossRef]
- Patel, R.; Klein, P.; Tiersten, A.; Sparano, J.A. An emerging generation of endocrine therapies in breast cancer: A clinical perspective. Npj Breast Cancer 2023, 9, 20. [Google Scholar] [CrossRef]
- Zattari, E.; Leporati, R.; Ligorio, F.; Vingiani, A.; Pruneri, G.; Vernieri, C. Hormone Receptor Loss in Breast Cancer: Molecular Mechanisms, Clinical Settings, and Therapeutic Implications. Cells 2020, 9, 2644. [Google Scholar] [CrossRef]
- Szostakowska, M.; Trębińska-Stryjewska, A.; Grzybowska, E.A.; Fabisiewicz, A. Resistance to endocrine therapy in breast cancer: Molecular mechanisms and future goals. Breast Cancer Res. Treat. 2019, 173, 489–497. [Google Scholar] [CrossRef]
- Anurag, M.; Punturi, N.; Hoog, J.; Bainbridge, M.N.; Ellis, M.J.; Haricharan, S. Comprehensive profiling of DNA repair defects in breast cancer identifies a novel class of endocrine therapy resistance drivers. Clin. Cancer Res. 2018, 24, 4887–4899. [Google Scholar] [CrossRef]
- Belachew, E.B.; Sewasew, D.T. Molecular Mechanisms of Endocrine Resistance in Estrogen-Positive Breast Cancer. Front. Endocrinol. 2021, 12, 599586. [Google Scholar] [CrossRef] [PubMed]
- Sadighi, S.; Zokaasadi, M.; Kasaeian, A.; Maghsudi, S.; Jahanzad, I.; Kamranzadeh Fumani, H. The effect of immunohistochemically detected p53 accumulation in prognosis of breast cancer; a retrospective survey of outcome. PLoS ONE 2017, 12, e0182444. [Google Scholar] [CrossRef]
- Yamamoto, M.; Hosoda, M.; Nakano, K.; Jia, S.; Hatanaka, K.C.; Takakuwa, E.; Hatanaka, Y.; Matsuno, Y.; Yamashita, H. P53 accumulation is a strong predictor of recurrence in estrogen receptor-positive breast cancer patients treated with aromatase inhibitors. Cancer Sci. 2014, 105, 81–88. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Ju, J.; Guo, L.; Ji, B.; Shi, S.; Yang, Z.; Gao, S.; Yuan, X.; Tian, G.; Liang, Y.; et al. Prediction of HER2-positive breast cancer recurrence and metastasis risk from histopathological images and clinical information via multimodal deep learning. Comput. Struct. Biotechnol. J. 2022, 20, 333–342. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.B.; Park, J.M.; Ahn, J.H.; Go, J.; Kim, J.; Park, H.S.; Kim, S.I.; Park, B.W.; Park, S. Ki-67 and breast cancer prognosis: Does it matter if Ki-67 level is examined using preoperative biopsy or post-operative specimen? Breast Cancer Res. Treat. 2022, 192, 343–352. [Google Scholar] [CrossRef]
- McHugh, M.L. Lessons in biostatistics interrater reliability: The kappa statistic. Biochem. Med. 2012, 22, 276–282. [Google Scholar] [CrossRef]
- Lorincz, A.M.; Sukumar, S. Molecular links between obesity and breast cancer. Endocr.-Relat. Cancer 2006, 13, 279–292. [Google Scholar] [CrossRef]
- Takahashi, H.; Watanabe, K.; Takahashi, M.; Taguchi, K.; Sasaki, F.; Todo, S. The impact of bilateral breast cancer on the prognosis of breast cancer: A comparative study with unilateral breast cancer. Breast Cancer 2005, 12, 196–202. [Google Scholar] [CrossRef]
- Betrucci, F.; Ng, C.K.Y.; Patsouris, A.; Droin, N.; Piscuoglio, S.; Carbuccia, N.; Soria, J.C.; Dien, A.T.; Adnani, Y.; Kamal, M.; et al. Genomic characterization of metastatic breast cancers. Nature 2019, 569, 560–564. [Google Scholar]
- Lee, H.B.; Han, W. Unique features of young age breast cancer and its management. J. Breast Cancer 2014, 17, 301–307. [Google Scholar] [CrossRef]
- Sleightholm, R.; Neilsen, B.K.; Elkhatib, S.; Flores, L.; Dukkipati, S.; Zhao, R.; Choudhury, S.; Gardner, B.; Carmichael, J.; Smith, L.; et al. Percentage of Hormone Receptor Positivity in Breast Cancer Provides Prognostic Value: A Single-Institute Study. J. Clin. Med. Res. 2021, 13, 9–19. [Google Scholar] [CrossRef]
- Allison, K.H.; Hammond, M.E.; Dowsett, M.; McKernin, S.E.; Carey, L.A.; Fitzgibbons, P.L.; Hayes, D.F.; Lakhani, S.R.; Chavez-MacGregor, M.; Perlmutter, J.; et al. Estrogen and progesterone receptor testing in breast cancer: ASCO/CAP guideline update. J. Clin. Oncol. 2020, 38, 1346–1366. [Google Scholar] [CrossRef]
- Thota, K.; Prasad, K.; Rao, M.V.B. Detection of cytochrome p450 polymorphisms in breast cancer patients may impact on tamoxifen therapy. Asian Pac. J. Cancer Prev. 2018, 19, 343–350. [Google Scholar] [PubMed]
- Nik-Zainal, S.; Davies, H.; Staaf, J.; Ramakrishna, M.; Glodzik, D.; Zou, X.; Martincorena, I.; Alexandrov, L.B.; Martin, S.; Wedge, D.C.; et al. Landscape of somatic mutations in 560 breast cancer whole genome sequences. Nature 2016, 534, 47–54. [Google Scholar] [CrossRef]
- Bischof, K.; Knappskog, S.; Hjelle, S.M.; Stefansson, I.; Woie, K.; Salvesen, H.B.; Gjertsen, B.T.; Bjorge, L. Influence of p53 Isoform Expression on Survival in High-Grade Serous Ovarian Cancers. Sci. Rep. 2019, 9, 5244. [Google Scholar] [CrossRef]
- Rivlin, N.; Brosh, R.; Oren, M.; Rotter, V. Mutations in the p53 tumor suppressor gene: Important milestones at the various steps of tumorigenesis. Genes Cancer 2011, 2, 466–474. [Google Scholar] [CrossRef]
- Dieci, M.V.; Griguolo, G.; Miglietta, F.; Guarneri, V. The immune system and hormone-receptor positive breast cancer: Is it really a dead end? Cancer Treat. Rev. 2016, 46, 9–19. [Google Scholar] [CrossRef]
- Khongthong, P.; Roseweir, A.K.; Edwards, J. The NF-KB pathway and endocrine therapy resistance in breast cancer. Endocr. Relat. Cancer 2019, 26, R369–R380. [Google Scholar] [CrossRef]
- Conciatori, F.; Bazzichetto, C.; Falcone, I.; Pilotto, S.; Bria, E.; Cognetti, F.; Milella, M.; Ciuffreda, L. Role of mTOR signaling in tumor microenvironment: An overview. Int. J. Mol. Sci. 2018, 19, 2453. [Google Scholar] [CrossRef]
- Chapman, N.M.; Chi, H. mTOR links environmental signals to T cell fate decisions. Front. Immunol. 2015, 5, 686. [Google Scholar] [CrossRef]
- Thorsson, V.; Gibbs, D.L.; Brown, S.D.; Wolf, D.; Bortone, D.S.; Yang, T.H.; Porta-Pardo, E.; Gao, G.F.; Plaisier, C.L.; Eddy, J.A.; et al. The Immune Landscape of Cancer. Immunity 2018, 48, 812–830.e14. [Google Scholar] [CrossRef] [PubMed]
- Goldberg, J.; Pastorello, R.G.; Vallius, T.; Davis, J.; Cui, Y.X.; Agudo, J.; Waks, A.G.; Keenan, T.; McAllister, S.S.; Tolaney, S.M.; et al. The Immunology of Hormone Receptor Positive Breast Cancer. Front. Immunol. 2021, 12, 674192. [Google Scholar] [CrossRef]
- Pellegrino, B.; Hlavata, Z.; Migali, C.; De Silva, P.; Aiello, M.; Willard-Gallo, K.; Musolino, A.; Solinas, C. Luminal Breast Cancer: Risk of Recurrence and Tumor-Associated Immune Suppression. Mol. Diagn. Ther. 2021, 25, 409–424. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Jiang, Z.; Gao, Y.; Wang, L.; Chen, C.; Wang, X. TP53 Mutations Promote Immunogenic Activity in Breast Cancer. J. Oncol. 2019, 2019, 5952836. [Google Scholar] [CrossRef]
- Guo, G.; Cui, Y. New perspective on targeting the tumor suppressor p53 pathway in the tumor microenvironment to enhance the efficacy of immunotherapy. J. Immunother. Cancer 2015, 3, 9. [Google Scholar] [CrossRef]
| Variable | Total Sample = 67 | With Primary ET Resistance n = 29 (44%) | Without Primary ET Resistance n = 38 (56%) | p-Value | |
|---|---|---|---|---|---|
| n | % | n (%) | n (%) | ||
| Age (year) | |||||
| <35 | 4 | 5.97 | 3 (75) | 1 (25) | 0.187 |
| ≥35 | 63 | 94.03 | 26 (41.27) | 37 (58.73) | |
| ER status | |||||
| Negative (<1%) | 0 | 0 | 0 | 0 | 0.685 |
| 1–20% | 6 | 8.96 | 3 (50) | 3 (50) | |
| 20–50% | 11 | 16.42 | 3 (27.27) | 8 (72.73) | |
| 50–80% | 14 | 20.9 | 6 (42.86) | 8 (57.14) | |
| ≥80 | 36 | 53.73 | 17 (47.22) | 19 (52.78) | |
| Progesterone Receptor Status | |||||
| Positive | 63 | 94.03 | 26 (41.27) | 37 (58.73) | 0.187 |
| Negative | 4 | 5.97 | 3 (75) | 1 (25) | |
| Stage | |||||
| Stage IIIa | 26 | 40 | 14 (53.85) | 12 (46.15) | 0.152 |
| Stage IIIb | 38 | 58.46 | 13 (34.21) | 25 (65.79) | |
| Stage IIIc | 1 | 1.54 | 1 (100) | 0 (0) | |
| Histopathological Grade | |||||
| I | 1 | 1,49 | 0 (0) | 1 (100) | 0.265 |
| II | 29 | 43.28 | 10 (34.48) | 19 (65.52) | |
| III | 37 | 55.22 | 19 (51.35) | 18 (48.65) | |
| Neo-adjuvant chemotherapy | |||||
| Taxane based | 10 | 14.93 | 5 (50) | 5 (50) | 0.061 |
| Doxorubicin based | 47 | 70.15 | 16 (34.04) | 31 (65.96) | |
| Taxan and doxorubicin combination | 6 | 8.96 | 5 (83.33) | 1 (16.67) | |
| Taxan and platinum combination | 4 | 5.97 | 3 (75) | 1 (25) | |
| Radiotherapy | |||||
| Yes | 7 | 10.45 | 4 (57.14) | 3 (42.86) | 0.457 |
| No | 60 | 89.55 | 25 (41.67) | 35 (58.33) | |
| Endocrine Therapy | |||||
| SERM | 26 | 38.81 | 7 (26.92) | 19 (73.08) | 0.296 |
| Aromatase inhibitor | 19 | 28.36 | 10 (52.63) | 9 (47.37) | |
| SERD | 1 | 1.49 | 1 (100) | 0 (0) | |
| SERM, aromatase inhibitor | 5 | 7.46 | 2 (40) | 3 (60) | |
| LHRH/GnRH agonist, SERM | 16 | 22.88 | 9 (56.25) | 7 (43.75) | |
| Variable | Total Sample = 67 | With Primary ET Resistance n = 29 (44%) | Without Primary ET Resistance n = 38 (56%) | p-Value | OR | 95% CI |
|---|---|---|---|---|---|---|
| n (%) | n (%) | n (%) | ||||
| p53 expression | ||||||
| Positive expression | 37 (55.22) | 22 (73.33) | 8 (26.67) | <0.0001 | 11.78 | 3.72–37.37 |
| Negative expression | 30 (45.45) | 7 (18.92) | 30 (81.08) | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Halim, F.; Azhar, Y.; Suwarman, S.; Wahjoepramono, E.J.; Hernowo, B. Positive p53 Expression Is Associated with Primary Endocrine Therapy Resistance in Locally Advanced Stage Luminal B HER2-Negative Breast Cancer Patients: A Cross-Sectional Study in Indonesia. Diagnostics 2023, 13, 1838. https://doi.org/10.3390/diagnostics13111838
Halim F, Azhar Y, Suwarman S, Wahjoepramono EJ, Hernowo B. Positive p53 Expression Is Associated with Primary Endocrine Therapy Resistance in Locally Advanced Stage Luminal B HER2-Negative Breast Cancer Patients: A Cross-Sectional Study in Indonesia. Diagnostics. 2023; 13(11):1838. https://doi.org/10.3390/diagnostics13111838
Chicago/Turabian StyleHalim, Freda, Yohana Azhar, Suwarman Suwarman, Eka Julianta Wahjoepramono, and Bethy Hernowo. 2023. "Positive p53 Expression Is Associated with Primary Endocrine Therapy Resistance in Locally Advanced Stage Luminal B HER2-Negative Breast Cancer Patients: A Cross-Sectional Study in Indonesia" Diagnostics 13, no. 11: 1838. https://doi.org/10.3390/diagnostics13111838





