Exploring the Potential Driver Gene Mutations That Promote Renal Cancer Cell Metastasis and Implantation Based on Circulating Tumor Cells Culture
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patients and Healthy Controls
2.2. Preparation of Synthetic Biological Scaffold
2.3. Culture of Peripheral Blood CTCs of RCC Patients
2.4. Handling of Blood from Healthy Subjects
2.5. Application of Cultured CTCs to Construct CDX Models
2.6. DNA Extraction, Whole Exome Sequencing and Analysis
3. Results
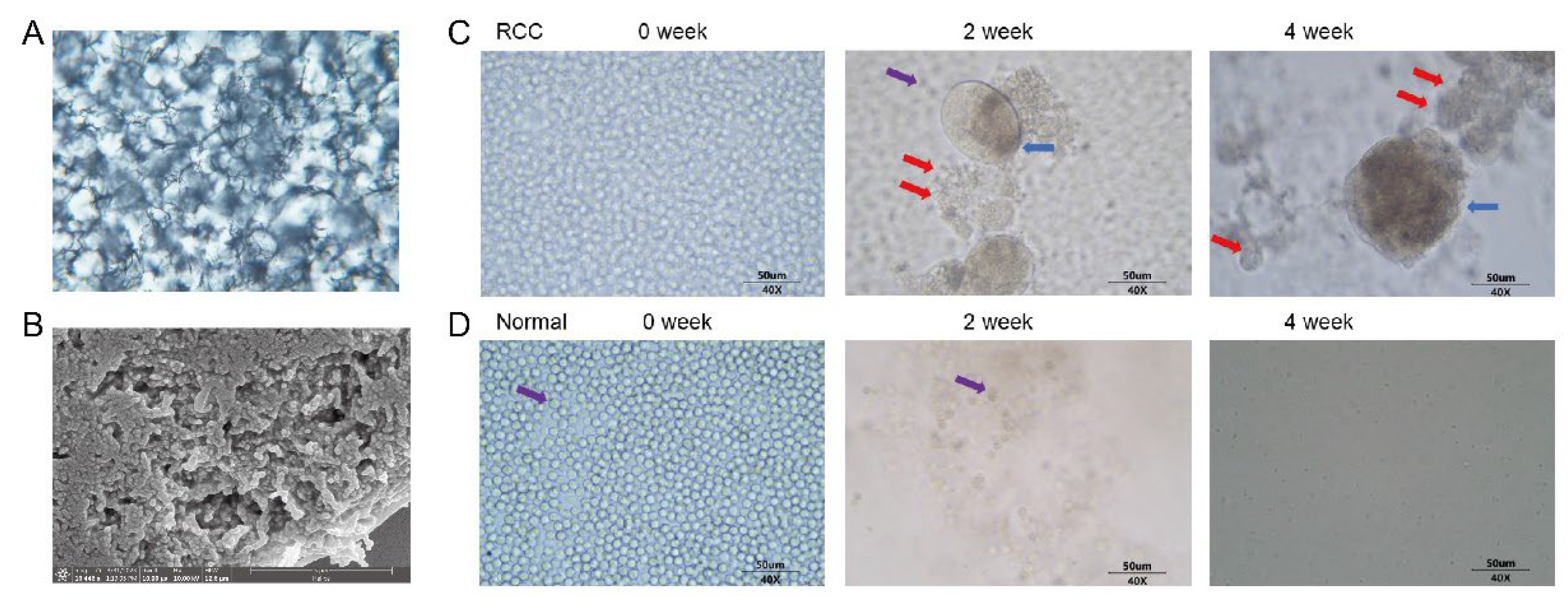
3.1. Successful Preparation of Synthetic Biological Scaffolds
3.2. Proliferation of Peripheral Blood CTCs in mRCC Patients and Healthy Subjects
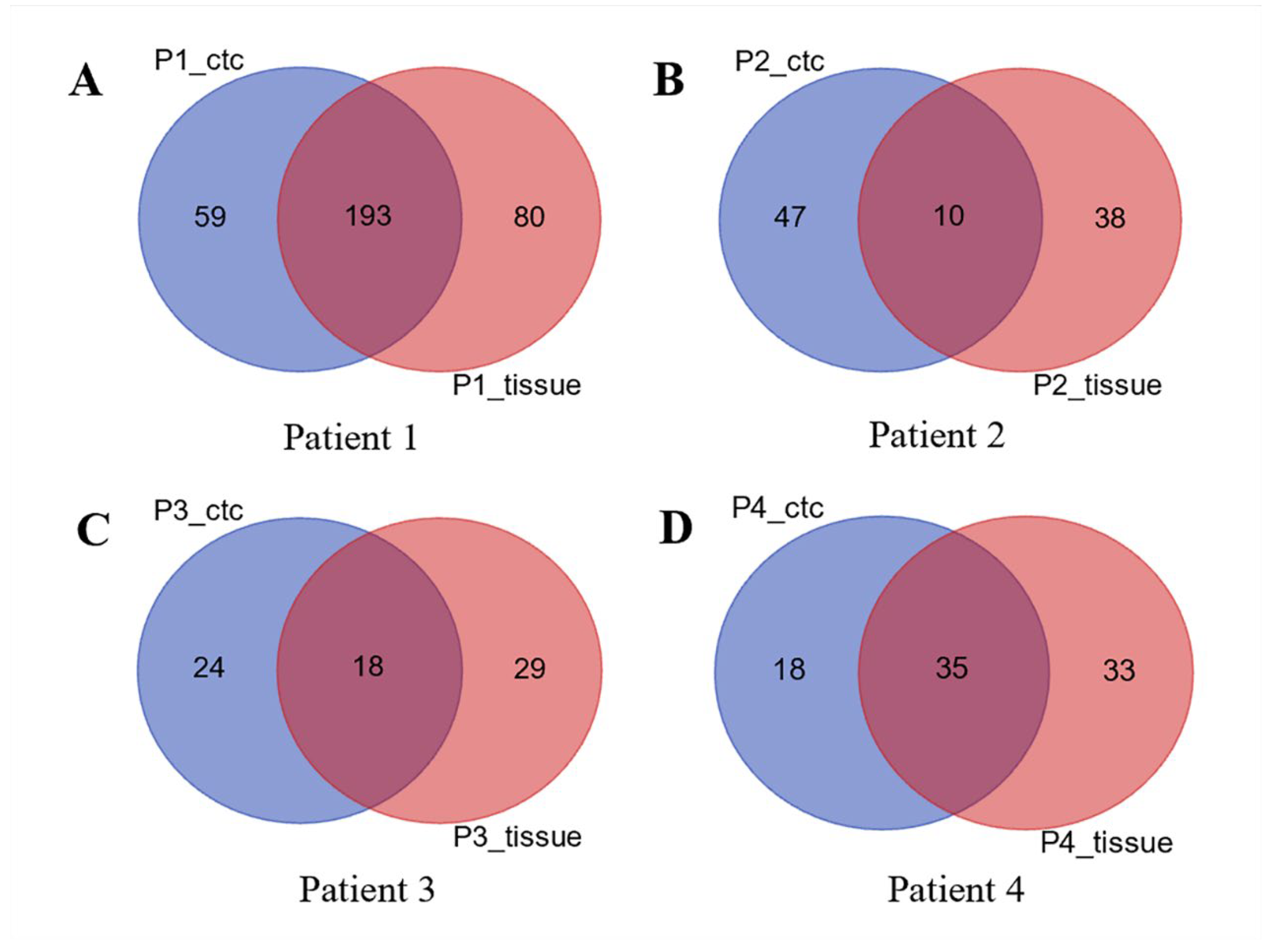
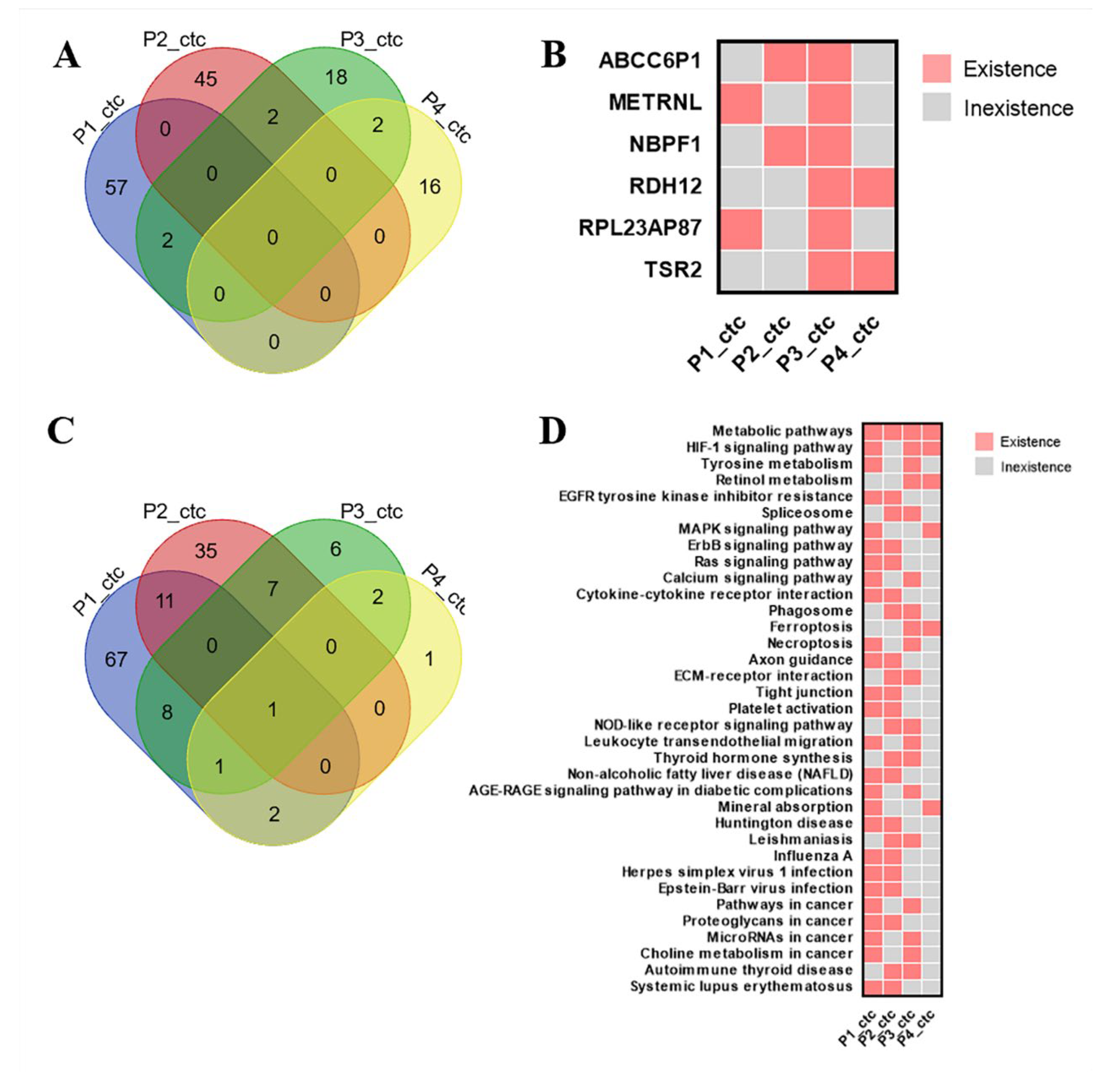
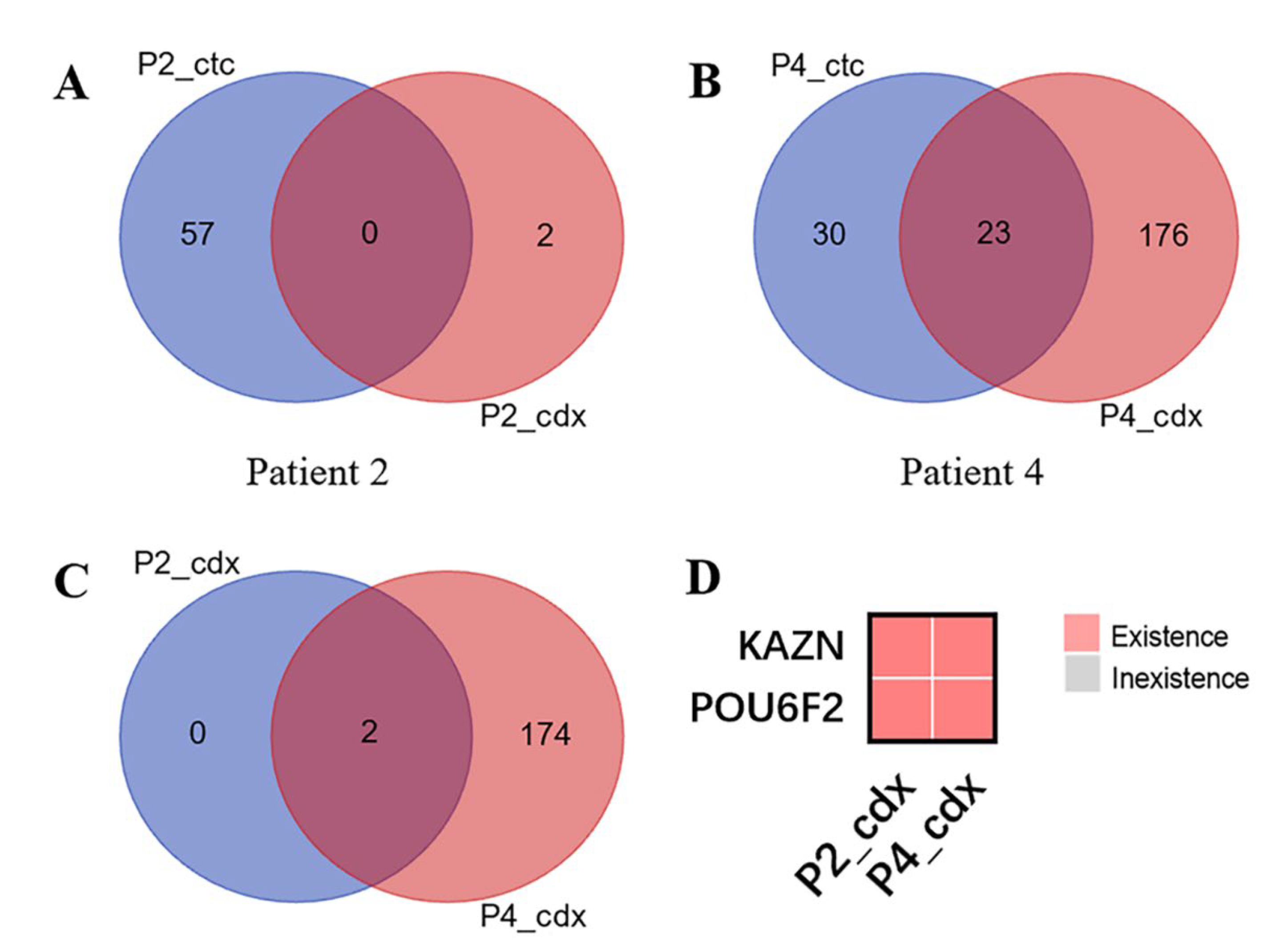
3.3. CDX Models Construction and Whole Exome Sequencing
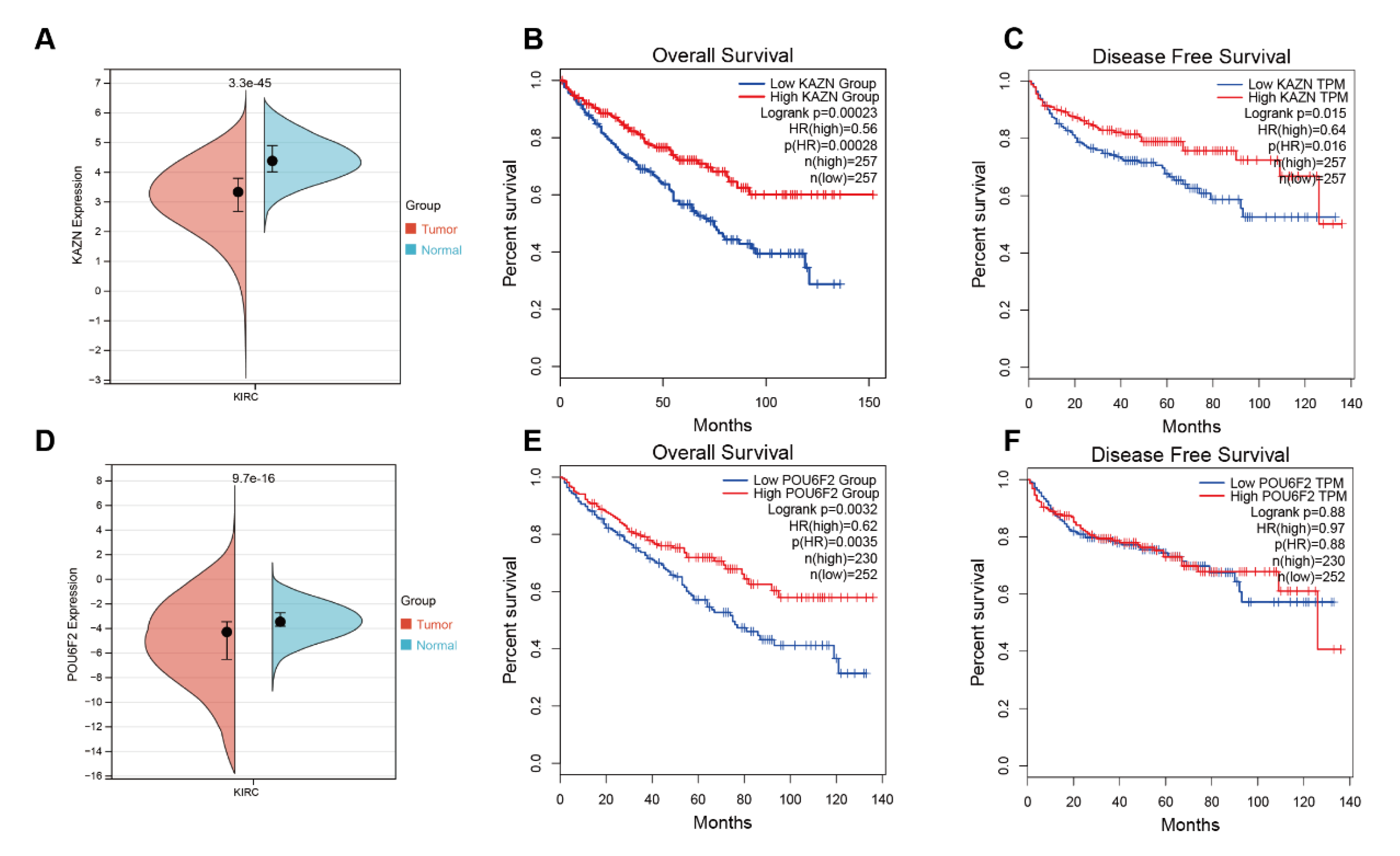
3.4. Bioinformatics Analysis Results of KAZN and POU6F2
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Wagle, N.S.; Jemal, A. Cancer statistics, 2023. CA Cancer J. Clin. 2023, 73, 17–48. [Google Scholar] [CrossRef]
- Klatte, T.; Rossi, S.H.; Stewart, G.D. Prognostic factors and prognostic models for renal cell carcinoma: A literature review. World J. Urol. 2018, 36, 1943–1952. [Google Scholar] [CrossRef] [PubMed]
- Psutka, S.P.; Heidenreich, M.; Boorjian, S.A.; Bailey, G.C.; Cheville, J.C.; Stewart-Merrill, S.B.; Lohse, C.M.; Atwell, T.D.; Costello, B.A.; Leibovich, B.C.; et al. Renal fossa recurrence after nephrectomy for renal cell carcinoma: Prognostic features and oncological outcomes. BJU Int. 2017, 119, 116–127. [Google Scholar] [CrossRef] [PubMed]
- Herout, R.; Graff, J.; Borkowetz, A.; Zastrow, S.; Leike, S.; Koch, R.; Draeger, D.-L.; Protzel, C.; Hakenberg, O.W.; Wirth, M.P.; et al. Surgical Resection of Locally Recurrent Renal Cell Carcinoma after Nephrectomy: Oncological Outcome and Predictors of Survival. In Urologic Oncology: Seminars and Original Investigations; Elsevier: Amsterdam, The Netherlands, 2018; Volume 36, pp. 11.e1–11.e6. [Google Scholar]
- Ljungberg, B.; Albiges, L.; Abu-Ghanem, Y.; Bedke, J.; Capitanio, U.; Dabestani, S.; Fernández-Pello, S.; Giles, R.H.; Hofmann, F.; Hora, M. European Association of Urology Guidelines on Renal Cell Carcinoma: The 2022 Update. Eur. Urol. 2022, 82, 399–410. [Google Scholar] [CrossRef] [PubMed]
- Kotecha, R.R.; Motzer, R.J.; Voss, M.H. Towards individualized therapy for metastatic renal cell carcinoma. Nat. Rev. Clin. Oncol. 2019, 16, 621–633. [Google Scholar] [CrossRef]
- Bedke, J.; Albiges, L.; Capitanio, U.; Giles, R.H.; Hora, M.; Ljungberg, B.; Marconi, L.; Klatte, T.; Volpe, A.; Abu-Ghanem, Y. The 2022 Updated European Association of Urology Guidelines on the Use of Adjuvant Immune Checkpoint Inhibitor Therapy for Renal Cell Carcinoma. Eur. Urol. 2023, 83, 10–14. [Google Scholar] [CrossRef]
- Stukalin, I.; Alimohamed, N.; Heng, D.Y.C. Contemporary treatment of metastatic renal cell carcinoma. Oncol. Rev. 2016, 10, 295. [Google Scholar] [CrossRef]
- Bu, J.; Nair, A.; Kubiatowicz, L.J.; Poellmann, M.J.; Jeong, W.-J.; Reyes-Martinez, M.; Armstrong, A.J.; George, D.J.; Wang, A.Z.; Zhang, T. Surface engineering for efficient capture of circulating tumor cells in renal cell carcinoma: From nanoscale analysis to clinical application. Biosens. Bioelectron. 2020, 162, 112250. [Google Scholar] [CrossRef]
- Bade, R.M.; Schehr, J.L.; Emamekhoo, H.; Gibbs, B.K.; Rodems, T.S.; Mannino, M.C.; Desotelle, J.A.; Heninger, E.; Stahlfeld, C.N.; Sperger, J.M.; et al. Development and initial clinical testing of a multiplexed circulating tumor cell assay in patients with clear cell renal cell carcinoma. Mol. Oncol. 2021, 15, 2330–2344. [Google Scholar] [CrossRef]
- Leitão, T.P.; Miranda, M.; Polido, J.; Morais, J.; Corredeira, P.; Alves, P.; Oliveira, T.; Silva, R.P.E.; Fernandes, R.; Ferreira, J.; et al. Circulating tumor cell detection methods in renal cell carcinoma: A systematic review. Crit. Rev. Oncol./Hematol. 2021, 161, 103331. [Google Scholar] [CrossRef]
- Baccelli, I.; Schneeweiss, A.; Riethdorf, S.; Stenzinger, A.; Schillert, A.; Vogel, V.; Klein, C.; Saini, M.; Bäuerle, T.; Wallwiener, M.; et al. Identification of a population of blood circulating tumor cells from breast cancer patients that initiates metastasis in a xenograft assay. Nat. Biotechnol. 2013, 31, 539–544. [Google Scholar] [CrossRef]
- Hou, J.M.; Krebs, M.; Ward, T.; Sloane, R.; Priest, L.; Hughes, A.; Clack, G.; Ranson, M.; Blackhall, F.; Dive, C. Circulating tumor cells as a window on metastasis biology in lung cancer. Am. J. Pathol. 2011, 178, 989–996. [Google Scholar] [CrossRef]
- Murlidhar, V.; Rivera-Báez, L.; Nagrath, S. Affinity Versus Label-Free Isolation of Circulating Tumor Cells: Who Wins? Small 2016, 12, 4450–4463. [Google Scholar] [CrossRef]
- Swami, U.; Nussenzveig, R.H.; Haaland, B.; Agarwal, N. Revisiting AJCC TNM staging for renal cell carcinoma: Quest for improvement. Ann. Transl. Med. 2019, 7 (Suppl. S1), S18. [Google Scholar] [CrossRef]
- Cheng, S.B.; Xie, M.; Xu, J.Q.; Wang, J.; Lv, S.-W.; Guo, S.; Shu, Y.; Wang, M.; Dong, W.-G.; Huang, W.-H. High-efficiency capture of individual and cluster of circulating tumor cells by a microchip embedded with three-dimensional poly (dimethylsiloxane) scaffold. Anal. Chem. 2016, 88, 6773–6780. [Google Scholar] [CrossRef]
- Cheng, S.B.; Xie, M.; Chen, Y.; Xiong, J.; Liu, Y.; Chen, Z.; Guo, S.; Shu, Y.; Wang, M.; Yuan, B.-F.; et al. Three-dimensional scaffold chip with thermosensitive coating for capture and reversible release of individual and cluster of circulating tumor cells. Anal. Chem. 2017, 89, 7924–7932. [Google Scholar] [CrossRef]
- Mitchell, J.W.; Yang, D. Microcarriers, Matrices and Scaffolds for Culturing Mammalian Cells and Methods of Manufacture. U.S. Patent 10,723,809, 28 July 2020. [Google Scholar]
- Cimadamore, A.; Gasparrini, S.; Massari, F.; Santoni, M.; Cheng, L.; Lopez-Beltran, A.; Scarpelli, M.; Montironi, R. Emerging molecular technologies in renal cell carcinoma: Liquid biopsy. Cancers 2019, 11, 196. [Google Scholar] [CrossRef]
- Ashworth, T.R. A case of cancer in which cells similar to those in the tumours were seen in the blood after death. Aust. Med. J. 1869, 14, 146. [Google Scholar]
- Pan, H.; Gray, R.; Braybrooke, J.; Davies, C.; Taylor, C.; McGale, P.; Peto, R.; Pritchard, K.I.; Bergh, J.; Dowsett, M. 20-year risks of breast-cancer recurrence after stopping endocrine therapy at 5 years. N. Engl. J. Med. 2017, 377, 1836–1846. [Google Scholar] [CrossRef]
- Cheng, M.L.; Pectasides, E.; Hanna, G.J.; Parsons, H.A.; Choudhury, A.D.; Oxnard, G.R. Circulating tumor DNA in advanced solid tumors: Clinical relevance and future directions. CA A Cancer J. Clin. 2021, 71, 176–190. [Google Scholar] [CrossRef]
- Su, Z.; Wang, Z.; Ni, X.; Duan, J.; Gao, Y.; Zhuo, M.; Li, R.; Zhao, J.; Ma, Q.; Bai, H.; et al. Inferring the evolution and progression of small-cell lung cancer by single-cell sequencing of circulating tumor cells. Clin. Cancer Res. 2019, 25, 5049–5060. [Google Scholar] [CrossRef] [PubMed]
- Keller, L.; Pantel, K. Unravelling tumour heterogeneity by single-cell profiling of circulating tumour cells. Nat. Rev. Cancer 2019, 19, 553–567. [Google Scholar] [CrossRef] [PubMed]
- Sperger, J.M.; Emamekhoo, H.; McKay, R.R.; Stahlfeld, C.N.; Singh, A.; Chen, X.E.; Kwak, L.; Gilsdorf, C.S.; Wolfe, S.K.; Wei, X.X.; et al. Prospective evaluation of clinical outcomes using a multiplex liquid biopsy targeting diverse resistance mechanisms in metastatic prostate cancer. J. Clin. Oncol. 2021, 39, 2926–2937. [Google Scholar] [CrossRef] [PubMed]
- Sperger, J.M.; Emamekhoo, H.; McKay, R.R.; Stahlfeld, C.N.; Singh, A.; Chen, X.E.; Kwak, L.; Gilsdorf, C.S.; Wolfe, S.K.; Wei, X.X.; et al. Circulating tumor cells are an independent predictor of shorter survival in patients undergoing resection for pancreatic and periampullary adenocarcinoma. Ann. Surg. 2020, 271, 549–558. [Google Scholar]
- Thanh Huong, P.; Gurshaney, S.; Thanh Binh, N.; Pham, A.G.; Nguyen, H.H.; Nguyen, X.T.; Pham-The, H.; Tran, P.-T.; Vu, K.T.; Duong, N.X.; et al. Emerging role of circulating tumor cells in gastric cancer. Cancers 2020, 12, 695. [Google Scholar] [CrossRef]
- Kolenčík, D.; Shishido, S.N.; Pitule, P.; Mason, J.; Hicks, J.; Kuhn, P. Liquid biopsy in colorectal carcinoma: Clinical applications and challenges. Cancers 2020, 12, 1376. [Google Scholar] [CrossRef]
- Piñeiro, R.; Martínez-Pena, I.; López-López, R. Relevance of CTC clusters in breast cancer metastasis. Circ. Tumor Cells Breast Cancer Metastatic Dis. 2020, 1220, 93–115. [Google Scholar]
- Xiao, W.; Ren, L.; Chen, Z.; Fang, L.T.; Zhao, Y.; Lack, J.; Guan, M.; Zhu, B.; Jaeger, E.; Kerrigan, L. Toward best practice in cancer mutation detection with whole-genome and whole-exome sequencing. Nat. Biotechnol. 2021, 39, 1141–1150. [Google Scholar] [CrossRef]
- Li, L.; Chen, S.; Tang, Y.; Wu, J.; He, Y.; Qiu, L. Oncogene or tumor suppressor gene: An integrated pan-cancer analysis of NBPF1. Front. Endocrinol. 2022, 13, 950326. [Google Scholar] [CrossRef]
- Rhodes, D.R.; Kalyana-Sundaram, S.; Mahavisno, V.; Varambally, R.; Yu, J.; Briggs, B.B.; Barrette, T.R.; Anstet, M.J.; Kincead-Beal, C.; Kulkarni, P.; et al. Oncomine 3.0: Genes, pathways, and networks in a collection of 18,000 cancer gene expression profiles. Neoplasia 2007, 9, 166–180. [Google Scholar] [CrossRef]
- Qin, Y.; Tang, X.; Liu, M. Tumor-suppressor gene NBPF1 inhibits invasion and PI3K/mTOR signaling in cervical cancer cells. Oncol. Res. 2016, 23, 13. [Google Scholar] [CrossRef]
- Gao, Y.; Zhu, H.; Mao, Q. Effects of neuroblastoma breakpoint family member 1 (NBPF1) gene on Akt-p53-Cyclin D pathway and growth of cutaneous squamous carcinoma cells. Neoplasma 2019, 66, 584–592. [Google Scholar] [CrossRef]
- Wu, L.; Wei, Y.; Zhou, W.B.; Zhang, S.; Chen, H.; Liu, X.; Zhu, P.; Zhou, J.; Yang, H.; Wang, M.; et al. Gene expression alterations of human liver cancer cells following borax exposure. Oncol. Rep. 2019, 42, 115–130. [Google Scholar] [CrossRef]
- Groot, K.R.; Sevilla, L.M.; Nishi, K.; Di Colandrea, T.; Watt, F.M. Kazrin, a novel periplakin-interacting protein associated with desmosomes and the keratinocyte plasma membrane. J. Cell Biol. 2004, 166, 653–659. [Google Scholar] [CrossRef]
- Sevilla, L.M.; Nachat, R.; Groot, K.R.; Watt, F.M. Kazrin regulates keratinocyte cytoskeletal networks, intercellular junctions and differentiation. J. Cell Sci. 2008, 121, 3561–3569. [Google Scholar] [CrossRef]
- Zhu, S.; Bao, H.; Zhang, M.-C.; Liu, H.; Wang, Y.; Lin, C.; Zhao, X.; Liu, S.-L. KAZN as a diagnostic marker in ovarian cancer: A comprehensive analysis based on microarray, mRNA-sequencing, and methylation data. BMC Cancer 2022, 22, 662. [Google Scholar] [CrossRef]
- Perotti, D.; De Vecchi, G.; Testi, M.A.; Lualdi, E.; Modena, P.; Mondini, P.; Ravagnani, F.; Collini, P.; Di Renzo, F.; Spreafico, F. Germline mutations of the POU6F2 gene in Wilms tumors with loss of heterozygosity on chromosome 7p14. Hum. Mutat. 2004, 24, 400–407. [Google Scholar] [CrossRef]
- Miao, Y.; Li, C.; Guo, J.; Wang, H.; Gong, L.; Xie, W.; Zhang, Y. Identification of a novel somatic mutation of POU6F2 by whole-genome sequencing in prolactinoma. Mol. Genet. Genom. Med. 2019, 7, e1022. [Google Scholar] [CrossRef]

| Patient ID | Age | Gender | TNM Staging | IMDC Score * | Grade | Culture Outcome |
|---|---|---|---|---|---|---|
| Patient-01 | 57 | male | T4N0M1 | 0 | 2 | fail |
| Patient-02 | 61 | male | T3N0M1 | 1 | 2 | fail |
| Patient-03 | 45 | male | T2N0M1 | 1 | 1 | fail |
| Patient-04 | 58 | male | T3N0M1 | 1 | 2 | succeed |
| Patient-05 | 50 | male | T3N0M1 | 1 | 3 | succeed |
| Patient-06 | 67 | male | T1N1M1 | 1 | 2 | fail |
| Patient-07 | 48 | male | T4N1M1 | 3 | 2 | succeed |
| Patient-08 | 73 | male | T2N0M1 | 1 | 4 | succeed |
| Patient-09 | 63 | male | TxN1M1 | 1 | 3 | succeed |
| Patient-10 | 70 | male | T3N1M1 | 1 | 2 | succeed |
| Patient-11 | 55 | male | TxN0M1 | 1 | 3 | succeed |
| Patient-12 | 54 | male | T4N1M1 | 2 | 2 | fail |
| Patient-13 | 73 | male | T3N0M1 | 1 | 4 | succeed |
| Patient-14 | 48 | male | T1aN1M1 | 1 | 4 | succeed |
| Patient-15 | 50 | male | T3N0M1 | 0 | 2 | succeed |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hong, B.; Zhang, X.; Du, X.; Yang, D.; Hu, Z.; Zhang, X.; Zhang, N. Exploring the Potential Driver Gene Mutations That Promote Renal Cancer Cell Metastasis and Implantation Based on Circulating Tumor Cells Culture. Diagnostics 2023, 13, 1855. https://doi.org/10.3390/diagnostics13111855
Hong B, Zhang X, Du X, Yang D, Hu Z, Zhang X, Zhang N. Exploring the Potential Driver Gene Mutations That Promote Renal Cancer Cell Metastasis and Implantation Based on Circulating Tumor Cells Culture. Diagnostics. 2023; 13(11):1855. https://doi.org/10.3390/diagnostics13111855
Chicago/Turabian StyleHong, Baoan, Xuezhou Zhang, Xin Du, Dazhi Yang, Zhiyuan Hu, Xiuli Zhang, and Ning Zhang. 2023. "Exploring the Potential Driver Gene Mutations That Promote Renal Cancer Cell Metastasis and Implantation Based on Circulating Tumor Cells Culture" Diagnostics 13, no. 11: 1855. https://doi.org/10.3390/diagnostics13111855




